Abstract
Towards addressing the knowledge gap of how bupropion interacts with the dopamine transporter (DAT) and nicotinic acetylcholine receptors (nAChRs), a ligand was synthesized in which the chlorine of bupropion was isosterically replaced with an iodine and a photoreactive azide was added to the 4′-position of the aromatic ring. Analog (±)-3 (SADU-3-72) demonstrated modest DAT and α4β2 nAChR affinity. A radioiodinated version was shown to bind covalently to hDAT expressed in cultured cells and affinity-purified, lipid-reincorporated human α4β2 neuronal nAChRs. Co-incubation of (±)-[125I]-3 with non-radioactive (±)-bupropion or (−)-cocaine blocked labeling of these proteins. Compound (±)-[125I]-3 represents the first successful example of a DAT and nAChR photoaffinity ligand based on the bupropion scaffold. Such ligands are expected to assist in mapping bupropion-binding pockets within plasma membrane monoamine transporters and ligand-gated nAChR ion channels.
Keywords: bupropion, dopamine transporter, nicotinic acetylcholine receptor, photoaffinity labeling
Bupropion (Wellbutrin, Zyban) ((±)-1, Figure 1) is a well-established antidepressant in use for more than two decades.1 With respect to treating nicotine abuse, a sustained release formulation of bupropion displays significant benefits as a smoking cessation agent.2 More recently, (±)-1 has garnered noteworthy attention as a treatment for both cocaine and methamphetamine dependence, showing greater efficacy in the latter.3 Given its clinical versatility, a number of analogs of (±)-1 and its major active metabolite, (2S,3S)-hydroxybupropion, have been recently disclosed in the search for improved pharmacotherapies for smoking cessation and cocaine addiction.4-7 Despite these well-documented therapeutic effects, the neurochemical mechanisms underlying bupropion’s action are still not well-defined, and the molecular determinants of how (±)-1 interacts with its major drug targets remain unknown.
Figure 1.
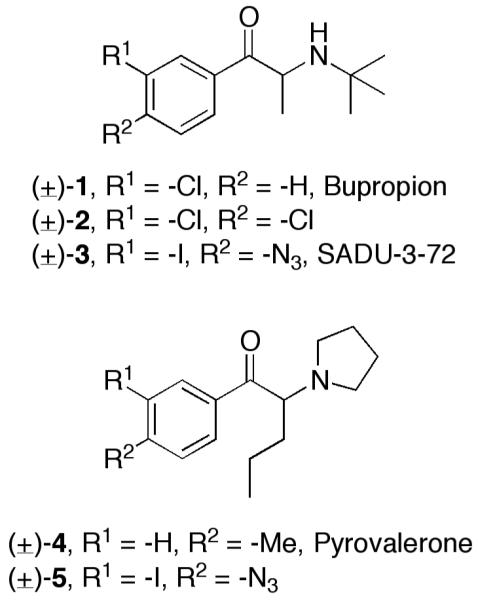
Structural comparison of aminoketone DAT inhibitors and photoprobe analogs.
Unlike many other antidepressants, (±)-1 has very little effect on serotonin reuptake. Instead, bupropion is traditionally described as a norepinephrine-dopamine reuptake inhibitor (NDRI) showing 8-fold selectivity for binding to the dopamine transporter (DAT).4 Additionally, (±)-1 noncompetitively inhibits a number of nicotinic acetylcholine receptors (nAChRs) including the α3β4*, α3β2, α4β2, and α7 subtypes.8 Multiple molecular targets are employed by (±)-1 in modulating the behavioral effects of nicotine and psychostimulants. Slight adjustments to the nAChR activity of an analog with a NDRI profile may be the key to developing more efficacious smoking cessation aids.7
With respect to the DAT, details regarding the transport inhibition mechanism and the discrete ligand-binding pockets remain poorly understood. Results from site-directed mutagenesis experiments and structure-activity relationship (SAR) studies imply that structurally heterogeneous inhibitors bind to different domains or binding sites within the DAT.9-11 Additionally, it has been suggested that the binding of inhibitors to distinct DAT domains or conformations could affect their behavioral profile in psychostimulant abuse animal models.12 As a result, molecular probes based on bupropion would represent important pharmacological tools for structure-function studies of nAChRs and the DAT towards developing enhanced addiction and depression therapeutics. Given the emergence of 3-D molecular models of these proteins, photoaffinity ligands based on (±)-1 are expected to aid in determining protein conformational states and mapping of bupropion-binding sites. This information might reveal how the DAT and nAChRs discriminate therapeutic versus abused compounds at the molecular level.
To date, the chemical development of DAT photoaffinity ligands has predominantly focused on tropane-based ligands and their conformationally flexible piperidine and piperazine analogs (see references within reference #13). In contrast, structurally heterologous non-tropane compounds have received significantly less attention in terms of their development into DAT irreversible probes.13,14 In particular, determination of the DAT and nAChR conformational states and binding sites for bupropion and structurally related compounds is in its early stages.15,16
Our interest in developing a photoaffinity ligand based on bupropion stemmed from initial DAT labeling success with photoprobe (±)-[125I]-5, a derivative of pyrovalerone ((±)-4).13 Bupropion and pyrovalerone belong to the aminoketone class of DAT inhibitors, bearing similar structures to cathinone and diethylpropion as stimulants, and to phenethylamines (e.g., amphetamines) in general (Figure 1). In addition to their chemical differences, (±)-1 and (±)-4 differ substantially in abuse potential, with the latter being a Schedule V controlled substance in the United States. This is in sharp contrast to bupropion, which is classified as “non-abusable” or having low abuse potential. Pyrovalerone (hDAT Ki = 8 ± 2 nM) displays 55-fold higher DAT affinity versus bupropion (hDAT Ki = 441 ± 174 nM) in N2A neuroblastoma cells.13 Although the notably lower DAT affinity represents a risk in developing a DAT photoprobe based on bupropion, pursuit of target photoprobe (±)-3 was justified based on its clinical significance and possible utilization in both DAT and nAChR photoaffinity labeling studies.
The design of pyrovalerone photoprobe (±)-[125I]-5 resulted from structure-activity relationships (SAR) indicating pyrovalerone’s aromatic ring is able to tolerate a wide range of substitutions with respect to retaining appreciable DAT affinity.17 As a result, photoprobe (±)-[125I]-5 was designed by replacing the 4′-methyl group of pyrovalerone with a photoreactive azide (−N3) and including an iodine at the 3′ position of the aromatic ring to facilitate future incorporation of 125I as a radiotracer tag.13 These substitutions resulted in a ~9.8-fold loss in DAT affinity for photoprobe (±)-5 (hDAT Ki = 78 ± 18 nM) when compared to pyrovalerone (hDAT Ki = 8 ± 2 nM). A 125I version of this compound was shown to bind covalently to rDAT and hDAT expressed in cultured cells.13
Target bupropion photoprobe (±)-3 was rationally designed in an analogous manner. Similar to pyrovalerone, monoamine transporter and nAChR SAR have been recently reported for bupropion analogs featuring a variety of substituents on the aromatic ring.4,5 In particular, analog (±)-2 (hDAT Ki = 472 ± 81 nM) containing hydrophobic chlorine atoms at positions 3′ and 4′ of the aromatic ring displayed improved DAT affinity (1.8-fold higher) versus bupropion (hDAT Ki = 871 ± 126 nM) in HEK cells.4 Thus, target photoprobe (±)-3 was designed by isosteric replacement of the 3′-chlorine in bupropion ((±)-1) with the larger hydrophobic halogen iodine, plus addition of a hydrophobic photoreactive azide to the 4′-position of the aromatic ring. Support for designation of an aryl azide as a hydrophobic functional group stems from work of Petukhov et al. in the area of histone deacetylase inhibitor photoprobes.18
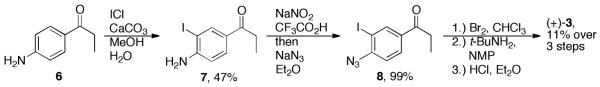
The synthesis of target bupropion photoprobe (±)-3 is depicted in Scheme 1. 4′-Aminopropiophenone (6) was first iodinated using ICl and CaCO3 analogous to Fujita et al.19 to provide iodo-aniline 7 in moderate yield (47%). The aniline was subsequently converted into an aryl azide (8) in excellent yield via diazotization and displacement with sodium azide. Access to target (±)-3 in its HCl salt form was then achieved using standard methodology for the synthesis of bupropion and its derivatives.4,5,20 First, ketone 8 was brominated to provide an α-bromo ketone derivative, followed by displacement of the bromide with tert-butyl amine and subsequent HCl salt formation. Although this strategy proved successful, it provided target photoprobe (±)-3 in rather low yield, 11% over three steps.
Scheme 1.
Synthesis of bupropion photoprobe (±)-3 (SADU-3-72).
Another synthetic route to target photoprobe (±)-3 was explored that also allowed formation of a desired 125I derivative (Scheme 2). This strategy featured introduction of the photoreactive azide group as the final step versus earlier in the synthetic sequence. Aniline 6 was first converted to its corresponding amide derivative (9) in essentially quantitative yield using acetyl chloride. The amide was then transformed into (±)-10 in 23% yield over three steps using the same chemistry previously described for synthesizing bupropion analogs. Amide hydrolysis then provided aniline bupropion analog (±)-11, which could be utilized to prepare a radioactive version of probe (±)-3 to determine if photoactivation produced covalent ligation to the DAT or a selected nAChR. In order to accomplish this task, a one-flask synthesis of (±)-[125I]-3 was performed using methodology previously described for the preparation of radioiodinated cocaine analogs as DAT photoaffinity labels.21 Briefly, electrophilic radioiodination of (±)-11 with [125I]-NaI (1.86 mCi) under no-carrier-added conditions using Chloramine-T as the oxidant was followed by diazotization and subsequent treatment with sodium azide. This sequence ending with reversed-phase HPLC isolation provided (±)-[125I]-3 in 65% yield, high purity (>99%), and high specific activity (2057 mCi/μmol). The ligand exhibited a chromatographic profile identical to that of non-radioactive (±)-3 (Supplemental Figure 1). Supplemental Figure 1A shows the preparative HPLC trace where (±)-[125I]-3 (tR = 16.6 min) was well resolved from radioactive and non-radioactive side products. The major non-radioactive materials are assigned as the azide (tR = 5.1 min) and chloroazide (tR = 10.6 min) congeners based upon model studies conducted in the presence and in the absence of Chloramine-T. Supplemental Figure 1B shows purified (±)-[125I]-3, while Figure 1C shows HPLC co-elution of purified (±)-[125I]-3 with a fully characterized sample of non-radioactive (±)-3.
Scheme 2.
Synthesis of (±)-[125I]-3 ((±)-[125I]-SADU-3-72).
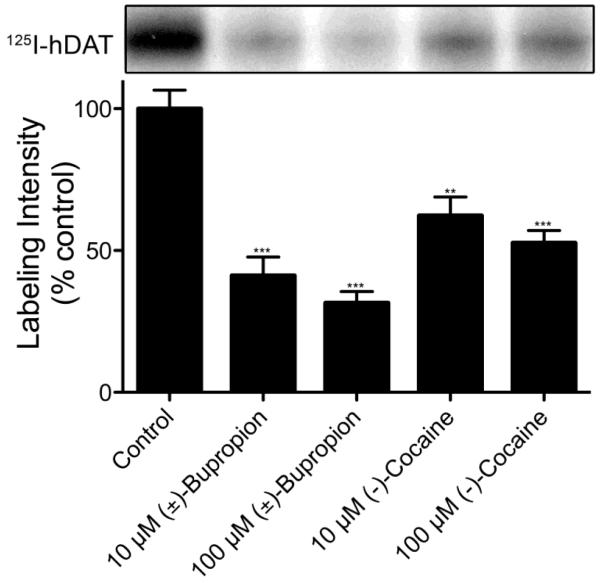
With both non-radioactive (±)-3 and (±)-[125I]-3 in hand, preliminary DAT and nAChR pharmacology and photoaffinity labeling experiments were initiated. DAT ligand affinities of (±)-3 and synthesized (±)-120 were determined via inhibition of [3H]-WIN-35,428 (a cocaine analog) binding to hDAT in N2A neuroblastoma cells. The DAT affinity for target compound (±)-3 (hDAT Ki = 3071 ± 497 nM) was sevenfold lower than bupropion (hDAT Ki = 441 ± 174 nM), but still bioactive in the range of parent compound (±)-1 such that further experimentation was justified. To determine if the DAT underwent irreversible labeling with (±)-[125I]-3, LLCPK1 cells expressing 6Xhis-hDAT were photoaffinity labeled with (±)-[125I]-3 in the absence or presence of 10 μM or 100 μM (±)-bupropion or (−)-cocaine. The cells were then detergent-solubilized and the lysates were immunoprecipitated with anti-his antibody and analyzed by SDS-PAGE/autoradiography analogous to previously described procedures.22 Labeled proteins of ~80 kDa were obtained from LLCPK1 hDAT cells (Figure 2), demonstrating the incorporation of (±)-[125I]-3 into the DAT. Incorporation of the ligand was blocked by 40-70% by either (±)-bupropion or (−)-cocaine in a dose-dependent manner, demonstrating the appropriate pharmacological specificity of (±)-[125I]-3 attachment to the DAT. Similar to results previously reported for tropane, GBR, and benztropine DAT photoaffinity ligands,23,24 analysis of total cell lysates showed that several proteins undergo adduction with (±)-[125I]-3 (not shown). However, these do not represent the DAT because they do not immunoprecipitate with DAT antibody as shown for the protein in Figure 2.
Figure 2.
Photoaffinity labeling of hDAT with (±)-[125I]-3. LLCPK1 cells expressing 6Xhis-hDAT were photoaffinity labeled with 10 nM (±)-[125I]-3 in the absence or presence of 10 μM or 100 μM (±)-bupropion or (−)-cocaine. Cells were solubilized and DATs were immunoprecipitated followed by analysis by SDS-PAGE and autoradiography. The relevant portion of a representative autoradiograph is pictured followed by a histogram that quantitates relative band intensities. (means ± SE of three independent experiments; ***P <0.0001 versus control; **P <0.001 versus control)
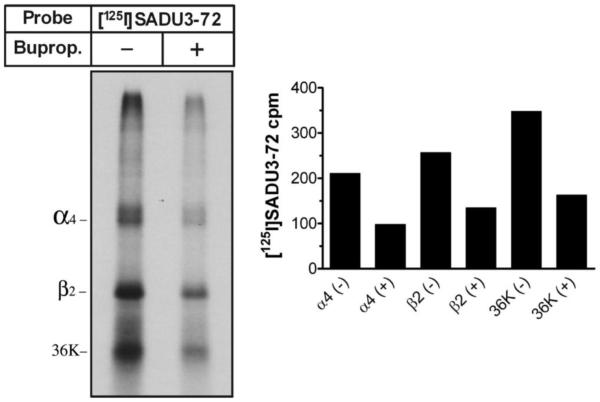
Likewise, nAChR pharmacology and photoaffinity labeling experiments were performed with (±)-[125I]-3. The affinity of (±)-[125I]-3 was approximated via inhibition by nonradioactive (±)-bupropion for binding to HEK-α4β2 nAChR cell membranes. (±)-Bupropion inhibited (±)-[125I]-3 binding to human α4β2 nAChRs with an IC50 value of 8.3 μM, a value consistent with those previously reported.8 Affinity-purified and lipid-reincorporated (DOPC/DOPA/CH-3:1:1) human α4β2 neuronal nAChRs (~45 μg) were photolabeled with 78 nM (±)-[125I]-3 ([125I]-SADU-3-72) in the absence or presence of 160 μM (±)-bupropion. Polypeptides were then gel-fractionated, visualized by Coomassie Blue staining, and processed for autoradiography (Figure 3). As evident from the significant reduction in (±)-[125I]-SADU-3-72 labeling in the presence of an excess of (±)-bupropion, (±)-[125I]-3 specifically photoincorporates into the α4β2 nAChR.
Figure 3.
Photoincorporation of (±)-[125I]-3 ((±)-[125I]-SADU-3-72) into the human α4β2 neuronal nAChR. UV irradiation at 365 nM proceeded for 10 min. Left panel, autoradiograph of an 8% SDS-polyacrylamide gel (1-week exposure) showing (±)-[125I]-3 photoincorporation into the α4 and β2 subunits, and into an ~36 kDa proteolytic fragment of the β2 subunit, in the absence (−) or presence (+) of 160 μM (±)-bupropion. Right panel, (±)-[125I]-3 photoincorporation of each band was quantified by gamma counting where inclusion (+) of (±)-bupropion (160 μM) inhibited labeling of each band by ~50%.
In summary, a photoaffinity ligand based on the well-known antidepressant and smoking cessation agent bupropion was designed, synthesized, and pharmacologically evaluated. Analog (±)-[125I]-3 represents the first successful example of a DAT and nAChR photoaffinity ligand based on the bupropion scaffold, thus representing an important contribution to the growing arsenal of probes useful for characterizing the function and 3-D structure of the DAT and nAChRs as therapeutically significant proteins. The binding affinities of compound (±)-3 for the DAT and α4β2 neuronal nAChR are reasonably similar to bupropion, suggesting this probe is accessing the biologically relevant site(s). In particular, the affinity of photoprobe (±)-3 for neuronal α4β2 nAChRs is similar to that of bupropion and other ligands that bind within the nAChR ion channel.8 In fact, recent pharmacological studies suggest the bupropion-binding site lies within the nAChR ion channel and not at the agonist-binding site;8,16 thus, probe (±)-3 is pharmacologically distinct from nAChR agonist photoprobes such as 5-azidoepibatidine. Furthermore, the modest nAChR binding affinity of (±)-3 does not preclude identification of the bupropion-binding site within neuronal nAChR ion channels.25 Iodine-125-labeled (±)-3 was shown to bind covalently to hDAT expressed in cultured cells and affinity-purified, lipid-reincorporated human α4β2 neuronal nAChRs. Successful adduction of (±)-[125I]-3 to these proteins suggests that this non-tropane ligand tolerates direct substitution of a photoreactive azido group on the aromatic ring of the inhibitor scaffold. This contrasts with the design of tropane-based DAT photoaffinity ligands, wherein the azide is placed at a distance (usually via a linker) from the inhibitor pharmacophore in order to achieve successful DAT labeling.26 As a result, compact photoaffinity ligands based on bupropion offer the advantage of a shorter tether between probe functional groups and protein amino acid residues in or near the inhibitor-binding site. Given the evidence that both DAT and nAChR inhibitors bind to nonidentical sites or conformations,8-12,16 this suggests that novel irreversible ligands based on bupropion may yield new nAChR and monoamine transporter structure-function information. Future directions include additional photoprobes based on bupropion and its major active metabolite, (2S,3S)-hydroxybupropion, their pharmacological characterization, binding site prediction via docking within DAT and nAChR computational models, and detailed elucidation of the binding domains within the DAT and selected nAChRs.
Supplementary Material
Acknowledgements
This work was funded by a Hunkele Dreaded Disease Award (D.J.L.), the Mylan School of Pharmacy at Duquesne University (D.J.L.), the Center of Membrane Protein Research (M.P.B.), Texas Tech University Health Sciences Center School of Medicine (M.P.B.), and NIH Grants DA27081 (D.J.L.), DA16604 (C.K.S.), and DA15175 (R.A.V.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:.
References and notes
- 1.).Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA. Prim. Care Companion J. Clin. Psychiatry. 2005;7:106. doi: 10.4088/pcc.v07n0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.).Wilkes S. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:45. doi: 10.2147/copd.s1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.).Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Neuropsychopharmacology. 2008;33:1162. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 4.).Carroll FI, Blough BE, Abraham P, Mills AC, Holleman A, Wolckenhauer SA, Decker AM, Landavazo A, McElroy KT, Navarro HA, Gatch MB, Forster MJ. J. Med. Chem. 2009;52:6768. doi: 10.1021/jm901189z. [DOI] [PubMed] [Google Scholar]
- 5.).Carroll FI, Blough BE, Mascarella SW, Navarro HA, Eaton JB, Lukas RJ, Damaj MI. J. Med. Chem. 2010;53:2204. doi: 10.1021/jm9017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.).Lukas RJ, Muresan AZ, Damaj MI, Blough BE, Huang X, Navarro HA, Mascarella SW, Eaton JB, Marxer-Miller SK, Carroll FI. J. Med. Chem. 2010;53:4731. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.).Carroll FI, Muresan AZ, Blough BE, Navarro HA, Mascarella SW, Eaton JB, Huang X, Damaj MI, Lukas RJ. J. Med. Chem. 2011;54:1441. doi: 10.1021/jm1014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.).Arias HR. J. Pediatric Biochem. 2010;1:185. [Google Scholar]
- 9.).Reith MEA, Berfield JL, Wang L, Ferrer JV, Javitch JA. J. Biol. Chem. 2001;276:29012. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- 10.).Newman AH, Kulkarni SS. Med. Res. Rev. 2002;22:1. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- 11.).Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S, Surratt CK. J. Pharmacol. Exp. Ther. 2005;314:575. doi: 10.1124/jpet.105.085829. [DOI] [PubMed] [Google Scholar]
- 12.).Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Mol. Pharmacol. 2008;73:813. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- 13.).Lapinsky DJ, Aggarwal S, Huang Y, Surratt CK, Lever JR, Foster JD, Vaughan RA. Bioorg. Med. Chem. 2009;17:3770. doi: 10.1016/j.bmc.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.).Lapinsky DJ, Velagaleti R, Yarravarapu N, Liu Y, Huang Y, Surratt CK, Lever JR, Foster JD, Acharya R, Vaughan RA, Deutsch HM. Bioorg. Med. Chem. 2011;19:504. doi: 10.1016/j.bmc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.).Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith MEA. J. Neurochem. 2008;107:928. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.).Arias HR, Gumilar F, Rosenberg A, Targowska-Duda KM, Feuerbach D, Jozwiak K, Moaddel R, Wainer IW, Bouzat C. Biochemistry. 2009;48:4506. doi: 10.1021/bi802206k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.).Meltzer PC, Butler D, Deschamps JR, Madras BK. J. Med. Chem. 2006;49:1420. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.).He B, Velaparthi S, Pieffet G, Pennington C, Mahesh A, Holze DL, Brunsteiner M, van Breemen R, Blond SY, Petukhov PA. J. Med. Chem. 2009;52:7003. doi: 10.1021/jm9005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.).Fujita K, Takahashi Y, Owaki M, Yamamoto K, Yamaguchi R. Org. Lett. 2004;6:2785. doi: 10.1021/ol0489954. [DOI] [PubMed] [Google Scholar]
- 20.).Perrine DM, Ross JT, Nervi SJ, Zimmerman RH. J. Chem. Ed. 2000;77:1479. [Google Scholar]
- 21.).Lever JR, Zou MF, Parnas ML, Duval RA, Wirtz SE, Justice JB, Vaughan RA, Newman AH. Bioconjugate Chem. 2005;16:644. doi: 10.1021/bc0497214. [DOI] [PubMed] [Google Scholar]
- 22.).Vaughan RA, Parnas ML, Gaffaney JD, Lowe MJ, Wirtz S, Pham A, Reed B, Dutta SM, Murray KK, Justice JB. J. Neuroscience Methods. 2005;143:33. doi: 10.1016/j.jneumeth.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 23.).Vaughan RA, Agoston GE, Lever JR, Newman AH. J. Neurosci. 1999;19:630. doi: 10.1523/JNEUROSCI.19-02-00630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.).Vaughan RA, Kuhar MJ. J. Biol. Chem. 1996;271:21672. doi: 10.1074/jbc.271.35.21672. [DOI] [PubMed] [Google Scholar]
- 25.).Experimental observation; a manuscript detailing mapping of (±)-[125I]-3 within the Torpedo nAChR ion channel is currently in preparation.
- 26.).Newman AH, Cha JH, Cao J, Kopajtic T, Katz JL, Parnas ML, Vaughan R, Lever JR. J. Med. Chem. 2006;49:6621. doi: 10.1021/jm0603973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.