Abstract
The limits of placental plasticity, i.e., the ability of the placenta to adapt and alter its growth trajectory in response to altered fetal requirements, are not known. We report fetal and placental hemodynamic adaptations in a novel non-human primate model in which the fetal inter-placental bridging vessels were surgically ligated. Doppler ultrasound studies showed that the rhesus placenta compensates for an approximate 40% reduction in functional capacity by increased growth and maintenance of umbilical volume blood flow. This unique experimental animal model has applications for mechanistic studies of placental plasticity and the impact on fetal development.
Introduction
Animal models of placental compromise have focused on fetal outcomes [1–3], while placental adaptations have been largely overlooked. Furthermore, interspecies differences in placental structure [4] and methodology to mimic vascular insufficiency (e.g., embolization [5] or carunclectomy [6]), limit the translational value of some animal models for human research. The hemochorial placenta of the rhesus monkey is similar to human, but is distinguished by two lobes [7]. The umbilical cord attaches centrally to a primary lobe (approximately 60% of the functional placental unit) and clusters of bridging vessels supply a structurally independent secondary lobe. The bi-lobed placenta can be exploited to provide a non-human primate (NHP) model of placental compromise by ligating the fetoplacental blood vessels that bridge the primary and secondary placentas, so called inter-placental bridging vessel ligation (IPVL). Myers and colleagues reported IPVL at 98–102 days gestation (dGA) in the NHP results in asymmetrical fetal growth restriction (FGR) [8]. In contrast, Novy et al. reported that earlier IPVL at 80–90dGA resulted in compensatory placental growth (increased DNA content) with an absence of FGR [9]. These observations suggest there may be a critical window for placental plasticity near midgestation which may compensate for relative utero-placental insufficiency and allow normal fetal growth. However, the impact of placental circulatory and metabolic adaptations on fetal development and the long-term consequences of fetal programming on adult onset disease remain to be elucidated [10, 11]. This model was originally developed prior to the knowledge of developmental programming, and before the availability of non-invasive vascular imaging techniques. Therefore, we have re-established this NHP model utilizing IPVL at two distinct gestational ages (80 and 110 days) followed by concurrent longitudinal ultrasound studies of maternal utero-placental and fetal hemodynamic adaptations. We report our methods and initial results to highlight the feasibility of this novel animal model and to suggest avenues for future research on the mechanisms which mediate adaptations to in utero vascular insults.
Methods
This protocol was approved by the Institutional Animal Care and Use Committee of the ONPRC, and humane animal care was followed. We performed inter-placental vessel ligation surgery at either early (80dGA, n=4) or late (110dGA, n=3) gestation [9]. Term gestation for the rhesus monkey is approximately 167 days. Hysterotomy was performed in the uterine fundus without exteriorization of the fetus. Amniotic fluid was aspirated and returned prior to uterine closure. Placental bridging vessels were visualized, grasped and clamped with the overlaying chorioamniotic membranes and doubly ligated (with 3-0 silk suture or microvascular clips). Prophylactic antibiotics, Cefazolin; 250mg IM, BID, and tocolytics, Terbutaline sulfate; 1mg SQ, TID and Indomethacin; 50mg PO, SID were administered for 3 days post-surgery to reduce uterine irritability.
Utero-placental and fetal hemodynamics were measured serially in experimental groups and in concurrent control animals (n=19) under light ketamine sedation (12mg/kg, IM) during ultrasound procedures. For conservation of non-human primate resources only one sham surgical control animal was studied to-date. The ultrasound data collected from this sham animal were entirely consistent with the nonsurgical control animal data. Maternal heart rate and SpO2% were monitored continuously. Image-directed pulsed and color Doppler equipment with a 5- to 9-MHz sector probe (GE Voluson) was used for ultrasonographic data collection. The lowest high-pass filter level was used (100Hz), and an angle of 15° or less between the vessel and Doppler beam was deemed acceptable. Blood flow velocity waveforms were obtained as previously published [12–14]. Statistical analyses comparing each ligation group to control were performed with unpaired t-tests; p<0.05 was considered significant.
Fetal necropsy was performed immediately after cesarean section at 140dGA. Fetal body weights, placental weights (trimmed of fetal membranes) and placenta dimensions were recorded, and aspect ratio (length/width) calculated for each lobe. Each placental lobe was divided into four quadrants and representative tissue sections were randomly taken from each quadrant, avoiding the umbilical insertion site and the outer edges. Fresh tissues were flash frozen and stored for future analysis (n=4/lobe/animal), or fixed in formalin for paraffin-embedding and histologic analysis using routine hematoxylin and eosin (H&E) staining (n=4/lobe/animal). Stained H&E placental tissue sections were reviewed by an expert clinical placental pathologist (T.K.M) while blinded to experimental group. Placental histology was assessed for evidence of oxidative stress or relative utero-placental insufficiency (villous calcifications, increased arborization, advanced villous maturation and infarction [15]). Photomicrographs were prepared using a Leica DFC320 camera and software (Lieca Microsystems, Switzerland).
Results and Discussion
The primary finding of this study is that inter-placental vessel ligation in the rhesus monkey results in a number of adaptive responses in the placenta, fetus and mother. Fetal growth tended to be diminished in the late group (312 ± 18g body weight) compared to the early group (340 ± 12g); predicted body weight for rhesus macaque fetuses at 140dGA is 361 ± 49g [8]. Compensatory changes in the primary (functional) placental lobe included an increase in mean weight (97g vs. 71g) and thickness (1.3 vs. 0.5cm) following early ligation compared to control primary placentas respectively. Using historical data [9] we can calculate a daily placental growth rate (both lobes as one functional unit) from 80dGA or 110dGA to 140dGA as 1.4g/day and 1.5g/day respectively. Following 80dGA IPVL, primary placental lobe growth increased to 2.2g/day, whereas following 110dGA IPVL, growth decreased to 0.8g/day, indicating a diminished placental capacity for adaptive growth in the late group compared to the early group. Interestingly, placental aspect ratio was altered after late vessel ligation compared to control (1.25 vs. 1.08). Emerging evidence suggests that irregular placental shapes (e.g., oval) are indicative of altered placental efficiency and may reflect sub-optimal branching of the vasculature. In humans, such irregularities in placental shape are predictive of lower birth weight and later adult onset disease [16–18].
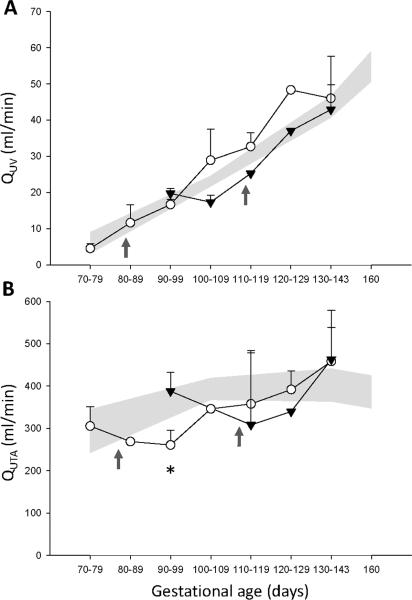
Despite an abrupt loss of approximately 40% of the total placental vascular bed following vessel ligation, there was a redistribution of umbilical volume blood flow (QUV) to the remaining primary lobe and overall maintenance of QUV (Figure 1A), with umbilical artery pulsatility index (PI) within the control range (Table 1). Our results suggest a substantial placental reserve capacity in the monkey placenta to increase volume blood flow. Supportive data from a sheep placental embolization model demonstrate that umbilical artery PI remains stable until >60% of the vascular bed is obliterated [19, 20]. Associated fetal hemodynamic changes included a shift in cardiac output from right to left ventricle, which favors blood flow to the fetal brain [21]. We observed a trend towards increased left hepatic vein pulsatility index together with evidence of decreased ductus venosus PI following ligation which suggests a redistribution of QUV away from the liver parenchyma (Table 1). Umbilical venous perfusion of the liver has an important influence on fetal growth [22, 23]. These fetal hemodynamic data did not reach statistical significance which is most likely due to the limitations of grouping ultrasound measurements over a 10-day gestational age range, in combination with the animal numbers in this formative data set.
Figure 1. A: Umbilical venous volume blood flow (QUV) and B: Uterine artery volume blood flow (QUTA).
The control group is presented as standard error of the mean (gray shaded areas, n=19). Early inter-placental vessel ligation (IPVL) at 80dGA, (open circles, n=4) and late IPVL at 110dGA (black triangles, n=3). Time of IPVL is indicated by the dark gray arrows. QUV = [0.5 × Vmax(cm/s) × cross sectional area × 60] where Vmax is the maximum velocity of intra-abdominal umbilical vein blood flow. QUTA = [Heart rate × VTI × cross sectional area] where VTI is the Velocity Time Integral of the uterine artery [19]. Ligation data are shown as the mean ± SD. *p<0.03 (Early IPVL vs. Control).
Table 1.
Fetal Regional and Cardiac Hemodynamics.
| Pulsatility Index | Control | Early | p-value | Late | p-value |
|---|---|---|---|---|---|
| n | 4 | 4 | 3 | ||
| Umbilical artery | 0.80 ± 0.19 | 0.75 ± 0.15 | 0.686 | 0.75 ± 0.15 | 0.687 |
| Descending aorta | 1.76 ± 0.08 | 1.56 ± 0.10 | 0.171 | 1.85 ± 0.11 | 0.543 |
| Middle cerebral artery | 1.60 ± 0.09 | 1.40 ± 0.03 | 0.093 | 1.46 ± 0.13 | 0.401 |
| Left hepatic vein | 1.47 ± 0.31 | 1.72 ± 0.20 | 0.541 | 1.69 ± 0.07 | 0.582 |
| Ductus venosus | 0.40 ± 0.04 | 0.30 ± 0.06 | 0.195 | 0.31 ± 0.13 | 0.488 |
| Aortic isthmus | 3.14 ± 0.49 | 2.47 ± 0.40 | 0.081 | 2.92 ± 0.54 | 0.583 |
|
|
|||||
| Cardiac parameters | |||||
|
|
|||||
| CCO (ml/min) | 180 ± 17 | 175 ± 21 | 0.867 | 176 ± 35 | 0.919 |
| RVCO (% of CCO) | 64 ± 3 | 58 ± 3 | 0.203 | 62 ± 5 | 0.785 |
| LVCO (% of CCO) | 36 ± 3 | 42 ± 3 | 0.203 | 38 ± 5 | 0.785 |
CCO, Combined Cardiac Output; RV and LV, Right and Left Ventricle
Measurements obtained by Doppler ultrasound (final scan obtained between 127–141 dGA). Early: vessel ligation at 80dGA; Late: vessel ligation at 110dGA. Data are mean ± SD. p values vs. control.
We report for the first time that an early response to inter-placental vessel ligation at 80dGA includes a statistically significant, albeit transient, decline in maternal QUTA (Figure 1B). Since QUTA includes both uterine and intervillous components it will be necessary in future studies to employ advanced imaging techniques (e.g., contrast enhanced ultrasound [24]) in order to characterize the temporal and spatial adaptations in both the maternal and fetal compartments to elucidate the mechanisms underlying placental plasticity.
Histologic analyses of placental tissue sections complemented our observations on the differences in placental growth and feto-placental blood flow after IPVL. After early ligation the primary lobe showed normal villous arborization in conjunction with increased placental weight and focal chorionic villous calcification compared to controls (Figure 2). Our group has recently reported increased villous calcification in the placentas of Japanese macaques fed high fat diets, which may be a consequence of oxidative stress at the utero-placental interface [14]. Villous calcification is evident in human gestational diabetes which is also associated with placental enlargement [25]. Late ligation cases did not increase their placental mass, but demonstrated chorionic villous arborization. Accelerated maturation with increased arborization is a feature seen in pregnancy complications associated with relative utero-placental insufficiency [15, 25]. The secondary placental lobe showed immature, avascular villi following IPVL in all experimental animals which was to be expected due to the loss of fetal circulation by the surgical manipulation.
Figure 2. Placental Histology.
Representative histologic sections from central full-thickness samples of the primary and secondary placental lobes taken in close proximity to the chorionic plate. A: Primary placental lobe from near-term (140dGA) sham control animal demonstrates normal villous architecture without calcification and fetal blood vessels (asterisk). B. Primary placental lobe after early inter-placental bridging vessel ligation (IPVL) demonstrates normal villous architecture albeit with focal areas of chorionic villous calcification (arrow). C. Primary placental lobe after late IPVL demonstrates accelerated villous maturation with a predominance of small caliber tertiary chorionic villi. D. Secondary placental lobe after IPVL demonstrates avascular villi (asterisk). Sections are stained with hematoxylin and eosin (H&E) and photographed using a Leica microscope and 20× objective.
In conclusion, our animal model has the potential to increase our understanding of the functional capacity of the primate placenta and its role in fetal development. Although the initial findings are based on a modest sample size, our data suggest that variations in placental plasticity are related to gestational age and imply that differences in fetal (and perhaps maternal) outcomes depend on the relative timing of utero-placental insufficiency. Although our experimental manipulation (in keeping with other animal models) does not reproduce a developmental abnormality (e.g., inadequate trophoblast invasion) our model lends itself to studying, in a non-human primate, the consequences of a discrete vascular insult on both maternal and fetal placental circulations concurrently. Furthermore, identification of potential fetal-maternal signaling mechanisms in the circulation of the primate placenta holds promise for developing interventional strategies and delivering therapeutic agents which may alter the course of human placental compromise.
Acknowledgments
Funding support: K99 HD055059, 4R00 HD055053, RR00163, R01 HL087710
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl. 2003;61:183–94. [PubMed] [Google Scholar]
- 2.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S99–107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 3.Ergaz Z, Avgil M, Ornoy A. Intrauterine growth restriction-etiology and consequences: what do we know about the human situation and experimental animal models? Reprod Toxicol. 2005;20(3):301–22. doi: 10.1016/j.reprotox.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–45. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 5.Louey S, Cock ML, Stevenson KM, Harding R. Placental insufficiency and fetal growth restriction lead to postnatal hypotension and altered postnatal growth in sheep. Pediatr Res. 2000;48(6):808–14. doi: 10.1203/00006450-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Mellor DJ, Mitchell B, Matheson IC. Reductions in lamb weight caused by pre-mating carunclectomy and mid-pregnancy placental ablation. J Comp Pathol. 1977;87(4):629–33. doi: 10.1016/0021-9975(77)90070-6. [DOI] [PubMed] [Google Scholar]
- 7.Benirschke K. Placentation. J Exp Zool. 1983;228(2):385–9. doi: 10.1002/jez.1402280221. [DOI] [PubMed] [Google Scholar]
- 8.Myers RE, Hill DE, Holt AB, Scott RE, Mellits ED, Cheek DB. Fetal growth retardation produced by experimental placental insufficiency in the rhesus monkey. I. Body weight, organ size. Biol Neonate. 1971;18(5):379–94. doi: 10.1159/000240380. [DOI] [PubMed] [Google Scholar]
- 9.Novy MJ, Aubert ML, Kaplan SL, Grumbach MM. Regulation of placental growth and chorionic somatomammotropin in the rhesus monkey: effects of protein deprivation, fetal anencephaly, and placental vessel ligation. Am J Obstet Gynecol. 1981;140(5):552–62. doi: 10.1016/0002-9378(81)90232-5. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ. The long-term outcome of retarded fetal growth. Schweiz Med Wochenschr. 1999;129(5):189–96. [PubMed] [Google Scholar]
- 11.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl 1):3–6. [PubMed] [Google Scholar]
- 12.Makikallio K, Erkinaro T, Niemi N, Kavasmaa T, Acharya G, Pakkila M, Rasanen J. Fetal oxygenation and Doppler ultrasonography of cardiovascular hemodynamics in a chronic near-term sheep model. Am J Obstet Gynecol. 2006;194(2):542–50. doi: 10.1016/j.ajog.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Acharya G, Sitras V, Erkinaro T, Makikallio K, Kavasmaa T, Pakkila M, Huhta JC, Rasanen J. Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet Gynecol. 2007;29(4):401–6. doi: 10.1002/uog.3977. [DOI] [PubMed] [Google Scholar]
- 14.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–64. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–60. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 16.Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, Thorp J. Modeling the variability of shapes of a human placenta. Placenta. 2008;29(9):790–7. doi: 10.1016/j.placenta.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, Eucker B, Thorp J. Placental surface shape, function, and effects of maternal and fetal vascular pathology. Placenta. 2010;31(11):958–62. doi: 10.1016/j.placenta.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54(2–3):525–30. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trudinger BJ, Stevens D, Connelly A, Hales JR, Alexander G, Bradley L, Fawcett A, Thompson RS. Umbilical artery flow velocity waveforms and placental resistance: the effects of embolization of the umbilical circulation. Am J Obstet Gynecol. 1987;157(6):1443–8. doi: 10.1016/s0002-9378(87)80241-7. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound Med Biol. 1990;16(5):449–58. doi: 10.1016/0301-5629(90)90167-b. [DOI] [PubMed] [Google Scholar]
- 21.Makikallio K, Jouppila P, Rasanen J. Retrograde aortic isthmus net blood flow and human fetal cardiac function in placental insufficiency. Ultrasound Obstet Gynecol. 2003;22(4):351–7. doi: 10.1002/uog.232. [DOI] [PubMed] [Google Scholar]
- 22.Tchirikov M, Kertschanska S, Sturenberg HJ, Schroder HJ. Liver blood perfusion as a possible instrument for fetal growth regulation. Placenta. 2002;23(Suppl A):S153–8. doi: 10.1053/plac.2002.0810. [DOI] [PubMed] [Google Scholar]
- 23.Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Venous liver blood flow and regulation of human fetal growth: evidence from macrosomic fetuses. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Keator CS, Lindner JR, Belcik JT, Bishop CV, Slayden OD. Contrast-enhanced ultrasound reveals real-time spatial changes in vascular perfusion during early implantation in the macaque uterus. Fertil Steril. 2011;95(4):1316–1321. e3. doi: 10.1016/j.fertnstert.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, Murtagh C, Cox S, Glenn G. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1997;121(5):449–76. [PubMed] [Google Scholar]