Abstract
Dipyrone is a common antipyretic drug and the most popular non-opioid analgesic in many countries. In spite of its long and widespread use, molecular details of its fate in the body are not fully known. We administered dipyrone orally to mice. Two unknown metabolites were found, viz. the arachidonoyl amides of the known major dipyrone metabolites, 4-methylaminoantipyrine (2) and 4-aminoantipyrine (3). They were identified by ESI-LC-MS/MS after extraction from the CNS, and comparison with reference substances prepared synthetically. The arachidonoyl amides were positively tested for cannabis receptor binding (CB1 and CB2) and cyclooxygenase inhibition (COX-1 and COX-2 in tissues and as isolated enzymes), suggesting that the endogenous cannabinoid system may play a role in the effects of dipyrone against pain.
Keywords: Dipyrone, analgesia, cyclooxygenase, arachidonic acid amides, cannabinoid receptors
1. Introduction
Dipyrone (1; metamizole sodium; chemical name, sodium N-(2,3-dimethyl-5-oxo-1-phenyl-3-pyrazolin-4-yl)-N-methylaminomethanesulphonate, usually as monohydrate) was first marketed in Germany in 1922.1,2 The low toxicity of dipyrone and its efficacy support its use in clinical practice. It has been banned in some countries on account of its potential to cause blood dyscrasias, however, reported estimates of the risk of agranulocytosis vary by several orders of magnitude and may depend on dose, duration and concomitant medication.3, 4
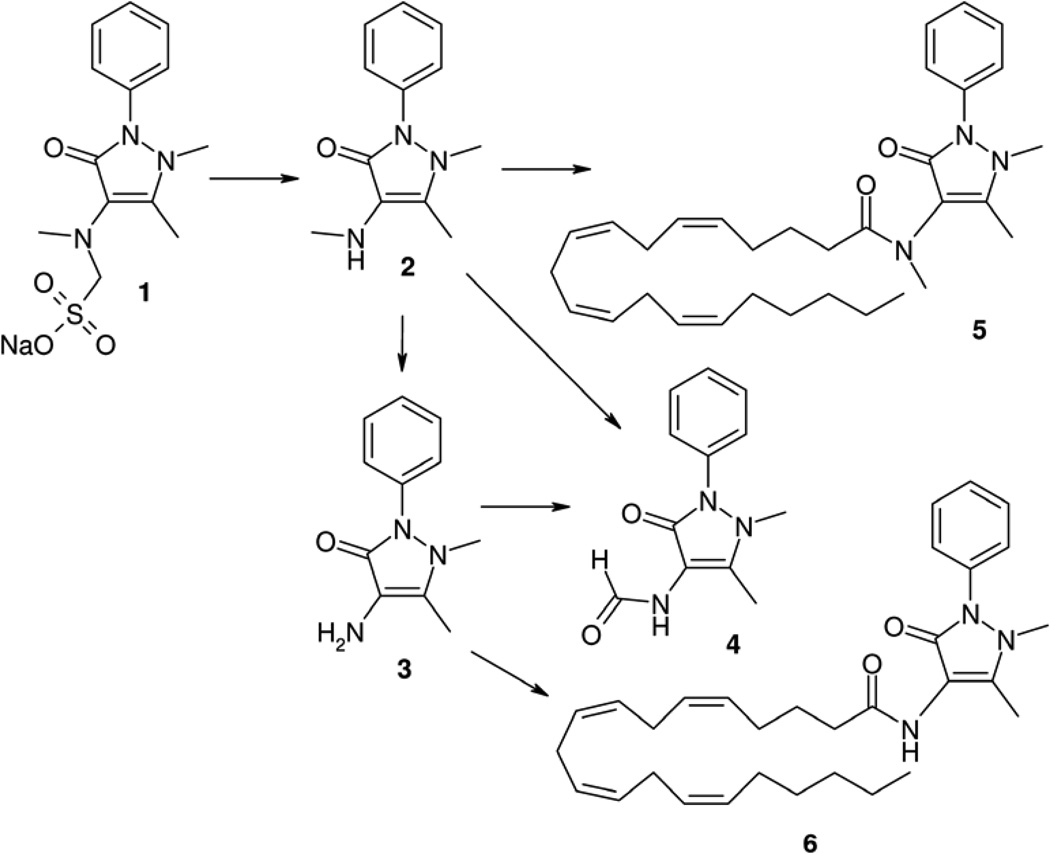
Dipyrone is detectable in the serum for only about 15 min following intravenous administration, and is not detectable after oral intake.5,6 The pharmacokinetics of dipyrone (Figure 1) are characterised by a rapid hydrolytic retro-Mannich reaction to 4-methylaminoantipyrine (2), which has 85% bioavailability after oral administration, and takes a short time to achieve maximal systemic concentrations (tmax of 1.2–2.0 hours). Compound 2 is further metabolised, with an average elimination half-life (t1/2) of 2.6–3.5 hours, to 4-aminoantipyrine (3) and 4-formylaminoantipyrine (4), which is an end-metabolite.6
Figure 1.
Metabolites of dipyrone (1)
The molecular mechanism of analgesic and antipyretic action of dipyrone has been under debate for a long time. Both central7,8 and peripheral inhibition of prostaglandin biosynthesis is presumed to be at the basis of dipyrone action.9,10 The onset and duration of the analgesic effects correlate with saliva concentrations of 2 and 3.11 Importantly, 2 was found to inhibit both cyclooxygenase (COX) isoforms, COX-1 and COX-2, at concentrations that were found in plasma after standard doses of dipyrone,12 probably by sequestering radicals that are necessary to initiate the catalytic activity of the COX enzymes.13 Although the lack of acidity was proposed to be responsible for the favourable gastrointestinal tolerability of dipyrone over acidic antiinflammatory drugs,12 the mechanism of action is still a matter of debate.14
We hypothesize that either unidentified alternative targets of 2, 3, and/or 4, or unidentified active metabolites contribute to the activity of dipyrone. Considering the pharmacokinetics, high dosage, and rather short duration of activity, unidentified active metabolites should be closely related to 2. We reasoned that central inhibition of COX-1 and COX-2 and activation of cannabinoid receptors of type 1 and 2 (CB1 and CB2), two new targets for the development of new analgesic drugs,15 might account for much of the clinical profile of dipyrone. Recently, Vazquez-Rodriguez et al. provided evidence that the antinociceptive effects of dipyrone following microinjection into ventrolateral periaqueductal grey matter16 were reversed by the CB1 antagonist AM251.17 Both 2 and 3 easily cross the blood-brain barrier, reaching concentrations of 5–30 µg ml−1 (2) and 1–7 µg ml−1 (3) in the cerebrospinal fluid.18 However, these compounds are very weak inhibitors of both COX isoenzymes with an IC50 of approximately 10−3 M.19 Another study reported comparatively low activity against purified COX-1 and COX-2, or COX-1 in intact bovine aortic endothelial cells and in human platelets, but higher activity against COX-2 in murine macrophages and primary human leukocytes, viz. approximately 12 and 21 µg ml−1.20 However, the authors stated that "the term metamizol (to) encompasses the entire set of pyrazolone derivatives" (dipyrone, 2, 3, 4), without specifying if they added dipyrone or a mixture of the pyrazolones to the various test systems. The different pyrazolones have differential effects and fate when added to systems with active enzymes. Since not even the relative ratio of the pyrazolones was reported, the results are difficult to interpret and compare with other findings.
Both 2 and 3 are weakly basic amines. We therefore assumed that they might be acylated within the CNS, leading to fatty acid amides. A related mechanism of action was proposed for acetaminophen (paracetamol).21, 22 The amide that could most likely exhibit COX inhibition is the arachidonoyl amide of 2 (5). As standards, we prepared the arachidonoyl amides 5 and 6 from 2 and 3 and arachidonoyl chloride. We then examined whether they are indeed produced in vivo from dipyrone and tested their activity in a variety of assays.
2. Results
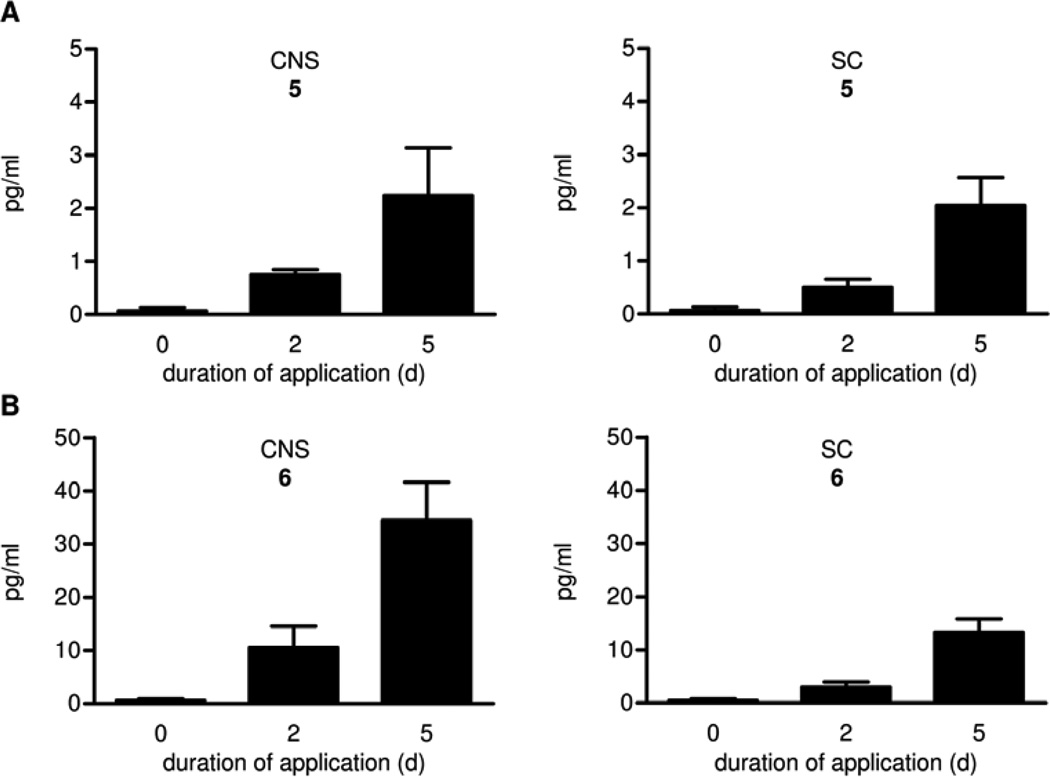
Wild-type mice fed dipyrone via drinking water possessed detectable levels of the arachidonoyl amides 5 and 6 in brain and spinal cord (SC) two days after the application of dipyrone, and slightly higher amounts after five days (Figure 2). The following data and considerations prove 5 and 6 to be the arachidonoyl amides of 2 and 3. In HPLC experiments with different eluants, retention times of the standards and material from animal tissue were identical. The LC-MS peak pattern of standards and samples was also identical. The product ions of greatest intensity (m/z 218.2 (5) and m/z 204.2 (6)) resulted from loss of arachidonic acid, as were clearly seen in tandem mass spectroscopy. Two other different ions were registered isochronically for 6 ((M)+ m/z 490.4, (M-H2O)+ m/z 472.4) and one for 5 ((M)+ m/z 504.5) (data not shown) and were found to be identical in retention time and relative intensities for both the standard and the sample. The amount of 5 and 6 detectable was directly associated with the feeding duration of dipyrone, and they were not present in negative controls.
Figure 2.
Concentrations of 5 (A) and 6 (B) in the brain (denoted CNS) and the spinal cord (SC) of C57BL/6 wild-type mice (n = 3 mice/group, mean ± SEM).
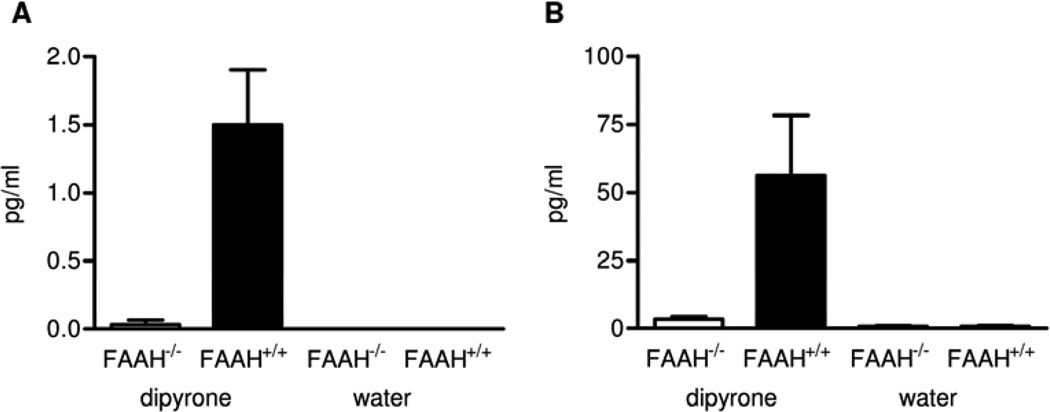
We investigated the involvement of fatty acid amide hydrolase (FAAH), an enzyme involved in both the hydrolysis and synthesis of some long chain fatty acid amides 23, 24 in the formation of 5 and 6. For this purpose, dipyrone was fed to wild-type and FAAH−/− mice25 for 5 days. The arachidonoyl amides 5 and 6 were detectable in substantial amounts in wild-type mice (FAAH+/+) after 2 days of the application of dipyrone, and in slightly higher amounts after 5 days of application. Compared to wild-type mice, less than 10% of the amides were formed in FAAH−/− mice. In controls (0 days) without addition of dipyrone to the drinking water, no substantial amount of 5 and 6 was observed (Figure 3).
Figure 3.
Concentrations of 5 (A) and 6 (B) in the brain of wild-type mice (FAAH+/+) and of FAAH knockout mice (FAAH−/−) after administration of dipyrone or water for five days (n = 3 mice/group, mean ± SEM).
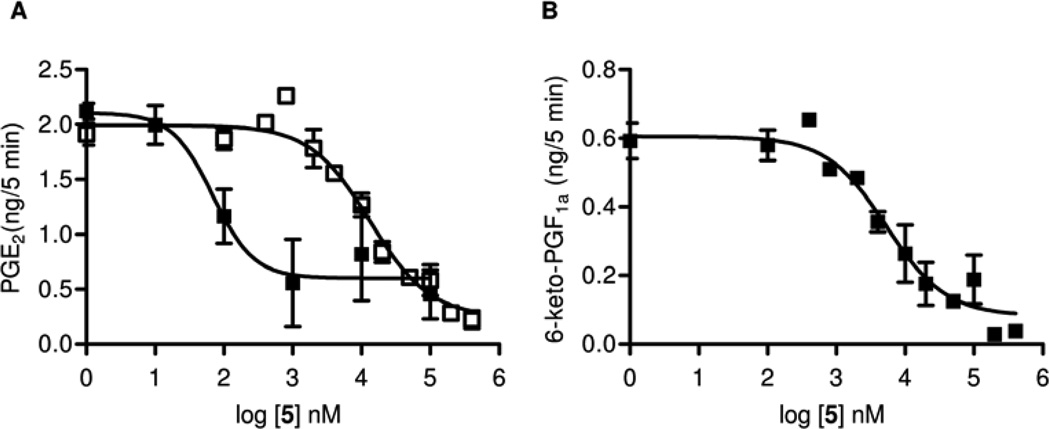
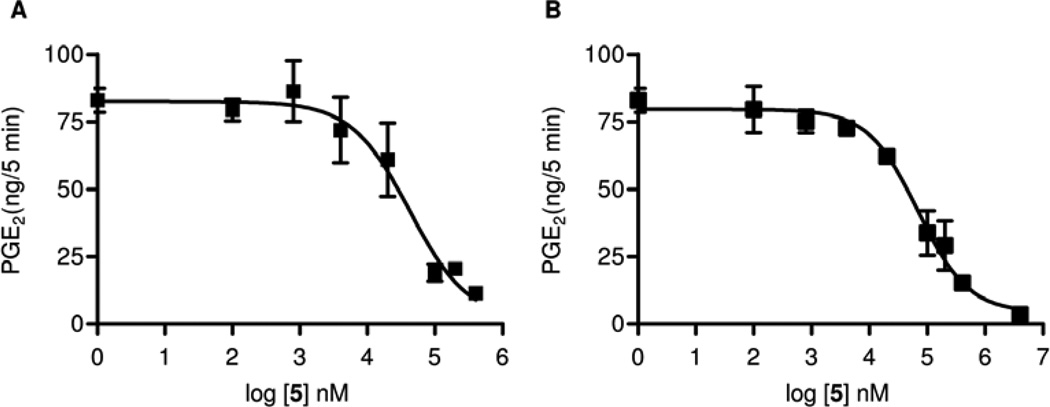
In the following set of experiments we characterized 5 and 6 in vitro. Derivatives and mimics of arachidonic acid are central metabolites in the COX and endocannabinoid (CB) systems,15 so we studied the inhibitory effect on COX. Both compounds inhibited the formation of COX products. Following addition of exogenous arachidonic acid to the brain homogenates, amide 5 inhibited the formation of PGE2 with an IC50 of 13.2 µM (Figure 4 A) and the formation of 6-keto-PGF1α, the stable hydrolysis product of PGI2, with an IC50 of 5.2 µM (Figure 4 B). In contrast, 6 exerted no effect on PGE2 formation by brain homogenates over a concentration range of 100 nM to 100 µM (Figure 5).
Figure 4.
Inhibition of PGE2 formation in brain homogenates in the presence ■ and absence □ of the FAAH inhibitor, 4-benzyloxyphenyl-n-butylcarbamate (URB532) (A) and inhibition of 6-keto-PGF1α formation in brain homogenates (B) by 5 (n = 4, mean ± SEM).
Figure 5.
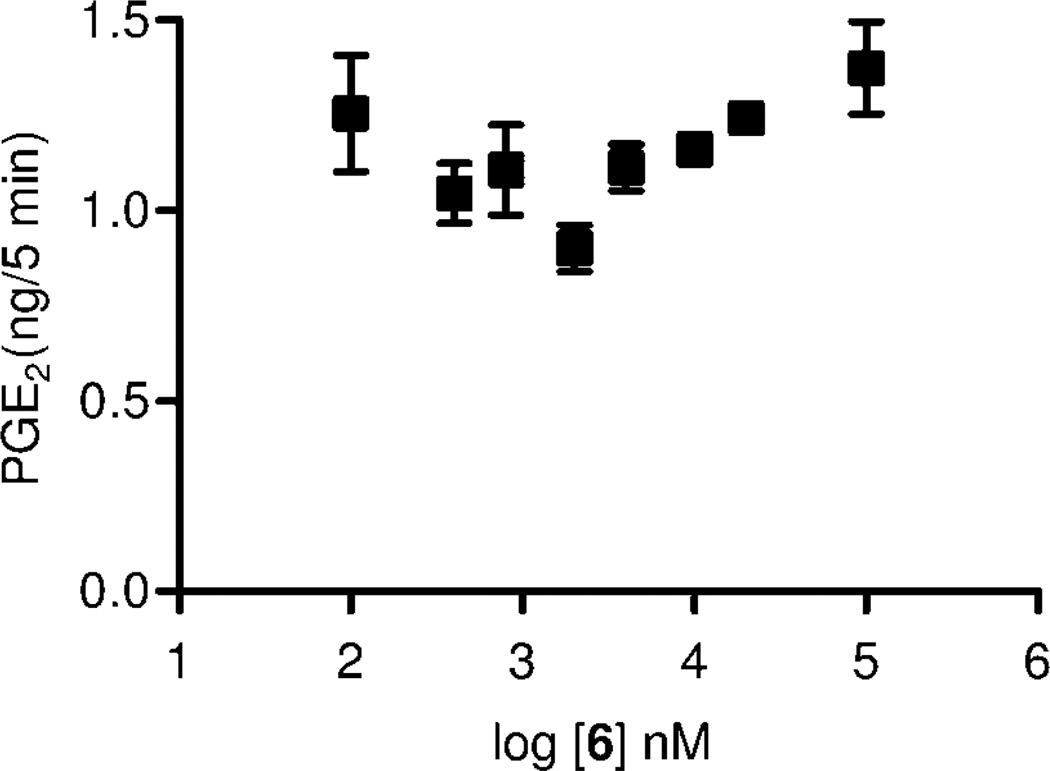
Inhibition of PGE2 formation in brain homogenate by 6 (n = 4, mean ± SEM).
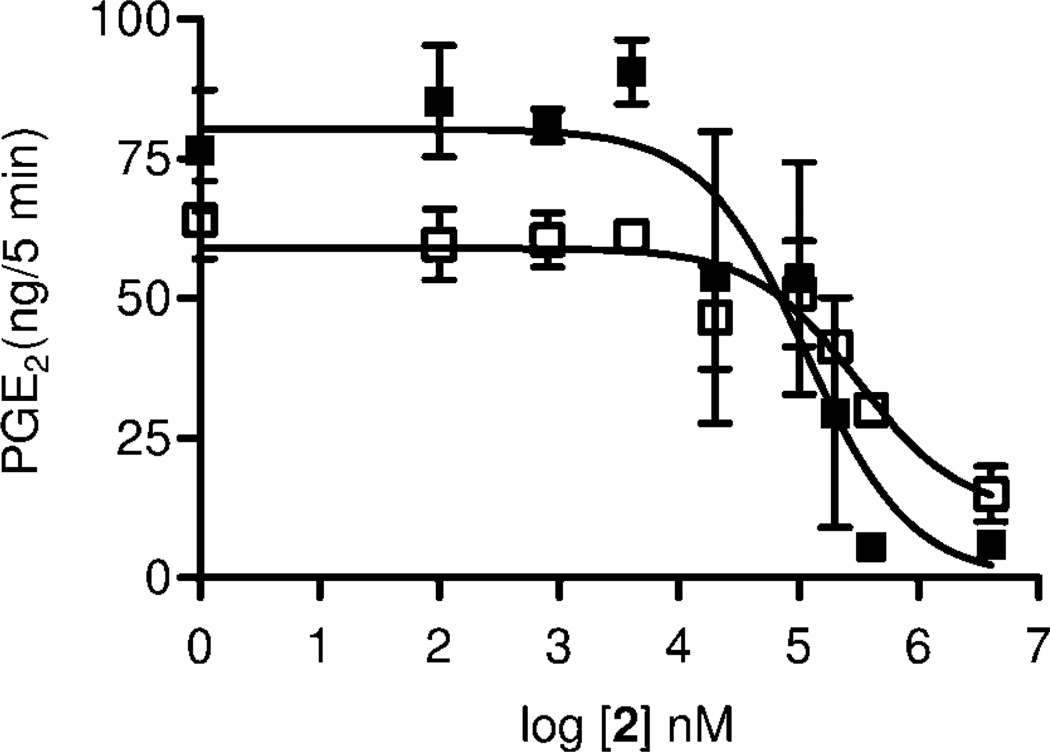
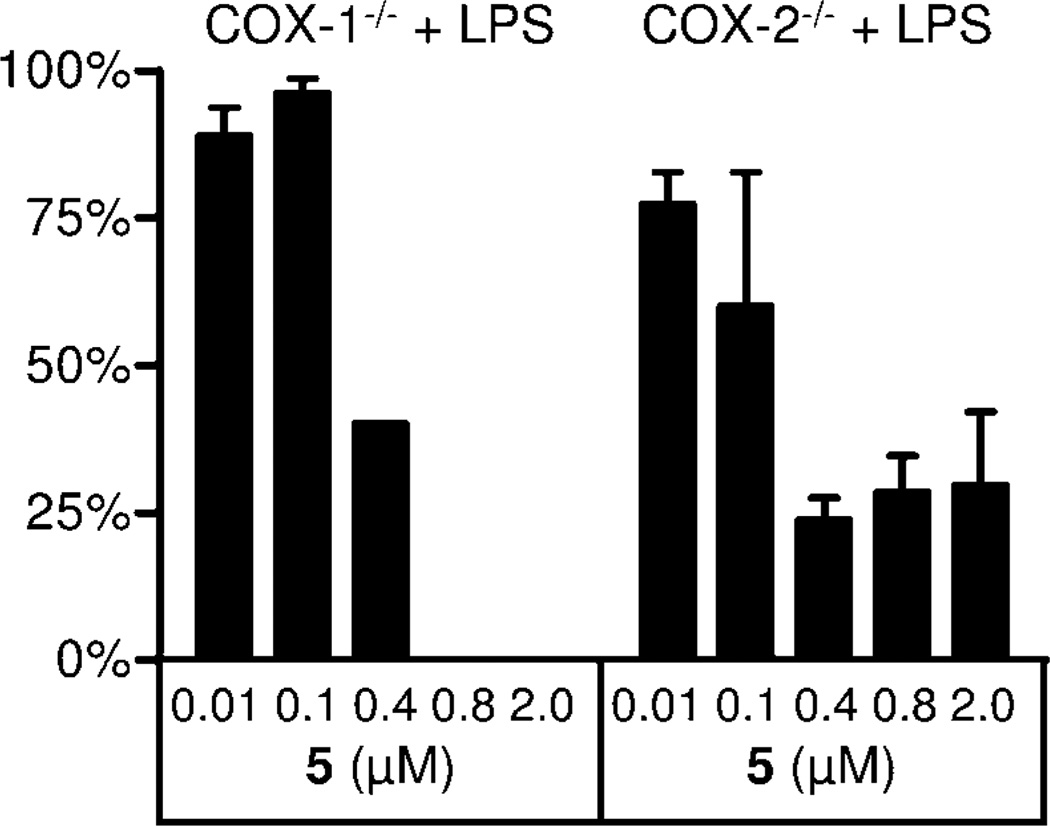
Therefore we determined the inhibitory potency of 5 on COX-1 and on COX-2 isolated enzyme activity, we also used isolated enzyme preparations. As shown in Fig. 6, both enzymes were blocked with similar potency. For COX-1, we found an IC50 of 42 µM (Figure 6 A), and for COX-2 ínhibition with an IC50 of 69 µM (Figure 6 B). The precursor of 5, the amine 2, displayed weaker inhibition at an IC50 of 115 µM for COX-1 and 307 µM for COX-2 (Figure 7). Furthermore, inhibition of PGE2 formation in brain homogenates of LPS stimulated COX-1−/− and COX-2−/− mice by 5 was assessed. We did not observe any formation of PGE2 in brain tissue from COX-1−/− mice with 0.8 µM 5 (IC50, 355 nM), while formation of PGE2 in brain tissue from COX-2−/− mice was reduced to 30% with 0.4 µM 5 (IC50 107 nM) (Figure 8). This can be interpreted by assuming suppression of residual PGE2 formation by COX-1 in COX-2−/− and of COX-2 in COX−/− mice by 5.
Figure 6.
Inhibition of COX-1 (isolated enzyme) (A) and COX-2 (isolated enzyme) (B) by 5 (n = 4, mean ± SEM).
Figure 7.
Inhibition of COX-1 ■ and COX-2 □ (isolated enzyme) by 2 (n = 4, mean ± SEM).
Figure 8.
Inhibition of PGE2 formation by 5 in brain homogenates of LPS stimulated COX1−/− and COX2−/− mice (n = 2, mean ± SEM).
The inhibitory potency of 5 was further analyzed in mouse brain homogenates in the presence or absence of the FAAH inhibitor 4-benzyloxyphenyl-n-butylcarbamate (URB532).26 The FAAH inhibitor shifted the dose-response curve for inhibition of PGE2 formation to the left to yield a calculated IC50 of 58 nM (Figure 4 A), thus suggesting that 5 might also be a FAAH substrate. This has implications for the analgesic activity of 1 – see Discussion below.
Since the amide of arachidonic acid N-arachidonoylethanolamide (anandamide) binds to cannabinoid CB1 and CB2 receptors,15 we tested the two compounds 5 and 6 on human recombinant CB1 and CB2 in specific binding assays. Compound 5 exhibited affinity for both CB1 (Ki = 7.8±0.8 µM) and CB2 (Ki = 3.0±0.4 µM) receptors. Similarly, compound 6 exhibited affinity for both CB1 (Ki = 2.9±0.3 µM) and CB2 (Ki = 5.4±0.6 µM) receptors (means ± SEM, N=3).
3. Discussion
Our findings suggest that the analgesic effect of dipyrone may be partly mediated by a dual mechanism of action: The inhibition of COX enzyme activity and the stimulation of CB receptors. Prerequisite for this combinatory action is the acylation of its primary metabolite 2 with arachidonic acid. Recently a similar analgesic mechanism has been shown for paracetamol (acetaminophen), which partly acts after metabolic conversion to N-arachidonoylaminophenol (AM 404) through a combination of COX inhibition and stimulation of CB1 receptors and transient receptor potential vanilloid type-1 (TRPV1) channels.21,27 Antinociception was observed after intracerebroventricular injection of 10 nmol of AM404. Presently both agonistic and antagonistic effects at TRPV1 are supposed to lead to antinociception.31 Possibly similar to the multiple mechanism of action of paracetamol, we herein report evidence that dipyrone is similarly activated metabolically by acylation of its primary metabolite 2 with arachidonic acid to give 5. With an IC50 at TRPV1 receptors of 300 nM, as reported by us32, 5 may well contribute to the analgesic activity of dipyrone. For the TRPV1 agonist AM404, we found an EC50 of 26 nM.33 The N-demethylated arachidonate, 6, had no activity at TRPV1.
In a recent paper on the different pharmacological actions of paracetamol and dipyrone, the possibility of formation of 5 was ruled out because 4-methylaminoantipyrine was stated to be a tertiary amine.14 In fact, it is a secondary amine, and we were able to acylate it efficiently with arachidonic acid. Our data show that the resulting amide 5 and the related amide 6 are indeed formed in vivo after administration of dipyrone and are thus metabolites of this drug substance. The arachidonoyl amides 5 and 6 bound to both human recombinant cannabinoid CB1 and CB2 receptors at micromolar concentrations, and 5 concentration-dependently inhibited both isolated COX isoforms as well as PGE2 and PGI2 and LPS-induced PGE2 formation in mouse brain homogenates at nano- or micromolar concentrations that are attainable at normal dosages of dipyrone, which are rather high (500–1000 mg single dose).
Because FAAH, the enzyme responsible for hydrolysis of several fatty acid amides, may also act in the reverse direction and catalyze the condensation between long chain fatty acids and amines, as shown for AM404,21 we examined whether this enzyme is involved in the formation and degradation of 5 and 6. Indeed, FAAH seems to be responsible for the formation of these metabolites because less than 2% formation of 5 and about 6% formation of 6 was found in brain tissue from FAAH−/− mice as compared to wild type mice. Furthermore, we found that the FAAH inhibitor URB532 shifted the dose-response curve of 5 for inhibition of PGE2 formation in mouse brain homogenates to the left resulting in a more than 200 fold lower IC50 value. This finding supported our hypothesis that 5 was gradually hydrolyzed in the brain homogenates in which FAAH is very abundant. Consequently, the lower IC50 value calculated in the presence of the FAAH inhibitor reflects the fact that 5 can not only be produced but also hydrolysed by FAAH. Since FAAH activity is highest in the CNS and liver, we hypothesize that an additive part of the analgesic and antipyretic activity of dipyrone is due to a conversion to its respective arachidonoyl amides in the CNS. This would also explain its very weak peripheral antiinflammatory activity.
4. Conclusions
The CB receptor agonist, Δ9-tetrahydrocannabinol, is known to have spasmolytic activity.28 With this in mind, it is intriguing to speculate that the spasmolytic effect of dipyrone perhaps is caused by formation of 5 and 6 in other tissues with resulting stimulation of CB receptors. Further studies will shed light on this aspect and on the details of the tissue distribution of the active metabolites 5 and 6 that we identified in the course of this study. We believe to have identified that dipyrone acts as prodrug for two substances that potentially elicit analgesic effects through the endocannabinoid system. With both paracetamol and dipyrone being activated through conversion to arachidonoyl amides, it seems likely that other drug substances might be converted to fatty acid derivatives that are responsible for at least part of their pharmacological activity. Moreover, knowledge of this dual mechanism of analgesia by arachidonoyl amides would allow deliberate development of pain killers with higher activity than dipyrone and paracetamol, allowing for lower dosages.
5. Experimental Section
5.1. Syntheses
Arachidonoyl-4-methylaminoantipyrin (5; (5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid (1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-methyl-amide). Arachidonic acid (0.200 g, 0.66 mmol) and dimethylformamide p.a. (48 mg, 0.66 mmol) were dissolved in benzene p.a. (5 ml). The mixture was cooled to 0°C, two equivalents of oxalyl chloride (0.168 g, 2.0 mmol) were added dropwise, and stirring was continued for 1 h at 0°C. After dilution with tetrahydrofurane p.a. (THF, 5 ml), methylaminoantipyrin (2) (0.717 g, 3.3 mmol) was added in 5 ml THF solution. The mixture was stirred for another 15 min, diluted with dichloromethane, and washed with aqueous 10% HCl, 1M NaOH and water. The organic phase was dried over anhydrous magnesium sulphate and evaporated to dryness. The residue was purified by flash chromatography on normal phase silica gel (40–63 µm), eluting with CHCl3/EtOH (9+1). Yield, 0.223 g (67%) of a slightly greenish oil. EI-MS (30 eV), m/z (%) = 218 (100), 504 (M+) (9.5). EI-MS (60 eV), m/z (%) = 56 (100), 97 (48.7), 159 (19.1), 218 (11.8), 187 (9.0).- 1H NMR (400 MHz, CDCl3), δ: 7.48-7.28 (m, 5H, 5 × Ar-H), 5.41-5.27 (m, 8H, 4 × CH=CH), 3.29 (s, N-CH3, peak of rotational isomer of the product), 3.13 (s, 3H, N-CH3), 3.11 (s, 3H, CO-N-CH3), 3.07 (s, CO-N-CH3, peak of rotational isomer of the product), 2.82-2.73 (m, 6H, 3 × CH=CH-CH2-CH=CH), 2.27 (dt, 2J = 15.0 Hz, 3J = 7.5 Hz, 1H, CH2-CH2-CO-N-CH3), 2.18 (s, 3H, C=C-CH3), 2.12 (dt, 2J = 15.0 Hz, 3J = 7.5 Hz, 1H, CH2-CH2-CO-N-CH3), 2.10 (s, C=C-CH3, peak of rotational isomer of the product), 2.07-2.00 (m, 4H, 2 × CH=CH-CH2), 1.67 (tt, 3J = 3J = 7.5 Hz, 2H, CH2-CH2-CO-N-CH3), 1.36-1.23 (m, 6H, CH2-CH2-CH2-CH3), 0.87 (t, 3J = 6.8 Hz, 3H, …-CH3); 13C NMR (100 MHz, CDCl3), δ: 174.3, 161.7, 151.7, 134.6, 130.5, 129.6, 129.3, 129.1, 128.6, 128.3, 128.1, 127.9, 127.6, 127.1, 124.1, 115.4, 36.0, 35.7, 32.8, 31.6, 29.4, 27.3, 26.8, 25.7, 25.0, 22.6, 14.1, 10.6. - C32H45N3O2 (503.77). Calc., C 76.29 H 9.00 N 8.34, found, C 76.41 H 8.89 N 8.46.
Arachidonoyl-4-aminoantipyrin (6; (5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid (1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)- amide). Arachidonic acid (0.200 g, 0.66 mmol) and dimethylformamide p.a. (48 mg, 0.66 mmol) were dissolved in benzene p.a. (5 ml). The mixture was cooled to 0°C, two equivalents of oxalyl chloride (0.168 g, 2.0 mmol) were added dropwise, and stirring was continued for 1 h at 0°C. After dilution with tetrahydrofurane p.a. (THF, 5 ml), aminoantipyrin (3) (0.671 g, 3.3 mmol) was added in 5 ml THF solution. The mixture was stirred for another 15 min, diluted with dichloromethane, and washed with aqueous 10% HCl, 1M NaOH and water. The organic phase was dried over anhydrous magnesium sulphate and evaporated to dryness. The residue was purified by flash chromatography on normal phase silica gel (40–63 µm), eluting with CHCl3/EtOH (9+1). Yield, 0.211 g (65%) of a slightly yellow oil. EI-MS (30 eV), m/z (%) = 204 (100), 472 (48.7), 490 (M+) (45.2).- 1H NMR (400 MHz, CDCl3), δ: 8.26 (br s, 1H, CO-NH), 7.45-7.25 (m, 5H, 5 × Ar-H), 5.42-5.27 (m, 8H, 4 × CH=CH), 3.04 (s, 3H, N-CH3), 2.84-2.76 (m, 6H, 3 × CH=CH-CH2-CH=CH), 2.29 (t, 3J = 7.5 Hz, 2H, CH2-CH2-CO-NH), 2.20 (s, 3H, C=C-CH3), 2.11-2.00 (m, 4H, 2 × CH=CH-CH2), 1.70 (tt, 3J = 3J = 7.5 Hz, 2H, CH2-CH2-CO-NH), 1.38-1.22 (m, 6H, CH2-CH2-CH2-CH3), 0.87 (t, 3J = 7.1 Hz, 3H, …-CH3); 13C NMR (100 MHz, CDCl3), δ: 172.1, 161.8, 149.6, 134.6, 130.4, 129.3, 129.2, 128.51, 128.48, 128.3, 128.1, 127.9, 127.5, 126.8, 124.2, 109.0, 36.2, 35.6, 31.5, 29.3, 27.2, 26.8, 25.7, 25.6, 22.6, 14.1, 12.5.- C31H43N3O2 (489.76). Calc., C 76.02 H 8.84 N 8.58, found, C 76.05 H 9.03 N 8.43.
5.2 Assays
5.2.1 Feeding experiments
C57BL/6 mice were purchased from Charles River, Sulzfeld, Germany, housed under controlled conditions, and fed a standard chow diet. FAAH knock out mice were kindly provided by Beat Lutz (University of Mainz, Germany). COX-1 and COX-2 knockout mice were breeded as described by us.29 The study was performed with permission of the regional animal welfare committee in Giessen (RP Giessen; Germany). Mice were housed under controlled conditions with standardized air conditioning at 20°–22°C, 50–57% relative humidity, and a 12-hour artificial day/night rhythm. They were given standard diet and water ad libitum.
Dipyrone (Novaminsulfon-Ratiopharm ampule, containing dipyrone-sodium monohydrate) was given to the drinking water. Drinking volume was monitored daily and addition of 1 to the drinking water was adjusted accordingly to result in an approximate daily dose of 600 mg kg−1 bodyweight. The animals were sacrificed on day 2 or day 5 resulting in time of drug administration of 48 h and 120 h, respectively. Mice were anesthetized with isoflurane, brains quickly removed and chilled in ice-cold H2O. Vertebral column was dissected and a median laminectomy under a dissecting microscope exposed the spinal cord. The cord was transected at the medullary-spinal junction (C1), gently removed in toto from the vertebral column, and chilled in ice-cold H2O. Tissue samples were immediately homogenized and extracted with diisopropyl ether/ethyl acetate (1:3, v/v). Following centrifugation, the solvents were evaporated and acetonitrile/water (9:1, v/v) was added. An aliquot of this solution was analyzed by LC-MS/MS. Different amounts of synthetic 5 and 6 were used as external calibration samples.
5.2.2 Determination of 5 and 6 by ESI-LC-MS/MS
Ethyl acetate was purchased from Promochem (Wesel, Germany), acetic acid was obtained from Aldrich (Taufkirchen, Germany), diisopropyl ether and acetonitrile from Merck (Darmstadt, Germany).
The HPLC system consisted of a Perkin Elmer series 200 pump, a CTC HTS PAL autosampler (CTC Analytics, Zingen, Switzerland) and an Applied Biosystems API 3000 triple-quad tandem mass spectrometer (Applied Biosystems, Thornhill, Canada) equipped with a turbo ion interface.
The isocratic mobile phase consisted of 60% acetonitrile in water with 0.2% acetic acid and was pumped at a flow-rate of 0.2 ml min−1. Chromatography was performed on a Polaris (Varian Deutschland GmbH, Darmstadt, Germany) C18-A 3 µm; 50×2 mm narrow bore analytical column. The total runtime was 25 min with 5 eluting at 16.5 min and 6 at 13.2 min.
Detection by tandem mass spectrometry was based on precursor ion transitions to the strongest intensity product ions. Instrumental conditions were optimized to afford best sensitivity (dwell time 400 ms, DP 50 V, FP 160 V, EP 9 V). For positive ionization mode, the first quadrupole, Q1, was set to monitor the protonated molecules (M+H)+ at m/z 504.6 for 5, and m/z 490.4 for 6 with collision-induced fragmentation at Q2 (collision gas nitrogen, collision energy 30 eV), and monitoring the product ions via Q3 at m/z 218.2 for 5 and m/z 472.4 for the first measured ion and m/z 204.2 for the second product ion of 6 (data not shown).
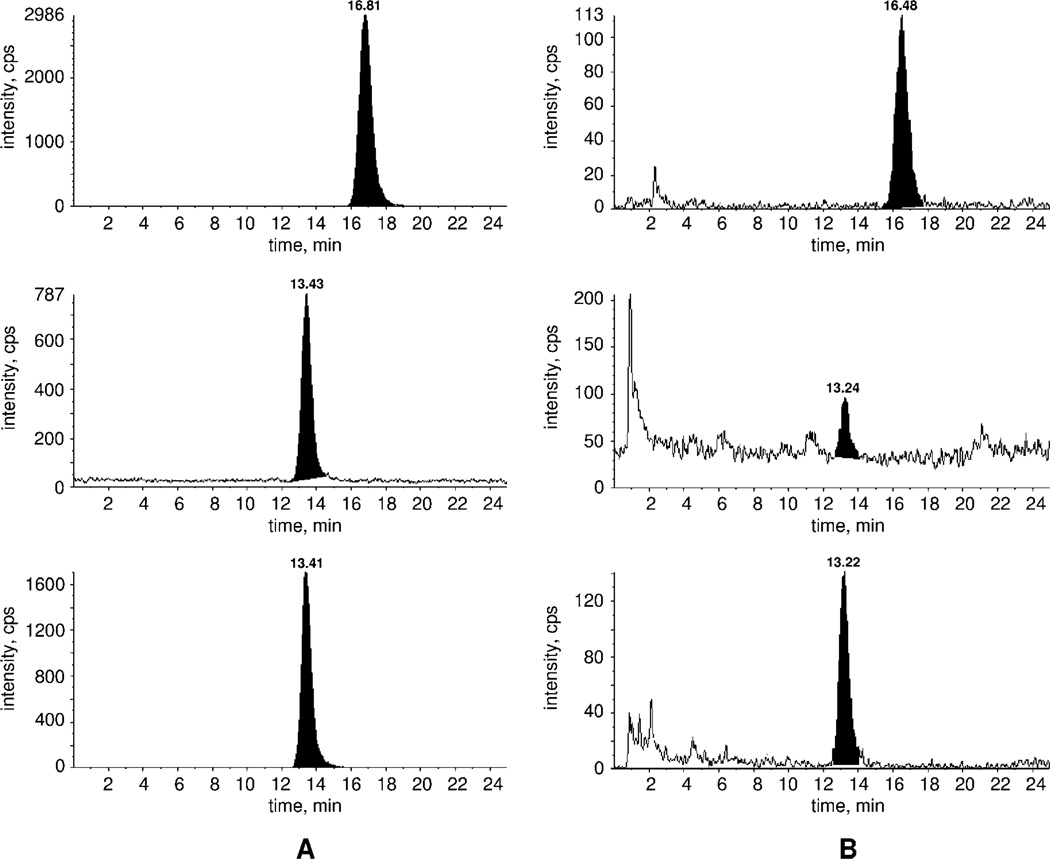
Cell homogenates (200 µl) were diluted with 500 µl water. External calibration samples were generated with 0, 10, 100 and 1000 pg of 5 and 6. The analytes were extracted with 1000 µl diisopropyl ether/ethyl acetate (1:3, v/v, recovery about 90%). The sample was centrifuged at 1000 g for 10 min. The solvent was evaporated and 100 µl acetonitrile/water (9:1, v/v) were added. The sample was sonicated and then centrifuged. A 10 µl aliquot of this solution was injected in the LC-MS/MS. Semi-quantification was achieved with external calibration samples (10, 100 and 1000 pg) (Figure 9).
Figure 9.
Representative chromatograms of 100 pg/sample extracted 5 and 6 standards (A), and mouse brain cell homogenates (B). Upper trace, 2.4 pg/sample 5 (m/z 504.6/218.2); middle trace, 6 (m/z 490.4/472.4) and lower trace, 6 (m/z 490.4/204.2) with 39 pg/sample.
5.2.3 Inhibition of COX activity in tissue extract
Brain tissue homogenates from C57BL/6 mice were prepared on ice in tissue buffer (50 mM Tris pH 7.4 buffer containing 1 mM phenol and 1x protease inhibitors (Calbiochem)). For the determination of COX activity, 20 µg of brain homogenate was diluted in tissue buffer containing different amounts of the inhibitors or vehicle. The reaction was started by the addition of 150 µM arachidonic acid at 37°C. After 5 min, the reaction was stopped by acidification to pH 2.5 with formic acid (1%) and immediately transferred onto ice. Prostanoids were extracted with ethyl acetate/hexane (7:1, v/v). PGE2 and 6-keto-PGF1α were determined by GC-MS/MS.30 In order to analyze inhibition of LPS-induced COX activity, we injected LPS i.p. (Sigma) at 50 µg kg−1 body weight. After 6 h, brain tissue homogenates were prepared, and COX activity was determined in the presence or absence of inhibitors.
5.2.4 Inhibition of COX activity in isolated enzymes
Isolated COX-1 and COX-2 enzymes (Cayman) were diluted in tissue buffer at a concentration of 0.53 U µl−1 for COX-1 and 0.51 U µl−1 for COX-2 in the presence of the inhibitors or vehicle. The reaction was started by the addition of 50 µM arachidonic acid at 37°C. After 5 min, the reaction was stopped by acidification to pH 2.5 with formic acid (1%). The samples were kept at 4°C. Prostanoids were extracted with ethyl acetate/hexane (7:1, v/v). PGE2 and 6-keto-PGF1α were determined by GC-MS/MS.29
5.2.5 Cannabinoid receptor binding assays
For CB1 and CB2 receptor binding assays, the new compounds were tested by using P2 membranes from HEK cells transfected with either the human recombinant CB1 or CB2 receptor and (3H)-(−)-cis-3-(2-hydroxy-4-(1,1-dimethylheptyl)-phenyl)-trans-4-(3-hydroxy-propyl)-cyclohexanol ((3H)CP-55,940) (Kd = 0.27 nM) as the high affinity ligand, as described by the manufacturer (Perkin Elmer, Italy). Displacement curves were generated by incubating drugs with 0.27 nM of (3H)CP-55,940. In all cases, Ki values were calculated by applying the Cheng-Prusoff equation to the IC50 values (obtained by GraphPad) for the displacement of the bound radioligand by increasing concentrations of the test compounds.
Acknowledgments
The authors wish to thank Prof. Dr. Beat Lutz, Department of Physiological Chemistry, Johannes-Gutenberg-Universität Mainz, Germany, and Prof. Dr. Benjamin F. Cravatt, Department of Cell Biology, The Scripps Research Institute, La Jolla, California, for providing the FAAH knockout mice. The LC/MS studies were supported by DFG grant SE 263/17-1. AHL acknowledges support by National Institutes of Health Grants P01DA009789, P01DA017259, F31DA026279 and T32DA007027. We thank Sanofi-Aventis Deutschland GmbH, Berlin, Germany, for a gift of 2 and 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Fendrich Z. Cas Lek Cesk. 2000;139:440. [Google Scholar]
- 2.Edwards JE, Meseguer F, Faura CC, Moore RA, McQuay HJ. Cochrane Database Syst Rev. 2001:CD003227. doi: 10.1002/14651858.CD003227. [DOI] [PubMed] [Google Scholar]
- 3.Maj S, Centkowski P. Med Sci Monit. 2004;10:PI93. [PubMed] [Google Scholar]
- 4.Ibanez L, Vidal X, Ballarin E, Laporte JR. Eur J Clin Pharmacol. 2005;60:821. doi: 10.1007/s00228-004-0836-y. [DOI] [PubMed] [Google Scholar]
- 5.Vlahov V, Badian M, Verho M, Bacracheva N. Eur J Clin Pharmacol. 1990;38:61. doi: 10.1007/BF00314805. [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Zylber-Katz E, Rosenkranz B. Clin Pharmacokinet. 1995;28:216. doi: 10.2165/00003088-199528030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson KH, Helmreich J, Jurna I. Pain. 1986;27:373. doi: 10.1016/0304-3959(86)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Tortorici V, Vanegas H. Eur J Neurosci. 1995;7:1857. doi: 10.1111/j.1460-9568.1995.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 9.Abbate R, Gori AM, Pinto S, Attanasio M, Paniccia R, Coppo M, Castellani S, Giusti B, Boddi M, Neri Serneri GG. Prostaglandins Leukot Essent Fatty Acids. 1990;41:89. doi: 10.1016/0952-3278(90)90059-t. [DOI] [PubMed] [Google Scholar]
- 10.Shimada SG, Otterness IG, Stitt JT. Agents Actions. 1994;41:188. doi: 10.1007/BF02001915. [DOI] [PubMed] [Google Scholar]
- 11.Rohdewald P, Drehsen G, Milsmann E, Derendorf H. Arzneimittelforschung. 1983;33:985. [PubMed] [Google Scholar]
- 12.Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF, Brune K. Faseb J. 2007;21:2343. doi: 10.1096/fj.06-8061com. [DOI] [PubMed] [Google Scholar]
- 13.Pierre SC, Schmidt R, Brenneis C, Michaelis M, Geisslinger G, Scholich K. Br J Pharmacol. 2007;151:494. doi: 10.1038/sj.bjp.0707239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezende RM, Franca DS, Menezes GB, dos Reis WG, Bakhle YS, Francischi JN. Br J Pharmacol. 2008;153:760. doi: 10.1038/sj.bjp.0707630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Marzo V, Bifulco M, De Petrocellis L. Nat Rev Drug Discov. 2004;3:771. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez-Rodriguez EEW, Ramirez K, Avila C, Vanegas H. 12th World Congress on Pain; Glasgow. 2008. [Google Scholar]
- 17.Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. Eur J Pharmacol. 1996;307:331. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- 18.Cohen O, Zylber-Katz E, Caraco Y, Granit L, Levy M. Eur J Clin Pharmacol. 1998;54:549. doi: 10.1007/s002280050511. [DOI] [PubMed] [Google Scholar]
- 19.Weithmann KU, Alpermann HG. Arzneimittelforschung. 1985;35:947. [PubMed] [Google Scholar]
- 20.Campos C, de Gregorio R, Garcia-Nieto R, Gago F, Ortiz P, Alemany S. Eur J Pharmacol. 1999;378:339. doi: 10.1016/s0014-2999(99)00477-x. [DOI] [PubMed] [Google Scholar]
- 21.Mallet C, Barrière DA, Ermund A, Jönsson BA, Eschalier A, Zygmunt PM, Högestätt ED. PLoS One. 2010;5:e12748. doi: 10.1371/journal.pone.0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Högestätt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. J Biol Chem. 2005;280:31405. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 23.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Nature. 1996;384:83. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 24.Kurahashi Y, Ueda N, Suzuki H, Suzuki M, Yamamoto S. Biochem Biophys Res Commun. 1997;237:512. doi: 10.1006/bbrc.1997.7180. [DOI] [PubMed] [Google Scholar]
- 25.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Proc Natl Acad Sci U S A. 2001;98:9371. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Nat Med. 2003;9:76. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 27.Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. Eur J Pharmacol. 2006;531:280. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Carlini EA. Toxicon. 2004;44:461. doi: 10.1016/j.toxicon.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Popp L, Haussler A, Olliges A, Nusing R, Narumiya S, Geisslinger G, Tegeder I. Eur J Pain. 2009;13:691. doi: 10.1016/j.ejpain.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Schweer H, Watzer B, Seyberth HW. J Chromatogr. 1994;652:221. doi: 10.1016/0378-4347(93)e0408-i. [DOI] [PubMed] [Google Scholar]
- 31.Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, Rossi FS, Woodward DF, Di Marzo V. Br J Pharmacol. 2007;150:766. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinning C, Watzer B, De Petrocellis L, Di Marzo V, Imming P. ChemMedChem. 2008;3:1956. doi: 10.1002/cmdc.200800271. [DOI] [PubMed] [Google Scholar]
- 33.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. FEBS Lett. 2000;13:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]