Abstract
Objectives
To explore how insomnia symptoms are hierarchically organized in individuals reporting daytime consequences of their sleep disturbances.
Methods
This is a cross-sectional study conducted in the general population of the states of California, New York and Texas. The sample included 8,937 individuals aged 18 years or older representative of the general population. Telephone interviews on sleep habits and disorders were managed with the Sleep-EVAL expert system and using DSM-IV and ICSD classifications. Insomnia symptoms and Global Sleep Dissatisfaction (GSD) had to occur at least three times per week for at least three months.
Results
A total of 26.2% of the sample had a GSD. Individuals with GSD reported at least one insomnia symptom in 73.1% of the cases. The presence of GSD in addition to insomnia symptoms considerably increased the proportion of individuals with daytime consequences related to insomnia. In the classification trees performed, GSD arrived as the first predictor for daytime consequences related to insomnia. The second predictor was nonrestorative sleep followed by difficulty resuming sleep and difficulty initiating sleep.
Conclusions
Classification trees are a useful way to hierarchically organize symptoms and to help diagnostic classifications. In this study, GSD was found to be the foremost symptom in identifying individuals with daytime consequences related to insomnia.
INTRODUCTION
For more than thirty years, the assessment of insomnia in epidemiologic studies was limited to the identification of insomnia symptoms in terms of their prevalence and associations with mental disorders and medical conditions [1]. The DSM-III-R [2], its successor the DSM-IV [3], and the second International Classification of Sleep Disorders (ICSD-II) [4] have represented important steps in the recognition and placement of each symptom. Progressively, four main insomnia symptoms were retained: Difficulty Initiating Sleep (DIS), Difficulty Maintaining Sleep (DMS), Early Morning Awakenings (EMA) and Non-Restorative Sleep (NRS). The assessment of daytime consequences was also mandatory in the DSM-III-R and subsequently in the DSM-IV and the ICSD-II.
Meanwhile, several studies have demonstrated that the prevalence of insomnia symptoms was dependent on their frequency during the week as opposed to the mere presence/absence of the symptoms [1]. Symptom duration (for example, one month or longer), however, was seldom investigated, although its importance has been outlined in classifications for nearly three decades. Current classifications, DSM-IV and ICSD-II, have arbitrarily set one month as the criterion for defining insomnia disorder. This poses a difficulty, since it is unlikely that a 1-month symptom duration characterizes a chronic health problem.
Some researchers have argued that the problem of insomnia prevalence, as defined until now, has resulted from the absence of crucial elements in the insomnia diagnosis. Indeed, some studies have shown that many individuals had insomnia symptoms without complaining about their sleep; i.e., when asked about specific insomnia symptoms they report having them but they remained satisfied with their sleep [5,6]. According to studies, a complaint of sleep quality or quantity was the foremost condition for an insomnia diagnosis and was prepotent in determining the hierarchy of insomnia symptoms [5-12]. Furthermore, some recent studies have demonstrated that insomnia as a pathology exists only when daytime consequences such as fatigue, excessive sleepiness, irritability, mood swings or cognitive difficulties are present [5,9,10].
Using a hierarchical classification method, we proposed, in this study, to explore how insomnia symptoms are organized within individuals reporting daytime consequences related to sleep disturbances. Which symptoms were prepotent in determining a clinical picture and in driving help seeking behaviour?
METHODS
Sample
The study was performed between 2003 and 2005 [13,14]. The target population was adults (18 years and older) living in the states of California, New York and Texas (USA). A total of 8,937 individuals aged 18 years or older, representative of the general population of these three states (3,243 subjects in California, 3,445 subjects in New York and 2,249 subjects in Texas), were interviewed by telephone. They represented a total of 62.8 million inhabitants. Rates of participation were 85.6% in California, 81.3% in New York and 83.2% in Texas.
Procedures
In the first stage, telephone numbers were randomly selected proportional to the population size of each county in California, New York and Texas. The selection was done within each state using a computerized residential phone book. In the second stage, during the telephone contact, the Kish method [15] was used to select one respondent per household. This method allowed for the selection of a respondent based on age and gender to maintain a sample representative of these two parameters. If the household member chosen declined to participate, the household was dropped and replaced with another number from the same area, and the process was repeated.
Interviewers explained the goals of the study to potential participants. They requested verbal consent before conducting the interview. The participants had the option of calling the principal investigator if they wanted further information. The study was approved by the Stanford University Institutional Review Board (IRB).
Subjects who declined to participate or who gave up before completing half the interview were classified as refusals. Excluded from the study were subjects who were not fluent in English (or Spanish), who suffered from a hearing or speech impairment, or who had an illness that precluded being interviewed. Phone numbers were dropped and replaced only after a minimum of 10 unsuccessful dial attempts were made at different times and on different days, including weekends. An added-digit technique, that is, increasing the last digit of a number by one, was employed to control for unlisted telephone numbers. The final sample included 21.4% unlisted telephone numbers.
The interviews lasted on average 74.5 (±37.8) minutes. An interview could be completed with more than one telephone call if longer than 60 minutes or at the request of the participant. Participants answered an average of 308 questions. The shortest interviews encompassed 110 questions and the longest 630 questions. The project manager or the team leaders also called nearly all the participants who completed the interview. During this 6-8 minute call, they asked a series of random questions related to the interview and also asked the participants how satisfied they were with the interviewer.
It was required that all the interviewers had no specific background in medicine and related sciences or in psychology. The interviewers were college students or had some college education. The training consisted of five 3-hour sessions that covered the study objectives, ethics in research, use of the Sleep-EVAL software and role-playing for interview situations. Interviewers were supervised by two or three team leaders with a ratio of one team leader for six interviewers.
Instrument
Interviewers used the Sleep-EVAL knowledge-based expert system [16,17] to conduct the interviews. This computer software is specially designed to administer questionnaires and conduct epidemiological studies in the general population.
The system is composed of a non-monotonic, level-2 inference engine, two neural networks, a mathematical processor, a knowledge base and a base of facts. Simply put, the interview begins with a series of questions asked of all the participants. It includes, in order of appearance: sociodemographic information, sleep/wake schedule, sleeping habits, sleep disturbance symptoms, medical and paramedical consultations and hospitalizations in the last 12-month period, physical diseases, use of prescribed and non-prescribed drugs, a health quality assessment scale, alimentation, fatigue scale, pain questionnaire, height and weight and, for women, questions on menopause. Questions were read out by the interviewer as they appeared on the screen. These questions were either close-ended (e.g., yes/no, 5-point scale, multiple choice) or open-ended (e.g., duration of symptom, description of illness).
Once this information was collected, the system began the diagnostic exploration of mental disorders. On the basis of responses provided by a subject to this questionnaire, the system formulated an initial diagnostic hypothesis that it attempted to confirm or reject by asking supplemental questions or by deductions. Concurrent diagnoses are allowed in accordance with the DSM-IV [3] and the International Classification of Sleep Disorders or ICSD [4]. The system terminated the interview once all diagnostic possibilities were exhausted.
The differential process is based on a series of key rules allowing or prohibiting the co-occurrence of two diagnoses. The questionnaire of the expert system is designed such that the decision about the presence of a symptom is based upon the interviewee’s responses rather than on the interviewer’s judgment. This approach has proved to yield better agreement between lay interviewers and psychiatrists on the diagnosis of minor psychiatric disorders [18]. The system has been tested in various contexts, in clinical psychiatry and sleep disorders clinics [19]. In psychiatry, kappas have ranged from .44 (schizophrenia disorders) to .78 (major depressive disorder). Agreement for insomnia diagnoses was obtained in 96.9% of cases (kappa 0.78)20.
Insomnia symptoms
Insomnia symptoms were defined as follows:
Global Sleep Dissatisfaction (GSD): Dissatisfaction with the sleep quality or quantity.
Difficulty Initiating Sleep (DIS): Difficulty falling asleep once in bed with the intention of sleeping.
- Difficulty Maintaining Sleep (DMS):
-
Frequent nocturnal awakenings in the same night (at least 3)OR
- Difficulty resuming sleep after an awakening (DRS).
-
Early Morning Awakenings (EMA): Premature awakening with an inability to resume sleep.
Non-Restorative Sleep (NRS): Feeling that the sleep is not refreshing even if the sleep duration is normal.
Severity and duration of each symptom were set at three nights or more per week and with a minimal duration of three months. We chose three months as the threshold criterion, rather than one month, in order to capture chronicity.
Daytime repercussions
Repercussions were assessed through 15 questions answered on a 5-point scale ranging from no impact to severe impact. These items covered cognitive functioning (memory, concentration, efficacy); affective tone (irritability, anxiety, depression); sensory irritability; fatigue; and excessive sleepiness.
Analyses
A classification tree was constructed based on the Exhaustive CHAID method (Chi-squared Automatic Interaction Detector), which is used to study the relationships between a dependent measure (in this study: daytime consequences) and a large series of possible predictor variables that themselves may interact (insomnia symptoms and GSD). Predictor variables are chosen using the Pearson’s chi-square test. The splitting point of the predictor variables is defined using a quadratic discriminant analysis. Subsequently, the data are split into two subsets based on the split point. This procedure is repeated for each of the two new subsets. The procedure stops when no significant predictor can be found. The significance level was p<0.05.
RESULTS
The participants in the sample were aged between 18 and 97 years; 54.1% of them were women. About half of the sample (51.4%) was married or living with a domestic partner and 30.4% of the participants were single. Retired individuals represented 17.1% of the sample, students 6.8%, daytime workers 39.6% and shift-workers 24.3%. Most of the participants were Whites (71.2%), 10.2% were Hispanics, 8% were Blacks, 3.5% were Asians and 6.4% were of mixed races. Interviews were conducted in Spanish for about 1% of the sample.
Association between GSD, insomnia symptoms and daytime consequences
A total of 26.2% of the sample was dissatisfied with either sleep quality or with sleep quantity (or both) (GSD). More specifically, 17.1% were dissatisfied with the quality of their sleep and 23.4% with the quantity of their sleep. As many as 73.1% of individuals with GSD reported at least one insomnia symptom. GSD without insomnia symptom was observed in 7.1% of the sample.
When at least two insomnia symptoms were present, more than 75% of the individuals also reported GSD (see Table 1). Proportion of individuals with GSD was significantly higher between individuals with only one insomnia symptom compared to those with two or more insomnia symptoms. Individuals with only nocturnal awakenings were less likely to report GSD. As shown in Table 2, the presence of GSD considerably increased the proportion of subjects with daytime consequences related to insomnia. In most cases, the association of one or several insomnia symptoms with GSD increased the proportion of individuals with daytime consequences to more than 80%.
Table 1.
Prevalence of insomnia complaints and symptoms within the population endorsing daytime consequences of sleep disturbances.
| Frequency (%) within individuals with daytime consequences of sleep disturbances |
% with GSD |
|
|---|---|---|
| GSD only | 10.1 | |
| DIS only | 3.5 | 55.8 |
| DMS only | ||
| NA | 2.9 | 28.4 |
| DRS | 6.6 | 41.6 |
| EMA only | 2.1 | 34.3 |
| NRS only | 9.2 | 67.0 |
| DIS+DMS | 3.2 | 75.5 |
| DIS+EMA | 0.3 | 90.9† |
| DIS+NRS | 3.5 | 87.6† |
| DMS+EMA | 2.4 | 60.5 |
| DMS+NRS | 5.0 | 77.2¶ |
| EMA+NRS | 1.3 | 68.3 |
| DIS+DMS+EMA | 3.0 | 87.0† |
| DIS+DMS+NRS | 3.7 | 94.1† |
| DIS+EMA+NRS | 1.1 | 97.1† |
| DMS+EMA+NRS | 3.8 | 84.4† |
| DIS+DMS+EMA+NRS | 4.7 | 96.0† |
DIS: Difficulty initiating sleep; DRS: Difficulty resuming sleep; EMA: Early morning awakenings; NA: Nocturnal awakenings without difficulty resuming sleep) NRS: nonrestorative sleep; GSD: Global sleep dissatisfaction
p<.01 with single symptom (DIS, DMS, EMA, NRS)
p<.01 with single symptom (DIS, DMS, EMA)
Table 2.
Prevalence and association between Global Sleep Dissatisfaction, insomnia symptoms and daytime consequences for the whole sample
| Prevalence (%) in the sample |
% with GSD |
% of GSD with the symptom |
% with daytime consequences |
||
|---|---|---|---|---|---|
| DIS only | Total | 2.4 | 46.8 | 4.3 | 60.8 |
| With GSD | 1.1 | 72.4† | |||
| No GSD | 1.3 | 50.5 | |||
| NA only | Total | 2.7 | 19.9 | 2.0 | 46.1 |
| With GSD | 0.5 | 65.9 | |||
| No GSD | 2.2 | 41.2 | |||
| DRS only | Total | 5.3 | 31.5 | 6.4 | 52.3 |
| With GSD | 1.7 | 69.0† | |||
| No GSD | 3.7 | 44.6 | |||
| EMA only | Total | 1.7 | 25.2 | 1.6 | 51.1 |
| With GSD | 0.4 | 69.7¶ | |||
| No GSD | 1.3 | 44.9 | |||
| NRS only | Total | 5.8 | 55.9 | 12.3 | 67.2 |
| With GSD | 3.2 | 80.6† | |||
| No GSD | 2.5 | 50.3 | |||
| DIS+DMS | Total | 1.7 | 68.9 | 4.5 | 77.3 |
| With GSD | 1.2 | 84.6¶ | |||
| No GSD | 0.5 | 61.0 | |||
| DIS+EMA | Total | 0.2 | 75.0 | 0.6 | 68.8 |
| With GSD | 0.2 | 83.3¶ | |||
| No GSD | 0.1 | 25.0 | |||
| DIS+NRS | Total | 1.7 | 85.9 | 5.5 | 88.3 |
| With GSD | 1.4 | 90.0 | |||
| No GSD | 0.2 | 77.8 | |||
| DMS+EMA | Total | 1.7 | 44.4 | 2.9 | 57.1 |
| With GSD | 0.8 | 78.0† | |||
| No GSD | 1.0 | 40.5 | |||
| DMS+NRS | Total | 2.4 | 77.2 | 7.1 | 88.0 |
| With GSD | 1.9 | 88.0 | |||
| No GSD | 0.5 | 88.1 | |||
| EMA+NRS | Total | 0.7 | 67.3 | 1.7 | 78.8 |
| With GSD | 0.5 | 80.0 | |||
| No GSD | 0.2 | 76.5 | |||
| DIS+DMS+EMA | Total | 0.9 | 81.8 | 2.7 | 81.8 |
| With GSD | 0.7 | 87.0¶ | |||
| No GSD | 0.2 | 58.3 | |||
| DIS+DMS+NRS | Total | 1.7 | 91.6 | 6.0 | 90.8 |
| With GSD | 1.6 | 93.3† | |||
| No GSD | 0.1 | 63.7 | |||
| DIS+EMA+NRS | Total | 0.5 | 94.7 | 1.8 | 89.5 |
| With GSD | 0.5 | 91.7 | |||
| No GSD | 0.0 | 50.0 | |||
| DMS+EMA+NRS | Total | 1.7 | 85.5 | 5.6 | 93.1 |
| With GSD | 1.5 | 92.0 | |||
| No GSD | 0.2 | 100.0 | |||
| DIS+DMS+EMA+NRS | Total | 2.2 | 94.0 | 7.8 | 90.4 |
| With GSD | 2.1 | 92.4¶ | |||
| No GSD | 0.1 | 60.0 |
DIS: Difficulty initiating sleep; DRS: Difficulty resuming sleep; EMA: Early morning awakenings; NRS: nonrestorative sleep; GSD: Global sleep dissatisfaction
p<.001
p<.01
Predictors of daytime consequences
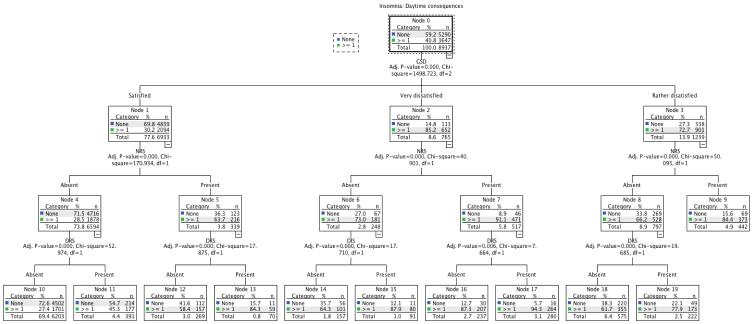
An exhaustive CHAID tree method was used to examine the relational hierarchy between GSD and insomnia symptoms in predicting daytime consequences associated with insomnia. The predictor variables entered in the tree modeling were: DIS, DMS (NA and DRS), EMA, NRS, GSD, difficulty getting started in the morning, sleep duration and sleep latency duration. The final result is presented in figure 1. The diagram represents a tree with progressive splits into smaller branches. The first split in the tree represents the most significant predictor. Each branch is further split until no significant difference is observed in the new split.
Figure 1.
Hierarchical tree for insomnia complaints and symptoms: Justification for Global Sleep Dissatisfaction with sleep quantity or quality (grouped into a single variable)
DIS: Difficulty initiating sleep; DRS: Difficulty resuming sleep; EMA: Early morning awakenings; NRS: nonrestorative sleep; GSD: Global sleep dissatisfaction
As it can be seen, the first split is with GSD. It is therefore the most important predictor for daytime consequences. As many as 72.7% of individuals who were rather dissatisfied (node 3) and 85.2% of those very dissatisfied (node 2) reported daytime consequences.
The second split is on NRS. Among individuals who were rather dissatisfied with their sleep, the addition of NRS increased the proportion of individuals with daytime consequences to 84.4% (node 9); being rather dissatisfied without NRS decreased the proportion to 66.2% (node 8); In very dissatisfied individuals, the second split was also on NRS: very dissatisfied individuals who also had NRS reported daytime consequences in 91.1% of cases (node 7), while in those very dissatisfied but without NRS daytime consequences were reported in 73% of cases (node 6). Among individuals without GSD (satisfied), the proportion with daytime consequences associated with insomnia was 30.2% (node 2). The second split was again with NRS. No GSD but with NRS increased the proportion of individuals with daytime consequences to 63.7% (node 5).
The third split on the tree branch involving individuals without GSD was with Difficulty Resuming Sleep (DRS), which was the strongest predictor of daytime repercussions in individuals satisfied with their sleep (nodes 11 and 13).
For individuals very dissatisfied with their sleep, the third split involved DIS and DRS. As it can be seen, in the absence of NRS, the presence of DIS increased the proportion of individuals with daytime consequences to 87.9% (node 15). When NRS was present, the addition of DRS slightly increased the proportion of individuals with daytime consequences (node 17).
For individuals rather dissatisfied with their sleep, the third split occurs with DRS. As it can be seen, the presence of DRS considerably increased the number of subjects with daytime consequences among those without NRS (node 19).
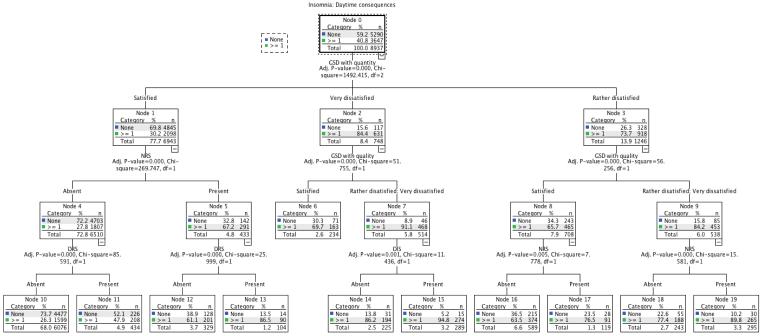
The same tree method was done again, this time using GSD with quantity and GSD with quality as two separated variables. As seen in Fig. 2, the results are not very different than the first tree. The first split is with GSD with quantity and the second split is on GSD with quality.
Figure 2.
Hierarchical tree for insomnia complaints and symptoms: Justification for Global Sleep Dissatisfaction with sleep quantity or quality (treated as two separated variables)
Legend: DIS: Difficulty initiating sleep; DRS: Difficulty resuming sleep; EMA: Early morning awakenings; NRS: nonrestorative sleep; GSD: Global sleep dissatisfaction
DISCUSSION
The main aim of this study was to explore how insomnia symptoms were organized within a general population sample and to determine the hierarchical importance of these symptoms to predict daytime consequences of sleep disturbances. The results clearly show that Global Sleep Dissatisfaction (GSD) with sleep quantity or sleep quality was the foremost symptom in identifying individuals with daytime consequences.
Are these findings surprising? At first glance, the answer is yes. However, many years before the appearance of the sleep disorders chapter in the DSM-III-R and the development of the International Classification of Sleep Disorders, the American Institute of Medicine, in 1979, had defined insomnia as being an unsatisfactory sleep [21]. Although the premises were good, such a definition was impractical and too vague; it is difficult to measure changes in symptoms when none are defined.
How the use of a general population sample enhances the design of a classification
Clinical samples are excellent to measure the efficacy of a treatment. However, as it comes to define symptoms of a disorder such as insomnia, the problem is that their patients already share one important characteristic: they are dissatisfied with their sleep. Therefore, actual definitions of classic insomnia symptoms are based on the implicit assumption that people reporting an insomnia symptom are dissatisfied with their sleep. It might be true in a clinical population, but in a general population sample it cannot be assumed: one of the consequences of such an assumption in the general population is that a large proportion of individuals with insomnia symptoms has no associated daytime consequences. This is clearly shown in our results (table 2): in the absence of GSD, less than half of individuals who reported insomnia symptom(s) had associated daytime consequences. This leads us to the question, “what are the differences between complaint, symptom, syndrome and disorder in sleep disorders?” A complaint refers to a patient reporting a problem bothering him or producing a handicap. A symptom is a clinical fact recognized by the clinician based on answers to a questionnaire or an examination.
A syndrome is a regrouping of symptoms and/or complaints in terms of criteria that can have an intensity, a duration, a frequency, an evolution and a specificity linked with age, gender or other categories.
One can already see a fundamental difference between epidemiological and clinical studies: epidemiological studies first look for symptoms identified a posteriori based on the answers to a questionnaire. In clinical studies, a participant is primarily selected based on a complaint and only after that will symptoms be explored. It is why it is so important to define our field very carefully using both general population and clinical samples. Our insomnia model could help the field in defining more specific diagnostic algorithms through the entire field of sleep.
The statistical method we have used may surprise many. The decision tree technique we used was developed in 1980 with the primary purpose to help classifications in the field of medical and psychiatric research [22]. The main problem is that it requires a large number of subjects because it can have multiple splits and the number of subjects can quickly become too small for reliable analysis. However, when the prerequisites are respected, the method is very valuable. It offers a useful way of summarizing data and shows the major natural divisions of the participants.
It should be kept in mind, however, that our results are based on subjective reports. Therefore, symptoms are described in the manner that the participants experienced them and may not correspond exactly to polysomnographic measures. Since ours is an epidemiological study, we did not conduct laboratory testing with respondents to confirm symptoms and diagnoses. In some cases, such as for disorders like Obstructive Sleep Apnea Syndrome, polysomnographic recording (PSG) is needed to confirm the diagnosis. It has been documented that individuals with insomnia tend to misjudge their sleep compared with PSG data [23,24].
Conclusions
To summarize, this study is the first one to underline and to document the strength and impact of GSD in the diagnosis making process of insomnia. GSD overrides all the other symptoms of insomnia: difficulty initiating sleep, nocturnal awakenings, early morning awakenings and nonrestorative sleep. In the classification tree, GSD emerges as the first symptom leading to the diagnosis of insomnia; associations with the other insomnia symptoms still remain, but they appear only on second or third levels in the classification tree.
We have studied how different parameters were playing in the identification of different categories of insomnia in the general population. We have shown that using a more hierarchical procedure inside of the diagnosis of insomnia is a way to not only identify people with insomnia more clearly but also to identify the ones who need to be treated with a greater chance of compliance.
Scientifically, this approach is easily reproducible and could lead to insomnia studies with greater comparability between themselves and to fewer variations in the definitions of patient groups.
The benefits in the appreciation of new medications could be decisive in terms of efficacy but also in terms of follow-up and evaluation of unexpected adverse events. In terms of educational purposes, the interest of a classification corresponding to the reality of the medical community’s work is a major advantage toward encouraging general practitioners to become more interested in recognizing and treating the sleep pathology of their patients.
Acknowledgments
This study was support by grants from the Bing Foundation (MMO), the Arrillaga Foundation (MMO) and the NIH # R01NS044199 (MMO)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work of the DSM-V workgroup on sleep wake disorders section
REFERENCES
- 1.Ohayon M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 3th Edition Revised APA; Washington DC: 1987. [Google Scholar]
- 3.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th Edition APA; Washington DC: 1994. [Google Scholar]
- 4.AASM (American Academy of Sleep Medicine) International classification of sleep disorders. 2nd edition. Westchester, Il: 2005. ICSD-2. [Google Scholar]
- 5.Ohayon MM, Roth T. What are the Contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–755. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM, Caulet M, Guilleminault C. Complaints about nocturnal sleep: how a general population perceives its sleep, and how this relates to the complaint of insomnia. Sleep. 1997;20:715–723. doi: 10.1093/sleep/20.9.715. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Zulley J. Correlates of global sleep dissatisfaction in the German population. Sleep. 2001;24:780–787. [PubMed] [Google Scholar]
- 8.Jacobs JM, Cohen A, Hammerman-Rozenberg R, Stessman J. Global sleep satisfaction of older people: the Jerusalem Cohort Study. J Am Geriatr Soc. 2006;54:325–329. doi: 10.1111/j.1532-5415.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–241. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Caulet M, Priest RG, Guilleminault C. DSM-IV and ICSD-90 insomnia symptoms and sleep dissatisfaction. Br J Psychiatry. 1997;171:382–388. doi: 10.1192/bjp.171.4.382. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM, Paiva T. Global sleep dissatisfaction for the assessment of insomnia severity in the general population of Portugal. Sleep Med. 2005;6:435–441. doi: 10.1016/j.sleep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Zilli I, Ficca G, Salzarulo P. Factors involved in sleep satisfaction in the elderly. Sleep Med. 2009;10:233–239. doi: 10.1016/j.sleep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Ohayon MM. Nocturnal awakenings and comorbid disorders in the American general population. J Psychiatr Res. 2008;43:48–54. doi: 10.1016/j.jpsychires.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–241. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kish L. Survey Sampling. John Wiley & Sons Inc.; New York: 1965. [Google Scholar]
- 16.Ohayon MM. Copyright Office, Canadian Intellectual Property Office. Industry Canada; Ottawa: 1994. Sleep-EVAL, Knowledge Based System for the Diagnosis of Sleep and Mental Disorders. [Google Scholar]
- 17.Ohayon MM. Improving decision-making processes with the fuzzy logic approach in the epidemiology of sleep disorders. J Psychosom Res. 1999;47:297–311. doi: 10.1016/s0022-3999(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 18.Lewis G, Pelosi AJ, Araya RC, Dunn G. Measuring psychiatric disorder in the community, a standardized assessment for use by lay interviewers. Psychol Med. 1992;22:465–486. doi: 10.1017/s0033291700030415. [DOI] [PubMed] [Google Scholar]
- 19.Ohayon MM, Guilleminault C, Zulley J, et al. Validation of the Sleep-EVAL system against clinical assessments of sleep disorders and polysomnographic data. Sleep. 1999;22:925–930. doi: 10.1093/sleep/22.7.925. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon M. Validation of expert systems: Examples and considerations. Medinfo. 1995;8:1071–1075. [PubMed] [Google Scholar]
- 21.Institute of Medicine . Sleeping Pills, Insomnia and Medical Practice. National Academy of Sciences; Washington, DC: 1979. [Google Scholar]
- 22.Kass GV. An Exploratory Technique for Investigating Large Quantities of Categorical Data. Applied Statistics. 1980;29:119–127. [Google Scholar]
- 23.Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Arch Gen Psychiatry. 1976;33:615–23. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- 24.Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62:474–482. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]