Abstract
Fetal hypoxia adversely affects the brain and heart development, yet the mechanisms responsible remain elusive. Recent studies indicate an important role of the extracellular matrix in fetal development and tissue remodeling. The matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitors of metalloproteinases (TIMPs) have been implicated in a variety of physiological and pathological processes in the cardiovascular and central nervous systems. This review summarizes current knowledge of the mechanisms by which fetal hypoxia induces the imbalance of MMPs, TIMPs and collagen expression patterns, resulting in growth restriction and aberrant tissue remodeling in the developing heart and brain. Collectively, this information could lead to the development of preventive diagnoses and therapeutic strategies in the fetal programming of cardiovascular and neurological disorders.
Physiological hypoxia (as compared with the arterial partial oxygen pressure in the mother) is a normal part of fetal life for all vertebrates and has a significant role in vasculogenesis, angiogenesis, hematopoiesis and chondrogenesis during fetal development [1]. The partial oxygen tension of a developing embryo is <10 mmHg, which is regarded as being hypoxic compared with normal tissue with an oxygen tension of 20–40 mmHg [2]. This suggests that the fetus is persistently hypoxic during organ formation, growth and maturation, and that fetal tissues have a lower threshold at which they reach a state of oxygen insufficiency [3]. Although the restricted oxygen supply is essential for intrauterine growth, excessive or severe hypoxia might compromise normal development and can adversely affect the fetus in various ways [4]. Pathophysiological hypoxia during pregnancy causes a redistribution of fetal blood flow to facilitate oxygen delivery to the vital organs, such as the brain and heart [5]; the occurrence of cardiac remodeling modifies the structure, function and gene expression in the fetal heart to compensate for the hypoxic stress. Cardiac hypertrophy and fibrosis are the major processes during the heart remodeling in the adaptive response to fetal hypoxia. Hypertrophy owing to the cardiomyocyte enlargement and hyperplasia might occur as a result of an increased myocardial workload [6–11]. Fibrosis is characterized by a disproportionate accumulation of fibrillar collagen, which stiffens the ventricles and causes the loss of compliance and the impairment of contraction and relaxation [12]. The adaptive alteration of the fetal heart might not change the basal cardiovascular function but might cause heightened vulnerability to ischemic injury in adulthood [13–15]. Fetal hypoxia also increases the risk of heart failure and other cardiovascular disease in later postnatal life [16]. In addition to the adverse effect on heart development, numerous studies have demonstrated that fetal hypoxia is one of the major causes of neurodevelopmental impairment and neurological deficits in the offspring [17–22].
Recent studies indicate that the timely breakdown of extracellular matrix (ECM) is crucial for normal fetal development [23]. ECM is a complicated microenvironment that includes a range of matrix proteins, signaling molecules, proteases and cell types involved in the tissue remodeling process [24]. Various factors that participate in the cardiac and cerebral remodeling have been revealed and matrix metalloproteinases (MMPs) are one of the most significant mediators in ECM turnover. MMPs are a family of zinc-dependent proteases. Together with tissue inhibitors of metalloproteinases (TIMPs), they have been implicated in a variety of physiological and pathological processes in the cardiovascular and central nervous systems, including the modulation of fibrillar collagen structure and deposition, and the regulation of cell proliferation and cell death. MMPs can be regulated at the transcriptional level and their activities can be inhibited by their endogenous inhibitors, the TIMPs.
In this review, we summarize current studies of the mechanisms by which hypoxia alters fetal cardiac and cerebral morphology, reprograms the related protein expression patterns, and leads to abnormal cell proliferation and cell death. We focus particularly on studies of how fetal hypoxia induces the imbalance of MMPs, TIMPs and collagens, resulting in growth restriction and aberrant tissue remodeling in the developing heart and brain (Figure 1).
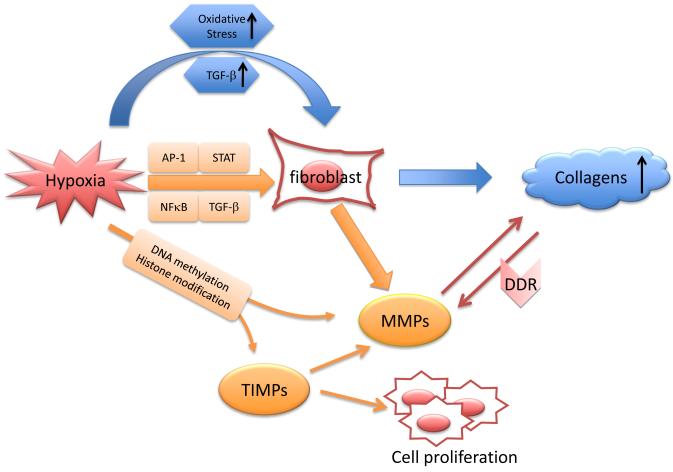
Figure 1.
Schematic mechanisms of hypoxia-induced collagen deposition. Hypoxia stimulates the production of collagens via oxidative stress or transforming growth factor beta (TGF-β) signaling pathway (in blue). Oxidative stress can also activate TGF-β, which might induce the expression of pro-fibrogenic genes, including those encoding collagens. Matrix metalloproteinases (MMPs) and the endogenous tissue inhibitors of metalloproteinases (TIMPs) can be regulated at transcriptional levels through epigenetic mechanisms (i.e. DNA methylation and histone modification) in response to hypoxia. In addition, hypoxia activates several transcriptional factors (e.g. nuclear factor-kappaB [NF-κB] , AP-1 [activating protein 1], STAT [signal transducers and activators of transcription-1] and TGF-β) that subsequently bind to some of the key transcriptional binding sites, regulating MMP gene expression (in orange). The MMPs digest collagens and reduce collagen deposition; as an autoregulation, collagens bind to their discoidin domain receptor (DDR) to upregulate MMPs levels. In addition to the inhibitory effect on MMPs, TIMPs also have a key role in cell proliferation and cell death.
Fetal hypoxia and abnormal heart and brain development
Large epidemiological and animal studies have indicated that intrauterine growth restriction with low birth weight results in an increased incidence of perinatal morbidity and mortality, and increases the risk of neurological deficits and cardiovascular disease later in adult life [25–28]. In humans, fetal hypoxia is one of the major causes of intrauterine growth restriction [29]. The fetus can experience prolonged hypoxia under various conditions, such as pregnancy at high altitude, pregnancy with cigarette smoke, drug abuse, anemia, pulmonary disease and hypertension [7]. Maternal hypoxia or insufficient delivery of oxygen to the fetus contributes to remarkable pathophysiological changes in fetal tissue remodeling. However, little is known about the fetal adaptive mechanisms involved and potential therapeutic approaches. Elucidating the various molecular mechanisms underlying the structure and functional changes in cardiac and cerebral growth is an area of ongoing research.
Fetal hypoxia and cardiac remodeling
Many studies have demonstrated that hypoxia results in a decrease in fetal body weight, but an increase in the heart:body weight ratio [30–34]. During fetal hypoxia, there is a redistribution of fetal cardiac output from the periphery to essential organs such as the brain and heart. This is induced by carotid chemoreflex and sustained by the local vasodilators nitric oxide and adenosine in the essential circulation and the peripheral vasoconstriction factors, catecholamines and neuropeptide Y [31]. This causes the retarded growth of nonessential organs and tissues, but maintains growth of the heart and brain. Nevertheless, fetal hypoxia causes apoptotic cell death in the heart and increases the number and size of binucleated cardiomyocytes [30]. In normal fetal heart development, cardiomyocytes first undergo hyperplasia before midgestation. In rats, the cardiomyocytes become binucleated and terminally differentiated in the first 2 weeks after birth [35]. The binucleated cardiomyocytes are no longer capable of proliferation and division. Fetal hypoxia interrupts the proliferation of myocytes prematurely and cardiomyocytes undergo hypertrophic growth to compensate for the reduced number of myocytes. In fact, the enlargement of the fetal heart following hypoxia has been demonstrated [30].
Although the mechanism of cardiac hypertrophy resulting from chronic hypoxia has not been well established, a study using long-term intermittent hypoxia has demonstrated that tumor necrosis factor-alpha (TNF-α), insulin-like growth factor II (IGF-II), phosphorylated p38 mitogen-activated protein kinase (p38 MAPK), signal transducers and activators of transcription-1 (STAT)-1 and STAT-3 are involved in 4-week hypoxia-induced hypertrophic myocardium and increased interstitial space [36]. In addition, interleukin-6 (IL-6), mitogen-activated protein kinase 5 (MEK5) and extracellular signal-regulated kinase 5 (ERK5) are activated with 8-week-hypoxia exposure [36], suggesting that a pro-inflammatory cytokine pathway is involved in cardiac hypertrophy triggered by long-term hypoxia. Studies of chronic anemic fetal sheep have illustrated increases in biventricular cardiac output and myocardial blood flow accompanied by fetal heart hypertrophy [37,38]. This is not unexpected because there is an association between prenatal hypoxia and the development of primary pulmonary hypertension in the offspring [39]. In this case, increased capillary blood supply and ventricular work are required to maintain cardiac function, and increasing myocardial vessel growth might be an adaptation to anemia–hypoxia insult.
It has been shown in fetal lambs that anemia and/or hypoxia increase hypoxia inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF) and cause capillary coronary vascular growth [38,40]. Additionally, HIF-1 also induces the transcription of glycolytic enzymes to maintain myocardial use of peripherally generated lactate as an energy substrate in the hypoxic condition [38]. Thereby, cardiac hypertrophy with angiogenesis stimulated by the pro-inflammatory cytokines, HIF-1 and VEGF, has an essential role in the adaptive mechanisms in response to chronic hypoxia. Interestingly, hypoxia during the early fetal development stage resulted in myocardial thinning in a rat model, which is different from the increased heart:body weight ratio or hypertrophy that occurred with hypoxia at late gestation [1]. This finding implies that the distinct duration and gestational periods of hypoxia might determine the nature and severity of abnormal heart development.
In addition to cardiomyocyte hypertrophy, alteration of ECM components, particularly interstitial collagens in the heart, is seen in cardiac remodeling caused by hypoxia. Type I (85% of cardiac interstitium) and type III (11% of cardiac interstitium) collagens are the major collagens in the connective tissue network of the vertebrate heart and form several distinct organized layers in the heart walls to provide rigidity and elasticity [41,42]. Human fetal heart expresses collagen III before collagen I during the second trimester and collagen III forms a major component of the collagen network in the fetal heart [43]. After birth, the ratios of total collagen to total protein as well as that of collagen I to III are high in neonatal hearts and they gradually decrease with age, which explains the relatively rigid and less compliant heart in the neonate as compared with that in the adult [44]. The ECM network of the heart is formed by a complex three-dimensional arrangement of glycoproteins and proteoglycans and is closely linked to cardiac function [45]. This elastic and stress-tolerant network is responsive to multiple pathophysiological signals, for example, myocardial hypoxia that stimulates the synthesis of collagens [45,46].
Generally, ECM turnover during development under both normoxic and hypoxic conditions is tightly controlled by coordinated degradation and synthesis of collagens and other ECM components. Excessive amounts of collagens released from cardiac fibroblasts might contribute to ventricular stiffness and impairment of diastolic filling after cardiac insults [47]. A study by Xu et al. confirmed that fetal hypoxia significantly enhances beta and/or alpha myosin heavy chain (MHC) isoform ratio and collagen I and III accumulation, yet reduces MMP-2 activity in the left ventricular of adult rat offspring; this suggests that impaired fetal development leads to dysregulated collagen deposition in the heart and alters the susceptibility of the heart to ischemia and/or reperfusion injury [32]. Fetal hypoxia alters the expression patterns of many genes, including the 70-kd heat shock protein (HSP70), endothelial nitric oxide synthase (eNOS), beta2-adrenoreceptor, protein kinase C epsilon isozyme and type 2 angiotensin II (AT2) receptors in the heart, and causes left ventricular remodeling [13,14,32,48–50]. In vitro studies of cultured cardiac fibroblasts have revealed that angiotensin II directly stimulates collagen synthesis and the expression of ECM proteins via type 1 angiotensin II (AT1) receptors, and indirectly stimulates collagen deposition via induction of transforming growth factor beta (TGF-β), endothelin 1 (ET-1), IL-6 and osteopontin [12]. Additionally, a study using knockout mice reported that angiotensin II failed to promote fibrosis in TGF-β1-deficient mice [51]. The sustained activation of TGF-β in mice overexpressing TGF-β can induce ventricular fibrosis in the heart [52]. Interestingly, collagen I in mouse cardiomyocytes was enhanced after hypoxia reoxygenation, and the release of reactive oxygen species (ROS) appeared to induce the genes for collagen [53]. The overexpression of TGF- β1 can block ROS and therefore inhibit collagen accumulation, implying an antifibrotic role for TGF- β1 [53]. Collectively, TGF- β1 might act as both a pro- and anti-fibrotic factor upon various stimuli and upon short-term or long-term stimuli. The primary effect of TGF- β1 in the fetal and neonatal heart following chronic hypoxia remains unclear. More studies demonstrating the direct effect of hypoxia on abnormal development of fetal and neonatal heart are therefore warranted.
Fetal hypoxia and brain development
Fetal hypoxia is also linked to brain growth retardation and neurological deficits during development. Evidence indicating the detrimental effects of in utero hypoxia on the fetal brain is also well documented. The reduced fetal cerebral oxygenation could result from a decrease in fetal cerebral perfusion owing to a failure of cerebral–hypoxic vasodilatation, cardiac decompensation or perinatal stroke [54]. Low brain oxygenation in fetuses is associated with abnormal neurovascular development and an increased risk of brain injury, such as cerebral palsy or periventricular leukomalacia (PVL) in newborns [55,56]. It has been shown that 2 h of transient hypoxia (9% oxygen) during late pregnancy reduced the level of brain-derived neurotrophic factor (BDNF) in the fetal brain, probably in relation to the impaired morphology of the hippocampus and cerebellum during development [57]. Hypoxia did not stimulate inflammation or cell death, but delayed neuronal migration by diminishing the proteins involved in the migration, such as Reelin, Disabled1 and amyloid precursor protein, in the fetal brain [19]. Hypoxia might regulate cell proliferation by altering the fetal cerebella gene expression, which is evidenced by a trend of upregulation in cell cycle-related genes seen 2 h after fetal hypoxia, followed by downregulation 24 h or 20 days after hypoxia [18]. In addition, key proteins in the gamma-aminobutyric acid (GABA) pathway were immediate repressed by transient hypoxia in the fetal cerebral cortex, which might result in an increased susceptibility to seizures and epilepsy in the offspring [17]. It is possible that hypoxia-induced fetal programming in the brain development is responsible for the increased vulnerability to cerebral insults in adulthood [58]. Meanwhile, GABA has a trophic effect during early brain development, and hypoxia might alter the function of GABAergic transmission during this period, thus compromising the development of neuronal wiring, plasticity of the neuronal network, and affecting the neural organization [58].
Interestingly, chronic fetal hypoxia appears to regulate brain tissue remodeling by a similar pathway. In a guinea pig model, the enhancement of oxidative stress and apoptosis caused by chronic fetal hypoxia is mediated by inflammatory cytokines activation in the fetal brain [59]; this finding is also supported by our own study that downregulation of TIMPs by chronic hypoxia might participate in increased apoptosis in the neonatal rat brain [22]. Taken together, insufficient oxygen alters fetal brain growth, resulting in abnormalities in fetal and neonatal cerebral structure. This abnormality probably involves a sustained reduction in neuronal proliferation and increased cell death.
The role of MMPs and TIMPs in hypoxia-induced abnormal heart and brain development
The MMP family [AU1]includes at least 25 members [60], which are generally classified into several classes according to their specific substrates: collagenases (MMP-1, -8, -13 and -18) are capable of degrading insoluble fibrillar collagens, especially collagen I and III; gelatinases (MMP-2 and -9) can digest gelatins as well as collagen IV in the basement membranes; membrane type 1–6 MMPs (MT1–MT6 MMPs) can digest collagens and activate other MMPs; stromelysins (MMP-3, -10 and -11) are active against some ECM components; and a heterogeneous group that includes MMP-7, -12, -20, -26 and -28 (Figure 2) [61,62]. An important control of MMP activity is through a group of specific MMP endogenous inhibitors, the TIMPs. There are four TIMPs identified so far, termed TIMP-1, -2, -3 and -4; these can bind noncovalently with high efficiency to active MMPs in a 1:1 molar ratio, therefore preventing access of the MMP catalytic domains to their substrates [24]. The most studied are TIMP-1 and TIMP-2, which bind MMP-9 and MMP-2 with a high affinity, respectively [63]. TIMP-3 was first proposed by Yang and Hawkes in 1992, and can bind directly to ECM proteins [24,64]. TIMP-4, as a recently identified member of TIMP family, was first discovered in the human heart in 1996, and has a high binding affinity to proMMP-2 [65,66].
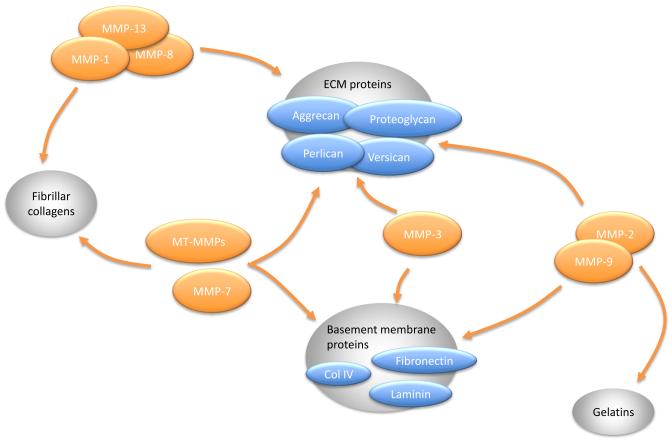
Figure 2.
Matrix metalloproteinases (MMPs) and their substrates in myocardium. Collagenases (MMP-1, -8 and -13) have high substrate specificity for fibrillar collagens (e.g. collagen I, II and III) and extracellular matrix (ECM) proteins (proteoglycans, perlican, aggrecan, versican, etc.). Stromelysins (MMP-3 and -7) can digest ECM proteins and basement membrane proteins (e.g. collagen IV and fibronectin). Additionally, MMP-7 also degrades fibrillar collagens. The substrates for gelatinases (MMP-2 and -9) include gelatins, basement membrane proteins and ECM proteins. Membrane-type (MT-) MMPs breakdown basement membrane components, fibrillar collagens and ECM proteins.
Hypoxia in the regulation of MMP protein expression patterns
Prenatal hypoxia induces abnormal heart growth and mental retardation in fetuses and offspring, and the alteration of MMPs has a crucial role in tissue remodeling in both the heart and brain. It has been reported that fetal hypoxia results in an overall tendency towards increased MMP-2 and -9 during fetal and neonatal periods. Given that MMP-2 and MMP-9 are the predominant MMPs in the cardiac ventricle, studies in fetal guinea pig hearts focused on both MMPs and showed that fetal hypoxia enhanced their mRNA levels, and upregulated the protein expression of MMP-9 but not MMP-2, although the MMP-2 activity was increased [67]. Our studies indicated that fetal hypoxia increased the MMP-2 and MMP-9 activity in the brain on postnatal days 0 and 4 neonatal rats, whereas no differences were seen afterwards [22]. Although the mechanisms of MMP upregulation by chronic prenatal hypoxia are not fully understood, it is known that MMPs can be regulated by transcription, pro-enzymatic activation and endogenous inhibition. MMPs are mainly regulated at the transcriptional level with relatively low basal levels during normal development [68]. Hypoxia might activate several transcriptional factors that subsequently bind to some of the key transcriptional binding sites, enhancing or repressing MMP gene expression. The activating protein 1 (AP-1) site, nuclear factor kappa B (NF-κB) site and STAT site are involved primarily in the regulation of MMP genes [68]. Increases in MMP-2 synthesis were found in a culture of rat cardiac fibroblasts exposed to 1% oxygen for 24 h, and a functional AP-1 site mediated MMP-2 transcription through the binding of distinctive Fra1-JunB and FosB-JunB heterodimers [69]. In vitro studies have shown a rapid activation in the binding of DNA to AP-1 during hypoxia, and AP-1 synthesis was enhanced to activate many AP-1 and AP-1 family-controlled genes [70] that possibly include various MMP genes. The study also suggested that the activation of NF-κB by hypoxia reoxygenation is slow, which implies that NF-κB is indirectly activated and probably relies on other gene products newly induced by hypoxia-inducible factors [70]. Chen et al. reported that the expression of MMP-1 in cardiac fibroblast is increased, accompanied by elevated ROS and NF-κB after the onset of hypoxia, and continues to be upregulated during prolonged hypoxia [71]. Given that the generation of ROS from hypoxia is believed to activate a range of intracellular signaling pathways, including NF-κB [72], this finding implies that oxidative stress is associated with hypoxia-triggered upregulation of MMP, potentially through the NF-κB binding site. Although hypoxia might activate STAT protein by various pathways [73], it is unclear whether STAT proteins can bind directly to the transcriptional regulatory region of MMP genes [74]; thus, the role of the STAT pathway in the upregulation of MMP transcription induced by hypoxia remains elusive.
MMPs are also regulated by Smad family proteins that repress or enhance TGF-β-mediated gene expression, implicating a dual role for TGF-β in modulating MMP genes in tissue remodeling and cancer [68]. It has been demonstrated in an in vitro study that hypoxia increased TGF-β expression [53], and TGF-β has been shown to inhibit MMP-1 after ischemic reperfusion in the heart [75]. Although TGF-β is implicated in regulating MMP expression, whether hypoxia-mediated TGF-β regulates MMPs at the transcriptional level remains to be elucidated. Moreover, how chronic fetal hypoxia transcriptionally regulates MMPs during tissue remodeling requires more investigation.
Imbalance of MMPs and TIMPs and aberrant tissue remodeling
Not only does the alteration of MMPs affect fetal and neonatal tissue growth and remodeling in the hypoxic condition, but the dynamic changes of MMPs and TIMPs together also contribute to aberrant remodeling in the developing fetus. Given that a wide range of neurovascular matrix and cell-surface proteins are the substrates for MMP-2 and -9, they have been extensively investigated in the brain [76,77]. MMP-2 and -9 are released mainly from astrocytes [63]. Studies in humans reported that serum levels of MMP-9 on the day of birth were significantly increased in perinatal asphyxiated neonates followed by a subsequent increase in serum TIMP-1 levels and a decrease in serum MMP-9 on the day after birth, suggesting that TIMP-1 increases in response to MMP-9 and it, in turn, suppresses MMP-9 [78]. The study also showed that the serum MMP-9:TIMP-1 ratio in asphyxiated neonates with neurological sequelae is significantly higher than in those without sequelae [78], indicating that the imbalance of MMP-9 and TIMP-1 is linked to prenatal hypoxia-induced neurological deficits during brain development. Further studies demonstrated that chronic maternal hypoxia alters the TIMP-1:MMP-9 and TIMP-2:MMP-2 ratios by suppressing TIMP expression, resulting in reduced cell proliferation and increased cell death in the neonatal brain [22]. Ryu et al. explored the influence of chronic hypoxia on the developmental expression profile of additional MMPs and TIMPs in the mouse lung [79]. Prolonged neonatal hypoxia resulted in an arrest in alveolarization, elevated MMP-2 and lowered TIMP-2 levels, whereas no significant changes were found in MMP-9, MT1-MMP, TIMP-1 and TIMP-3 [79]. This study confirmed that chronic hypoxia breaks the delicate balance of proteolytic and anti-proteolytic forces during lung development, which accounts for several pulmonary pathologies, including pulmonary fibrosis. The TIMP-1:MMP-1 ratio was also remarkably increased in patients with severe left ventricular hypertrophy, showing that the interaction of TIMP-1 with another MMP besides MMP-9 also has a significant role in the pathophysiological remodeling [80]. The disruption of fine dynamics of MMP–TIMPs by acute hypoxia is also supported by studies using a neonatal hypoxic ischemia (HI) model. Activation of MMP-9 was followed by the delayed elevation of TIMP-1, and the mismatch of MMP-9–TIMP-1 elevation and the increased MMP-9:TIMP-1 ratio might be responsible for the blood brain barrier (BBB) breakdown that occurs after hypoxic injury in developing brain [81]. The temporal profiles of -2–TIMP-2 MMP were not altered dramatically after HI, distinct to the findings in the brain after chronic hypoxia. This discrepancy implies that MMP-2 and TIMP-2 are not fully responsible for hypoxia-induced brain remodeling. In addition, changes in the MMP:TIMP ratio might have different roles in acute and prolonged tissue remodeling after hypoxia. In the rapid response to hypoxia, MMPs are released by early gene expression and cause the BBB breakdown and cell death, whereas in the prolonged phase after hypoxia, MMPs and TIMPs might participate in neurogenesis and angiogenesis to recover from the brain injury by different pathways.
Role of MMPs in fibrillar collagen deposition
The primary effect of MMPs, which was first discovered by Gross and colleagues in 1962, is to digest a wide range of collagens [82]; therefore, MMPs are key regulators of ECM homeostasis. Collagen degradation is a major step in tissue remodeling, and MMP-1, -13 and MT1-MMP are the primary enzymes that digest fibrillar collagen [83]. Generally, MMPs cleave the native triple helix of collagen at a Gly-Leu or Gly-Ile site, leading to an unstable conformation at body temperature and further degradation by nonspecific proteases [62]. Enhanced cardiac fibrosis attributes to excessive synthesis of fibrillar collagen (mainly type I) and a reduced degradation of collagens, causing ventricular stiffening and impaired diastolic filling. The reduced digesting of collagens does not necessarily result from the low levels of MMPs; in fact, increased collagens can activate its discoidin domain receptor (DDR) and therefore upregulate MMP-1 expression [84]. In the heart, DDR2 is primarily expressed on cardiac fibroblasts, which appear to be the major source of ECM components and MMPs [47,85]. The role of DDR2 in fibroblast proliferation and migration has been demonstrated by primary cell culture from DDR2-knockout mice, and the reduced growth of fibroblast from DDR2-null mice is probably the result of the reduced expression of MMP-2 [86]. An in vitro study of DDR1-null smooth muscle cells also indicated a decreased proliferative and migratory response and the involvement of both MMP-2 and MMP-9 expression [87]. It has been reported that DDR2 expression is observed during early cardiac development under normal conditions, whereas hypoxia, as well as other pathologies, might regulate DDR1 and DDR2 expression by a variety of pathways [88–90]. Studies carried out by Hu and colleagues proposed that hypoxia–reoxygenation increases the signals for collagen I and MMPs, and the elevated levels of MMPs are the result of an autoregulatory response to collagen I signal in cardiac fibroblasts [53]. However, how MMPs are autoregulated by collagen I was not determined in the study. Taken together, in addition to the inadequate inhibition by TIMPs [91], collagen receptors DDR1 and DDR2 might have an essential role in hypoxia-induced adverse tissue remodeling and might serve as an adaptive mechanism for the upregulation of MMPs by collagens after chronic hypoxia.
In brain tissue, collagens are generally rare and exist principally at the meninges, the basement membranes and the sensory end organs [92]. Increasing amounts of data have shown that collagens have an active role during the development of the nervous system, including axon guidance and synaptogenesis in the establishment of the architecture of the brain [92]. DDR1 was found to be expressed prenatally in neurons of the proliferative areas, and yet was not detectable postnatally during brain development in mouse [93]. Whether MMPs can be regulated by collagens and whether DDR participates in the regulation of MMPs in the fetal brain are not fully understood at present; it is also necessary to determine further the role of DDR in fetal hypoxia-promoted brain remodeling.
Role of TIMPs in cell proliferation and cell death
TIMPs are linked to MMP-independent mechanisms, such as cell proliferation and apoptosis, in addition to their inhibitory effect on specific MMPs in hypoxia-induced tissue development. In some non-expressing MMP cell lines, TIMP-1 promotes cell proliferation, as measured by DNA content [94]. The reductive alkylated TIMP-1 and -2 have no MMP inhibitory activity, but both significantly enhance cell proliferation. Moreover, the activity is not seen with the complex of proMMP2–TIMP-2 or proMMP9–TIMP-1 [95]. These studies demonstrate that the cell-proliferating activity of TIMPs is independent of their inhibition of MMP activity. The cell-proliferation activity might be the result of a TIMP-1 sequence that is homologous with human granulocyte-macrophage colony-stimulating factor, whereas TIMP-2 seems to be devoid of such a sequence [94]. However, subsequent studies demonstrated that the cell surface receptor might be present for TIMP-2 for its cell-proliferating action [95]. The anti-apoptotic effect of TIMP-1 has also been confirmed in tissue remodeling, and TIMP-1 levels suppressed by fetal hypoxia associates with a significant increase in cell death in the CA1 hippocampal area after birth [22]. The MAPK pathway and cAMP–protein kinase A (PKA) pathway might be related to TIMP growth-promoting activity [96]. More details of the regulation between chronic hypoxia and TIMPs in the tissue remodeling process need to be determined. By contrast, TIMP-3 has been shown to inhibit several MMPs and to be expressed at high levels in cardiac tissue. It is unique within in the TIMP family, because it binds firmly to ECM and has pro-apoptotic effects [97]. The direct binding to ECM might stabilize the MMP–TIMP complex within the interstitial space [24]. The gene encoding TIMP-3 has no TATA sequence, but contains many Sp1 binding sites in its promoter region, which suggests a potential epigenetic modification through DNA methylation. TIMP-3 can inhibit neonatal cardiomyocyte proliferation possibly by the epidermal growth factor receptor (EGFR)/c-Jun NH2-terminal kinase (JNK)–SP-1–p27 signaling pathway [98]. TIMP-3 therefore appears to have a detrimental effect on cardiac remodeling after insults and, together with other TIMPs, it might comprehensively modulate cardiac or cerebral tissue remodeling in response to fetal hypoxia.
Epigenetic mechanisms of hypoxia-induced abnormal tissue remodeling
Several MMPs have shown intransient expression upon external stimuli and, therefore, are generally considered to be inducible genes. Although the mechanisms underlying different stimuli in inducing MMPs expression are not fully understood and are complex, increasingly amounts of new data have demonstrated that tumor and peri-tumor cells in cancer can constitutively express MMPs at high levels, suggesting that epigenetic modification is involved in MMP regulation [68]. Epigenetic modification, including the methylation of cytosine in CpG dinucleotides and the post-translational modification of histone, is crucial to normal mammalian development and differentiation, and enables an organism to respond to the environment by changing gene expression patterns [13,99–101]. Animal studies have suggested that abnormal DNA methylation is related to many morphological and functional deficits and has the capacity to act as an epigenetic mutation [102–104]. Patterson et al. clearly demonstrated that maternal chronic hypoxia can alter protein kinase c epsilon (PKCε) gene promoter DNA methylation patterns, resulting in downregulation of PKCε protein and mRNA in the fetal heart and heightened heart ischemic vulnerability in adult male offspring [13]. Other than hypoxia, maternal cocaine and fetal nicotine exposure in rat models also resulted in PKCε gene repression in the fetal heart; these studies have therefore shown a link between maternal chronic insults and reprogramming of certain genes in the fetal heart and the resulting alteration of their expression patterns in adult life [105,106].
DNA methylation and MMP and TIMP gene expression
Although the exact epigenetic mechanisms involved in hypoxia-induced MMP and TIMP reprogramming have not yet been established, several possible mechanisms have been elucidated by recent studies. Given that methylation of CpG islands in the promoter region of genes is generally an efficient mechanism of repressing transcription, it is plausible that hypoxia changes methylation patterns of certain cis-elements in MMP gene promoter regions, leading to promoter hypo- or hypermethylation and, therefore, enhancement or inhibition of MMP gene expression. An example of the epigenetic control of MT1-MMP, MMP-2 and MMP-3 is seen in cancer cells. It has been shown that the MT1-MMP and MMP-2 promoter regions are hypermethylated in MCF-7 cells that express low levels of MT1-MMP and MMP-2; in addition, the lack of two crucial DNA methyltransferases, Dmt-1 and Dmt3b, increases MMP-3 expression in a colon cancer cell line, suggesting that epigenetic mechanisms silence the synthesis of these MMPs [107,108]. The low levels of methylation of the MMP-9 promoter have been shown to favor the constitutive expression of MMP-9. Notably, methylation at the Sp1 binding site can significantly affect MMP-9 transcription, as the core binding site for Sp1, 5′-GGGCGG, contains a potentially methylatable CpCpG site. Using bisulfite genomic mapping of the MMP-9 to analyze the status of MMP-9 promoter methylation, Chicoine et al. showed that the heavily methylated promoter, particularly at Sp1 sites, suppresses MMP-9 gene transcription and expression. Meanwhile, the treatment of 5-aza-2′-deoxycytidine, a DNA demethylating agent, triggers MMP-9 expression in non-MMP-9-expressing cells [109]. Interestingly, Sp1 has been shown to be insensitive to methylated CpG, whereas two consecutive cytosines at CpCpG were required for the inhibition of Sp1 binding. Another study confirmed that aberrant methylation of the outer cytosine CpCpG blocked Sp1 binding, thus acting in a similar way to a mutation at the Sp1 site; this could further induce CpG methylation of the rest of the island [110]. Therefore, Sp1 binding is crucial for maintaining CpG islands in an unmethylated state and might be involved in regulating the appropriate expression of MMP-9 and other MMPs that harbor Sp1 sites.
Other than MMPs, CpG islands have been found in TIMP-1, TIMP-2, TIMP-3 and TIMP-4 promoters. The DNA methylation status of the TIMP-2 gene promoter in several cancer cell lines has been determined; in particular, a 1.35-kb-long CpG island located at −0.5 to −0.85 kb region from the transcription start site was found to not be heavily methylated, whereas a 0.5-kb-long CpG island located in the −1.7 to −1.2 region was hypermethylated, which implies that the ratio of these two CpG islands has a pivotal role in the epigenetic regulation of TIMP-2 and that this regulation is different from that of MMPs in cancer cell lines [107]. By contrast, hypoxia has been shown to induce the hypomethylation of genomic DNA in human fibroblasts (40% reduction of 5-mC) as well as cell lines originally derived from primary tumors; this was the first study to demonstrate that 5-mC levels might be changed along with hypoxia [111]. This finding suggests an epigenetic mechanism that could explain our own data that chronic hypoxia significantly increased MMP-2 and -9 expression and reduced TIMP-1 and -2 expression in developing brain in neonatal rats [22]. To date, the kinetics of alteration in MMP and TIMP promoter methylation by chronic hypoxia and whether such change is tissue specific are not clear and deserve further investigation. Determining the binding sites at MMP and TIMP promoter regions that undergo methylation or demethylation after hypoxia and how the changes in methylation patterns affect the interaction with transcription factors in regulating the gene activities are questions that remain.
Histone modification, and MMP and TIMP gene expression
In addition to DNA methylation, histone modifications might contribute to the reprogramming of MMP and TIMP gene expression patterns. Histone modifications include histone methylation and acetylation or deacetylation, which inhibit or facilitate the access of transcription factors and other transcriptional machinery to the promoter regions. The acetylation level of histones is mediated by histone acetylase (HAT) and histone deacetylase (HDAC) [103]. An effect of chromatin remodeling on the control of MMP and TIMP expression has been reported for some MMP and TIMP genes. It has been shown that inhibition of DNA methylation alone induces demethylation of the MT1-MMP promoter, yet does not increase its expression [107]. The levels of histone modification in MT1-MMP and MMP-2 promoter regions have been further determined by looking at the epigenetic marks H3K27me3, H3K4me2 and H3K4me3; H3K27me3 modification is increased in transcriptionally silent MMP genes in MCF-7 cells. Because H3K27me3 reflects the inactive genomic loci, these findings suggest that histone methylation, along with DNA methylation, are required to silence MMP-2 and MT1-MMP transcription in MCF-7 cells [107]. A microarray-based epigenetic profile of MMP-related genes revealed high levels of H3ac and H3K4me2 and low levels of H3K27me3 in the TIMP-1 gene promoter, which is related to the active status of TIMP-1 gene transcription in U251 and MCF-7 cells. Similar findings have been demonstrated downstream of the transcription initiation site of the TIMP-3 gene in MCF-7 cells, which is also associated with the transcriptional activity. Conversely, the repressive H3K27me3 mark is increased in the same region, which is associated with downregulation of TIMP-3 in U251 cells [112]. HDAC and HAT are essential for the modulation of chromatin remodeling, because they are able to remove or add acetyl groups to histone and, consequently, cause gene repression and heterochromatin or unpacking of DNA from nucleosome that are necessary for transcription [68].
Recently, a role of HDAC4, a member of the class IIa HDAC superfamily, was uncovered in MMP gene silencing during tissue fibrosis. It was demonstrated that impaired histone acetylation occurs at the MMP promoters during transdifferentiation in hepatic stellate cells, and HDAC4 is found to accumulate correspondingly with histone deacetylation and MMP repression. Given that aberrant expression of HDAC4 in normal hepatic stellate cells can repress MMP genes, the study clearly demonstrated that the silencing of MMP genes is epigenetically mediated by HDAC4 upregulation in liver fibrosis [113]. This study proposed a recruitment of HAT activity upon stimulation of injury signals, which might enable the upregulation of MMPs, such as MMP-9 and MMP-13, by various external stimuli. In addition, other studies have suggested that class IIa HDAC has an anti-hypertrophic effect in cardiocytes, perhaps by inhibiting cardiac-specific transcription factors in the heart [114]. Further studies by Jeon and Lee indicate that HDAC levels are increased in several cancer tissues and become elevated after hypoxia [115]. Taken together, it is possible that stress such as hypoxia or injury triggers heart remodeling, including hypertrophy, fibrosis and apoptosis, via HAT- and HDAC-mediated epigenetic regulation of MMPs transcription and expression. Undoubtedly, MMPs and TIMPs have a key role in the maintenance of normal heart and brain development, and reprogramming of their expression patterns induced by hypoxia is likely to have a crucial role in heart and brain tissue remodeling in an adaptive response to epigenetic effectors.
Possible interventions in fetal hypoxia-induced abnormal development
Antifibrotic therapies might be useful in attenuating ventricle wall stiffening and improving cardiac function of the diseased heart. A study by Zeisberg and colleagues demonstrated that cardiac fibrosis is linked to the occurrence of fibroblasts that originate from endothelial cells, termed ‘endothelial–mesenchymal transition’ [116]. Administration of recombinant human bone morphogenic protein-7 (rhBMP-7) can effectively inhibit endothelial–mesenchymal transition and the progression of cardiac fibrosis in animal models [116], suggesting that intervening in this transition is a potential treatment for heart fibrosis. In this study, TGF-β1 was used to induce fibrosis in endothelial cell culture, which was confirmed by Sakata’s study using TGF-β-overexpressing mice, which showed that sustained activation of TGF-β induced ventricular fibrosis in the heart [52]. Based on the fact that TGF-β is implicated in collagen production seen after hypoxia [117], these findings suggest another possible intervention, in that inhibition of TGF- β might blunt the process of fibrosis. Another study reported that angiotensin II fails to promote fibrosis in TGF- β1-deficient mice [51], indicating that targeting TGF- β1 could have a central role in antifibrotic therapies. It is known that hypoxia enhances the synthesis of collagen through oxidative stress [53]; therefore, the use of antioxidants might be a potential treatment for fibrosis.
Taken together, targeting for effectively normalizing collagen levels in cardiac tissue remodeling might be a promising approach to inhibiting cardiac fibrosis. In the fetal heart, it appears that hypoxia enhances MMP levels, indicating that the degradation of collagens is facilitated, but this fails to catch up with increased synthesis. In this case, pharmacological manipulation of MMPs in the heart seems to be irrelevant compared with collagens. By contrast, because collagen is not the major type of protein in the ECM of the brain, targeting MMPs might be more beneficial for neuroprotection in this organ. Studies have shown that MMPs levels in the immature brain are increased after prenatal hypoxia or neonatal hypoxic-ischemia [22,81]. MMPs are the major mediators involved in the disruption of the BBB after stroke [118], and MMP inhibitors have been suggested to have a neuroprotective effect after ischemia insults in different animal models [81,119,120]. Although TIMP-1 is believed to be an endogenous inhibitor of MMPs, and the overexpression of TIMPs is neuroprotective in both in vivo and in vitro studies [121], TIMPs have not been reported to be appropriate for pharmacological approach, owing to their short half life in vivo [122].
Concluding remarks
To date, the fundamental mechanisms of prenatal hypoxia in cardiac and cerebral development are still not fully understood. The reprogramming of the expression patterns of MMP and ECM proteins has been clearly shown to link to fetal hypoxia and to have a central role in normal growth and tissue remodeling in the immature heart and brain. Collagens are a major type of protein in the ECM, and their synthesis can be enhanced by fetal hypoxia. Meanwhile, the alteration of MMPs and TIMPs might be initiated to compensate for the accumulation and deposition of collagens; however, the interruption of the fine balance between MMPs and TIMPs after hypoxia might eventually decompensate and impair the fetal heart and brain morphology and function. In addition, TIMPs might have an important role in MMP-independent pathways, such as cell proliferation and cell death, suggesting that TIMPs per se regulate fetal development. Growth abnormalities following fetal hypoxic insult might not appear until exposure to a secondary insult in the offspring, and fetal programming of certain genes is believed to contribute to the heightened susceptibility to challenges later in life. The epigenetic modification is likely to be involved in reprogramming of MMPs and TIMPs that predispose offspring to heart disease and brain injury. Understanding the precise mechanisms by which hypoxia epigenetically modifies these gene expression patterns is important and deserves further attention. Given that ECM and related proteins are the key mediators in tissue remodeling after fetal hypoxia, possible interventions might be to target collagens and MMPs to restore normal morphology and function. Furthermore, whether, and to what extent, the alteration of the expression patterns of MMPs and TIMPs in the fetus persist long term into adulthood require further investigation, and identifying the underlying mechanisms could provide useful insights into future clinical therapeutic approaches.
Acknowledgments
This work was supported, in part, by NIH grants HL82779 (LZ), HL83966 (LZ), HL89012 (LZ), and HD31226 (LZ). We apologize to all authors whose work could not be cited owing to space constraints.
Biography

Lubo Zhang Lubo Zhang is Professor of Pharmacology and Physiology at Loma Linda University School of Medicine. He received his PhD in pharmacology from Iowa State University in 1990, and was the President of the US Western Pharmacology Society in 2008. He has been a member of various study sections of the grant review boards for the US National Institutes of Health and American Heart Association for more than 15 years. Dr Zhang is the author or co-author of over 460 scientific articles, book chapters and abstracts. His research interests focus on the epigenetic mechanisms in developmental programming of adult cardiovascular disease.

Wenni Tong Wenni Tone received her BSc in biotechnology from Wuhan University, China, in 2006. She is currently carrying out research for her doctorate at Loma Linda University School of Medicine. Her research focuses on the effect of maternal hypoxia on matrix metalloproteinase expression patterns in the developing brain and heart. She is the author or coauthor of several papers related to neuroscience and cardiovascular research. She attended the Society for Neuroscience conference in 2008 and received an award at the Experimental Biology meeting in 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ream M, et al. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R583–R595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res. C. 2007;81:215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 3.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr. Mol. Med. 2010;10:653–666. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornburg KL, et al. The role of growth in heart development. Nestle Nutr. Workshop Ser. Pediatr. Program. 2008;61:39–51. doi: 10.1159/000113169. [DOI] [PubMed] [Google Scholar]
- 5.Teitel D, Rudolph AM. Perinatal oxygen delivery and cardiac function. Adv. Pediatr. 1985;32:321–347. [PubMed] [Google Scholar]
- 6.Dasgupta C, Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today. 2011;16:22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L. Prenatal hypoxia and cardiac programming. J. Soc. Gynecol. Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Sundgren NC, et al. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- 9.Sundgren NC, et al. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J. Physiol. 2003;548:881–891. doi: 10.1113/jphysiol.2003.038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraud GD, et al. Estrogen-induced left ventricular chamber enlargement in ewes. Am. J. Physiol. 1993;264:E490–496. doi: 10.1152/ajpendo.1993.264.4.E490. [DOI] [PubMed] [Google Scholar]
- 11.Jonker SS, et al. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp. Physiol. 2010;95:131–139. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manabe I, et al. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ. Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 13.Patterson AJ, et al. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ. Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Q, et al. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc. Res. 2011;89:300–308. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J. Pharmacol. Exp. Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tintu AN, et al. Hypoxia disturbs fetal hemodynamics and growth. Endothelium. 2007;14:353–360. doi: 10.1080/10623320701746347. [DOI] [PubMed] [Google Scholar]
- 17.Louzoun-Kaplan V, et al. Prenatal hypoxia down regulates the GABA pathway in newborn mice cerebral cortex; partial protection by MgSO4. Int J Dev Neurosci. 2008;26:77–85. doi: 10.1016/j.ijdevneu.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Haramati O, et al. Magnesium sulfate treatment alters fetal cerebellar gene expression responses to hypoxia. Int. J. Dev. Neurosci. 2010;28:207–216. doi: 10.1016/j.ijdevneu.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Golan MH, et al. Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology. 2009;57:511–522. doi: 10.1016/j.neuropharm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan SM, et al. Structural remodeling of gray matter astrocytes in the neonatal pig brain after hypoxia/ischemia. Glia. 2010;58:181–194. doi: 10.1002/glia.20911. [DOI] [PubMed] [Google Scholar]
- 21.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 22.Tong W, et al. Maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPs in the brain of neonatal rats. Dev. Neurobiol. 2010;70:182–194. doi: 10.1002/dneu.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 24.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 26.Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 27.Gluckman PD, et al. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 29.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am. J. Public Health. 1997;87:1003–1007. doi: 10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae S, et al. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 31.Camm EJ, et al. Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am. J. Obstet. Gynecol. 2010;203:e24–e34. doi: 10.1016/j.ajog.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, et al. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, et al. Hypoxia during pregnancy in rats leads to early morphological changes of atherosclerosis in adult offspring. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1321–H1328. doi: 10.1152/ajpheart.00440.2008. [DOI] [PubMed] [Google Scholar]
- 34.Xiao D, et al. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J. Soc. Gynecol. Invest. 2000;7:279–283. [PubMed] [Google Scholar]
- 35.Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum. Dev. 2005;81:745–751. doi: 10.1016/j.earlhumdev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Chen LM, et al. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp. Physiol. 2007;92:409–416. doi: 10.1113/expphysiol.2006.036590. [DOI] [PubMed] [Google Scholar]
- 37.Mascio CE, et al. Myocardial vascular and metabolic adaptations in chronically anemic fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1736–R1745. doi: 10.1152/ajpregu.00278.2005. [DOI] [PubMed] [Google Scholar]
- 38.Martin C, et al. Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am. J. Obstet. Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 39.Rueda-Clausen CF, et al. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc. Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 40.Cartwright JE, et al. Hypoxia and placental remodelling. Adv. Exp. Med. Biol. 2007;618:113–126. doi: 10.1007/978-0-387-75434-5_9. [DOI] [PubMed] [Google Scholar]
- 41.Carver W, et al. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat. Rec. 1993;236:511–520. doi: 10.1002/ar.1092360311. [DOI] [PubMed] [Google Scholar]
- 42.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J. Am. Coll. Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 43.Jackson M, et al. Development of the collagen network of the human fetal myocardium: an immunohistochemical study. Int. J. Cardiol. 1993;41:77–86. doi: 10.1016/0167-5273(93)90139-8. [DOI] [PubMed] [Google Scholar]
- 44.Marijianowski MM, et al. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 1994;23:1204–1208. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 45.Carver W, et al. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ. Res. 1991;69:116–122. doi: 10.1161/01.res.69.1.116. [DOI] [PubMed] [Google Scholar]
- 46.Wikman-Coffelt J, et al. The cardiac hypertrophy process. Analyses of factors determining pathological vs. physiological development. Circ. Res. 1979;45:697–707. doi: 10.1161/01.res.45.6.697. [DOI] [PubMed] [Google Scholar]
- 47.Kania G, et al. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc. Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Li G, et al. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1712–H1719. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- 49.Li G, et al. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J. Soc. Gynecol. Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 50.Thornburg KL. Foetal programming reveals the dark side of AT(2)R. Cardiovasc. Res. 2011;89:260–261. doi: 10.1093/cvr/cvq387. [DOI] [PubMed] [Google Scholar]
- 51.Schultz JJ, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakata Y, et al. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res. Cardiol. 2008;103:60–68. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu CP, et al. Blockade of hypoxia-reoxygenation-mediated collagen type I expression and MMP activity by overexpression of TGF-beta1 delivered by AAV in mouse cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1833–H1838. doi: 10.1152/ajpheart.00488.2007. [DOI] [PubMed] [Google Scholar]
- 54.Rees S, et al. An adverse intrauterine environment: implications for injury and altered development of the brain. Int. J. Dev. Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Hallak M, et al. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstet. Gynecol. 2000;96:124–128. doi: 10.1016/s0029-7844(00)00844-9. [DOI] [PubMed] [Google Scholar]
- 56.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Golan H, et al. Maternal hypoxia during pregnancy induces fetal neurodevelopmental brain damage: partial protection by magnesium sulfate. J. Neurosci. Res. 2004;78:430–441. doi: 10.1002/jnr.20269. [DOI] [PubMed] [Google Scholar]
- 58.Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum. Dev. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 59.Guo R, et al. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod. Sci. 2010;17:540–548. doi: 10.1177/1933719110364061. [DOI] [PubMed] [Google Scholar]
- 60.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phatharajaree W, et al. Matrix metalloproteinases and myocardial infarction. Can. J. Cardiol. 2007;23:727–733. doi: 10.1016/s0828-282x(07)70818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Armiento J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends Cardiovasc. Med. 2002;12:97–101. doi: 10.1016/s1050-1738(01)00160-8. [DOI] [PubMed] [Google Scholar]
- 63.Vaillant C, et al. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang TT, Hawkes SP. Role of the 21-kDa protein TIMP-3 in oncogenic transformation of cultured chicken embryo fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10676–10680. doi: 10.1073/pnas.89.22.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greene J, et al. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J. Biol. Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 66.Bigg HF, et al. Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J. Biol. Chem. 1997;272:15496–15500. doi: 10.1074/jbc.272.24.15496. [DOI] [PubMed] [Google Scholar]
- 67.Oh C, et al. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am. J. Obstet. Gynecol. 2008;199:e1–e6. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Fanjul-Fernández M, et al. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Bergman MR, et al. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem. J. 2003;369:485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-kappa B. Eur. J. Biochem. 1995;234:632–640. doi: 10.1111/j.1432-1033.1995.632_b.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen K, et al. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J. Cardiovasc. Pharmacol. 2004;44:682–687. doi: 10.1097/00005344-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 72.Nanduri J, et al. Transcriptional responses to intermittent hypoxia. Respir. Physiol. Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joung YH, et al. Hypoxia activates signal transducers and activators of transcription 5 (STAT5) and increases its binding activity to the GAS element in mammary epithelial cells. Exp. Mol. Med. 2003;35:350–357. doi: 10.1038/emm.2003.46. [DOI] [PubMed] [Google Scholar]
- 74.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J. Cell. Physiol. 2007;213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 75.Chen H, et al. TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1612–H1617. doi: 10.1152/ajpheart.00992.2002. [DOI] [PubMed] [Google Scholar]
- 76.Tian L, et al. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sulik A, Chyczewski L. Immunohistochemical analysis of MMP-9, MMP-2 and TIMP-1, TIMP-2 expression in the central nervous system following infection with viral and bacterial meningitis. Folia Histochem. Cytobiol. 2008;46:437–442. doi: 10.2478/v10042-008-0058-8. [DOI] [PubMed] [Google Scholar]
- 78.Sunagawa S, et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in perinatal asphyxia. Brain Dev. 2009;31:588–593. doi: 10.1016/j.braindev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Ryu J, et al. Differential expression of matrix metalloproteinases and their inhibitors in human and mouse lung development. Thromb. Haemost. 2005;94:175–183. doi: 10.1160/TH04-10-0656. [DOI] [PubMed] [Google Scholar]
- 80.Deschamps AM, Spinale FG. Disruptions and detours in the myocardial matrix highway and heart failure. Curr. Heart Fail. Rep. 2005;2:10–17. doi: 10.1007/s11897-005-0002-6. [DOI] [PubMed] [Google Scholar]
- 81.Chen W, et al. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J. Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- 82.Gross J. How tadpoles lose their tails: path to discovery of the first matrix metalloproteinase. Matrix Biol. 2004;23:3–13. doi: 10.1016/j.matbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Ohuchi E, et al. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 84.Vogel W, et al. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 85.Goldsmith EC, et al. Organization of fibroblasts in the heart. Dev. Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 86.Olaso E, et al. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J. Biol. Chem. 2002;277:3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- 87.Hou G, et al. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ. Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- 88.Chen SC, et al. Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochem. Biophys. Res. Commun. 2008;374:662–667. doi: 10.1016/j.bbrc.2008.07.092. [DOI] [PubMed] [Google Scholar]
- 89.Ferri N, et al. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am. J. Pathol. 2004;164:1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldsmith EC, et al. The collagen receptor DDR2 is expressed during early cardiac development. Anat. Rec. 2010;293:762–769. doi: 10.1002/ar.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polyakova V, et al. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J. Am. Coll. Cardiol. 2004;44:1609–1618. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 92.Hubert T, et al. Collagens in the developing and diseased nervous system. Cell. Mol. Life Sci. 2009;66:1223–1238. doi: 10.1007/s00018-008-8561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roig B, et al. Expression of the tyrosine kinase discoidin domain receptor 1 (DDR1) in human central nervous system myelin. Brain Res. 2010;1336:22–29. doi: 10.1016/j.brainres.2010.03.099. [DOI] [PubMed] [Google Scholar]
- 94.Hayakawa T, et al. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 95.Hayakawa T, et al. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J. Cell. Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 96.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci. Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woessner JF., Jr That impish TIMP: the tissue inhibitor of metalloproteinases-3. J. Clin. Invest. 2001;108:799–800. doi: 10.1172/JCI13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammoud L, et al. Tissue inhibitor of metalloproteinase-3 inhibits neonatal mouse cardiomyocyte proliferation via EGFR/JNK/SP-1 signaling. Am. J. Physiol. Cell. Physiol. 2009;296:C735–745. doi: 10.1152/ajpcell.00246.2008. [DOI] [PubMed] [Google Scholar]
- 99.Reik W, et al. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 100.Dean W, et al. DNA methylation in mammalian development and disease. Birth Defects Res. C. 2005;75:98–111. doi: 10.1002/bdrc.20037. [DOI] [PubMed] [Google Scholar]
- 101.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Scarano MI, et al. DNA methylation 40 years later: its role in human health and disease. J. Cell. Physiol. 2005;204:21–35. doi: 10.1002/jcp.20280. [DOI] [PubMed] [Google Scholar]
- 103.Turek-Plewa J, Jagodziński PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell. Mol. Biol. Lett. 2005;10:631–647. [PubMed] [Google Scholar]
- 104.Meyer K, Zhang L. Fetal programming of cardiac function and disease. Reprod. Sci. 2007;14:209–216. doi: 10.1177/1933719107302324. [DOI] [PubMed] [Google Scholar]
- 105.Lawrence J, et al. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc. Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang H, et al. Maternal cocaine administration causes an epigenetic modification of protein kinase C epsilon gene expression in fetal rat heart. Mol. Pharmacol. 2007;71:1319–1328. doi: 10.1124/mol.106.032011. [DOI] [PubMed] [Google Scholar]
- 107.Chernov AV, et al. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J. Biol. Chem. 2009;284:12727–12734. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Couillard J, et al. The role of DNA hypomethylation in the control of stromelysin gene expression. Biochem. Biophys. Res. Commun. 2006;342:1233–1239. doi: 10.1016/j.bbrc.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 109.Chicoine E, et al. Evidence for the role of promoter methylation in the regulation of MMP-9 gene expression. Biochem. Biophys. Res. Commun. 2002;297:765–772. doi: 10.1016/s0006-291x(02)02283-0. [DOI] [PubMed] [Google Scholar]
- 110.Clark SJ, et al. Sp1 binding is inhibited by (m)Cp(m)CpG methylation. Gene. 1997;195:67–71. doi: 10.1016/s0378-1119(97)00164-9. [DOI] [PubMed] [Google Scholar]
- 111.Shahrzad S, et al. Induction of DNA hypomethylation by tumor hypoxia. Epigenetics. 2007;2:119–125. doi: 10.4161/epi.2.2.4613. [DOI] [PubMed] [Google Scholar]
- 112.Chernov AV, et al. Microarray-based transcriptional and epigenetic profiling of matrix metalloproteinases, collagens, and related genes in cancer. J. Biol. Chem. 2010;285:19647–19659. doi: 10.1074/jbc.M109.088153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin L, Han YP. Epigenetic repression of matrix metalloproteinases in myofibroblastic hepatic stellate cells through histone deacetylases 4: implication in tissue fibrosis. Am. J. Pathol. 2010;177:1915–1928. doi: 10.2353/ajpath.2010.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kee HJ, Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J. Biomed. Biotechnol. 2011 doi: 10.1155/2011/928326. DOI: 10.1155/2011/928326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeon HW, Lee YM. Inhibition of histone deacetylase attenuates hypoxia-induced migration and invasion of cancer cells via the restoration of RECK expression. Mol. Cancer Ther. 2010;9:1361–1370. doi: 10.1158/1535-7163.MCT-09-0717. [DOI] [PubMed] [Google Scholar]
- 116.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 117.Agocha A, et al. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-beta1, thyroid hormone, angiotensin II and basic fibroblast growth factor. J. Mol. Cell. Cardiol. 1997;29:2233–2244. doi: 10.1006/jmcc.1997.0462. [DOI] [PubMed] [Google Scholar]
- 118.Rosenberg GA, et al. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 119.Yang Y, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 120.Gu Z, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tejima E, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma. 2009;26:1935–1941. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heeneman S, et al. The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J. Pathol. 2003;200:516–525. doi: 10.1002/path.1395. [DOI] [PubMed] [Google Scholar]