Abstract
Diffusion tensor imaging can provide unique and detailed information about white matter anatomy following stroke. Fiber tract reconstruction using tract-based techniques and cross-sectional region of interest delineation are two common approaches to quantify white matter integrity. After stroke, white matter tract integrity can be affected both locally and distally to the primary lesion location. It has been shown that tract disruption is associated with degree of functional impairment and response to skill training in participants with stroke. However, the reliability and validity of these approaches has not been systematically evaluated nor have the two approaches been directly compared in individuals with chronic stroke.
Ten well-recovered individuals with chronic, right-sided, ischemic stroke in the sub-cortex and ten age-, gender- and handedness-matched healthy participants were studied. Semi-automated tractography of the ipsi- and contralesional corticospinal tract and cross-sectional region of interest drawing of the posterior limb of the internal capsule were performed bilaterally. Fractional anisotropy (FA) values and the hemispheric asymmetry in FA were the primary measures of tract integrity. Two raters performed each analysis method twice to evaluate inter- and intra-rater reliability. Participants with stroke were compared to healthy individuals to determine validity of each analysis approach. Correlational analyses were conducted to examine the relationships between the two approaches and the association between approaches and upper extremity motor impairment.
Both analyses methods generally demonstrated good to excellent intra- and inter-rater reliability in each group (p<0.05). Stroke participants demonstrated lower mean FA values in both ipsi- and contralesional tract integrity, and larger FA hemispheric asymmetry as compared with healthy individuals (p<0.05). Comparison between the analysis approaches revealed significant associations between approaches across both groups and within each group (p<0.05). In stroke, individual tract integrity was not correlated between approaches for ipsilesional (r=0.26) or contralesional (0.15) tracts, nor was FA hemispheric asymmetry (r=0.18). Additionally, contralesional mean FA quantified with the cross-sectional approach correlated with upper extremity motor impairment (r=0.69).
Importantly, this study is the first to systematically characterize the reliability of tract-based and cross-sectional DTI analysis approaches in well-recovered individuals with chronic stroke and matched healthy participants. Results suggest both tract-based and cross-sectional approaches to evaluate white matter tract integrity are reliable, can differentiate between groups of stroke and healthy participants, and are associated with one another. However, only mean FA values for the contralesional side derived using the cross-sectional approach were related to upper extremity impairment. Our findings suggest that each approach provides complimentary rather than redundant information regarding integrity and support the use of both approaches in combination in future investigations in well-recovered individuals with stroke.
Keywords: Diffusion Tensor Imaging, Stroke, Reproducibility, White Matter, Integrity, Tractography
1. Introduction
Magnetic resonance (MR) imaging of the brain can be used to three-dimensionally evaluate white matter tract integrity using diffusion tensor imaging (DTI) techniques. Orientation of specific tracts can be displayed on two-dimensional color maps based on eigenvalues and eigenvectors of the diffusion tensor ellipsoid that determine the shape and orientation respectively at each image pixel (Mori et al. 1999). One approach to DTI analysis employs cross-sectional identification of a tract of interest by drawing regions of interest (ROIs) for the specified volume. Fractional anisotropy (FA) can be derived from the calculated tensor for each voxel within the ROI as a quantitative measure of the directionality of water diffusion, thereby providing an assessment of microstructural white matter tract integrity of the given ROI volume (Basser et al., 1996). White matter tractography is another tool to identify specific white matter tracts (Mori et al., 1999). This tract-based method to reconstruct a desired fiber tract utilizes an algorithm to group pixels based on their tensor properties; in turn this approach allows a given tract to be delineated and tract integrity measured (for review: Mori et al., 2002). Currently, the FA value is the most common metric used to quantify the structural properties of white matter tracts in individuals with stroke (Jang, 2010).
These two types of DTI-based analysis approaches have been utilized to demonstrate reduced integrity, indicated by lower FA values, of the corticospinal tract (CST) (Schaechter et al., 2009; Werring et al., 2000) and total motor tract fiber number (Lindenberg et al., 2010) in individuals with stroke. Reduced integrity can occur within the primary lesion location due to local tissue damage or remotely as a consequence of anterograde (Wallerian) and/or retrograde axonal degeneration (Werring et al., 2000). Interhemispheric asymmetries in FA have been associated with reduced motor recovery following stroke (Jang et al., 2005; Lindenberg et al., 2010; Watanabe et al., 2001), reduced skill improvement in response to training (Stinear et al., 2007), and may be a more useful marker of motor dysfunction than functional MR imaging (fMRI) after stroke (Qiu et al., 2011).
Although these findings are encouraging, previous research has yet to systematically evaluate the reliability and validity of DTI-based measurement of white matter integrity in well-recovered individuals with stroke in the chronic stage of recovery after stroke. Additionally, the heterogeneity of lesion location and motor dysfunction in most experimental stroke groups makes interpretation and extrapolation of previous findings challenging. As such, past reports must be interpreted cautiously and further investigation in well-recovered patients in the chronic stage of recovery is needed. Within past work, inter-rater reliability has been reported for both semi-automated tractography (Wakana et al., 2007) and cross-sectional region of interest (ROI) measurement (Qiu et al., 2011). Also, good agreement between tract-based and cross-sectional approaches for quantifying white matter integrity has previously been demonstrated in healthy subjects (Zhang et al., 2008) and in patients in the acute recovery phase after stroke (Tang et al., 2010).
To date, direct comparison between tract-based and cross-sectional approaches has not yet been conducted concurrently for either healthy individuals or for individuals with chronic stroke. As DTI-based approaches to quantify tract integrity in clinical populations become more common, due to promising preliminary work and an increasing number of easy-to-use, freely available software packages, it is essential to characterize the measurement properties of this imaging technique. Therefore, the primary purposes of this work were to determine the inter- and intra-rater reliability, content validity and association between two approaches to white matter integrity quantification in well-recovered individuals with similar stroke pathology in the chronic stage of recovery and age-matched healthy participants.
2. Material and methods
2.1 Subjects
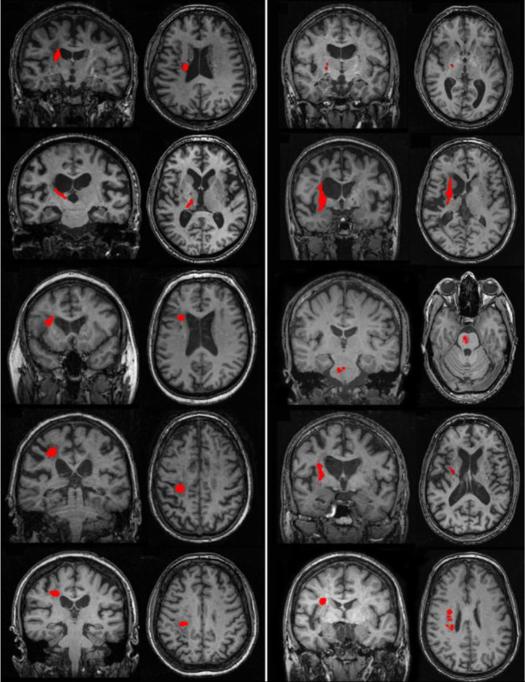
Ten well-recovered chronic ischaemic stroke (ST) participants with a first-time, right-sided, subcortical infarct (Figure 1) and ten healthy control (HC) participants (mean age ± SD=62.1 ± 7.8y, 5F) were recruited from University and local community postings (Table 1). Each participant's consent was obtained according to the Declaration of Helsinki; the research ethics boards at the University of British Columbia approved all aspects of the study. Level of physical impairment was quantified using the upper extremity portion of the Fugl-Meyer (FM) motor scale (0–66) for each ST participant, where higher scores indicate less physical impairment (Fugl-Meyer AR, 1975). Each FM assessment was conducted by a single licensed physical therapist. Exclusion criteria for both ST and HC participants included: (1) a score below the 25th percentile on the Mini-Mental Status Exam using age adjusted norms (Crum et al., 1993); (2) left hand dominance (Oldfield, 1971); (3) any contraindications to MRI. For the HC group, exclusion was deemed if participants exhibited any frank or clinically evident signs of neurological impairment or disease (Lundy-Ekman, 1998).
Figure 1.
Coronal and axial image of lesion location (outlined in red) for each individual in the stroke group.
Table 1.
Patient demographic information
| Subject ID | Age (y) | Gender | Dominant Hand | Time Since Onset (mo) | MMSE | FM UE Score |
|---|---|---|---|---|---|---|
| S1 | 65 | M | R | 20 | 28 | 51 |
| S2 | 72 | M | R | 169 | 29 | 32 |
| S3 | 59 | F | R | 42 | 30 | 61 |
| S4 | 74 | M | R | 65 | 29 | 36 |
| S5 | 55 | F | R | 19 | 30 | 51 |
| S6 | 64 | M | R | 29 | 30 | 66 |
| S7 | 65 | F | R | 90 | 29 | 60 |
| S8 | 58 | M | R | 17 | 30 | 55 |
| S9 | 63 | M | R | 28 | 23 | 55 |
| S10 | 65 | F | R | 24 | 29 | 60 |
|
| ||||||
| Mean ± SD: | 64.0 ± 5.9 | 50.3 ± 47.8 | 29.3 ± 0.7 | 54.9 ± 12.3 | ||
MWSE: Mini-Mental Status Exam, FM UE: Fugl-Meyer Upper Extremity, SD: standard deviation, y: years, mo: months
2.2 MR Data Acquisition
MR acquisition was conducted at the UBC MRI Research Centre on a Philips Achieva 3.0T whole body MRI scanner (Phillips Healthcare, Andover, MD) using an eight-channel sensitivity encoding head coil (SENSE factor=2.4) and parallel imaging. Participants underwent a single high-resolution anatomical scan (TR = 12.4 ms, TE = 5.4 ms, flip angle θ = 8°, FOV = 256 mm, 170 slices, 1 mm thickness). Diffusion weighted data were collected with an echo-planar imaging (EPI) sequence with a single shot readout (TR = 7465 ms, TE = 75 ms, FOV = 212 × 212mm, 60 slices, 2.2 mm slice thickness, voxel dimension =2.23mm). Diffusion weighting was performed across 15 different non-collinear orientations (b = 1000 s/mm2) with an additional minimal diffusion weighted image acquired (b=0). A gradient table was computed using imaging parameters of the diffusion-weighted scan (Farrell et al., 2007).
2.3 MR Data Analysis
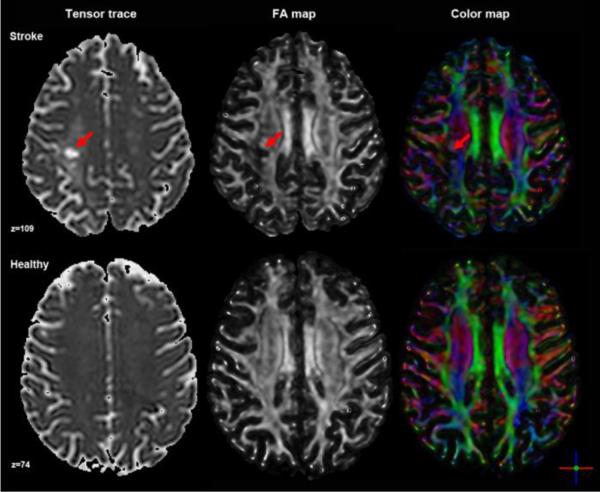
Two DTI analysis approaches (tract-based and cross-sectional) and two raters (K.W. and M.B) were selected to assess the intra- and inter-reliability of DTI-based quantification of white matter tract integrity. Both raters had previous MR data acquisition and analysis experience and were provided DTI-specific training using the DTI Studio software package (www.MriStudio.org) (Jiang et al., 2006) from an experienced user (J.Z.) and video-based tutorials. Integrity was quantified by FA, a unit-less measure that yields values between 0 and 1, based on the magnitude of diffusivity in three defined orientations and the mean diffusivity of each (Basser et al., 1996). Color-coded orientation maps were used to visualize the principal fiber orientation within each pixel (red: right-left, blue: superior-inferior, green: anterior-posterior). Prior to tensor calculation, the quality of the raw images were visually inspected for excessive motion artifact or instrumental noise using a slice-by-slice procedure; if an image was deemed corrupt, it was removed prior to final tensor calculation (Jiang et al., 2006). The number of images removed did not differ significantly between raters and less than 2% of images were removed across all subjects. Regardless of the analysis approach used, the same calculated tensor, FA map and color-coded orientation map for each subject was used for each subsequent analysis step (Figure 2).
Figure 2. Axial images generated from tensor calculation: tensor trace, FA map and color map.
An example from each group is presented and with lesion location noted with a red arrow in each panel.
2.3.1 Semi-automated tract-based quantification
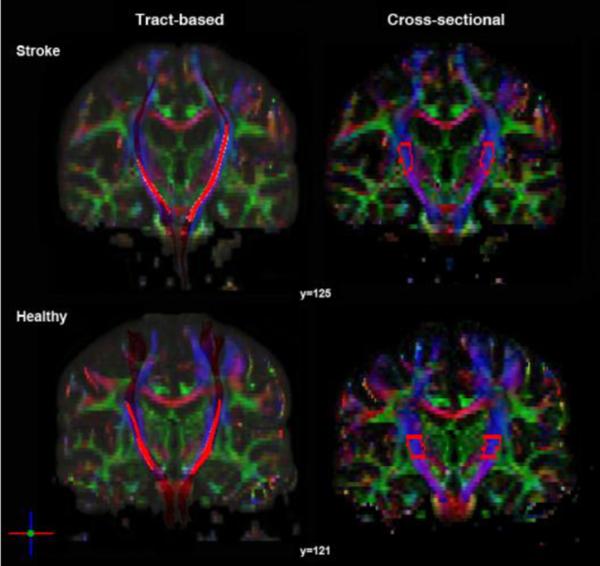
Using DTI Studio, fiber tracts were reconstructed using the fractional anisotropy continuous tracking (FACT) algorithm with the brute-force fiber tracking approach (Jiang et al., 2006; Mori et al., 1999). Tracts were removed if the FA values were less than 0.2 or if the track turn angle threshold exceeded 40° (Wakana et al., 2007). The CST was delineated bilaterally and ROIs were first drawn for each participant using the primary eigenvalue color map using a polygonal drawing tool for posterior limb of the internal capsule (PLIC) at the level of the anterior commissure. Next, the logical “And” command was selected to retain fibers that penetrated both the PLIC and the ipsilateral precentral gyrus and associated white matter. Lastly, the anterior aspect of the cerebral peduncle was retained as well. These three regions were determined a priori based on previous studies (Lindenberg et al., 2010; Wakana et al., 2007) and known anatomical landmarks (Kretschmann, 1988). After the ROIs were delineated and the fiber tracts were reconstructed, aberrant fibers were manually removed using the logical “Not” command to only include fibers within the desired tract (Figure 3). FA values from the remaining fibers were stored for subsequent statistical analysis.
Figure 3. Coronal color map image of white matter tracts quantified for tract-based and cross-sectional approaches in an individual with stroke and a healthy participant.
Tract-based results demonstrate both participants have an intact segment of white matter tract from primary motor cortex to cerebral peduncle with reduced fiber bundle size bilaterally in the stroke participant. The PLIC ROI used for cross-sectional DTI analysis is outlined for each participant demonstrating similar volume size between hemispheres and between individuals.
2.3.2 Cross-sectional ROI quantification
The ROIEditor software program (www.MriStudio.org) was used to perform cross-sectional quantification of PLIC integrity. The PLIC was delineated bilaterally, beginning at the level of the anterior commissure and terminating at the inferior border of the corona radiata (Stinear et al., 2007). The manually defined ROIs were drawn consulting FA maps, color maps and a white matter atlas (Oishi et al., 2011). Lesions penetrating the PLIC were not excluded from the drawing procedure. If there was complete destruction of the affected PLIC, the region was estimated as a mirror volume to the contralateral PLIC (Stinear et al., 2007). Mean FA values for each region were then computed removing pixels with zero or negative FA values and the mean FA from the remaining voxels within the manually-defined ROI mask for the PLIC were recorded for subsequent statistical analyses (Figure 3).
2.4 Data Analysis
To assess the intra- and inter- reliability of each analysis method, intraclass correlation coefficients (ICCs) for hemispheric mean FA and for mean FA hemispheric asymmetry (FAcontralesional – FAipsilesional / FAcontralesional + FAipsilesional) were calculated for each rater and analysis. Separate two-way mixed effects models, where rater and ratings effects were random and measures effects of individual ratings were fixed, were used to calculate ICCs within and between raters for each approach (Shrout and Fleiss, 1979):
whereby BMS= between subject mean square; EMS= error mean square; k= number of comparisons (Portney and Watkins 2000, Kimberley et al 2009). This model was chosen because raters were not randomly selected from the population and it is an appropriate model to assess intrarater reliability with multiple values from a rater (Portney and Watkins, 2000; Shrout and Fleiss, 1979). ICC values greater than 0.75 were considered to demonstrate excellent reliability while values between 0.40 to 0.75 indicated fair to good reliability and less than 0.40 were indicative of poor reliability (Rosner, 2005). The dispersion, or relative variation, of mean FA values was quantified by calculating the coefficient of variation (CV) for each participant and median CV was determined for each group across raters and analyses for both cross-sectional and tract-based methods (Portney and Watkins, 2000). Content validity of the analysis methods for evaluating tract integrity of individuals with stroke was assessed using multivariate analysis of variance (MANOVA) to compare ipsi- and contra-lesional mean FA and FA hemispheric asymmetry between ST and HC separately for each approach. Post-hoc pairwise comparisons were performed with Bonferroni correction for multiple comparisons. Correlational analyses were conducted to measure the degree of association between the two analysis methods across all subjects for mean FA and FA asymmetry. Additionally, mean FA for ipsi- and contra-lesional tracts and FA asymmetry were assessed for ST subjects. In the ST group, correlational analyses were also performed to assess the relationship between mean FA and FA asymmetry and FM score for each approach. All statistical analyses were conducted using SPSS software (19.0 SPSS Inc, Chicago, USA).
3. Results
3.1 Reliability
Results for each ICC analysis are summarized in Table 2. For the tract-based method, both inter- and intra-rater reliability were found to be good to excellent across both groups (ICC range: 0.59–0.89, p<0.0005). Similar inter- and intra-rater reliability was found for the cross-sectional method (ICC range: 0.46–0.95, p<0.05) in both groups with one exception: inter-rater FA asymmetry in the HC group was found to be poor (0.12, p=0.30).
Table 2.
Inter- and intra-rater reliability of each analysis method for both groups
| Analysis | Reliability | Cronbach's α | ICC (±95% Cl) | F test | df | p value |
|---|---|---|---|---|---|---|
| Tract-based | ||||||
| Stroke | ||||||
| Mean FA total | Inter-rater | 0.90 | 0.82 (0.67–0.91) | 10.24 | 32 | <0.0001* |
| Contralesional | 0.74 | 0.59 (0.17–0.83) | 3.91 | 16 | 0.005* | |
| Ipsilesional | 0.89 | 0.81 (0.53–0.93) | 9.43 | 16 | <0.0001* | |
| FA asymmetry | 0.95 | 0.90 (0.74–0.86) | 19.15 | 16 | <0.0001* | |
| Mean FA total | Intra-rater | 0.92 | 0.84 (0.71–0.92) | 11.74 | 32 | <0.0001* |
| Contralesional | 0.94 | 0.89 (0.73–0.96) | 17.63 | 16 | <0.0001* | |
| Ipsilesional | 0.88 | 0.78 (0.48–0.92) | 8.13 | 16 | <0.0001* | |
| FA asymmetry | 0.87 | 0.78 (0.47–0.92) | 7.88 | 16 | <0.0001* | |
| Healthy | ||||||
| Mean FA | Inter-rater | 0.75 | 0.60 (0.32–0.78) | 3.94 | 31 | <0.0001* |
| FA asymmetry | 0.83 | 0.71 (0.34–0.89) | 5.79 | 15 | 0.001* | |
| Mean FA | Intra-rater | 0.85 | 0.74 (0.53–0.86) | 6.57 | 31 | <0.0001* |
| FA asymmetry | 0.54 | 0.73 (0.37–0.89) | 6.26 | 15 | <0.0001* | |
| Cross-sectional | ||||||
| Stroke | ||||||
| Mean FA total | Inter-rater | 0.83 | 0.71 (0.50–0.84) | 5.85 | 35 | <0.0001* |
| Contralesional | 0.54 | 0.37 (−0.11–0.71) | 2.16 | 17 | 0.06 | |
| Ipsilesional | 0.84 | 0.72 (0.41–0.89) | 6.36 | 17 | <0.0001* | |
| FA asymmetry | 0.77 | 0.62 (0.23–0.84) | 4.26 | 17 | 0.003* | |
| Mean FA total | Intra-rater | 0.97 | 0.94 (0.86–0.98) | 29.76 | 35 | <0.0001* |
| Contralesional | 0.85 | 0.74 (0.44–0.90) | 6.80 | 17 | <0.0001* | |
| Ipsilesional | 0.97 | 0.95 (0.88–0.97) | 38.97 | 17 | <0.0001* | |
| FA asymmetry | 0.97 | 0.94 (0.86–0.98) | 35.19 | 17 | <0.0001* | |
| Healthy | ||||||
| Mean FA | Inter-rater | 0.83 | 0.71 (0.48–0.85) | 5.81 | 31 | <0.0001* |
| FA asymmetry | 0.28 | 0.16 (−0.35–0.60) | 1.39 | 15 | 0.27 | |
| Mean FA | Intra-rater | 0.92 | 0.84 (0.71–0.92) | 11.85 | 31 | <0.0001* |
| FA asymmetry | 0.81 | 0.67 (0.29–0.87) | 5.14 | 15 | 0.001* | |
FA: fractionalanisotropy, ICC: Intraclass correlation coefficient, Ct: confidence interval,df: degrees of freedom.
p<0.05
3.2 Relative variation
Relative variation of ipsilesional mean FA across raters and ratings for the ST group was similar for the cross-sectional (median CV= 26.71%, range: 19.80–73.97%) and tract-based (median CV= 28.84%, range: 22.17–36.43%) methods. Relative variation of mean FA for right-sided white matter integrity for the HC group using both the cross-sectional (CV=21.17%, range: 15.87–28.38%) and tract-based (CV=24.36%, range: 20.76–32.25%) approaches were lower than observed for the ST group (Table 3).
Table 3.
Coeffcient of variation (CV) of Mean FA for each analysis approach and group
| Analysis | Region of Interest | Median CV of Mean FA (Range) |
|---|---|---|
| Tract-based | ||
| Stroke | Total CST | 27.94 (21.34–36.43) |
| Contralesional CST | 28.00 (21.34–30.76) | |
| Ipsilesional CST | 28.84 (22.17–36.43) | |
| Healthy | Total CST | 24.95 (16.34–32.25) |
| Left CST | 25.30 (16.34–29.60) | |
| Right CST | 24.36 (20.76–32.25) | |
| Cross-sectional | ||
| Stroke | Total PLIC | 25.47 (18.83–73.97) |
| Contralesional PLIC | 23.63 (18.83–31.67) | |
| Ipsilesional PLIC | 26.71 (19.80–73.97) | |
| Healthy | Total PLIC | 21.17 (15.87–28.38) |
| Left PLIC | 21.31 (16.87–28.27) | |
| Right PLIC | 21.17 (15.87–28.38) |
FA: fractional anisotropy, CST: corticospina! tract, PUC: posterior limb internal capsule
3.3 Content validity
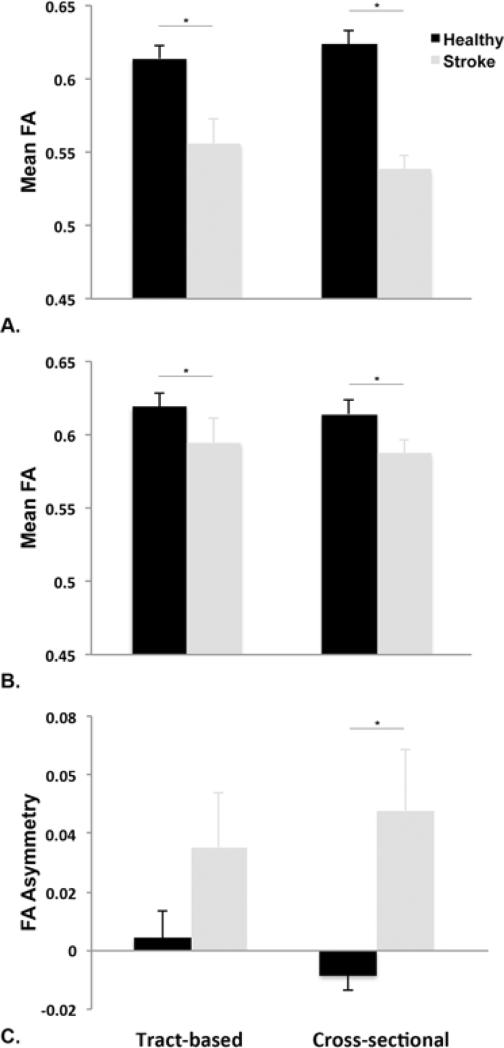
There was a significant difference between tract integrity between the ST and HC group using both tract-based (Wilks' λ =0.58, F(3,16)=3.87, p=0.03) and cross-sectional (Wilks' λ =0.49, F(3,16)=5.52, p=0.008) approaches. Post-hoc test indicated that ipsilesional mean FA was significantly reduced in the ST group compared to HC right-sided tract integrity for both tract-based (mean difference: 0.06 ± 0.02, p=0.007) and cross-sectional (mean difference: 0.05 ± 0.01, p=0.001). Mean FA was also significantly lower contralesionally in the ST group compared to the left side in the HC group using both the tract-based (mean difference: 0.03 ± 0.01, p=0.04) and cross-sectional (mean difference: 0.03 ± 0.01, p=0.01) approaches. FA asymmetry was significantly greater for the ST group compared to HC group using the cross-sectional (mean difference: 5.64 ± 1.90%, p=0.008) but not the tract-based (mean difference: 3.07 ± 0.95%, p=0.12) approach (Figure 4).
Figure 4. Mean FA and FA asymmetry values between participants with stroke and healthy individuals.
A. Ipsilesional mean FA was significantly lower in the stroke group compared to healthy individuals using either tract-based or cross-sectional approach. B. Contralesional mean FA was also lower in the stroke group compared to healthy controls for both approaches. C. FA asymmetry was significantly higher in the stroke group, indicating increased tract disruption in the ipsilesional tract compared to the contralesional tract, using the cross-sectional, but not the tract-based, approach. *p<0.05.
3.4 Association between measurement approaches
Across ST and HC groups, mean FA for both approaches were significantly correlated (r =0.53, p<0.0005). In the ST group, mean FA for the tract-based and cross-sectional approaches demonstrated a significant relationship (r=0.38, p<0.0005). Evaluating tract integrity individually in the ST group, contralesional mean FA was not correlated between approaches (r =0.15, p=0.33) nor was ipsilesional mean FA (r=0.26, p=0.10). FA asymmetry was correlated between approaches across both groups (r=0.28, p=0.01) but not in the ST (r =0.18, p=0.29) or HC (r =−0.07, p=0.68) groups individually.
3.5 Association between measurement approaches and upper extremity motor impairment
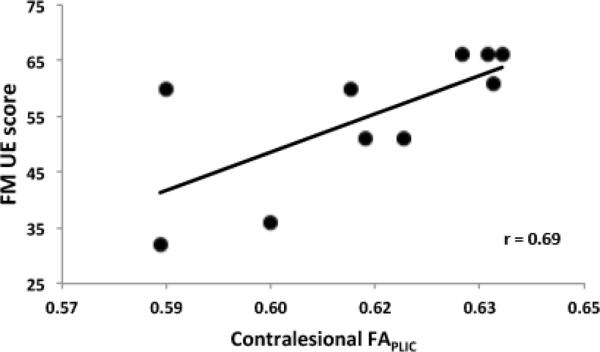
The second set of ratings was used from rater two to perform correlational analyses between mean FA and FA asymmetry for both approaches and FM score. A significant correlation between mean FA and FM score was observed for contralesional FA using the cross-sectional (r=0.69, p=0.03) but not tract-based (r=0.40, p=0.25) approach (Figure 5). Significant correlations between FM score and ipsilesional FA were not observed for either approach (tract-based: r=0.40, p=0.25, cross-sectional: r=0.13, p=0.71). Upper extremity impairment and FA asymmetry were also not significantly associated for either approach (tract-based: r=−0.13, p=0.73, cross-sectional: r=0.02, p=0.97).
Figure 5. Scatterplot demonstrating the relationship between upper extremity motor impairment and contralesional white matter integrity.
A significant correlation between Fugl-Meyer (FM) Upper Extremity (UE) Motor Score and contralesional mean FA of the PLIC using the cross-sectional approach was observed (p=0.03)
4 Discussion
The results of this work indicate that both tract-based and cross-sectional approaches to quantify white matter tract integrity are reliable, can differentiate between individuals with stroke and healthy participants, and are associated with one another. However, the results from these different approaches do not strongly correlate when evaluating individual tract integrity in stroke and only cross-sectionally defined contralesional PLIC integrity was associated with upper extremity motor impairment. This study is the first to systematically characterize the reliability of tract-based and cross-sectional DTI analysis approaches between raters and between ratings for a sample of well-recovered individuals with chronic stroke and healthy participants. Our findings suggest that both methods are appropriate to evaluate white matter integrity in certain populations of individuals with chronic stroke, but that each approach may evaluate different aspects of bilateral tract disruption thus providing important insights into neuroanatomical changes following brain damage.
Previous investigations of white matter integrity in stroke have demonstrated a relationship between upper extremity function and fiber tract disruption within the PLIC (Qiu et al., 2011; Stinear et al., 2007), and throughout the entire corticofugal motor projection system from cortex to brainstem (Lindenberg et al., 2010). It is not clear whether cross-sectional ROI delineation of the PLIC or tractography of the CST from cortex to distal brainstem is the preferred DTI-based quantification approach as there presently is no gold standard for accuracy of integrity quantification (Wakana et al., 2007). Previous work has shown excellent reliability for tractography and ROI-based approached applied in a sample of participants with stroke three months after onset using diffusion spectrum imaging (Tang et al., 2010). Direct comparison of the reproducibility between the DTI approaches examined here have not been studied previously in individuals with chronic stroke.
Presumably, these two approaches evaluate different aspects of white matter integrity based on methodological differences and the extent of tract evaluated. The tract-based approach is sensitive to significant anatomical deformations and requires at least a portion of the tract of interest to be intact from start to finish. Therefore, in individuals with stroke that have complete lesions of at a least a portion of the CST, the tract-based approach would not be appropriate. The cross-sectional approach commonly examines a representative section of the motor output tracts, such as the PLIC, and provides information regarding the entire delineated volume. This method can be used in cases of severe tract disruption but is does not typically measure specific tracts in the brain from origination to termination. Increasing the size of the ROI could, in part, mitigate this limitation but may be offset by increased analysis time and subjectivity of ROI determination. It should also be noted that both approaches employed predominately evaluate CST integrity but other corticofugal fibers that do not project to the spinal cord may also have been included (Lindenberg et al., 2010) in the analysis; however, this distinction should not alter the reported results. Here, both approaches were found to be reliable and able to distinguish between healthy individuals and well-recovered participants with chronic stroke. This finding suggests that both approaches are sufficiently sensitive to detect tract disruption following stroke but each may provide unique information regarding lesion effects on white matter integrity in different populations that may be clinically significant based on previous literature (Lindenberg et al., 2010; Qiu et al., 2011; Stinear et al., 2007; Tang et al., 2010).
Although both approaches were found to be reliable between- and within-raters, a range of ICC values and relative variation was observed. As expected, the tract-based approach was the most reliable across comparisons likely due to the higher level of automaticity and lower level of subjectivity compared to the cross-sectional approach. It could be argued that reliability was lower for cross-sectional quantification PLIC integrity due to encroachment of the lesion creating a discrepancy between subjects' images and the standard atlases used for ROI delineation. This does not appear to have affected reliability but may explain the large range of CV values observed for the ipsilesional PLIC.
While good reproducibility was demonstrated, there are potential factors that could further improve the reliability and sensitivity of both analysis approaches. Here, MR images were not normalized to standard space (e.g., Talairach or MNI); therefore, each rater delineated ROIs individually for each participant for each approach. This is often important consideration when studying stroke-affected brain regions due to anatomical deformations that may be present after infarct and be magnified by distortions associated with normalization to a template brain. Thus, in the present work normalization was not employed to minimize variability in our analysis procedure (Zhang et al., 2009). Future work could examine recently proposed methods for normalization of DTI data (Zhang et al., 2009) to assess the impact of this factor.
In the present work fifteen gradient directions were acquired; this is fewer than has been collected in other work (Schaechter et al., 2009; Tang et al., 2010), but in line with other recent investigations in individuals with stroke (Qiu et al., 2011; Sterr et al., 2010; Stinear et al., 2007). Lastly, collecting more than one DWI scan during a single data acquisition session has been recommended as a method to minimize noise artifact (Wakana et al., 2007). Here, reliability and sensitivity were not compromised when analyzing a single fifteen direction scan for each participant, but there is the possibility that increasing the number of orientations collected could improve tensor determination within each voxel potentially improving ROI identification for each approach. Increasing the number of scans could mitigate data collection artifacts, e.g. subject head motion, which could also reduce signal quality and affect subsequent data analyses. Multiple DWI scans with more gradient directions may further improve the reliability and sensitivity of DTI-based analyses of tract integrity.
We also noted that mean FA values were more reliable and sensitive as compared to FA asymmetry. Further, the interhemispheric asymmetry values observed in the present study were smaller in comparison to other work examining people with stroke (Lindenberg et al., 2010; Stinear et al., 2007). There are number of potential explanations for the differences between studies. Participant demographics including lesion location, time post-stroke, age, gender, handedness and functional impairment could all contribute to differences in integrity values between studies (Buchel et al., 2004; Hsu et al., 2008; Pfefferbaum et al., 2000; Stinear et al., 2007). Here, lesion characteristics (all right-sided, subcortical, first-time infarct) and handedness (all right-hand dominant) were relatively homogenous between participants. Importantly, no participant demonstrated complete disruption of the ipsilesional tract regardless of analysis method employed. On average, in comparison to subjects in related investigations (Lindenberg et al., 2010; Stinear et al., 2007), our participants demonstrated higher Fugl-Meyer scores indicating reduced upper extremity impairment. This could explain the lower magnitude of FA asymmetry observed. Previous work has shown that lower FA asymmetry values were associated with higher Fugl-Meyer scores (Qiu et al., 2011; Stinear et al., 2007). Additionally, FA asymmetry values were in line with recent results from participants with comparable levels of upper extremity impairment (Qiu et al., 2011). These data suggest mean FA values of ipsi- and contralesional tracts provide a sensitive and reliable estimate of white matter integrity in well-recovered individuals with stroke.
In our sample, mean FA was able to identify tract disruption using both approaches while FA asymmetry was not significantly different between the two groups using the tract-based approach. It is important to note that contralesional integrity was also reduced after stroke in this patient sample for both approaches. This bilateral reduction in integrity may explain why FA asymmetry values were not different between groups using the tract-based approach. Bilateral disruptions in tract integrity have not been reported previously using the approaches described here and may offer unique insights into the interhemispheric effects of stroke. It has been shown previously that the physiologic balance of activity between hemispheres may be disrupted following stroke (Murase et al., 2004; Ward and Cohen, 2004). These interhemispheric imbalances have been associated with poorer recovery and rehabilitation approaches aimed at restoring this balance have shown positive effects in some cases (Fregni et al., 2006; Murase et al., 2004; Nowak et al., 2008).
Additionally, related investigations have demonstrated reduced contralesional white matter integrity in chronic stroke (Buffon et al., 2005) that has been associated with poorer motor skill recovery (Schaechter et al., 2009). In patients with better motor recovery, elevated FA values were reported bilaterally in comparison to healthy controls (Schaechter et al., 2009). It is difficult to directly compare level of physical impairment between patient samples but our findings of reduced FA in the contralesional motor output tract for well-recovered individuals using both approaches appear to contrast these findings. Despite methodological and patient characteristic differences, taken together our results demonstrate modified microstructural properties bilaterally after stroke, which may be associated with level of recovery.
Specific to our work, the preliminary results reported here indicate that contralesional tract integrity can be reliably measured and is reduced in a sample of well-recovered individuals following stroke. Also, contralesional FA was associated with upper extremity motor impairment using the cross-sectional, but not tract-based approach while ipsilesional integrity was not correlated with function regardless of approach. This may be the result of the relatively low levels of motor dysfunction observed in this chronic stroke patient sample. Previous work has shown increasing FA levels with longer time since stroke (Wang et al., 2006) and contralesional tract integrity may be associated with better motor recovery (Schaecter et al. 2009). The clinical significance of this reduced integrity and association with upper extremity motor impairment in well-recovered individuals in the chronic phase of stroke recovery is not well understood and merits further inquiry. Additionally, it should be noted that a patient sample with greater stroke severity, and larger and more complex lesions may pose greater challenges to the reliability of each of these approaches. This population warrants future study.
5. Conclusions
This is the first study to demonstrate the inter- and intra-rater reliability of two commonly utilized DTI-based approaches to quantify white matter integrity in well-recovered individuals in the chronic recovery stage from stroke. By studying well-recovered individuals with chronic, right-sided, subcortical stroke without complete disruption of the affected CST, the reproducibility of both approaches was demonstrated and each was found to be appropriate to quantify white matter disruption. This work is also the first to report reduced integrity in the less affected hemisphere using these approaches, and shows an association between contralesional tract integrity and motor impairment. These findings support increased investigation into contralesional white mater integrity that may provide novel insights into the mechanisms underlying motor recovery after stroke. Future inquiry should examine the relationships between these two approaches and 1) measures of functional recovery following stroke and 2) motor learning in response to rehabilitation paradigms in chronic stroke.
4. Highlights.
We conducted two data analysis approaches of white matter integrity in stroke.
-
>
The reliability of these methods was confirmed between two raters and two ratings.
-
>
Approaches differentiated bilateral tract integrity between stroke and healthy individuals.
-
>
Approaches may evaluate different aspects of white matter integrity disruption.
-
>
Good reproducibility of both approaches supports use in future inquiry in stroke and stroke recovery.
Acknowledgements
Support was provided to LAB by the Canada Research Chairs and the Michael Smith Foundation for Health Research, and the National Sciences and Engineering Council to KPW. The authors would also like to thank Jill Zwicker, OT, PhD (J.Z.), Alex Rauscher, PhD and the UBC MRI Research Centre for software program and data acquisition assistance.
Funding This work was supported by the National Institutes of Health [NS051714 to L.A.B.] and the American Heart Association [0530022N to L.A.B.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cerebral Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Buffon F, Molko N, Herve D, Porcher R, Denghien I, Pappata S, Le Bihan D, Bousser M-G, Chabriat H. Longitudinal diffusion changes in cerebral hemispheres after MCA infarcts. Journal of Cerebral Blood Flow & Metabolism. 2005;25:641–650. doi: 10.1038/sj.jcbfm.9600054. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993;269:2386–2391. [PubMed] [Google Scholar]
- Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, Mori S. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. Journal of Magnetic Resonance Imaging. 2007;26:756–767. doi: 10.1002/jmri.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR JL, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scandanivan Journal of Rehabilitation. 1975;7:13–31. [PubMed] [Google Scholar]
- Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. Neurorehabilitation. 2010;27:367–372. doi: 10.3233/NRE-2010-0621. [DOI] [PubMed] [Google Scholar]
- Jang SH, Cho S-H, Kim Y-H, Han BS, Byun WM, Son S-M, Kim SH, Lee SJ. Diffusion anisotrophy in the early stages of stroke can predict motor outcome. Restorative Neurology & Neuroscience. 2005;23:11–17. [PubMed] [Google Scholar]
- Jiang H, van Zijl PCM, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods & Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ. Localisation of the corticospinal fibres in the internal capsule in man. Journal of Anatomy. 1988;160:219–225. [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy-Ekman L. Neuroscience: Fundamentals for Rehabilitation. WB Saunders; Philadelphia: 1998. [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, Fink GR. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Archives of Neurology. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, van Ziji P, Mori S. MRI Atlas of Human White Matter. 2nd ed Academic Press; 2011. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance in Medicine. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Portney L, Watkins M. Foundations of Clinical Research: Applications to Practice. Second Edition ed Appleton & Lange; Norwalk, CN: 2000. [Google Scholar]
- Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabilitation & Neural Repair. 2011;25:275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. Duxbury Press; Belmont, CS: 2005. [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human Brain Mapping. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P, Fleiss J. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological Bulletin. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabilitation & Neural Repair. 2010;24:413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Tang PF, Ko YH, Luo ZA, Yeh FC, Chen SH, Tseng WY. Tract-specific and region of interest analysis of corticospinal tract integrity in subcortical ischemic stroke: reliability and correlation with motor function of affected lower extremity. Ajnr: American Journal of Neuroradiology. 2010;31:1023–1030. doi: 10.3174/ajnr.A1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Archives of Neurology. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Honda Y, Fujii Y, Koyama M, Matsuzawa H, Tanaka R. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. Journal of Neurosurgery. 2001;94:955–960. doi: 10.3171/jns.2001.94.6.0955. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Li X, Zhang J, Luft A, Hanley DF, van Zijl P, Miller MI, Younes L, Mori S. Landmark-referenced voxel-based analysis of diffusion tensor images of the brainstem white matter tracts: application in patients with middle cerebral artery stroke. Neuroimage. 2009;44:906–913. doi: 10.1016/j.neuroimage.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Olivi A, Hertig SJ, van Zijl P, Mori S. Automated fiber tracking of human brain white matter using diffusion tensor imaging. Neuroimage. 2008;42:771–777. doi: 10.1016/j.neuroimage.2008.04.241. [DOI] [PMC free article] [PubMed] [Google Scholar]