Abstract
Influenza viruses are important pathogens that cause respiratory infections in humans and animals. In addition to vaccination, antiviral drugs against influenza virus play a significant role in controlling viral infections by reducing disease progression and virus transmission. Plant derived polyphenols are associated with antioxidant activity, anti-carcinogenic, and cardio- and neuro-protective actions. Some polyphenols, such as resveratrol and epigallocatechin gallate (EGCG), showed significant anti-influenza activity in vitro and/or in vivo. Recently we showed that quercetin and isoquercetin (quercetin-3-β-D-glucoside), a glucoside form of quercetin, significantly reduced the replication of influenza viruses in vitro and in vivo (isoquercetin). The antiviral effects of isoquercetin were greater than that of quercetin with lower IC50 values and higher in vitro therapeutic index. Thus, we investigated the synthesis and antiviral activities of various quercetin derivatives with substitution of C3, C3’, and C5 hydroxyl functions with various phenolic ester, alkoxy, and aminoalkoxy moieties. Among newly synthesized compounds, quercetin-3-gallate which is structurally related to EGCG showed comparable antiviral activity against influenza virus (porcine H1N1 strain) to that of EGCG with improved in vitro therapeutic index.
Keywords: antiviral activity, influenza virus, porcine H1N1 strain, quercetin-3-gallate, substituted quercetins
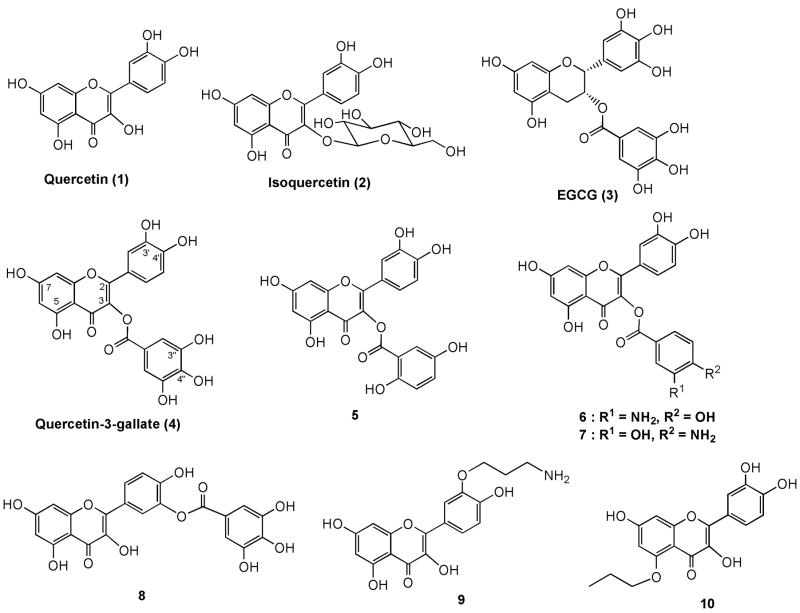
Influenza infections are responsible for over 3 million cases of illness and up to a half of million deaths per year.1 Although antiviral drugs against influenza infections are available, the emergence of viral resistance to existing antiviral drugs emphasizes the demand for development of new antiviral drugs against influenza infections. In the past few years, polyphenols including quercetin (1) and its analogs,2–4 quercetin-3-β-galactoside,5 quercetin 3-rhamnoside,6 quercetin 3-β-D-glucoside or isoquercetin (2)7 (Figure 1) were reported effective against influenza infections. Among them, isoquercetin (2) demonstrated a lower IC50 value against influenza viruses and higher therapeutic index compared to that of quercetin.7 (-)-Epigallocatechin-3-gallate (EGCG; 3) is the major polyphenol in green tea and reported to have anti-influenza virus activity8,9 and organic anion-transporting enhancing property.10 Quercetin-3-gallate (4) has been reported to be effective for the treatment of inflammatory bowel disease by inhibiting Na+-K+-ATPase and/or Na+/H+ exchange activities.11 However, the synthesis and anti-influenza virus activities of 4 and its hydroxyl aryl analogs have not been reported. A hybrid of quercetin and gallate or its analogs may show improved antiviral activities. Therefore antiviral activities of derivatives of quercetin with various substitutions at the hydroxyl functions were investigated. Herein, we report the synthesis and anti-influenza virus activities of compounds 4 – 10 containing C3-dihydroxybenzoate, C3-aminohydroxybenzoates, C3’-gallate, C3’-aminopropyloxy, and C5-propyloxy functions. Among the derivatives, quercetin-3-gallate (4) which is structurally related to EGCG showed comparable antiviral activity against influenza virus porcine H1N1 as that of EGCG with improved in vitro therapeutic index.
Figure 1.
Quercetin, Isoquercetin, EGCG, quercetin-3-gallate and synthesized quercetin anologs
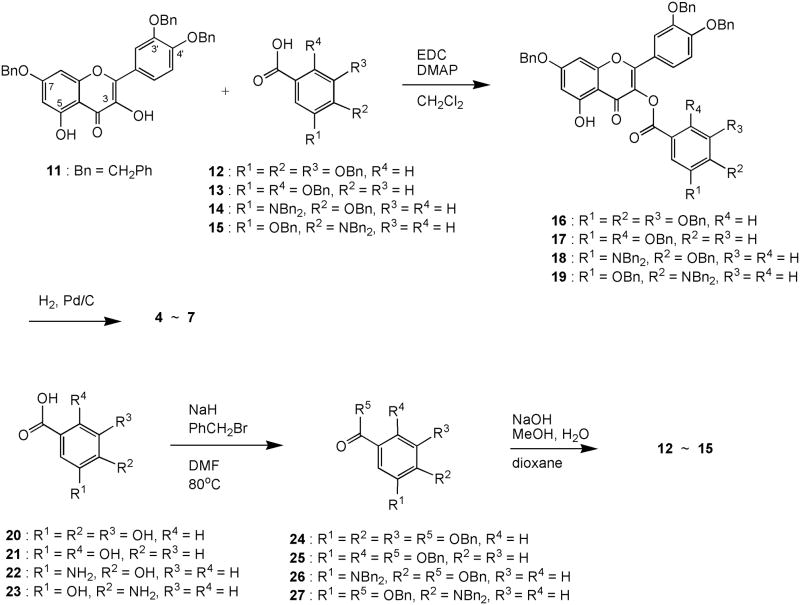
For the synthesis of C3-O-ester analogs of quercetin, a reported selective protection of the three most reactive hydroxyl functions at C7, C3’, and C4’ was adapted.12 Hence, 3’,4’,7-triO-benzylquercetin (11), derived from tribenzylation of rutin with potassium carbonate and benzyl bromide in DMF followed by removal of the C3-rutinose with hydrochloric acid and ethanol, was condensed with 3,4,5-tribenzylgallic acid (12),13 N-ethyl-N’-(3-dimethylaminopropyl)carbodiimide (EDC), and a catalytic amount of 4-dimethylaminopyridine (DMAP) in dichloromethane to give ester 16 in 64% yield (Scheme 1). Removal of the benzyl ether protecting groups of 16 with hydrogen (15 psi) and 5% palladium over carbon afforded quercetin gallate 4 (68% yield). It should be noted that C5-hydroxyl function is less reactive than C3-hydroxyl due to intramolecular hydrogen bonding and resonance conjugation with C4-keto function.
Scheme 1.
Synthesis of quercetin 3-O-analogs 4 – 7
Similarly, condensation of compound 11 with 2,5-dibenzyloxybenzoic acid (13),14,15 4-benzyloxy-3-(N,N-dibenzylamino)benzoic acid (14), and 3-benzyloxy-4-(N,N-dibenzylamino)benzoic acid (15) separately gave benzyl esters 17, 18, and 19 (83%, 75%, and 71% yield), respectively, which upon hydrogenation furnished respectively quercetin analogs 5, 6, and 7 (61%, 58%, and 70% yield) (Scheme 1). Compounds 12 and 13 are known and were prepared from a modified procedure of benzylation of gallic acid (20) and 2,5-dihydroxybenzoic acid (21), respectively, with sodium hydride (or sodium carbonate) and benzyl bromide in DMF13,15 followed by basic hydrolysis with sodium hydroxide (Scheme 1). Carboxylic acids 22 and 23 were similarly tetrabenzylated and hydrolyzed to give benzylated arylcarboxylic acids 14 and 15 in 76% and 87% overall yield, respectively.
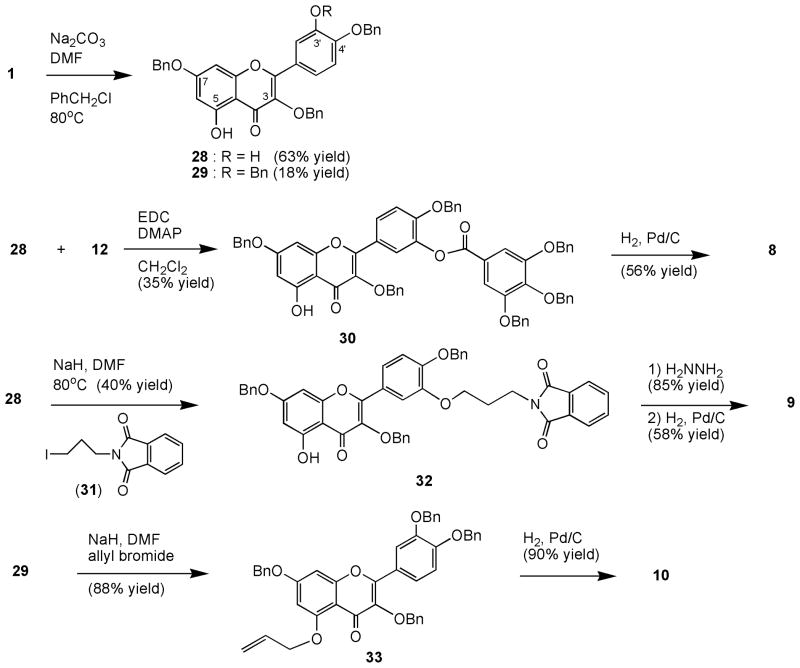
To access quercetin analogs containing C3’-O- and C5-O-substituents, we prepared hydroxyl-protected quercetins, compounds 28 and 29, as described previously16 from 1 and sodium carbonate and 3 equiv. of benzyl chloride in DMF (Scheme 2). A mixture of tribenzyl 28 (63% yield) and tetrabenzyl 29 (18 % yield) were isolated after column chromatographic separation. As mentioned above, due to intramolecular hydrogen bonding, C5-hydroxyl function is less reactive toward an electrophile than C3’-hydroxyl, hence selective esterification of 28 with tribenzyl gallic acid 12, EDC, and DMAP afforded C3’-gallic ester 30. Deprotection of the benzyl ether functions of 30 with hydrogen and palladium gave quercetin C3’-gallate 8 (Scheme 2).
Scheme 2.
Syntheses of quercetin C3' and C5 analogs
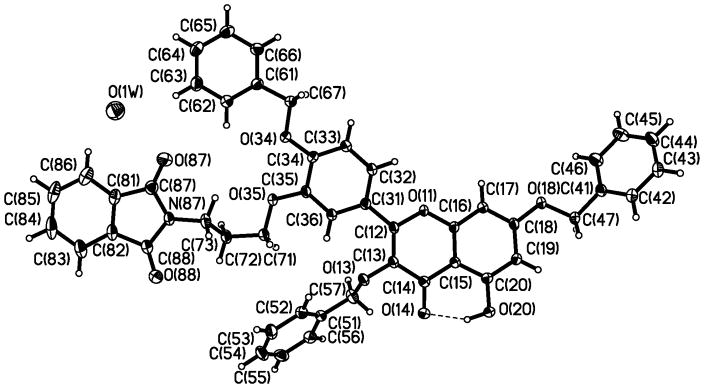
Selective C3’-alkylation can similarly be carried out. Hence, alkylation of 28 with sodium hydride and N-(3-iodopropyl)phthalimide (31)17,18 gave compound 32 (40% yield), which was subjected to reduction with hydrazine followed by hydrogen and palladium afforded 3-aminopropyl quercetin 9 (49% overall yield). The structure of compound 32 was unequivocally determined by a single-crystal x-ray analysis (Figure 2).18 The crystal is triclinic, space group of P-1, and the R factor of 0.051. A mole of water was revealed in the crystal structure of 32.
Figure 2.
An ORTEP drawing of X-ray crystallographically determined structure of compound 32.
Among five hydroxyl moieties, C5-hydroxyl function is the least reactive hydroxyl group of quercetin. We were unable to esterify C5-hydroxyl function of tetrabenzyl protected quercetin 29 with either tribenzyl protected gallic acid 12 or tribenzyl protected galloyl chloride and pyridine or sodium hydride at elevated temperature. Likely, the bulky 3,4,5-tribenzyloxybenzoyl group could not fit into the C5-hydroxyl function. However, smaller electrophiles such as allyl bromide can be attached to C5-hydroxyl group of 29. Alkylation of quercetin 29 with sodium hydride and allyl bromide gave C5-O-allyl quercetin 33 in 88% yield, which upon reduction with hydrogen and palladium furnished C5-O-propyl derivative 10 (90% yield). Allyl bromide was chosen as the alkylating agent instead of propyl iodide because a higher yield was produced.
The inhibition of influenza A virus (A/swine/OH/511445/2007[H1N1], Oh7) by compounds 1 – 10 and 29 was evaluated following a procedure reported previously.7 In brief, Madin-Darby canine kidney (MDCK) cells were infected with influenza virus Oh7 and incubated with each compound at various concentrations from 0.1 – 150 μM in the presence of trypsin (10 μg/mL) for up to 4 days. Cytopathic effects (CPE) of the cells were observed for each compound. The effective doses for 50% virus reduction (ED50) and toxic doses at 50% cell death (TD50) of the above compounds are summarized in Table 1. Viral replication was also confirmed by immunofluorescence assay and Western blot analysis with antibodies to the viral nucleoprotein or whole influenza virus, respectively. Among the tested compounds, isoquercetin, EGCG, quercetin-3-gallate appear to have similar ED50 values against H1N1 virus, and isoquercetin (2) is the most active compound with a ED50 value of 1.2 μM. The ED50 of EGCG and quercetin-3-gallate was determined at 8 and 9.5 μM, respectively, and therapeutic index (TI) value of quercetin-3-gallate of 9.9 is slightly better than that of EGCG of 5.5. C3-Dihydroxyl and hydroxylaminobenzoate analogs, compounds 5 – 7, have ED50 values of 20 – 24 μM and similar TD50 values as that of EGCG indicating modification of the gallate moiety (of EGCG and quercetin-3-gallate) retains the anti-influenza activity. Quercetin (1), without gallate function, is less effective with ED50 and TD50 values of 48.2 and 83.4 μM, respectively. Despite having a gallate function at C3’, compound 8, and C3’-3-aminopropyloxy analog 9 and C5-propyloxy compound 10 are not effective up to 50 μM. Benzylated analogs such as compound 29 are not effective up to 50 μM. Therefore the TD50 values of compounds 8 – 10 and 29 were not determined. It appears that derivatization of C3’ and C5 led to lower antiviral activity.
Table 1.
Values of effective dose required to reduce the replication of virus by 50% (ED50) and toxic dose for 50% cell death (TD50) of various natural and synthetic compounds.
| Compound | ED50 value in μM | TD50 value in μM | Therapeutic index (TI) |
|---|---|---|---|
| Quercetin (1) | 48.2 | 83.4 | 1.7 |
| Isoquercetin (2) | 1.2 | 45.1 | 37.6 |
| EGCG (3) | 8.3 | 45.5 | 5.5 |
| Quercetin-3-gallate (4) | 9.1 | 90.2 | 9.9 |
| 5 | 19.4 | 45.2 | 2.3 |
| 6 | 22.6 | 60.1 | 2.7 |
| 7 | 24.1 | 54.8 | 2.3 |
| 8 | >50 | ND* | - |
| 9 | >50 | ND | - |
| 10 | >50 | ND | - |
| 29 | >50 | ND | - |
ND: not determined due to high ED50 values.
Note: Quercetin, isoquercetin and EGCG were purchased from Sigma-Aldrich (St. Louis, MO).
In conclusion, various C3, C3’, and C5 substituted quercetins were synthesized and their anti-H1N1 activities were examined. C3-Substituted quercetins were derived from a carbodiimide-activated coupling reaction of C3’,C4’,C7-O-tribenzyl-protected quercetin 11 and various arylcarboxylic acids, and C3’- and C5-substituted quercetins were synthesized from C3,C4’,C7-O-tribenzyl- and C3,C3’,C4’,C7-O-tetrabenzyl-protected quercetin, respectively. Subsequent global removal of benzyl protecting groups afforded substituted quercetins. C5-Esterification with gallic acid 12 failed, but alkylation was possible. The synthetic sequence is short and amenable to large scale synthesis, and the synthesized C3-analogs have comparable antiviral activity as that of EGCG implying further modification at C3 is possible in improving efficacy.
Supplementary Material
Acknowledgments
This work has been supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (U01 AI081891).
Footnotes
Synthetic procedure, analysis data, and protocols for antiviral evaluation are included. Supplementary data associate with this article can be found in the online version, at …
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, Gust ID, Hampson AW, Hay AJ, Hurt AC, de Jong JC, Kielso A, Klimov AI, Kageyama T, Komadina N, Lapedes AS, Lin YP, Mosterin A, Obuchi M, Odagiri T, Osterhaus AD, Rimmelzwaan GF, Shaw MW, Skepner E, Stohr K, Tashiro M, Fouchier RA, Smith DJ. Vaccine. 2008;26(Suppl 4):D31. doi: 10.1016/j.vaccine.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 2.Liu A-L, Wang H-D, Lee SM, Wang Y-T, Du G-H. Bioorg & Med Chem. 2008;16:7141. doi: 10.1016/j.bmc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Lin C-W, Tsai F-J, Tsai C-H, Lai C-C, Wan L, Ho T-Y, Hsieh C-C, Chao P-DL. Antiviral Res. 2005;68:36. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu YB, Kim JH, Park S-J, Chang JS, Rho M-C, Bae K-H, Park KH, Lee WS. Bioorg & Med Chem Lett. 2010;20:971. doi: 10.1016/j.bmcl.2009.12.106. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Li J, Luo C, Liu H, Xu W, Chen G, Liew OW, Zhu W, Puah CM, Shen X, Jiang H. Bioorg & Med Chem. 2006;14:8295. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JJ, Song JH, Park KS, Kwon DH. Eur J Pharmaceutical Sci. 2009;37:329. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Narayanan S, Chang K-O. Antiviral Res. 2010;88:227. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Song J-M, Lee K-H, Seong B-L. Antiviral Res. 2005;68:66. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Nagle DG, Ferreira D, Zhou Y-D. Phytochem. 2006;67:1849. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Timmermann BN, Hagenbuch B. Drug Metabolism & Disposition. 2011;39:920. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares-Da-Silva PMVA. 2004/084886. PCT Int Appl WO. 2004:36.
- 12.Huang H, Jia Q, Ma J, Qin G, Chen Y, Xi Y, Lin L, Zhu W, Ding J, Jiang H, Liu H. Eur J Med Chem. 2009;44:1982. doi: 10.1016/j.ejmech.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belin F, Barthelemy P, Ruiz K, Lacombe JM, Pucci B. Helv Chim Acta. 2003;86:247. [Google Scholar]
- 14.Hayashi K, Hamada Y, Shioiri T. Tetrahedron Lett. 1992;33:5075. [Google Scholar]
- 15.Yamada K, Ikezaki M, Umino N, Ohtsuka H, Itoh N, Ikezawa K, Kiyomoto A, Iwakuma T. Chem Pharm Bull. 1981;29:744. doi: 10.1248/cpb.29.744. [DOI] [PubMed] [Google Scholar]
- 16.Wang RE, Kao JL-F, Hilliard CA, Pandita RK, Roti Roti JL, Hunt CR, Taylor J-S. J Med Chem. 2009;52:1912. doi: 10.1021/jm801445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaji E, Zen S. Bull Chem Soc Jpn. 1973;46:337. [Google Scholar]
- 18.Shi A, Nguyen TA, Battina SK, Rana S, Takemoto DJ, Chiang PK, Hua DH. Bioorg & Med Chem Lett. 2008;18:3364. doi: 10.1016/j.bmcl.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The authors have deposited atomic coordinates for the structures, compound 32 with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 175022. The coordinates can be obtained on request, from the Director, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, U.K.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.