Abstract
The cortisol awakening response (CAR) is presumed critically important for healthy adaptation. The current literature, however, is hampered by systematic measurement difficulties relative to awakening, especially with young children. While reports suggest the CAR is smaller in children than adults, well-controlled research in early childhood is scarce. We examined whether robust CARs exist in 2- to 4-year-old children and if sleep restriction, wake timing, and napping influence the CAR (n=7). During a 25-day in-home protocol, researchers collected four salivary cortisol samples (0, 15, 30, 45 min post-wake) following five polysomnographic sleep recordings on non-consecutive days after 4 hrs (morning nap), 7 hrs (afternoon nap), 10 hrs (evening nap), 13 hrs (baseline night), and 16 hrs (sleep restriction night) of wakefulness (20 samples/child). The CAR was robust after nighttime sleep, diminished after sleep restriction, and smaller but distinct after morning and afternoon (not evening) naps. Cortisol remained elevated 45 min after morning and afternoon naps.
Keywords: CAR, Cortisol, Children, Sleep, Polysomnography
Cortisol, the primary hormonal product of the hypothalamic-pituitary-adrenal (HPA) axis, plays an essential role in an organism's ability to cope with everyday stressors (Gunnar 1989). Cortisol secretion follows a circadian rhythm, with the highest levels post-awakening, a sharp decrease during morning hours, and a more gradual decline throughout the day (Van Cauter 2005). A striking feature of the daily distribution of cortisol is the precipitous increase in levels 30-45 min after awakening (Pruessner, Wolf et al. 1997; Wust, Federenko et al. 2000). Findings with adults suggest this cortisol awakening response (CAR) is greatest during the morning hours when the adrenal cortex is most sensitive to light inputs from the suprachiasmatic nuclei (Leproult, Colecchia et al. 2001). While the CAR is modulated by the circadian system, it represents a distinct response reflecting phasic activation of the HPA axis during the sleep-to-wake transition (Wilhelm, Born et al. 2007). Comprehensive assessment of the CAR entails measurement of two different dimensions, overall cortisol secretory activity and the dynamic of the response (Pruessner, Kirschbaum et al. 2003; Clow, Thorn et al. 2004; Thorn, Hucklebridge et al. 2006).
Although the CAR is an established biomarker of adrenocortical activity (Pruessner, Wolf et al. 1997), its function or functions are not well understood. The CAR is thought to reflect anticipation of the metabolic and postural challenges of starting a new day (Kunz-Ebrecht, Kirschbaum et al. 2004; McEwen 2006), thereby providing a physiological “boost” for anticipated demands based on prior experience (Adam, Hawkley et al. 2006). Individual differences in the magnitude CAR are associated with a variety of psychological, stress, and health variables (Clow, Thorn et al. 2004). For example, an elevated CAR was observed in individuals with amplified chronic stress (Steptoe, Cropley et al. 2000; Schlotz, Hellhammer et al. 2004) and chronic anxiety (Greaves-Lord, Ferdinand et al. 2007), whereas a blunted response was reported in children with comorbid attention-deficit hyperactivity disorder and oppositional defiant disorder (Freitag, Hanig et al. 2009) and in adults with insomnia (Backhaus, Junghanns et al. 2004).
Although published reports examining associations between sleep-related parameters (e.g., sleep duration, wake timing, napping) and the CAR are accumulating, further study is needed. To date, several reports on adults indicate the CAR is neither related to nocturnal sleep duration (Pruessner, Wolf et al. 1997; Wust, Wolf et al. 2000; Federenko, Wust et al. 2004) nor to wake timing (Pruessner, Wolf et al. 1997; Wust, Wolf et al. 2000), while a handful of other studies suggest associations between wake time and CAR parameters (Edwards, Evans et al. 2001; Kudielka and Kirschbaum 2003; Federenko, Wust et al. 2004). Uncertainty also exists about the presence of the CAR following diurnal sleep periods (naps). Based upon one report of no CAR after a short early evening nap in college students (Federenko, Wust et al. 2004), it was suggested that either the CAR is produced after only longer periods of nighttime sleep (Fries, Dettenborn et al. 2009) or that the dampened response is associated with the timing of the circadian nadir of cortisol (Federenko, Wust et al. 2004). Interpreting the existing literature on sleep and the CAR is complicated because the majority of findings have been based upon correlational and/or quasi-experimental studies with subjective measurement of sleep (bedtime, wake time, sleep duration) and incomplete control over potential confounds (Pruessner, Wolf et al. 1997; Wust, Wolf et al. 2000; Federenko, Wust et al. 2004). Furthermore, data have been collected primarily by participants in their own homes, and reliance on participant compliance is inherently problematic (Clow, Thorn et al. 2004). For example, if the first sample is delayed too long after wake time (>15 min), the CAR will appear “flat” (Dockray, Bhattacharyya et al. 2008; Okun, Krafty et al. 2010). This measurement issue is likely magnified in young children as they are unable to collect their own saliva samples and may wake earlier than their parents with the result being a “missed” CAR.
To date, very little is known about the CAR in young children, including its typical characteristics, confounds, and correlates. Findings from available reports of pre-pubertal children suggest the dynamic of the CAR may be smaller in children than previously documented in adults and that older children are more likely to be “responders” (show a positive post-wake response) than younger children (Schmidt, Fox et al. 1997; Kudielka and Kirschbaum 2003; O'Connor, Ben-Shlomo et al. 2005; Rosmalen, Oldehinkel et al. 2005; Hatzinger, Brand et al. 2007; DeCaro and Worthman 2008; Freitag, Hanig et al. 2009; Saridjan, Huizink et al. 2010). Careful examination of these reports indicates none have employed objective measures of wake time, thus, making it difficult to differentiate between measurement error and true developmental change (Clow, Thorn et al. 2004). Furthermore, in the handful of studies including children, most obtained cortisol samples at only two time points (i.e., 0 and 30 min post-wake), thereby limiting current understanding of overall morning cortisol secretory activity in children. Research on the CAR in preschool children is extremely rare (Schmidt, Fox et al. 1997; Saridjan, Huizink et al. 2010).
To our knowledge, no published reports on both dimensions of the CAR (i.e., total cortisol secretory activity, dynamic of the response) using objective sleep measures in children under the age of 5 years exist. We are also unaware of any well-controlled experimental research on the effects of sleep restriction, wake timing, and napping on the CAR. In the present study, we studied healthy, typically developing 2- to 4-year old children with a rigorous protocol to address a number of methodological and measurement concerns. During the 25-day study, children followed an individualized, strict sleep-wake schedule verified by continuous wrist actigraphy monitoring. Researchers collected all salivary cortisol samples in the child's home environment, and polysomnography (PSG) was used to ensure accurate information about children's wake time and sleep duration. We documented the timing of each saliva sample and monitored activity levels and food and beverage intake. We examined the CAR after a baseline full night of sleep, after a night of acute sleep restriction due to a delayed bedtime, and on days where naps occurred in the late morning, mid-afternoon, and early evening (with at least 2 recovery days/nights between assessments). We used these data to address three main questions: (a) What is the effect of acute nighttime sleep restriction (sleep duration) on the CAR in children; (b) Do differences exist in the CAR depending on the time of waking (i.e., after baseline night sleep, morning nap, afternoon nap, evening nap); and (c) Is the CAR evident after daytime naps?
Method
Recruitment and Screening of Participants
Participants were 7 healthy children aged 30- to 48-months (5 females). Families were recruited by posting flyers, laboratory website advertising, and personal contact at community events. Screening involved parents completing a brief telephone interview followed by a set of questionnaires. Study inclusion required children to be 30- to 48-months-old and regularly following a biphasic sleep schedule (nighttime sleep period of a least 10.5 hrs and one daytime nap of at least 45 min time in bed) in which they fell asleep at least 2 days per week during their nap opportunity. Exclusionary criteria included the following: (a) daily/nightly cosleeping; (b) a bedtime/wake time sleep schedule varying more than 2 hrs between weekday and weekends; (c) travel beyond 2 time zones within 3 months prior to assessments; (d) regular use of medications affecting sleep, daytime alertness, the circadian system, and/or HPA axis activity; (e) sleep problems; (f) developmental disabilities, epilepsy, neurologic/metabolic disorders, chronic medical conditions, lead poisoning, and head injury involving loss of consciousness; (g) pre-term or post-term delivery (term= 35-45 weeks); (h) low birth weight (<5.5lbs); or (i) a family history (first degree) of diagnosed narcolepsy, psychosis, or bipolar disorder.
Of the 78 children screened, 10 met all criteria. Exclusions were due to children not having a daily nap opportunity (n=48), not napping (sleeping) at least 2 days per week (n=6), sleep problems (n=4), an inconsistent sleep-wake schedule (n=2), medication use (n=4), preterm delivery (n=1), chronic medical conditions (n=2), and a family history of bipolar disorder (n=1). Seven of the 10 enrolled children completed the entire protocol. Incomplete assessments were a consequence of children not providing adequate saliva samples (n=1), skin sensitivity to electrodes (n=1), or noncompliance with the study protocol (n=1). All families signed an institutional review board approved consent form. Parents received $120 in cash, and children received small non-monetary gifts following each assessment and a $200 savings bond at study completion.
Protocol
During the 25-day study, children were required to sleep at home (own bed, lights-out, no other activities) and to refrain from using caffeine. Children followed a prescribed sleep schedule, and compliance was verified with actigraphy, sleep diary entries, and daily email/telephone contact with parents. The protocol included two phases:
Phase I: Stabilization
Children followed a structured sleep schedule on study days 1-5. This “stabilization” period stems from the need to minimize sleep restriction and to entrain the circadian system. It also allowed us to tailor schedules for individual children and provided consistency prior to the experimental phase of the study so effects on CAR parameters were well-controlled. The stabilization schedule included a minimum sleep opportunity of 12.5 hrs per 24-hr day.
Phase II: Varied Prior Wakefulness
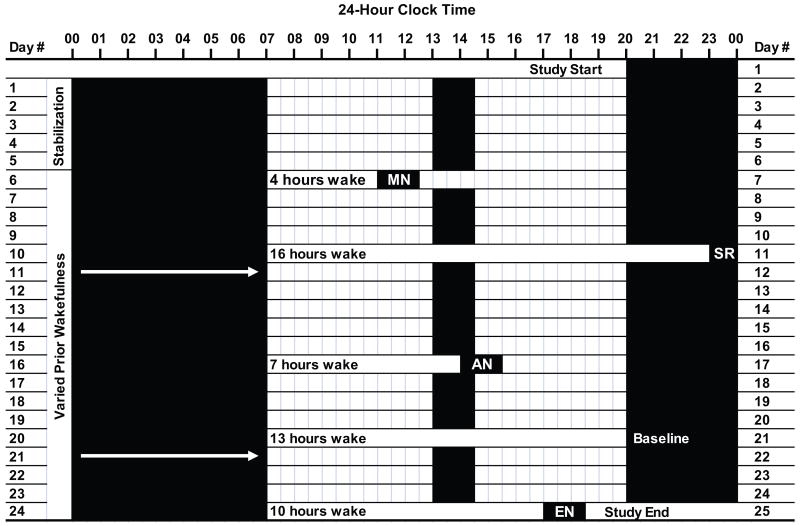
During the next three weeks, children followed their stabilization sleep schedule; however, on five days, we varied the duration of wakefulness prior to the sleep period for the purpose of assessing effects on the CAR. PSG sleep recordings were performed after 4 hrs (morning nap), 7 hrs (afternoon nap), 10 hrs (evening nap), 13 hrs (baseline night), and 16 hrs (sleep restriction night) of wakefulness. Thus, a child with a 90 min nap opportunity (13:00-14:30), a 20:00 bedtime, and a 7:00 wake time (12.5 hours total time in bed) during the stabilization phase would have sleep recordings scheduled at 11:00, 14:00, and 17:00, 20:00, and 23:00 (Figure 1). When prior wakefulness was at or past the regular bedtime (13 and 16 hours), sleep recordings continued throughout the night. Children slept until spontaneous wake from all day and night sleep recordings except the evening nap; in this condition, we awakened children who were still asleep after 60 min or at completion of their first full nonrapid eye-movement (NREM)/rapid eye-movement (REM) cycle due to concerns that longer sleep would disrupt the subsequent nighttime sleep period (n=3). Sleep recordings occurred on non-consecutive days of the protocol (order randomly determined), with at least 2 intervening recovery nights of sleep (average = 4.3 nights).
Figure 1.
A 25-day sample protocol for a child following a strict sleep-wake schedule with a 20:00 bedtime, a 07:00 wake time, and a 13:00-14:30 afternoon nap (12.5 hours time in bed/24-hour period). Days 1-5 represent the stabilization phase; days 6-25 included five polysomnographic randomly-ordered sleep recordings following 4, 7, 10, 13, and 16 hours of prior wakefulness. For this child, recordings were performed at 11:00 (morning nap: MN), 14:00 (afternoon nap: AN), 17:00 (evening nap: EN), 20:00 (baseline night; Baseline), and 23:00 (sleep restriction night: SR). Solid black bars represent time in bed; white bars represent periods of wakefulness. Arrows indicate an overnight sleep recording.
Field-Based Measures
Polysomnography (PSG)
PSG was performed with a portable Vitaport 3:16 channel EEG recorder (Temec Instruments, Kerkrade, The Netherlands). Recordings were made of referential (contralateral mastoid) EEG from C3, C4, O1 and O2 electrodes measured according to the standard 10-20 system (Jasper 1958). PSGs were visually scored as wake, REM sleep, Stages 1, 2, 3, and 4 in NREM sleep, or movement time in 30-sec epochs according to standard criteria (Rechtschaffen and Kales 1968). Measures obtained from PSG included sleep period (lights-off to lights-on) and sleep duration (sleep onset to sleep offset). PSG also provided an objective measure of nap and morning wake time, which were used to compute latency (Sekine, Yamagami et al.) between wake and the time of the first post-wake saliva sample.
Actigraphy
Actigraphy is widely accepted as a noninvasive means to estimate sleep patterns under non-laboratory conditions (Acebo and LeBourgeois 2006). The actigraph (model AW2) is worn on the child's non-dominant wrist and provides continuous recordings of sleep/wake states by measurement of motor activity (Philips Respironics, Murrysville, PA). The Actiware-Sleep V5.02 software analyzes 1 min epochs for sleep and wake by modifying the activity count during a single epoch by activity level in the surrounding 2 min time period. This algorithm is applied to portions of the record identified as sleep through a combination of diary reports and actigraph event markers at “lights out” and “lights on.” We used actigraphy to verify sleep schedule compliance and as an additional objective measure of wake time.
Sleep Diary
Parents completed a 26-item daily sleep diary. Evening questions documented children's mood, stress level, nap time(s), caffeine/medicine intake, actigraph off times, bedtime, and lights-out time. Morning questions documented children's night awakenings, sleep quality, and wake time. We used sleep diary entries to verify study compliance with study rules and schedules and to support scoring of actigraphy data.
CAR Assessments
Cortisol concentration was determined from saliva samples following well-established procedures (Gunnar and White 2001; Watamura, Donzella et al. 2003). Milk products were withheld for 2 hrs before sampling, and all food and liquids were withheld 15 min before sampling. All samples were obtained with the assistance of a researcher (not parents) in the home setting (e.g., bedroom, family room). While seated, children mouthed a 6 inch dental cotton roll for 1 to 2 min. Any excess was cut off by the researcher before inserting the cotton into a tube. Samples were tightly capped, centrifuged, and refrigerated on-site until transported to the laboratory where they were frozen within 6 hrs of assessment completion (-20° C). Cortisol samples were analyzed in duplicate by high sensitivity enzyme immunoassay kit (Salimetrics, PA); range of sensitivity was .007-1.2 ug/dl (intra and inter-assay coefficients of variation were 4.13% and 8.89%, respectively). All samples from the same child were stored and analyzed together to decrease potential error variance associated with assay kit differences. In order to measure the CAR, salivary cortisol was collected at 4 points after five PSG-recorded sleep episodes (morning nap, afternoon nap, evening nap, baseline night, sleep restriction night): 0 (wake time), 15, 30, and 45 minutes after wake time. One sampling day was carried out per sleep condition. Thus, a total of 20 salivary cortisol samples were collected from each child during the 25-day protocol.
Analysis Plan
Analyses were performed with SPSS Statistics Version 17.0 and multilevel models using HLM Version 6.0. Of the 161 possible data points, data were missing for six (3.7%) due to participant non-compliance. We replaced all missing values with the mean of remaining participants from respective time points (McKnight, McNight et al. 2007). We employed standard measures to assess both dimensions of the CAR. Total cortisol secretion was computed as area under the curve with respect to ground [AUCg = (S1+S2/2)+(S2+S3/2)+(S3+S4/2); (Pruessner, Kirschbaum et al. 2003)]. The dynamic of the CAR was computed as the difference between the waking (0 min) and maximum post-wake value (15, 30, 45 min; Wake-Max Change). It should be noted that of the 35 possible assessments, two (different children) produced negative Wake-Max Change measures due to cortisol concentration at 0 min being higher than at 15, 30, and 45 min (no increase observed). In consideration of this well-known issue, we set these values to 0 (Pruessner, Kirschbaum et al. 2003).
To examine sleep restriction effects on the CAR (Question 1), we compared standard CAR parameters after the baseline night of sleep (13 hrs prior wake) to those after the sleep restriction night (16 hrs prior wake) with paired t-tests. To better characterize sleep restriction effects on the CAR, we then used a 3-level model to examine whether the CAR (4 time points taken at 0, 15, 30, and 45 min) differed between Baseline and Sleep Restriction Night Conditions. Because of the nature of the CAR, we estimated both linear and quadratic effects. Note that this 3-level modeling approach has been used successfully by other authors to examine similar processes ((Adam, Hawkley et al. 2006)) and is recommended for data of this type (Hruschka, Kohrt et al. 2005). The estimated model had the following form (Raudenbush, Bryk et al. 2004):
| Level 1: | Yti = π0i + π1i*(CORTIDti) + π2i*(CORTIDti2) + eti |
| Level 2: | π0i = β00 + β01*(DAYi) + r0i |
| π1i = β10 + β11*(DAYi) + r1i | |
| π2i = β20 + β21*(DAYi) + r2i | |
| Level 3: | β00 = γ000 + u00 |
| β01 = γ010 | |
| β10 = γ100 | |
| β11 = γ110 | |
| β20 = γ200 | |
| β21 = γ210 |
where Yti is the outcome variable, cortisol level, for person i at time t; π0i is the intercept; π1i is the linear effect of the main variable sampling time and π2i is the quadratic effect of sampling time; and eti is the residual variance. The cortisol intercept, linear slope, and quadratic slope were modeled as random coefficients (i.e., allowing for day-to-day variability in these coefficients) that vary as a function of the type of day (e.g., baseline vs. sleep restriction). Furthermore, we accounted for clustering within individuals, by allowing between-person variability in cortisol intercepts u00 (but due to the small sample size we did not allow for between-person variability in linear or quadratic slopes).
To address the effects of wake timing on the CAR (Question 2), we compared standard CAR parameters using repeated measures ANOVA (baseline, morning nap, afternoon nap, evening nap); this analysis also enabled us to examine whether a nap was sufficient to produce a CAR (Question 3). Again, we further examined difference in the CAR between the baseline night and the three naps (morning, afternoon, and evening) using a 3-level hierarchical model with a comparable approach to that reported in the previous HLM model.
We used ANOVA with a Greenhouse-Geiser correction to produce a valid F-ratio with corrected degrees of freedom (p<.05). Post-hoc comparisons were performed using a Bonferroni correction (p<.008). Summary measures were presented as mean ± standard deviation. Effect sizes were reported as d or η2.
Results
Sleep Protocol Verification
The mean morning wake time on the day of all sleep recordings was 6:28 (:28), and average lights-out time for the five sleep conditions was consistent with the expectations of the experimental protocol. To examine the effects of acute nighttime sleep restriction on measures of the CAR, we manipulated sleep duration by holding morning wake time constant and extending wakefulness by 3 hours (13 hrs on Baseline to 16 hrs on Sleep Restriction). As expected, sleep period was longer (118.7±40.6 minutes), t(6)=9.71; d=5.25; p<.001, and sleep duration was greater (108.9±29.7 minutes), t(6) = 7.74; d = 4.29; p < .001 in the Baseline than in the Sleep Restriction condition (Table 1). We also tested differences in CAR parameters as a function of nap timing while holding nap duration constant. Averaged sleep duration between the Morning, Afternoon, and Evening Nap conditions did not differ (Table 1).
Table 1. Sleep parameters, latency to first saliva sample, and awakening cortisol response (ACR) measures presented as mean (SD) for 7 young children.
Sleep recordings were performed on non-consecutive days after 4 hrs (Morning Nap), 7 hrs (Afternoon Nap), 10 hrs (Evening Nap), 13 hrs (Baseline Night), and 16 hrs (Sleep Restriction Night) of prior wakefulness.
| Sleep Parameters (PSG) | NIGHT SLEEP | DAYTIME SLEEP | |||
|---|---|---|---|---|---|
| Baseline | Sleep Restriction | Morning Nap | Afternoon Nap | Evening Nap | |
| Lights-Out Time a | 20:06 (0:37) | 22:47 (0:37) | 11:05 (0:28) | 13:54 (0:24) | 16:38 (0:25) |
|
|
|
|
|
|
|
| Wake Time a | 6:57 (0:35) | 7:25 (0:41) | 13:05 (0:18) | 15:31 (0:26) | 17:54 (0:34) |
|
|
|
|
|
|
|
| Sleep Period (min) a | 630.71 (37.54) | 512.00 (17.83) | 87.07 (28.06) | 82.14 (38.93) | 64.36 (13.98) |
|
|
|
|
|
|
|
| Sleep Duration (min) a | 602.35 (26.89) | 493.50 (14.56) | 86.14 (28.24) | 81.36 (38.63) | 60.08 (10.16) |
|
|
|
|
|
|
|
| Latency to First Sample | |||||
|
|
|
|
|
|
|
| Min to Sample 1 (PSG) | 9.12 (8.25) | 3.34 (2.55) | 4.87 (4.01) | 3.85 (2.36) | 3.89 (2.36) |
|
|
|
|
|
|
|
| Min to Sample 1 (ACT) | 12.00 (6.71) | 8.00 (3.70) | 10.00 (3.58) | 7.86 (3.85) | 10.00 (4.32) |
|
|
|
|
|
|
|
| ACR Measures | |||||
|
|
|
|
|
|
|
| AUCg (μg/dl) a, b, c, d, e | 1.89 (0.58) | 1.53 (0.32) | 0.89 (0.21) | 0.91 (0.60) | 0.21 (0.17) |
|
|
|
|
|
|
|
| Wake-Max Change (μg/dl) e | 0.14 (0.11) | 0.19 (0.16) | 0.21 (0.08) | 0.17 (0.14) | 0.04 (0.04) |
Note: PSG = polysomnography; Sleep Period = lights-off to lights-on time; Sleep Duration = sleep onset to sleep offset; Minutes to Sample 1(PSG) = latency from sleep offset to the first post-wake sample defined by PSG; Minutes to sample 1 (ACT) = latency from sleep offset to the first post-wake sample defined by actigraphy; AUCg = area under the curve with respect to ground; Wake-Max Change = difference between awakening sample (0 min) and maximum sample at 15, 30, 45 min. Significant pairwise comparisons include:
(Baseline ≠ Sleep Restriction);
(Baseline ≠ Morning Nap);
(Baseline ≠ Afternoon Nap);
(Baseline ≠ Evening Nap);
(Morning Nap ≠ Evening Nap).
Latency from Awakening to First Saliva Sample
An important aspect of this study was to ensure salivary cortisol samples were collected as close to wake time as possible. On average, the first sample for all five sleep conditions was collected before the 15 min threshold as measured by PSG and actigraphy (Dockray, Bhattacharyya et al. 2008; Okun, Krafty et al. 2010) and did not differ between conditions (Table 1). In the Baseline condition, two children had transient early morning wakings, thus, making it difficult to accurately define wake time via online PSG monitoring. In these cases, the first morning sample was taken beyond the 15 min threshold; however, both children showed a robust positive CAR.
Group CAR Profiles following Daytime Naps and Nighttime Sleep
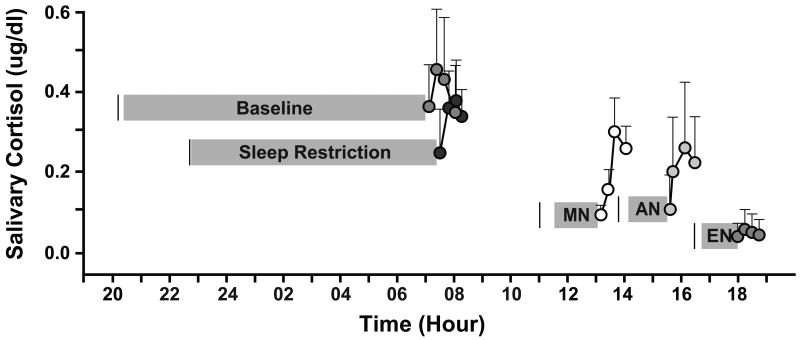
Salivary cortisol levels rose from 0 to 15/30 minutes post-wake and then decreased from 30 to 45 minutes in the Morning Nap, Afternoon Nap, Baseline, and Sleep Restriction conditions (Figure 2). After an evening nap, however, we did not detect change in average cortisol levels. Salivary cortisol values of the CAR declined throughout the day, with the first morning wake-up values after the baseline and sleep restriction nights being higher than those following the morning, afternoon, and evening naps, F(2.5,15.3) = 25.71, η2 = .81, p < .001.
Figure 2.
Awakening Cortisol Response (ACR) Profiles following Nighttime Sleep and Daytime Naps (n=7). Salivary cortisol samples obtained after a morning nap (MN; lights-out 4 hrs post morning wake time), an afternoon nap (AN; lights-out 7 hrs post morning wake time), an evening nap (EN; lights-out 10 hrs post morning wake time), a typical all-night sleep period (Baseline; lights-out 13 hrs post morning wake time), and a restricted all-night sleep period (Sleep Restriction; lights-out 16 hrs post morning wake time). Averaged morning wake time on days of sleep recordings was 6:28 (:28). Solid vertical black lines represent lights-out time. Grey bars indicate sleep duration (sleep onset to sleep offset) based upon polysomnographic recordings. Error bars represent standard deviations.
Sleep Restriction effects on CAR Measures
A two-tailed paired samples t-test showed overall cortisol secretory activity (AUCg) after awaking was higher after the Baseline than after the Sleep Restriction Night condition, t(6) = 2.77; d = 0.78; p = .03. No difference in the CAR dynamic (Wake-Max Change) was observed (Table 1).
With regard to the 3-level heirarchical model, we first ran the unconditional model that estimated day-to-day variability in the CAR without the effects of sleep restriction. Results indicated that all three growth parameters (cortisol intercept, linear slope, and quadratic slope) had day-to-day variability (SD = .12, p < .001 for the intercept, SD = .17, p < .001 for the linear slope, and SD = .05, p < .001 for the quadratic slope, respectively). Next we added the effect of sleep restriction on the CAR. Sleep restriction was significantly associated with variability in the intercept but not the variability in linear or quadratic slopes. Participants had significantly lower cortisol intercepts (waking value) following the sleep restriction night than the baseline night, b = -.13, p = .01. Sleep restriction accounted for 41% of the day-to-day variance in cortisol waking values (i.e. intercept). Thus, after only one night of sleep restriction, evidence of an altered CAR (i.e., reduced overall cortisol secretory activity, dampened first morning cortisol production) is apparent in young children.
Wake Time effects on CAR Measures after Daytime Naps and Nighttime Sleep
We compared standard CAR parameters following four sleep conditions (Baseline Night, Morning Nap, Afternoon Nap, and Evening Nap) using repeated measures ANOVA (Table 1). Overall cortisol secretory activity (AUCg) differed by condition, F(2.1,12.4) = 33.84, η2 = .85, p < .001. Post-hoc analysis indicated AUCg was greater after a full night of sleep (Baseline) than after a morning (p = .012), afternoon (p = .029), and evening nap (p < .001). AUCg was also lower after an evening than after a morning nap (p < .001). With regard to the CAR dynamic, the Wake-Max Change in cortisol was influenced by wake time, F(2.6,15.8) = 6.20, η2 = .51, p = .007. Post-hoc analysis revealed the Wake-Max Change was larger after the morning than after the evening nap (p = .008). Therefore, wake time in young children impacts the CAR as measured by both the overall cortisol secretory activity (greatest after a full night of sleep) and the dynamic of the response (most dramatic after a morning nap).
A 3-level hierarchical model showed a significant difference in the CAR intercept and linear slope after the baseline night as compared to after the three naps. The intercept (waking value) was .29 ug/dL lower following naps than following morning awakening, p < .001; however, the linear increase was .06 units steeper following naps than following the baseline night, p < .01. Modeling the baseline night versus the nap accounted for 79% of the day-to-day variance in waking values and 2% of the day-to-day variance in linear slopes (variance in linear slopes was calculated using an alternative formula as suggested by Xu, 2003). We next compared the CAR across the three nap conditions. Napping in the morning and afternoon as compared to the evening resulted in a greater linear rise in cortisol after awakening, B = .06, p < .01 and B = .03, p < .05, respectively. However, the CAR after a morning nap did not differ from the CAR following an afternoon nap.
Discussion
To our knowledge, this study is the first to describe dimensions of the CAR in healthy, normally developing 30- to 48-month old children after a full night of sleep, a night of sleep restricted by about 2 hours, and a late morning, mid-afternoon, and early evening nap. A combination of tightly-controlled procedures minimizing sources of error variance, a repeated-measures design, and large effect sizes led to several significant findings in this relatively small sample: (a) As evidenced by both overall cortisol secretory activity and the response dynamic, young children exhibit a robust CAR after nighttime sleep; (b) striking CARs following morning and afternoon naps and a flattened CAR after an evening nap indicate short sleep periods are of sufficient duration to produce CARs, at least when the timing of wake does not coincide with the circadian cortisol nadir; (c) one night of sleep restriction in young children attenuates overall cortisol secretion but may produce little change in the CAR dynamic; (d) while total hormonal production is greater after waking from a full night of sleep than after daytime naps, the dynamic of response is most striking after a morning nap.
In ambulatory studies of children using parent-reported wake time, within-study findings suggest age-related increases in “responders.” Close examination of several developmental studies (DeCaro and Worthman 2008; Freitag, Hanig et al. 2009; Saridjan, Huizink et al. 2010), however, reveals substantial variability in positive CAR rates and no clear trend associated with age. Whether true CAR “non-responders” exist is debated in the literature, and understanding factors influencing flat or negative CARs has implication for the analysis, interpretation, and significance of reported CAR data. In the current study, 100% of children elicited a positive CAR, which builds upon results of Wilhelm et al.(Wilhelm, Born et al. 2007), who found 100% of in-lab (PSG-defined wake) compared to 75% of in-home participants showed a positive CAR. This suggests a notable proportion of non-responders are either inaccurate reporters of morning wake time, perhaps due to transient awakenings, or that variable ambient conditions in the home environment may influence results. We believe the likelihood of non-responders resulting from a “missed” first morning sample is likely heightened in young children because they may wake before their parents and are too young to collect their own saliva samples.
Prior suggestions of a dampened CAR in healthy children as compared to adults stem from findings on the magnitude of the dynamic, specifically the absolute difference and the percent change in cortisol from wake to 30 min post-wake (Pruessner, Wolf et al. 1997; Rosmalen, Oldehinkel et al. 2005; Freitag, Hanig et al. 2009). Following both nighttime sleep periods, mean salivary cortisol values in our sample of healthy young children increased on average 50% (.18 μg/dl or 5.00 nmol/l) from 0 to 15/30 minutes post-awakening and then declined from 30 to 45 minutes, which is consistent with findings from an ambulatory study of school-aged children (increase of 50%, 5.63 nmol/l)(Pruessner, Wolf et al. 1997). However, results from the most well-controlled adult laboratory study (e.g., PSG recordings, waking light level, habitual wake time, activity level) indicate an increase of 6.3 nmol/l (108%) in the first 30 min post waking (Wilhelm, Born et al. 2007). Taken together, our findings suggest young children do exhibit a robust yet smaller CAR than adults; however, future well-controlled, longitudinal research is needed to ascertain developmental change.
In comparing our findings with existing reports showing no significant association between sleep duration and the CAR, it is important to note our repeated-measures design provided tight control of individual differences. Furthermore, while the few existing studies examining sleep associations measured both CAR dimensions, all but one reported solely on the dynamic of the response (Federenko, Wust et al. 2004). Using PSG, our findings replicate those of others revealing self-reported sleep duration is not related to the CAR dynamic (Pruessner, Wolf et al. 1997; Wust, Wolf et al. 2000; Federenko, Wust et al. 2004) and extend this small body of work to the early childhood period. With regard to total cortisol concentration after waking, Federenko and colleagues (Federenko, Wust et al. 2004) found no relationship between self-reported sleep duration and AUC in a sample of nurses and college students. The current results, however, provide strong evidence that even one night of shortening children's sleep duration by about 2 hrs dampens overall cortisol secretion during the 45 min post-awakening period. Whether such an effect would be observed in older children, adolescents, or adults is unknown, and the novelty of this finding warrants replication with individuals across the lifespan.
We considered several possible reasons for sleep restriction-induced changes in the CAR, including variations in sleep intensity, sleep fragmentation, and light exposure surrounding the sleep period. As expected, a paired t-test showed the percent time children spent in slow-wave sleep (SWS) was higher in the Sleep Restriction (25.9%) than in the Baseline condition (21.9%; t(6) = -3.58, d = 1.87, p = .012). Given the suspected inhibitory influences of SWS on HPA secretory activity during the night (Spath-Schwalbe, Uthgenannt et al. 1993; Gronfier, Luthringer et al. 1997), it is plausible the overall dampened post-awakening cortisol production we observed following shortened nighttime sleep in children is accounted for by changes in sleep intensity. Because awakenings during nighttime sleep induce cortisol secretory pulses and lead to higher morning cortisol levels (Spath-Schwalbe, Gofferje et al. 1991; Omisade, Buxton et al. 2010; Stamatakis and Punjabi 2010), we also wondered whether children experienced greater sleep fragmentation on the baseline night. We found the percent time in wake after sleep onset as measured with PSG was the same (Baseline: 3.1%, Sleep Restriction: 2.7%), suggesting the effect of sleep restriction on the CAR in young children was not associated with differences in sleep fragmentation. Finally, the CAR is more pronounced with morning light exposure (Scheer and Buijs 1999; Leproult, Colecchia et al. 2001; Thorn, Hucklebridge et al. 2004). We cannot rule out the possibility that children experienced more light following baseline sleep than sleep restriction because light levels were neither controlled nor measured.
In the present study, children took a late morning, mid-afternoon, and early evening nap. We found robust (and indistinguishable) CARs after both morning and afternoon naps, but we were unable to detect a CAR after the evening nap. These findings replicate Federenko and colleagues' (Federenko, Wust et al. 2004) demonstration that a nap in the evening was insufficient to produce an CAR in adults. We did find a short nap produces a CAR if the time of wake is not positioned at the circadian cortisol nadir, suggesting the circadian system may “gate” opportunity for phasic activation of the HPA axis in response to the transition from sleep to wakefulness. Additionally, evidence for an influence of self-reported awakening time on measures of the CAR by some (Edwards, Evans et al. 2001; Kudielka and Kirschbaum 2003; Federenko, Wust et al. 2004) and no relationship between the two by others (Pruessner, Wolf et al. 1997; Wust, Wolf et al. 2000) has promoted the suggestion for further investigation of the wake time-CAR link using well-controlled methods (Clow, Thorn et al. 2004; Wilhelm, Born et al. 2007; Fries, Dettenborn et al. 2009). Our results suggest different patterns as a function of assessed CAR dimension. While we observed a gradual decrease in overall cortisol secretory activity across the day (i.e., night sleep larger than morning and afternoon naps, which were larger than evening nap), the dynamic of the CAR was similar after a full night of sleep and a nap, with the morning nap showing the largest increase. We know of no study to date combining the objective measurement and experimental manipulation of sleep, and our findings provide strong support for wake time effects on the CAR, including both overall cortisol secretory activity and the dynamic of the CAR.
The findings of the present study have several implications. The CAR has been hypothesized to reflect the anticipation of stress or “allostatic load” (McEwen, 2006; Kunz-Ebrecht, Kirschbaum, Marmot, & Steptoe, 2004). When a nighttime sleep period is shortened in young children, overall levels of cortisol secreted after awakening are reduced, potentially resulting in a decreased ability to deal with environmental stressors. Additionally, while the diminished CAR following an early evening nap in preschoolers may lead to difficulties in dealing with evening stressors after waking up, it may also serve an adaptive function in preserving low arousal before bedtime, thus facilitating sleep onset. Furthermore, individual variability exists in the CAR and may subsequently relate to differences in children's abilities to cope with environmental stressors post-awakening throughout the day. The present study also indicates that a nap alone is sufficient to elicit a cortisol awakening response, which has important implications for research procedures conducted in young children, highlighting the need to take naps and their effects into account when designing experimental protocols. Specifically, current understanding of stress reactivity and the maturation of daytime cortisol patterns across early childhood is based on protocols typically requiring children to be awake after naps for at least 30 min before afternoon cortisol sampling. Our findings, however, provide strong evidence that the CAR may still be affecting cortisol levels as late as 45 minutes after nap wake time. Finally, while PSG provides on-line accurate determination of sleep duration and wake time, it is labor-intensive and costly. Our findings support the idea that actigraphy offers a relatively cost-effective way to estimate sleep parameters and wake time in ambulatory studies of the CAR (Dockray, Bhattacharyya et al. 2008); thus, future studies would increase control over sample timing errors and other confounds by including this objective measure in their protocols.
Although this study employed a tightly-controlled protocol, it is not without limitations. Notably, the use of a repeated-measures design with a small sample of children somewhat limits the generalizability of our findings. A second limitation is the fact that although we have many cortisol samples for each child across each condition, we did not obtain morning, afternoon, and evening samples on non-nap and each of the nap manipulation days. Thus, we are limited in our understanding of whether the CAR following morning and afternoon naps disrupts the diurnal cortisol pattern in early childhood. Now that we have established these procedures, we intend to collect additional matched samples from a larger sample of children. As noted previously, light levels surrounding the sleep periods were neither controlled nor assessed, which may have produced error variance in our CAR measures, especially in the mornings following nocturnal sleep. Finally, we do not know if the robust CARs in young children were in part due to collecting post-awakening samples while removing electrodes; however, this is unlikely given the fact we observed flat CARs following an evening nap and no behavioral evidence of a stress response.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (K01MH74643; LeBourgeois). Mary A. Carskadon, Ph.D. and Ronald Seifer, Ph.D. provided valuable advice in designing the study. Julia Dmitrieva, Ph.D. assisted with the multi-level modeling. We are most grateful to the children and families for their generousity, time, and effort in making this study possible.
Contributor Information
Colleen E. Gribbin, Email: ceg2160@columbia.edu.
Sarah Enos Watamura, Email: swatamura@psy.du.edu.
Alyssa Cairns, Email: alyssa_cairns@brown.edu.
John R. Harsh, Email: john.harsh@usm.edu.
Monique K. LeBourgeois, Email: monique.lebourgeois@colorado.edu.
References
- Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, et al. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, et al. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29(9):1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, et al. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- DeCaro JA, Worthman CM. Return to school accompanied by changing associations between family ecology and cortisol. Dev Psychobiol. 2008;50(2):183–195. doi: 10.1002/dev.20255. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, et al. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33(1):77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, et al. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26(6):613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, et al. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29(2):174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Hanig S, et al. Cortisol awakening response in healthy children and children with ADHD: impact of comorbid disorders and psychosocial risk factors. Psychoneuroendocrinology. 2009;34(7):1019–1028. doi: 10.1016/j.psyneuen.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, et al. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Ferdinand RF, et al. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatr Scand. 2007;116(2):137–144. doi: 10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, et al. Temporal relationships between pulsatile cortisol secretion and electroencephalographic activity during sleep in man. Electroencephalogr Clin Neurophysiol. 1997;103(3):405–408. doi: 10.1016/s0013-4694(97)00013-1. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Studies of the human infant's adrenocortical response to potentially stressful events. New Dir Child Dev. 1989;(45):3–18. doi: 10.1002/cd.23219894503. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, White BP. Salivary cortisol measures in infant and child assessment. In: Zeskind PS, editor. Biobehavioral assessment of the newborn infant. New York: Guilford Press; 2001. pp. 167–189. [Google Scholar]
- Hatzinger M, Brand S, et al. Hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: importance of gender and associations with behavioral/emotional difficulties. J Psychiatr Res. 2007;41(10):861–870. doi: 10.1016/j.jpsychires.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, et al. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federa- stimulation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28(1):35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, et al. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29(4):516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, et al. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86(1):151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55(10 Suppl 2):S20–23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McKnight P, McNight K, et al. Missing Data. New York: The Guilford Press; 2007. [Google Scholar]
- O'Connor TG, Ben-Shlomo Y, et al. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Okun ML, Krafty RT, et al. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35(3):460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omisade A, Buxton OM, et al. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99(5):651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, et al. HLM 6: Hierarchical Linear and Nonlinear Modeling. Chicago, IL: Scientific Software International; 2004. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, et al. Determinants of salivary cortisol levels in 10-12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30(5):483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, et al. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Horm Behav. 2010;57(2):247–254. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84(9):3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Hellhammer J, et al. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004;66(2):207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, et al. Behavioral and neuroendocrine responses in shy children. Dev Psychobiol. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sekine M, Yamagami T, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28(2):163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Gofferje M, et al. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29(6):575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Uthgenannt D, et al. Corticotropin-releasing hormone-induced adrenocorticotropin and cortisol secretion depends on sleep and wakefulness. J Clin Endocrinol Metab. 1993;77(5):1170–1173. doi: 10.1210/jcem.77.5.8077308. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Cropley M, et al. Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosom Med. 2000;62(2):286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, et al. The effect of dawn simulation on the cortisol response to awakening in healthy participants. Psychoneuroendocrinology. 2004;29(7):925–930. doi: 10.1016/j.psyneuen.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, et al. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31(8):1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. In: Endocrine Physiology Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. W.B. Saunders Company; 2005. pp. 269–270. [Google Scholar]
- Watamura SE, Donzella B, et al. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: age differences and behavioral correlates. Child Dev. 2003;74(4):1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, et al. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, et al. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, et al. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]