Abstract
The present investigation worked to understand whether patients with mild Alzheimer's disease (AD) could use general or self-referential mental imagery to improve their recognition of visually presented words. Experiment 1 showed that, unlike healthy controls, patients generally did not benefit from either type of imagery. To help determine whether the patients' inability to benefit from mental imagery at encoding was due to poor memory or due to an impairment in mental imagery, subjects then performed four imagery tasks, with varying imagery and cognitive demands. Experiment 2 showed that patients successfully performed basic visual imagery, but degraded semantic memory, coupled with visuospatial and executive functioning deficits, impaired their ability to perform more complex types of imagery. Given that patients with AD can perform basic mental imagery, our results suggest that episodic memory deficits likely prevent AD patients from storing or retrieving general mental images generated during encoding. Overall, the results of both experiments suggest that neurocognitive deficits do not allow patients with AD to perform complex mental imagery, which may be most beneficial to improving memory. However, our data also suggest that intact basic mental imagery and rehearsal could possibly be helpful if used in a rehabilitation multi-session intervention approach.
Keywords: mental imagery, visual imagery, recognition memory, cognitive rehabilitation
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that gradually destroys one's ability to learn, reason, and carry out daily activities. The disease will develop in 5% to 11% of people over the age of 65, and it will affect as many as 40% of those over the age of 851. The Alzheimer's Association reported in 2010 that over 5.3 million Americans are currently diagnosed with AD and that the current direct and indirect costs for care are estimated at $172 billion annually. The hallmark of AD, and often the presenting complaint, is impairment in episodic memory. Memory problems can drastically compromise one's ability to live independently and, along with dementia, are a reason commonly cited for nursing home placement2–4. A relatively recent study showed that a $4 billion savings could be realized by delaying nursing home placement by only one month5. Therefore, it is critical to identify ways to improve memory or to develop strategies that will help individuals compensate for memory loss and live at home or in the community longer.
One possible strategy that has shown benefit to healthy adults in the cognitive aging literature is using mental imagery at the time of encoding6–8. A distinct cognitive process in itself, mental imagery has become one of the single most important memory-training techniques used over the last 25 years9. Research has shown that training in the use of mental imagery leads to substantial improvements in memory performance in healthy young7 and older adults10. Further, these improvements are reliable and can be maintained over time in these populations11. Self-referential imagery, in which participants envision themselves interacting with the item of interest, has been shown to have particular benefit6,12. Many studies involving older adults have demonstrated the success of self-referential imagery in improving memory10,12–16. However, there has been no investigation examining the use of mental imagery to improve memory encoding in patients with AD. Given that healthy older adults show such robust improvement, even a slight improvement in recognition for patients with AD could potentially enhance independent functioning.
At the foundation of the effectiveness of mental imagery to improve memory for words is the picture superiority effect. This effect is a consistent empirical finding showing that stimuli presented as pictures are better remembered than stimuli presented as words17. It has been hypothesized that in addition to a verbal code, words can evoke an image code (likely a prototypical stored semantic or mental representation) through spontaneous mental imagery18. These verbal and image codes are stored in partly interconnected, but functionally independent, symbolic systems in memory19. While the encoding of words regularly activates prefrontal and temporoparietal regions20, work has shown that performing mental imagery to turn these words into pictures activates primary visual areas18. Though not all investigations of mental imagery suggest activation of primary visual areas21, more recent work neuroimaging work has confirmed that activity in primary visual cortex reflects spontaneous memory-related visual imagery during encoding of words22,23. These findings certainly have implications for patients with AD, as primary visual areas tend to be affected later in the disease course than temporoparietal or prefrontal regions24.
Given evidence that primary visual areas tend to remain intact until later in the disease course, in addition to the fact that the picture superiority effect remains rather robust in patients with mild AD25, we hypothesized that using mental imagery may be an effective intervention for improving verbal memory performance early in AD. To test this hypothesis, patients with mild AD and healthy age, gender, and education matched controls participated in three study-test conditions involving words. In the first condition, subjects were asked to make like/dislike judgments. In the second condition, subjects were asked to come up with a general mental image of the word on the screen. Finally, in the last condition, we asked subjects to generate a “self-referential” mental image in which they mentally interacted or engaged with the item on the screen. Based on previous work suggesting that patients with AD can perform basic mental imagery26,27, we hypothesized that, like healthy older adults, patients would demonstrate better discrimination for the two imagery encoding conditions than for the standard like/dislike encoding condition. Further, we hypothesized that patients and controls would perform better on the self-referential imagery condition compared to the general imagery condition.
2. Experiment 1
2.1. Method
2.1.1. Participants
Eighteen patients with a diagnosis of probable mild AD as determined by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria (NINCDS-ADRDA28) and seventeen healthy older controls were recruited for this experiment. The participants with AD were recruited from the Boston University Alzheimer's Disease Center in Boston, Massachusetts and from previous patients that had participated in studies from our laboratory. Patients with AD were excluded if their Mini Mental State Examination29 (MMSE) score fell below 21. Healthy older adults were excluded if they had a first-degree relative with AD, another neurodegenerative disorder, or dementia. They were also excluded if they scored 1.5 standard deviations below the mean on any element of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Memory test30,31 (memory, recall, and recognition), or if they scored below 28 on the MMSE. All participants were excluded from participation if they were characterized as having clinically significant depression, alcohol or drug abuse, cerebrovascular disease, history of traumatic brain injury, or if English was not their native language.
Each participant completed a brief neuropsychological battery in a 30 to 45-minute session either directly following their participation or during a separate visit. This battery included the MMSE29, the CERAD word list memory test30, Trail Making Tests Part B32, Verbal Fluency33, and the 15-item Boston Naming Test34. Demographic and neuropsychological data for the two groups are presented in Table 1. Fisher F-tests revealed no significant differences in age [F(1, 28) = 1.35, p = .254] or years of education [F(1, 28) = 2.90, p = .100] between the AD and healthy older adult groups. Chi-square analysis also revealed no differences in gender between groups [χ2(1) = .536, p = .464]. The human subjects committees of the Edith Nourse Rogers Memorial Veterans Hospital and Boston University approved this study. Written informed consents were obtained from all participants and from their caregivers, where appropriate, and participants were compensated at $10/hr for their time. Three patients with AD were excluded from analysis due to poor performance greater than two standard deviations from the group mean on two or more of the three conditions. Additionally, two healthy older adults were excluded from the analysis due to performance on neuropsychological testing that suggested mild cognitive impairment.
Table 1.
Experiment 1 means and standard deviations (in parentheses) for age, education, and neuropsychological performance for the Alzheimer's disease (AD) and health older adult (OC) groups.
| AD | OC | |
|---|---|---|
| Subjects | n = 15 (9F) | n = 15 (8F) |
| MMSE | 25.4 (2.6) | 29.3 (0.8) |
| ** | 21 – 28 | 28 – 30 |
| Age | 77.5 (6.3) | 75.5 (7.9) |
| 65–87 | 65–89 | |
| Education | 14.1 (3.1) | 15.6 (2.3) |
| 11–18 | 12–18 | |
| CERAD encoding | 10.8 (2.8) | 21.8 (5.2) |
| ** | 6–15 | 16–30 |
| CERAD recall | 0.6 (1.3) | 7.4 (2.0) |
| ** | 0–4 | 6–10 |
| CERAD recognition | 6.3 (2.6) | 9.5 (1.0) |
| ** | 3–9 | 9–10 |
| Trails B | 168 (95.7) | 86.8 (29.9) |
| ** | 74–300 | 54–104 |
| FAS | 34.5 (14.4) | 50.1 (12.7) |
| ** | 12–60 | 30–68 |
| CAT | 31.0 (9.3) | 47.6 (10.0) |
| ** | 8–56 | 38–67 |
| Boston Naming Test | 12.4 (2.8) | 14.5 (0.9) |
| * | 9–15 | 13–15 |
FAS = total score letter fluency for letters F, A, and S. CAT = total scor semantic fluency for categories Animals, Vegetables, and Fruits. Asterisks signify significant differences between the two groups
less than .01
less than .05.
2.1.2. Stimuli
The stimuli were 300 high imagability concrete nouns from the University of Western Australia MRC Psycholinguistic Database (http://www.psy.uwa.edu/au/MRCDataBase/uwa_mrc.htm). These word stimuli were randomly divided into six lists of 50 words. The presentation of these lists was counterbalanced across participants in each group such that each list was used equally often in each of the conditions (i.e., like/dislike, general imagery, self-referential imagery) and as studied or unstudied items.
2.1.3 Design and procedure
Each participant was tested individually in a single session. Stimuli were presented on a Dell Inspiron 640 m laptop computer via E-Prime software (Psychology Software Tools Inc.; www.pst-net.com/eprime). The procedure consisted of three study-test blocks. To help prevent ceiling effects in healthy older adults, study and test sessions were separated by a 10-minute delay with a filler task. Patients had a one-minute delay with no filler task between study and test sessions. Before the first block was presented, participants were instructed verbally that they would be studying lists of words for a subsequent recognition memory test. In the study phase of the standard encoding block, participants studied 50 words for 3 seconds under deep encoding conditions by making a like/dislike judgment about each item. For both of the imagery conditions, study words were presented for 3 seconds to all participants as in the standard like/dislike condition, while imagery responses were self-paced. The experimenter would not move forward to the next study item until the subject reported being finished performing their mental image. Following the study-test delay, participants were instructed that they would see old words that they had previously studied and new words that they had not studied during this session, and that they would be asked to make an old/new (i.e., old = studied and new = not studied) judgment about each word presented.
2.1.4 Imagery Conditions
The general imagery block was conducted in a similar format to the standard encoding block (i.e., study 50 words and tested on 100) except that participants were instructed to create a general visual image of the object denoted by the presented word during study to aid recognition on a subsequent memory test. A card was placed at the top of the computer monitor that read, “Create a mental image of the object”, which served as a reminder of the task. Participants were further instructed to not envision themselves interacting with the object during the general imagery block; rather, to only create an image of the object itself. During the test phase, another card was placed on top of the monitor, which reminded participants to “Use the image you created of the object at study to help you remember.”
For the self-referential imagery block, participants were instructed to create a visual image, in which they were engaging or interacting with the object denoted by the presented word to aid recognition on a subsequent memory test. A card instructing the participant to “Create a mental image where you are interacting or engaging with the object” was placed on top of the monitor during the study phase. During the test phase, participants were again given a reminder card that was placed on top of the monitor to remind them to “Use the image you created of yourself interacting with the object at study to help you remember” to help you remember.
For the imagery conditions, two practice trials were administered for each study task. On these practice trials, once the participant indicated that s/he had created an image, the experimenter would ask the participant to detail the image s/he created in order to determine if s/he were completing the task correctly and gave feedback if appropriate. To further ensure that the patients were staying on task throughout the study phases of the general imagery and self-referential blocks, the experimenter would stop after every fifth item presented to query the participants about the image they created for that item and would give feedback if appropriate. The order of the three study-test blocks was counterbalanced across subjects. It should be noted that the order of presentation made no difference on performance [F(1, 27) < 1]. To confirm this, we also performed t-tests on the differences in performance based on which condition was administered first. Here, there was no difference when like/dislike was given first compared to when general imagery was given first [t(16) = .454, p = .656] or when self-referential imagery was given first [t(18) = .930, p = .365].
2.2. Results
To begin our examination of recognition performance, we calculated d' as a measure of discrimination and C as a measure of response bias35. We choose these measures because Snodgrass and Corwin (1988) note that models of d' and C demonstrate independence between discrimination and bias. Greater d' indicates greater discrimination; 0 indicates chance performance (range −4.67 to 4.67). Positive values of C indicate a conservative response bias, negative values indicate a liberal response bias, and 0 indicates a neutral bias (range = −1 to 1). Discrimination (d') and response bias (C) means and standard deviations for healthy older adults and patients with AD for the three conditions can be seen in Table 2.
Table 2.
Means, standard deviations (in parentheses), and range of scores (below) for patients with Alzheimer's disease (AD) and healthyolder controls (OC) on the like dislike (L/D), general imagery (General), and self-referential imagery (Self-Ref) conditions.
| Discrimination (d') | ||||||
|---|---|---|---|---|---|---|
| L/D | General | Self-Ref | ||||
| AD | 1.36 (0.78) | 1.50 (1.07) | 1.47 (0.79) | |||
| 0.28 – 2.84 | 0.11 – 2.63 | 0.38 – 3.14 | ||||
| Hit | FA | Hit | FA | Hit | FA | |
| .71 | .28 | .68 | .25 | .70 | .26 | |
| OC | 2.76 (0.87) | 2.81 (0.71) | 3.26 (0.67) | |||
| 1.43 – 4.67 | 1.52 – 3.99 | 2.03 – 4.22 | ||||
| Hit | FA | Hit | FA | Hit | FA | |
| .90 | .10 | .91 | .09 | .94 | .05 | |
| Response Bias (C) | ||||||
| AD | 0.01 (0.37) | 0.09 (0.61) | 0.06 (0.50) | |||
| −0.72 – 0.59 | −1.02 – 1.23 | −1.09 – .65 | ||||
| OC | 0.00 (.31) | 0.00 (0.28) | 0.002 (0.27) | |||
| −0.42 – 0.51 | −0.55 – 0.23 | −0.42 – 0.49 | ||||
Hit = hit rate. FA = false alarm rate
To compare discrimination (d') between groups on the three encoding tasks, a repeated-measures ANOVA with factors of Group (older controls, AD) and Condition (like/dislike, general imagery, self-referential imagery) was performed. The results of the ANOVA revealed an effect of Group [F(1, 28) = 31.41, p < .001] due to better overall discrimination for the older controls than for patients with AD, as well as an effect of Condition [F(2,56) = 3.82, p = .028] and an interaction of Group and Condition [F(2,56) = 3.62, p = .033]. This interaction was present because the older controls performed better on the self-referential imagery condition compared to the general imagery [t(14) = 2.43, p = .029] and Like/Dislike [t(14) = 2.26, p = .040] conditions. In contrast, there were no significant differences between the three conditions for the AD group.
In addition to discrimination, we also examined recognition response bias to determine if mental imagery had an effect on response criterion in these populations. Response bias means and standard deviations for all three groups can be seen in Table 2. To compare response bias (C) between groups for the three conditions, a repeated-measures ANOVA with the factors of group (older controls, AD) and condition (Like/Dislike, General Imagery, Self-referential Imagery) was performed. The ANOVA revealed no main effect of Group [F(1, 28) < 1] or Condition [F(2, 56) < 1], and there was no interaction of Group and Condition [F(2, 56) < 1].
To determine whether the failure to improve memory performance with mental imagery possibly resulted from lack of engagement in the encoding task, we calculated separate d' values for the general and self-referential imagery condition trials that were queried by the experimenter (every fifth trial). Although this new discrimination value (1.72) did not significantly differ from the overall d' value for the general imagery condition (1.50) [t(14) = 1.43, p = .175], it did differ significantly from the like/dislike d' value (1.36) [t(14) = 2.90, p = .012]. A similar trend was found for the self-referential condition. Here, the queried trials for the recalculated d' value (1.64) was not significantly different from the overall d' value (1.47) [t(14) = 1.46, p = .166], but there was a trend towards better performance compared to the like/dislike d' value (1.36) [t(14) = 1.82, p = .090].
2.3 Discussion
Our goal in this experiment was to determine whether patients with mild AD could benefit from the use of mental imagery to improve memory for words. We hypothesized that, like healthy older adults, patients with AD would show modest benefit from the general mental imagery condition, and even greater benefit from the self-referential condition. Contrary to these hypotheses, patients with AD showed no benefit from either condition. In contrast, healthy older adults showed significant improvement after engaging in self-referential mental imagery at encoding. The fact that patients did not benefit from the mental imagery conditions left us with two main hypotheses of interest: 1) despite the prior literature26,27, patients with AD cannot perform mental imagery, or 2) the patients can perform mental imagery at the time of encoding but their memory impairments are such that that the mental images cannot either be encoded, stored, or retrieved, so that mental imagery cannot be used as a successful aid to memory.
Although it has been speculated that patients with AD can perform mental imagery there has been no systematic investigation. A recent study of semantic memory and knowledge of famous names showed that using mental imagery, patients with AD did not generate as many semantic details about famous people compared to controls36. Borg et al. (2010) concluded that mental imagery is likely lowered in patients with AD due to semantic memory problems. Similarly, an investigation examining the early stages of visual perception and cognition (e.g., image segmentation, shape consistency, object memory, lexical access, and visual imagery) suggested that semantic memory deficits impaired patients' ability to perform complex mental imagery compared to healthy older adults37. Although Tippet and colleagues (2003) acknowledged that their data do not allow for a precise characterization of mental imagery ability in patients with AD, it certainly leaves the impression that patients may be able to perform mental imagery. Indeed, the patients in Tippett et al. (2003) were significantly more impaired than those in Experiment 1. Tippett and colleagues used an MMSE range of 10–19, which places patients in the moderate stage of the disease. Our patients in Experiment 1 were in the mild to very mild range, with MMSE scores ranging from 21–26. Therefore, we set out with the primary goal of Experiment 2 to determine whether the patients with AD from Experiment 1 could in fact perform mental imagery, helping to answer the question of whether patients with AD gain no benefit at encoding because they cannot perform mental imagery or because they cannot use the mental images to improve their memory.
3. Experiment 2
Mental imagery is a well-studied and characterized cognitive phenomenon. It has been described as a set of representations that gives rise to the experience of viewing a stimulus in the absence of appropriate sensory input18. Two main types of mental imagery have been discussed in the literature: visual and spatial38,39. Visual mental imagery is by definition, a representation specific to the visual modality and can be dissociated from spatial representations based on the inclusion of intrinsically visual information (e.g., color, shape, size)38. In contrast, spatial mental imagery relates to rotating representations of objects, scanning spatial characteristics of representations of objects and images, and mentally determining relationships between objects and individuals in space38. Although debate exists as to whether visual and spatial imagery are completely separate entities relying on two separate “visual systems” (see40), studies involving individuals who are congenitally blind show that they can successfully perform spatial imagery tasks, but cannot perform visual mental imagery41–44 suggesting that some stored visual representation is needed to perform visual imagery but not spatial imagery.
Less clear is the understanding of how imagery occurs in the brain. There have been a number of models to explain the process of mental imagery. Kosslyn (1980) proposed separable “components” and “processes” in the imagery system45. The main components consist of a long-term visual memory storage and a visual buffer. In the visual buffer, stored information is manipulated via various processes. Kosslyn (1980) proposed three main imagery processes of the visual buffer44. The first process, generation, creates the image in the buffer from information in long-term memory. The second process, inspection, explores representations to retrieve information such as color and shape. Finally, the third process, transformation, can rotate, reduce, etc., images in the buffer.
Helping to understand how mental imagery occurs, recent neuroimaging work has shed light on where imagery occurs in the brain. Primary visual areas V1 and V2 have been implicated in the generation of basic mental images46–49, whereas slightly higher-level visual processing areas V3, V4, and V5 have been implicated in the inspection and transformation of mental images50–53. In their review, Thompson and Kosslyn (2000) proposed that the generation process is highly reliant on medial occipital cortex including visual areas V1 and V2, the inspection process is highly reliant on medial occipital cortex and inferotemporal cortex, and the transformation process is heavily reliant on posterior parietal cortex. These brain regions involved have implications regarding which types of mental imagery may or may not be able to be performed by patients with AD.
Alzheimer's disease affects numerous brain structures throughout the course of the disease. Areas in medial temporal regions associated with new learning and episodic memory are typically the first areas to be affected by neurofibrillary tangle pathology54. However, more recent neuropathology investigation has suggested that association areas, such as posterior parietal and visual area V5, are affected early in the disease by amyloid plaque pathology55. It has been speculated that deterioration of V5 in patients with AD may lead to visual processing problems that recruit the dorsal pathway56. However, it has been suggested that primary visual areas remain relatively spared until later in the disease course24. Further, investigations have found that neuronal function along the dorsal pathway was affected prior to function along the ventral pathway in AD56. Based on these neuropathological findings, we hypothesize that patients with AD can likely perform basic mental imagery that involves generation, but may be impaired on types of mental imagery that have heavy inspection or transformation processing.
To further support these hypotheses, neuropsychological work has found impairments in cognitive domains likely critical to mental imagery. At the fore, neuropsychological findings suggest that episodic memory and visuospatial functioning can be impaired in very early AD57. In fact, a recent longitudinal study of patients with mild cognitive impairment (MCI) found that episodic memory recall, visuospatial, and semantic performance predicted those that ultimately developed AD58. In line with the predictions made based on the neuropathologic evidence, we hypothesized based on the neuropsychological findings that patients with AD can likely perform basic mental imagery, but might be impaired on those types of mental imagery that are heavily dependent on visuospatial, episodic memory, or semantic processing.
To determine whether the patients with AD from Experiment 1 could perform visual or spatial imagery, four mental imagery tasks were created based on modifications of previously used tasks. These tasks, as well as the imagery and cognitive processes involved, can bee seen in Table 4. Our a priori hypotheses, based on neuropathology and neuropsychology work, as well as previous imagery in AD findings (also seen in Table 4), were that patients would perform similarly to controls on the Letter Curves and Taller Wider tasks, which have lower spatial and semantic demands, as well as relatively low inspection and transformation imagery processing. In contrast, we predicted that patients would be impaired on the Animal Tails and Clock Angles tasks, which have relatively higher spatial and semantic demands, and also rely heavily on inspection and transformation imagery processing.
Table 4.
The four mental imagery tasks used in Experiment 2, the imagery and cognitive processes thought to be involved in these tasks, as well as our apriori hypotheses for Experiment 2.
| Task | Imagery Process | Cognitive Process | Hypothesis | |
|---|---|---|---|---|
| Letter Curves | image letters of alphabet and determine whether there is a curve in the printed version | visual imagery; generation | basic mental imagery | intact in patients |
| Taller-Wider | image an object and determine whether it is taller than it is wider | visual Imagery; generation; Inspection | basic semantic memory | intact in patients |
| Animal Tails | image an animal to determine whether its tail is long or short relative to its body | spatial imagery; generation; inspection | detailed semantic -episodic memory; working memory | impaired in patients |
| Clock Angles | image a clock and determine whether hands would results in an angle +/− 90 degrees | spatial imagery generation; inspection; transformation | semantic memory; visuospatial functioning; working memory; executive functioning | impaired in patients |
3.1. Method
3.1.1. Participants
All patients with AD and healthy older controls from Experiment 1 were recruited for Experiment 2. For various reasons of attrition (e.g., relocation for winter, declined) we were able to follow up with 9 patients with AD from Experiment 1. Although this is a rather small number of subjects, we thought it was preferable to emphasize continuity with the subjects from Experiment 1 and maintain a within-subjects design. All participants completed Experiment 2 within six months of Experiment 1, with a mean delay of 1.4 months. Because this delay was relatively short, neuropsychological testing was not repeated. To match for age, gender, and education, we selected 9 healthy older adults from Experiment 1 to act as control subjects in Experiment 2. Once again, we performed Fisher F-tests to assess group differences in age and years of education and Chi-Square to assess differences in gender. The results revealed that there were no significant differences in age [F(1, 16) = 1.72, p = .208], gender [F(1, 16) = 1.44, p = .247], years of education [F(1, 16) < 1], or gender [χ2(1) = .254, p = .614] between the AD and healthy older adult groups (see Table 3).
Table 3.
Experiment 2 means, standard deviations (in parentheses), and ranges (below) for age, education, and neuropsychological performance for the Alzheimer's disease (AD) and health older adult (OC) groups.
| AD | OC | |
|---|---|---|
| Subjects | n = 9 (5F) | n = 9 (5F) |
| MMSE | 26.6 (1.5) | 29.0 (1.1) |
| ** | 21 – 28 | 28–30 |
| Age | 76.1 (6.5) | 73.7 (8.7) |
| 65–87 | 65–89 | |
| Education | 14.5 (3.0) | 15.2 (1.9) |
| 11–18 | 12–18 | |
| CERAD encoding | 9.5 (1.9) | 23.2 (5.2) |
| ** | 6–15 | 18–30 |
| CERAD recall | 0.5 (1.1) | 7.9 (1.6) |
| ** | 0–4 | 6–10 |
| CERAD recognition | 6.25 (2.9) | 9.8 (0.4) |
| ** | 3–9 | 9–10 |
| Trails B | 141.0 (65.2) | 73.4 (19.1) |
| ** | 74–300 | 57–104 |
| FAS | 35.6 (13.7) | 44.3 (10.8) |
| ** | 12–60 | 31–68 |
| CAT | 29.5 (6.5) | 50.8 (10.0) |
| ** | 8–56 | 39–67 |
| Boston Naming Test | 11.5 (3.1) | 14.8 (0.4) |
| * | 9–14 | 14–15 |
FAS = total score letter fluency for letters F, A, and S. CAT = total scoresemantic fluency for categories Animals, Vegetables, and Fruits. Asterisks signify significant differences between the two groups
less than .01
less than .05.
3.1.2. Stimuli and Procedure
Four mental imagery tasks from the literature were slightly modified for use in patients with AD. Behavioral data were obtained in a single session with the order of these tasks counterbalanced across participants. Each task consisted of two conditions. One condition required mental imagery to successfully perform the task. A second condition consisted of a perceptual version of each imagery task to assure that any failure of a mental imagery trial was not secondary to a problem with perceptual functioning. As to not interfere with the imagery condition and aid performance, the perceptual task was given prior to the imagery task for the Clock Angles task only. For all other conditions, the perceptual task was given last.
In the Clock Angles task, based on that of Grossi et al., (1994) but modified to simplify the procedure and minimize the cognitive steps necessary to complete the task, participants were asked to imagine the angle created by the hands on a clock, set to a certain time, and determine whether that angle was less than 90 degrees. Participants first saw 40 clock faces (hands included) without numbers and were asked to make a judgment about whether the angle between the hands was less than 90 degrees. Participants next viewed digitally represented clock times and were asked to imagine the angle between the minute and hour hands (as represented on an analog clock) and make a judgment about whether that angle was less than 90 degrees. In the Animal Tails task38, participants viewed 29 animal names and were asked to imagine each animal and make a judgment about whether its tail was long or short relative to its body size. In the perceptual condition, they viewed pictorial referents of the animals with the tail exposed and were asked to make the same judgment. In the Taller/Wider task59, participants were asked to generate an image of an object presented as a word on the screen and make a judgment about whether the item is taller than it is wide. In the perceptual condition they were then shown pictorial referents of those words and asked to make the same judgment. Finally, in the Letter Curves task38, participants heard the 26 letters of the alphabet (both lower and upper case forms specified) presented in random order and were asked to create a mental image of a printed block letter to determine whether that letter contained any curved lines or all straight lines. In the perceptual condition of this experiment they viewed the same letters and were asked to make the same judgment.
3.2. Results
To analyze accuracy (percent correct) for the four imagery conditions (Table 5), we performed a repeated-measures ANOVA with the factors of Group (older controls, AD) and Task (Animal Tails, Taller/Wider, Letter Curves, Clock Angles). The ANOVA revealed an effect of Group [F(1,16) = 6.75, p = .019] because overall, the older controls performed better on the imagery tasks than patients with AD. There also was an effect of Task [F(3,48) = 13.46, p < .001], but no interaction of Group and Task [F(3,48) = 1.96, p = .133]. The effect of Task was present because all participants performed better on the Taller/Wider task than on the Animal Tails [t(17) = 5.17, p < .001] and Clock Angles [t(17) = 4.45, p < .001] tasks. They also performed better on the Letter Curves task than on the Animal Tails task [t(17) = 3.76, p = .002], and on the Clock Angles task [t(17) = 3.34, p = .004].
Table 5.
Mean percent correct, standard deviations (in parentheses), and range of score (below) for the Alzheimer's disease (AD) and healthy older controls (OC) on the Clock Angles (CA), Animal Tails (AT), Taller/Wider (TW), and Letter Curves (LC) imagery and perceptual tasks.
| Imagery Task | ||||
|---|---|---|---|---|
| CA | AT | TW | LC | |
| AD | .68 (.20) | .71 (.11) | .92 (.06) | .90 (.05) |
| .48 – 1.00 | .55 – .90 | .79 – 1.00 | .85 – 1.00 | |
| OC | .81 (.18) | .86 (.10) | .94 (.05) | .92 (.08) |
| .50 – 1.00 | .69 – .93 | .85 – 1.00 | .77 – 1.00 | |
| Perceptual Task | ||||
| AD | .94 (.15) | .91 (.06) | .97 (.04) | .97 (.02) |
| .57 – .98 | .79 – 1.00 | .88 – 1.00 | .94 – .98 | |
| OC | .95 (.12) | .93 (.02) | .96 (.06) | .96 (.03) |
| .63 – 1.00 | .92 – .96 | .83 – 1.00 | .92 – 1.00 | |
To compare the patients and controls on the perceptual conditions, we performed a repeated-measures ANOVA with the factors of Group (older controls, AD) and Task (Animal Tails, Taller/Wider, Letter Curves, Clock Angles) similar to that of the imagery conditions. The ANOVA revealed no effects of Group [F(1, 16) = 0.11, p = .742] or Task [F(3, 48) = 1.20, p = .318] and no interaction of Group and Task [F(3, 48) = .250, p = .861].
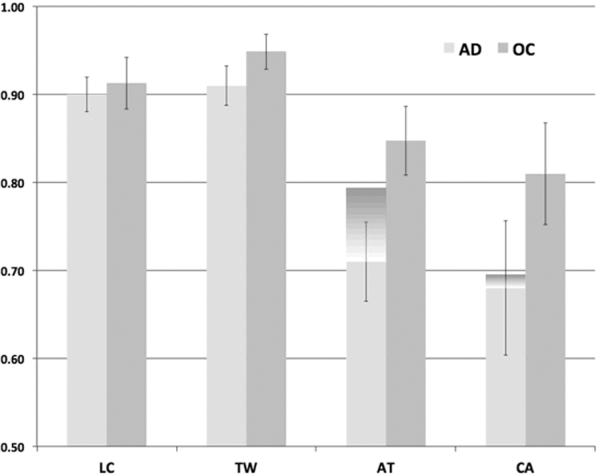
Because previous work has implicated confrontation naming as a possible limitation to imagery ability in patients with moderate AD37, and our patients with AD performed significantly worse on the Boston Naming Test than the healthy controls [t(16) = 3.14, p = .006], we elected to run a separate repeated-measures ANOVA with Boston Naming Test (BNT) performance included as a covariate on the imagery performance data. When naming performance was included, the ANOVA revealed an effect of Task [F(3, 45) = 5.69, p = .002] and interactions of Group and Task [F(3, 45) = 2.96, p = .042] and Task and BNT score [F(3, 45) = 5.73, p = .002]. In contrast to the original ANOVA, there was only a trend towards an effect of Group [F(1, 15) = 3.49, p = .081]. To follow up on the interaction of Group and Task, we ran a multivariate ANOVA with the covariate of BNT score. The ANOVA revealed a difference in group performance only on the Clock Angles task [F(1, 15) = 4.85, p = .044]. There were no group differences on the Animal Tails task [F(1, 15) < 1], the Taller/Wider task [F(1, 15) = 2.68, p = .123], or the Letter Curves task [F(1, 15) < 1]. The ANOVA also revealed a significant impact of BNT score on the Animal Tails task [F(1, 15) = 6.78, p = .020], but on no other task. Finally, to fully understand the impact of naming ability on each of the mental imagery tasks, we elected to perform pairwise comparisons between groups without the covariate. Here, the groups did not differ on the Taller/Wider task [t(16) = 1.17, p = .261] or the Letter Curves Task [t(16) < 1], but patients with AD performed significantly worse than controls on the Animal Tails [t(16) = 2.56, p = .021] and Clock Angles tasks [t(16) = 3.02, p = .008]. Figure 1 shows performance on the four imagery tasks with and without the covariate of Boston Naming score.
Figure 1.
Mean percent correct for the Clock Angles (CA), Animal Tails (AT), Taller/Wider (TW) and Letter Curves (LC) imagery tasks for the healthy older adults and patients with AD. The additional dark shaded area above the AD bars represents the means percent correct on these tasks with Boston Naming scores added in the ANOVA as a covariate.
3.3. Correlation analyses
To further understand the relationship between the data from both experiments and between our behavioral results and the neuropsychological data, we performed two sets of correlation analyses. First, we performed a bivariate correlation analysis to determine whether performance on the imagery tasks of Experiment 2 were related to performance on the memory tasks in Experiment 1. The results showed no relationship between performance on any of the imagery tasks and performance on the memory tasks (all correlation coefficients < .360), suggesting that variance in memory ability outweighs any effect of imagery ability. In other words, patients' significant variance in their poor memory likely outweighs the ability for imagery to improve their memory. Alternatively, correlations may not have reached significance due to such a small number of subjects. In contrast, performance on the memory and imagery tasks did correlate with performance on neuropsychological testing measures. Executive functioning ability, as measured by the Trail Making Test Part B (TMT-B), correlated significantly with performance on the General [r = −.425, p = .022] and Self-Referential Imagery [r = −.409, p = .028] memory tasks and with performance on the Clock Angles imagery task [r = −.861, p < .001]. However, only the correlation between TMT-B and the Clock Angles imagery task remained significant after Bonferroni correction for multiple comparisons with an adjusted alpha of .05/7 (p = .007).
3.4. Discussion
In Experiment 2, we examined whether the patients with AD from Experiment 1 could perform a series of mental imagery tasks with varying cognitive and imagery demands. Over the four tasks, patients with AD were generally unable to perform mental imagery in a manner comparable to healthy older adults, despite similar perceptual functioning. However, due to significant differences in task difficulty and the imagery and cognitive processes underlying each of the four tasks, we elected to perform pairwise comparisons between groups to test our specific hypotheses. In line with our hypotheses, the results showed that patients performed similarly to controls on the Letter Curves and Taller/Wider tasks, but significantly worse on the Animal Tails and Clock Angles tasks. Interestingly, when we used analysis methods similar to Tippett and colleagues (2003) and controlled for confrontation naming ability (BNT performance), the overall group differences were reduced to only a trend. Further, performance remained similar for the Letter Curves and Taller/Wider tasks in the new analysis, but now there was no difference in performance on the Animal Tails task between groups.
Out of the four imagery tasks, patients with AD demonstrated clear impairment on the Clock Angles task. Patients' performance on the Clock Angles task was reduced compared to healthy older controls, whether or not naming was entered as a covariate in the analysis. There are several likely reasons for impaired performance on the Clock Angles task in our patient group. First, in neuropsychological work, the Clock Drawing Test is often used to test visuospatial functioning60. Neuroimaging work investigating a variety of Clock Angle tasks using functional magnetic resonance imaging (fMRI) suggests that left parietal cortex is heavily involved in these tasks51,61. Further, a study examining the comparison of two clock angles in mind using mental imagery showed that the left parietal lobe was involved in generating the mental image of the clock while the right parietal lobe specialized in comparing multiple angles62. Given evidence suggesting impaired visuospatial performance in AD patients early in the disease63 and that Alzheimer plaque pathology may develop in posterior parietal regions very early in the disease course55, our current finding of impairment on the Clock Angles spatial imagery task is not surprising.
Executive dysfunction in patients with AD could also be contributing to the impaired performance on the Clock Angles task. TMT-B is used to test executive functioning ability, which is also often impaired in early AD64. In the current study, performance on the TMT-B, which was impaired for patients relative to controls, correlated with performance on the Clock Angles task. That is, the worse patients performed on the TMT-B, the worse they performed on the Clock Angles task. Being able to hold the clock and time in mind to determine the angle requires significant demand on working memory and executive skills62. As patients lose executive abilities as the disease progresses, their ability to perform spatial/transformational imagery likely deteriorates as well.
One final explanation for the impaired performance on the Clock Angles task in the patients with AD is semantic memory impairment. Recent work has suggested that poor performance on the neuropsychological measure, Clock Drawing Test, not only arises due to visuospatial and executive dysfunction in patients with AD, but also due to semantic memory failure65. Patients in the earliest stages of the disease have shown degraded semantic ability associated with cortical atrophy in anterior temporal and inferior prefrontal regions66. In the current study, we see slight improvement in the Clock Angles task once naming ability is covaried in the analysis (see Figure 1). Although performance improved after controlling for naming ability, it remained reduced compared to healthy older adults, suggesting that semantic impairment likely did not play a significant role in the poor performance seen our patient group.
In should be noted that our findings on the Clock Angles tasks are somewhat discordant with Grossi et al. (1994), who found that patients with mild AD could perform spatial imagery using clock angles in a similar manner to healthy older controls. The major difference however, is that Grossi and colleagues (1994) preselected participants from a patient pool that performed within normal limits on the neuropsychological assessment measure Clock Drawing Test, suggesting that patients without visuospatial or executive deficits can perform spatial imagery without problem. However, given that clock performance is typically impaired in the majority of patients with AD65, these deficits likely prevent patients from being able to perform spatial mental imagery. These results also suggest, as hypothesized, that the mental imagery process of transformation is impaired in patients with AD.
From the results of the Clock Angles task and the follow-up analyses, it is clear that visuospatial, executive, and semantic memory impairment hinder patients with AD from performing some types of mental imagery as well as their peers. Although semantic memory ability appeared to affect the Clock Angle task only slightly, it played a significant role in the Animal Tails task. In the original ANOVA, performance on the Animal Tails tasks was significantly reduced for patients compared to healthy older adults. However, our follow-up ANOVA revealed that when naming ability was entered as a covariate, performance did not differ between patients and controls (see Figure 1). Farah, Hammond et al. (1988) state that the Animal Tails task is a mental imagery task that requires detailed semantic knowledge of animals, with relatively little visuospatial influence. It has also been hypothesized that episodic memory likely plays a role to some degree37. The task requires participants to conjure a visual image and inspect this image for a specific detail. That patient performance was reduced without the covariate of naming is not unexpected given the progressive loss of detailed semantic and episodic memory ability in the course of AD67. Further, holding an image in mind to inspect for specific details likely places increased demands on working memory and executive functioning. As stated earlier, as these processes deteriorate in AD, tasks that rely on these processes likely suffer as well.
The results of the Animal Tails task suggest that some aspects of the inspection process of mental imagery may be impaired in patients with AD. As Kosslyn (1980) proposed, when determining whether a fox has a long tail compared to its body, the individual must construct or generate an image of a fox, then scan the surface representation to examine the tail. This process depends on both the long-term memory representation of a fox as well as holding the image of the fox in the working memory visual buffer while it is scanned, placing heavy demands on posterior parietal cortex48. At this time, it is unclear whether degraded long-term memory representation or impairment in the visual buffer contributes to the impaired performance seen in patients for the Animal Tails task. The literature has reported impairment in both68,69. Reports of Alzheimer pathology early in the disease affecting visual regions V4 and V555,56 as well as posterior parietal regions55 lend support to the hypothesis of visual buffer impairment. However, it has also been previously suggested that perhaps patients with AD would have difficulty in the inspection process due to degraded long-term representations rather than a deficit in the visual buffer70, consistent with the fact that patients' performance on the Animal Tails task is relatively spared when naming deficits are covaried.
The hypothesis suggesting that patients would have difficulty in the inspection process due to degraded long-term representations rather than a deficit in the visual buffer68 is also supported by the Taller/Wider task results. Here, patients did not differ in performance relative to controls, with or without the covariate of naming ability. The Taller/Wider task requires participants to generate a visual mental image of an object presented as a word on the screen, and determine whether the object is greater in height or width. This task, originally used by Kosslyn and colleagues (1985) with patient V.P., is reported to rely on basic semantic knowledge or familiarity of an object, but not on specific detailed information or holding an object in the visual buffer and scanning for a specific detail as in the Animal Tails task37. It is suggested that the semantic and episodic demand is lower on the Taller/Wider task than on the Animal Tails task37,38, and places relatively low demand on posterior parietal regions mediating the visual buffer during the inspection process48. That patient performance is unimpaired on this task suggests that at least some aspects of the inspection process of mental imagery remain intact.
The results of Experiment 2 suggest that, in general, basic visual imagery remains intact in patients with AD. That performance on the Taller/Wider and Letter Curves tasks was similar between groups, even without covarying for naming performance, suggests that the imagery processes of generation and inspection remain intact in patients with AD. Given that patients' performance on the Taller/Wider task remained intact, but performance on the Animal Tails task was impaired, makes it difficult to determine whether all aspects of the process of inspection are unimpaired. Patients appear able to inspect their mental images, but degraded semantic representations and impaired working memory make inspecting these images for specific details impaired. In contrast to the processes of generation and inspection, it appears as though the process of transformation is impaired. Patients performed worse on the Clock Angles task than controls whether or not we controlled for naming ability. The Clock Angles task is generally more difficult and has increased demands on visuospatial and executive ability, which likely contributed to the poor performance seen in our patient group.
4. General Discussion
Alzheimer's disease tends to affect all domains of cognition as the disease progresses. However, in the earliest stages, medial temporal structures responsible for new learning are most involved. A great deal of research has recently been focused on helping patients with mild cognitive impairment or very mild AD use novel techniques to enhance new learning71,72. These findings, in addition to work showing that patients can use metacognitive strategies to improve memory performance73,74, have led researchers to develop salubrious cognitive rehabilitation programs for these individuals75. As part of cognitive rehabilitation to improve memory, many investigators have used mental imagery, which has shown great improvement in healthy older adults14.
The results of our investigation suggest that patients with AD can perform basic visual imagery. Specifically, patients perform very similarly to healthy older adults on tasks that rely on the imagery processes of generation and basic inspection. However, patients cannot perform more complex or spatial mental imagery that involves heavy visuospatial, executive, or semantic demands, or imagery that requires holding an object in working memory for detailed inspection or transformation. We speculate that the impairment in spatial inspection and transformation likely prevents patients from benefiting from complex types of mental imagery, such as self-referential imagery, to improve verbal memory. Buckner and Carroll (2007) report that mentally projecting oneself into a situation relies on a network of parietal, frontal, and medial temporal structures (default network, episodic memory network)76. This network of structures is typically heavily affected in AD, and is thought to be responsible for the significant episodic memory deficits in patients77. That patients have difficulty using self-referential imagery is also consistent with behavioral work showing that patients with AD lack the ability to mentally project themselves into future situations78. That is, patients struggle with the ability to place themselves into a made up past or future scenario, which may be very similar to envisioning themselves interacting with an object in a mental imagery scene. Using the current study design, patients with AD showed little benefit from the self-referential strategy, even when rehearsed with the experimenter on every fifth trial.
Although patients' ability to perform basic mental imagery did not translate into a benefit to verbal recognition in the current study, there are a number of possibilities that suggest that mental imagery may actually help patients with AD. First, our methodology was designed to have patients and controls perform the same task, while keeping patients off of functional floor and controls off of ceiling. While we certainly accomplished this, the task of recognizing 50 studied items from each encoding condition was likely difficult for patients, and not realistic of everyday requirements of memory. Future work can examine the effect of mental imagery in patients using shorter study lists or more functional tasks (e.g., grocery lists, medications, etc.). Indeed, mental imagery has shown to be of great benefit to patients who have suffered a stroke needing to relearn activities of daily living79. Second, when we re-analyzed the data for every fifth trial where the experimenter verified the subject's general mental image, performance was significantly better compared to the baseline condition. This benefit was likely the result of deeper encoding due to rehearsal and verbalization. Future work could focus on using training in mental imagery or interaction with caregivers to scaffold new learning using mental imagery. These techniques could help to overcome patient apathy or poor attention that may contribute to encoding failure and resultant poor memory. Finally, the current investigation was an initial examination into whether patients with AD could use mental imagery to improve memory with no training. In the literature, studies examining the effects of cognitive rehabilitation in healthy and cognitively impaired populations provide training and strategies over many sessions75,79. Further, memory rehabilitation studies suggest that multiple sessions of training have shown that patients with AD can learn and successfully use new techniques to improve memory80. Incorporating what we have learned from the present study with current knowledge in the cognitive rehabilitation field could potentially provide meaningful strategies for improving memory functioning in patients with mild AD.
Acknowledgments
Funding Support This research was supported by National Institute on Aging grants K23 AG031925 and R01 AG038471 to BAA and R01 AG025815 to AEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer's disease in the U.S. population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2).Andel R, Hyer K, Slack A. Risk factors for nursing home placement in older adults with and without dementia. Journal of Aging and Health. 2007;19:213–228. doi: 10.1177/0898264307299359. [DOI] [PubMed] [Google Scholar]
- 3).Black BS, Rabins PV, German PS. Predictors of Nursing Home Placement Among Elderly Public Housing Residents. The Gerontologist. 1999;39:559–568. doi: 10.1093/geront/39.5.559. [DOI] [PubMed] [Google Scholar]
- 4).Cohen CA, Gold DP, Shulman KI, Wortley JT, McDonald G, Wargon M. Factors determining the decision to institutionalize dementing individuals: A prospective study. The Gerontologist. 1993;33:714–720. doi: 10.1093/geront/33.6.714. [DOI] [PubMed] [Google Scholar]
- 5).Clipp E. US Department of Veterans Affairs HSR&D Study NRI 95–218, Informal Caregivers of Veterans with Dementia: Cost, QOL, and Service Use. 2005. [Google Scholar]
- 6).De Beni R, Pazzaglia F. Memory for different kinds of mental images: Role of contextual and autobiographic variables. Neuropsychologia. 1995;33:1359–1371. doi: 10.1016/0028-3932(95)00069-f. [DOI] [PubMed] [Google Scholar]
- 7).Richardson JTE. Mental imagery and human memory. Macmillan; London: 1980. [Google Scholar]
- 8).West RL. Compensatory strategies for Age-Associated Memory Impairment (AAMI) In: Baddeley AD, Wilson BA, Watts FN, editors. Handbook of memory disorders. John Wiley; London: 1995. pp. 481–500. [Google Scholar]
- 9).Liu KPY, Chan CCH. Metacognitive Mental Imagery Strategies for Training of Daily Living Skills for People with Brain Damage: The Self-Regulation and Mental Imagery Program. In: Soderback I, editor. International Handbook of Occupational Therapy Interventions, Part 3. Springer Science + Business Media, LLC; New York: 2009. pp. 233–239. [Google Scholar]
- 10).Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journals of Gerontology. 1993;48:157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- 11).Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).West RL, Crook TH. Video training of imagery for mature adults. Applied Cognitive Psychology. 1992;6:307–320. [Google Scholar]
- 13).Floyd M, Scogin F. Cognitive-behavior therapy for older adults: How does it work? Psychotherapy: Theory, Research, Practice, Training. 1998;35:459–463. [Google Scholar]
- 14).Palladino P, DeBeni R. When mental images are very detailed: Image generation and memory performance as a function of age. Acta Psychologica. 2003;113:297–314. doi: 10.1016/s0001-6918(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 15).Yesavage JA, Rose TL, Bower GH. Interactive imagery and affective judgments improve face-name learning in the elderly. Journal of Gerontology. 1983;38:197–203. doi: 10.1093/geronj/38.2.197. [DOI] [PubMed] [Google Scholar]
- 16).Zarit SH, Cole KD, Guider RL. Memory training strategies and subjective complaints of memory in the aged. Gerontologist. 1981;21:158–164. doi: 10.1093/geront/21.2.158. [DOI] [PubMed] [Google Scholar]
- 17).Shepard RN. Recognition memory for words, sentences, and pictures. Journal of Verbal Learning and Verbal Behavior. 1967;6:156–163. [Google Scholar]
- 18).Kosslyn SM. Reflective thinking and mental imagery: A perspective on the development of posttraumatic stress disorder. Development and Psychopathology. 2005;17:851–863. doi: 10.1017/S0954579405050406. [DOI] [PubMed] [Google Scholar]
- 19).Paivio A. The empirical case for dual coding. In: Yuille JC, editor. Imagery, memory and cognition. Erlbaum; Hillsdale, NJ: 1983. pp. 307–332. [Google Scholar]
- 20).Grady CL, McIntosh AR, Rajah MN, Craik FI. Neural correlates of the episodic encoding of pictures and words. Proceedings of the National Academy of Sciences U S A. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Gardini S, De Beni R, Cornoldi C, Bromiley A, Venneri A. Different neuronal pathways support the generation of general and specific mental images. Neuroimage. 2005;27:544–552. doi: 10.1016/j.neuroimage.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 22).Foley MA, Foy J. Pictorial encoding effects and memory confusions in the Deese-Roediger-McDermott paradigm: Evidence for the activation of spontaneous imagery. Memory. 2008;16:12–27. doi: 10.1080/09658210802220054. [DOI] [PubMed] [Google Scholar]
- 23).Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, Zhou Y, Xu L, Song M, Li K. Spontaneous activity associated with primary visual cortex: A resting-state FMRI study. Cerebral Cortex. 2007;18:697–704. doi: 10.1093/cercor/bhm105. [DOI] [PubMed] [Google Scholar]
- 24).Macedo MN, Kim E, Seeley WW. Neuropathology of dementia. In: Miller BL, Boeve BF, editors. The Behavioral Neurology of Dementia. Cambridge University Press; New York: 2009. pp. 142–160. [Google Scholar]
- 25).Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Breuil V, De Rotrou J, Forette F, Tortrat D, Ganansia-Ganem A, Frambourt A, Moulin F, Boller F. Cognitive stimulation of patients with dementia: Preliminary results. International Journal of Geriatric Psychiatry. 1994;9:211–217. [Google Scholar]
- 27).Grossi D, Becker JT, Trojano L. Visuospatial imagery in Alzheimer's disease. Perceptual and Motor Skills. 1994;78:867–874. doi: 10.1177/003151259407800338. [DOI] [PubMed] [Google Scholar]
- 28).McKhann G, Drachman D, Folstein M, Katzman R, Price D. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:285–297. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29).Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30).Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C, the CERAD investigators The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31).Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Archives of Neurology. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- 32).Adjutant General's Office . Army Individual Test Battery: Manual of Directions and Scoring. War Department; Washington, D.C.: 1944. [Google Scholar]
- 33).Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 34).Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer's disease. Journal of Gerontology. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- 35).Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 36).Borg C, Thomas-Anterion C, Bogey S, Davier K, Laurent B. Visual imagery processing and knowledge of famous names in Alzheimer's disease and MCI. Aging, Neuropsychology, and Cognition. 2010;17:603–614. doi: 10.1080/13825585.2010.481357. [DOI] [PubMed] [Google Scholar]
- 37).Tippett LJ, Blackwood K, Farah MJ. Visual object and face processing in mild-to-moderate Alzheimer's disease: From segmentation to imagination. Neuropsychologia. 2003;41:453–68. doi: 10.1016/s0028-3932(02)00140-9. [DOI] [PubMed] [Google Scholar]
- 38).Farah MJ, Hammond KM, Levine DN, Calvanio R. Visual and spatial mental imagery: Dissociable systems of representation. Cognitive Psychology. 1988;20:439–462. doi: 10.1016/0010-0285(88)90012-6. [DOI] [PubMed] [Google Scholar]
- 39).Kosslyn SM, Ganis G, Thompson WL. Mental imagery and the human brain. In: Jing Q, Rosenzweig MR, D'Ydewalle G, Zhang H, Chen H-C, Zhang K, editors. Progress in Psychological Science Around the World, Vol. 1: Neural, Cognitive and Developmental Issues. Psychology Press; London: 2006. pp. 195–209. [Google Scholar]
- 40).Ungerleider LG, Mortimer M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. The Massachusetts Institute of Technology; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- 41).Carpenter PA, Eisenberg P. Mental rotation and the frame of reference in blind and sighted individuals. Perception and Psychophysics. 1978;23:117–124. doi: 10.3758/bf03208291. [DOI] [PubMed] [Google Scholar]
- 42).Jonides J, Kahn R, Rozin P. Imagery instructions improve memory in blind subjects. Bulletin of the Psychonomic Society. 1975;5:424–426. [Google Scholar]
- 43).Kerr NH. The role of vision in “visual imagery” experiments: Evidence from the congenitally blind. Journal of Experimental Psychology General. 1983;112:265–277. doi: 10.1037//0096-3445.112.2.265. [DOI] [PubMed] [Google Scholar]
- 44).Marmor GS, Zaback LA. Mental rotation by the blind: Does mental rotation depend on visual imagery? Journal of Experimental Psychology. Human Perception and Performance. 1976;2:515–521. doi: 10.1037//0096-1523.2.4.515. [DOI] [PubMed] [Google Scholar]
- 45).Kosslyn SM. Image and mind. Harvard University Press; Cambridge, MA: 1980. [Google Scholar]
- 46).Chen W, Kato T, Zhu XH, Ogawa S, Tank DW, Ugurbil K. Human primary visual cortex and lateral geniculate nucleus activation during visual imagery. NeuroReport. 1998;9:3669–3674. doi: 10.1097/00001756-199811160-00019. [DOI] [PubMed] [Google Scholar]
- 47).Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM. The role of area 17 in visual imagery: Convergent evidence from PET and rTMS. Science. 1999;284:167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 48).Thompson WL, Kosslyn SM. Neural systems activated during visual mental imagery: A review and meta-analyses. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Systems. Academic Press; San Diego: 2000. pp. 535–560. [Google Scholar]
- 49).Slotnick SD, Thompson WL, Kosslyn SM. Visual mental imagery induces retinotopically organized activation of early visual areas. Cerebral Cortex. 2005;15:1570–1583. doi: 10.1093/cercor/bhi035. [DOI] [PubMed] [Google Scholar]
- 50).Amedi A, Malach R, Pascual-Leone A. Negative BOLD differentiates visual imagery and perception. Neuron. 2005;48:859–872. doi: 10.1016/j.neuron.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 51).Conson M, Cinque F, Trojano L. The two sides of the mental clock: The Imaginal HemiSpatial Effect in the healthy brain. Brain and Cognition. 2008;66:298–305. doi: 10.1016/j.bandc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 52).Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 53).Knauff M, Kassubek J, Mulack T, Greenlee MW. Cortical activation evoked by visual mental imagery as measured by fMRI. Neuroreport. 2000;11:3957–3962. doi: 10.1097/00001756-200012180-00011. [DOI] [PubMed] [Google Scholar]
- 54).Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Patholology. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 55).McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA. Visual association pathology in preclinical Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- 56).Bokde AL, Lopez-Bayo P, Born C, Ewers M, Meindl T, Teipel SJ, Möller HJ, Hampel H. Alzheimer disease: Functional abnormalities in the dorsal visual pathway. Radiology. 2010;254:219–226. doi: 10.1148/radiol.2541090558. [DOI] [PubMed] [Google Scholar]
- 57).Dickerson BC, Eichenbaum H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Nordlund A, Rolstad S, Gothlin M, Edman A, Hansen S, Wallin A. Cognitive Profiles of Incipient Dementia in the Goteborg MCI Study. Dementia and Geriatric Cognitive Disorders. 2010;30:403–410. doi: 10.1159/000321352. [DOI] [PubMed] [Google Scholar]
- 59).Kosslyn SM, Holtzman JD, Farah MJ, Gazzaniga MS. A computational analysis of mental image generation: Evidence from functional dissociations in split-brain patients. Journal of Experimental Psychology General. 1985;114:311–341. doi: 10.1037//0096-3445.114.3.311. [DOI] [PubMed] [Google Scholar]
- 60).Mendez MF, Ala T, Underwood KL. Development of scoring criteria for the clock drawing task in Alzheimer's disease. Journal of the American Geriatrics Society. 1992;40:1095–1099. doi: 10.1111/j.1532-5415.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 61).Formisano E, Linden DE, Di Salle F, Trojano L, Esposito F, Sack AT, Grossi D, Zanella FE, Goebel R. Tracking the mind's image in the brain I: Time-resolved fMRI during visuospatial mental imagery. Neuron. 2002;35:185–194. doi: 10.1016/s0896-6273(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 62).Sack AT, van de Ven VG, Etschenberg S, Schatz D, Linden DE. Enhanced vividness of mental imagery as a trait marker of schizophrenia? Schizophrenia Bulletin. 2005;31:97–104. doi: 10.1093/schbul/sbi011. [DOI] [PubMed] [Google Scholar]
- 63).Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Chen T, Chen Y, Cheng T, Hua M, Liu H, Chiu M. Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Human Brain Mapping. 2009;30:3826–3836. doi: 10.1002/hbm.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Leyhe T, Saur R, Eschweiler GW, Milian M. Clock test deficits are associated with semantic memory impairment in Alzheimer disease. Journal of Geriatric Psychiatry and Neurology. 2009;22:235–245. doi: 10.1177/0891988709335798. [DOI] [PubMed] [Google Scholar]
- 66).Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, Lacombe J, Goldstein R, Chayer C, Kergoat MJ. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer's disease. Neuropsychologia. 2010;48:978–988. doi: 10.1016/j.neuropsychologia.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 67).Huff FJ, Corkin S, Growdon JH. Semantic impairment and anomia in Alzheimer's disease. Brain and Language. 1986;28:235–249. doi: 10.1016/0093-934x(86)90103-3. [DOI] [PubMed] [Google Scholar]
- 68).Budson AE, Price BH. Memory dysfunction. New England Journal of Medicine. 2005;352:692–699. doi: 10.1056/NEJMra041071. [DOI] [PubMed] [Google Scholar]
- 69).Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della SS. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- 70).Kosslyn SM, Dror IE. A cognitive neuroscience of Alzheimer's disease: what can be learned from studies of visual imagery? In: Christen Y, Churchland P, editors. Neurophilosophy and Alzheimer's Disease. Springer-Verlag; New York: 1992. [Google Scholar]
- 71).Hampstead BM, Stringer AY, Stilla RF, Deshpande G, Hu X, Moore AB, Sathian K. Activation and Effective Connectivity Changes Following Explicit-Memory Training for Face-Name Pairs in Patients With Mild Cognitive Impairment: A Pilot Study. Neurorehabilitation and Neural Repair. doi: 10.1177/1545968310382424. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Simmons-Stern NR, Budson AE, Ally BA. Music as a memory enhancer in patients with Alzheimer's disease. Neuropsychologia. 2010;48:3164–3167. doi: 10.1016/j.neuropsychologia.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Budson AE, Dodson CS, Daffner KR, Schacter DL. Metacognition and false recognition in Alzheimer's disease: Further exploration of the distinctiveness heuristic. Neuropsychology. 2005;19:253–258. doi: 10.1037/0894-4105.19.2.253. [DOI] [PubMed] [Google Scholar]
- 74).Waring JD, Chong H, Wolk DA, Budson AE. Preserved metamemorial ability in patients with mild Alzheimer's disease: Shifting response bias. Brain and Cognition. 2008;66:32–39. doi: 10.1016/j.bandc.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Clare L, Linden DE, Woods RT, Whitaker R, Evans SJ, Parkinson CH, van Paasschen J, Nelis SM, Hoare Z, Yuen KS, Rugg MD. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: A single-blind randomized controlled trial of clinical efficacy. American Journal of Geriatric Psychiatry. 2010;18:928–939. doi: 10.1097/JGP.0b013e3181d5792a. [DOI] [PubMed] [Google Scholar]
- 76).Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 77).Buckner RL, Snyder AZ, Shannon BJ, LaRosa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer's disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Liu KP, Chan CC, Lee TM, Hui-Chan CW. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2004;85:1403–1408. doi: 10.1016/j.apmr.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 80).Bier N, Van Der Linden M, Gagnon L, Desrosiers J, Adam S, Louveaux S, Saint-Mleux J. Face-name association learning in early Alzheimer's disease: A comparison of learning methods and their underlying mechanisms. Neuropsychological Rehabilitation. 2008;18:343–371. doi: 10.1080/09602010701694723. [DOI] [PubMed] [Google Scholar]