Abstract
Purpose
To conduct a meta-analysis on the rates of myopia progression in urban children of Asian and predominately European ethnicities who are corrected with traditional single-vision spectacles.
Methods
A search of the National Library of Medicine’s PubMed literature database for articles on myopia progression was conducted using the terms ‘myopi*progression’ and MeSH terms ‘myopia’ and ‘disease progression’, and limited to publications from January 1990 and only for papers reporting data for humans < 16 years of age. Studies were excluded if they were non-randomized, did not use cycloplegic autorefraction, had a sample size less than 30 individuals, examined high myopia (worse than −6.0D) or special subject groups, presented myopia as part of a syndrome or condition, were retrospective, or used controls wearing optical corrections other than spectacles.
Results
Of 175 articles identified, 20 remained after applying the exclusion criteria. The estimated myopia progression at a mean age of 9.3 years after one year of follow-up was −0.55 D (95% C.I. −0.39 to −0.72 D) for populations of predominantly European extraction and −0.82 D (95% C.I. −0.71 to −0.93 D) for Asians. The estimated progression rates were dependent on baseline age, with decreasing progression as age increased. The rates also varied with gender. For an average baseline age of 8.8 years, estimated annual progression (combined ethnicities) was −0.80 D/year for females (95% CI: −0.51 to −1.10), and a significantly slower (p<0.01) −0.71 D/year for males (95% CI: −0.42 to −1.00).
Conclusions
In children wearing single-vision spectacles, higher myopia progression rates were found in urban Asians compared to urban populations of predominantly European descent. Younger children and females demonstrated greater annual rates of progression of myopia.
Keywords: myopia, progression, children, meta-analysis, spectacles
Myopia, as a global health and quality of life issue, has attracted considerable attention, not only with respect to the visual impairment and consequent impact of refractive errors on daily life, but also because of the morbidity associated with this ametropia. Myopia is a risk factor for cataract,1 glaucoma,2 retinal detachment,3 and myopic retinopathy.4 Juvenile-onset myopia typically begins in the school years5 and affected individuals usually confront a lifetime of dependence on optical corrections and the associated financial burden consequent upon this provision.
There is growing evidence that the prevalence of myopia is increasing in many parts of the world, in particular in Taiwan,6 Japan,7 Hong Kong,8 Singapore,9 and the United States.10 Higher prevalence rates have historically been reported in urban than in rural regions, and the prevalence of myopia varies with ethnicity even within the same geographic zone.11,12 Clearly, myopia has become an important public health issue, evidenced by the fact that it is one of the five ocular conditions listed as immediate priorities by the World Health Organization’s Global Initiative for the Elimination of Avoidable Blindness.13
While there is a substantial body of data concerning both myopia prevalence and progression rates in the literature, difficulties are encountered when attempting to compare results between individual studies. Differing recruitment methods, refraction techniques, data presentation and population ethnicity mixes, all potentially confound comparisons. The purpose of this paper is to present a meta-analysis of published rates of myopia progression with respect to ethnicity, age, and years of follow-up in children wearing single-vision spectacles. A set of inclusion/exclusion criteria was applied to the published data in order to ensure uniformity.
METHODS
Literature search strategy
Data on myopia progression rates were obtained from published randomized or quasi-randomized studies, including longitudinal population and school-based surveys and the control groups in myopia intervention trials.
Studies and Databases Searched
The National Library of Medicine PubMed literature database was initially searched on October 23, 2009 for articles with a published date from January, 1990 to October, 2009 using the term ‘myopi* progression’, with limits of ‘humans’, ‘randomized controlled trial’, ‘clinical trial’, ‘English abstract’ and ‘all child: 0–18 years’. Ninety-five articles were identified. A further search was undertaken on October 28, 2009 using the following search terms selected from the National Library of Medicine's controlled vocabulary: ‘Myopia AND Disease Progression NOT Keratomileusis, Laser In Situ NOT surgery AND humans AND Clinical Trial OR Randomized Controlled Trial OR Controlled Clinical Trial OR English Abstract OR Journal Article AND infant OR child OR adolescent’. This search yielded 135 articles. Of the 95 articles found by the initial search strategy, 43 were not found by the MeSH term search, including most notably the COMET report of 2003.14 Of the 135 articles found by the second search strategy, 83 were not found by the first. Fifty-two articles were common to both searches. The total number of individual articles found by the searches was 178.
The abstract of each of the 178 articles (138 English language; 40 non-English language) was assessed with reference to the exclusion criteria listed in Table 1, and was classified as (i) To be included, (ii) Further assessment required, or (iii) To be excluded. Although several exclusion criteria may have applied to a single article, only one was recorded. The full text of those classified as (ii) was reviewed with subsequent re-classification of the manuscript as (i) or (iii). Based on the criteria listed in Table 2, 118 of the 138 English language articles were rejected and none of the 40 non-English language articles was found suitable. Twenty articles 14–33 met the inclusion criteria. Fourteen reported on myopia intervention trials with single-vision, spectacle-wearing controls,14–16, 18–21, 25–28, 30–31, 33 and six were longitudinal surveys.17, 22–24, 29, 32 Of the twenty articles, one30 was a 12-month study documenting the subsequent progression of myopia following 2 years of treatment with atropine that had been reported in a previous paper.15 Control group data from these two papers were combined in the analysis to derive myopia progression of the control group over a three-year, rather than a two-year period. Although two papers reporting progression rates of COMET children were included,14, 20 only one20 was used for the age-specific analysis. With the exception of those from one article14 that reported only adjusted data, unadjusted data were used.
Table 1.
Exclusion criteria.
| (1) | Baseline and subsequent refractions not obtained under cycloplegia. |
| (2) | Refractive measurements not obtained using automated refraction. |
| (3) | Data not from urban populations |
| (4) | Control groups of myopia intervention studies that were not randomized or quasi-randomized. |
| (5) | Groups that did not wear single-vision spectacles for correction. |
| (6) | Data obtained from exclusive populations that might not be representative of the population as a whole. |
| (7) | Myopia as part of a syndrome, associated with ocular or systemic disease, surgery or injury. |
| (8) | Data on populations less than 30 individuals (excluding sub-set data). |
| (9) | Subjects older than 16 years at baseline. |
| (10) | Studies with a retrospective component or review papers. |
| (11) | Articles that were reports on part of another study used in the meta-analysis where the former added no new usable data. |
Table 2.
Number of English and non-English language articles rejected.
| Reason for exclusion | English | Non-English |
|---|---|---|
| No progression data or data unsuitable | 43 | 2 |
| Retrospective component or review paper | 21 | 8 |
| Syndrome, disease, surgery or injury | 15 | 13 |
| Refractive data without cycloplegia | 11 | - |
| Data on less than 30 individuals | 8 | - |
| Control groups using other than S.V. spectacles | 4 | - |
| Unsuitable or no control group | 4 | - |
| Refractive data by other than autorefraction | 3 | - |
| Only subjects older than 16 years at baseline | 3 | - |
| Exclusive or special populations | 3 | - |
| No new data on study already in the meta-analysis | 2 | - |
| Non-urban population | 1 | - |
| English abstract insufficient to determine suitability | – | 16 |
| No English abstract available | – | 1 |
| Total | 118 | 40 |
Statistical Methods
From each publication that met the exclusion/inclusion requirements, the mean myopia progression was ascertained and used for further analyses. Mean progression data were entered together with information relating to follow-up visits, and categorized based on demographic factors including age, gender and ethnicity. The sample size at the start of each study and at each follow-up visit was also recorded and used to weight the mean progression data in the statistical model. The mean myopia progression was estimated with its 95% confidence limits by age, years of follow-up, ethnicity and gender. Data were analyzed using weighted linear mixed models with random intercepts where each publication was factored as a random effect. Demographic variables and follow-up visits were fixed effects. Covariate terms such as age and follow-up time were tested for linear and quadratic effects. The estimated means from this model were plotted and the best fitting curve was derived. For those studies that reported only cumulative progression for greater than 1 year, the annual progression was estimated using the method described under Progression and Ethnicity in the results section. Studies were classified into two broad groupings: (1) Those with data on subjects of predominantly European descent (referred to subsequently as “European”), and (2) Those with data on predominantly East-Asian subjects (referred to subsequently as “Asian” for ease of discussion). The “European” studies originated in the United States, with the mean percentages of ethnicities reported as White, 59%; African- American, 15%; Hispanic, 16%; Asian, 5%; and other, 5%. In the Asian studies, the mean percentage of ethnicities other than Chinese or Japanese was 6%, which included children of Malay, Indian and other non-Chinese ethnicities.
RESULTS
Progression by Baseline Age
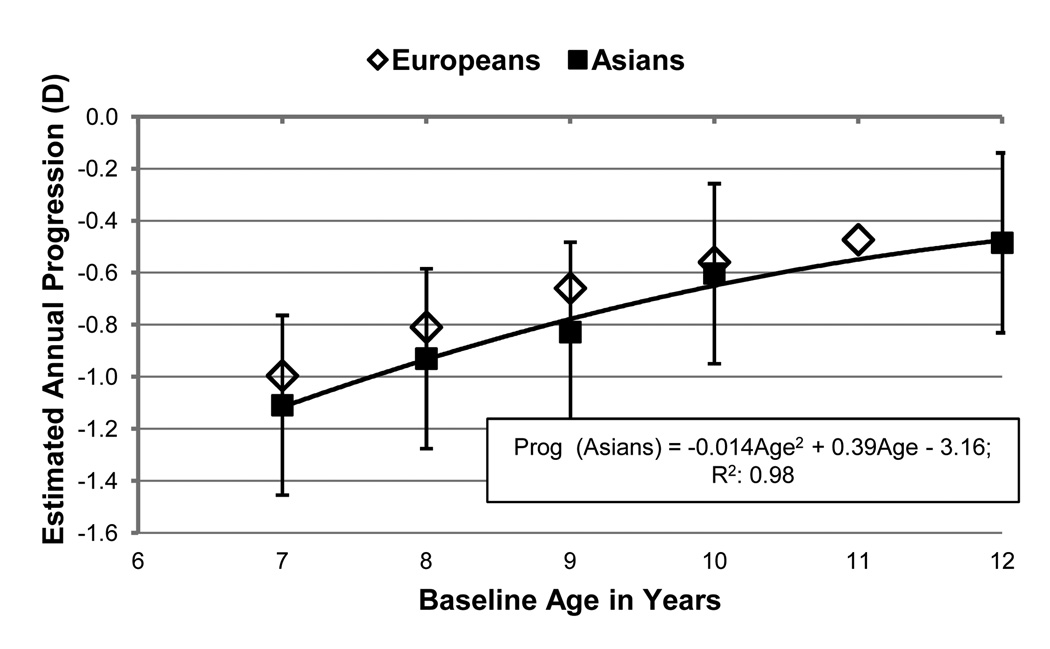
There was a total of 2194 subjects with progression rates reported for baseline ages between 7 and 12 years, with the largest data set (581 subjects) for 7 year-olds and the smallest (265 subjects) for 10 year-olds. Figure 1 shows the estimated annual progression at specific baseline ages (black, square markers) for Asian children, and the predicted relationship between annual progression in diopters and median age at baseline. Error bars denote 2× the standard error of the estimates. The relationship between progression and baseline age is negative, described curvilinearly, and modeled by a quadratic equation. Only one study 20 provided age-specific progression data by baseline age for Europeans, and these data points are shown as diamonds. In general, for both broad ethnic groupings, older children exhibited lower myopia progression, with the rate of progression decelerating with age.
Figure 1.
Black, square markers indicate estimated annual myopia progression for Asian children at specific baseline ages. Error bars denote 2× the standard error. Diamonds plot values for the one study that provided age-specific, progression data for European children.
Progression by Gender
The studies that reported progression by gender did so for all ages combined. Data were available on 408 females and 461 males with a mean age of 8.8 ± 0.6 years (range 8–10 years). The estimated annual myopia progression was slightly higher for females (−0.80 D, 95% CI: −0.51 to −1.10) compared to males (−0.71 D, 95% CI: −0.42 to −1.00) at a mean baseline age of 8.8 years (p < 0.01). Only one study 20 reported gender-specific data for Europeans. With these data removed, estimated annual progression for Asian females was −0.86 D (95% CI: −0.42 to −1.29), and for Asian males −075 D (95% CI: −0.32 to −1.19).
Progression and Ethnicity
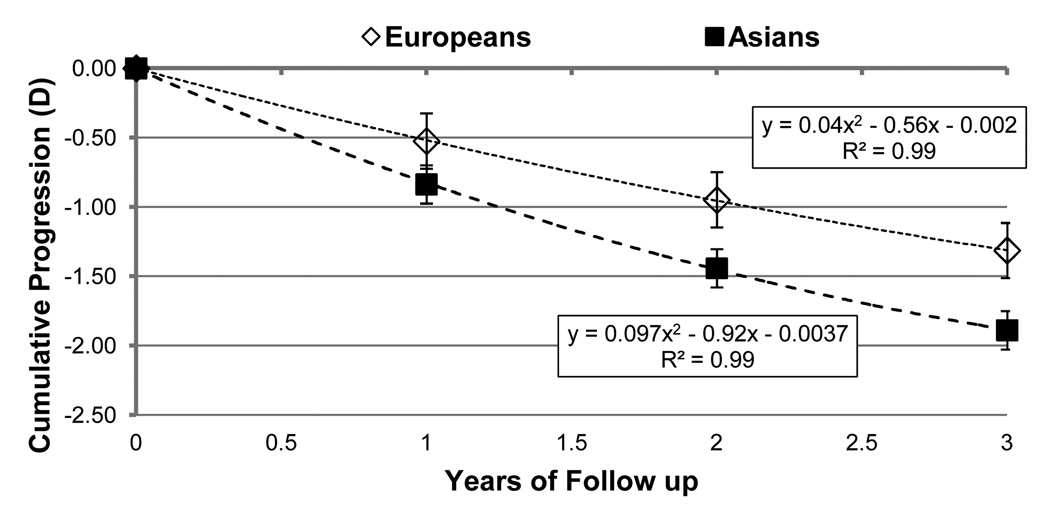
To compare differences between ethnicities, data for 1, 2 and 3 years of follow-up were used. The mean follow-up period for Europeans was 1.8 ± 0.8 years, and for Asians 1.5 ± 0.8 years. There was a significant difference in progression rates between the two broad ethnic groups (p=0.002), however it was observed that the differences between ethnicities interacted with the year of follow-up (p=0.001). This suggests that the rate of progression over the 3 years of follow-up was different for the two groups. The estimated cumulative progressions for 1, 2 and 3 years of follow-up for a child of 9.3 years at baseline are shown in Figure 2. The age of 9.3 years represents the mean age of the total sample. The data indicate that the cumulative difference between Asians and Europeans increases with years of follow up. The cumulative progression for Asians was higher than Europeans by −0.31 D, −0.49D and −0.58D at 1, 2 and 3 years of follow-up.
Figure 2.
Black, square markers indicate estimated cumulative myopia progression for Asian children at specific years of follow-up. Estimates for European children are indicated by diamonds. Error bars denote 2× the standard error.
The mean cumulative progression for subsequent years of follow-up was compared to that of the first year. Due to the age-related decline in progression rate, the mean cumulative progression over two years for Europeans was found to be 1.8× that of the first year, while the mean cumulative progression over three years was 2.5× that of the first. For Asians, the factors were slightly different, being 1.7× for cumulative 2-year progression and 2.3× for 3 years.
A limitation of our progression and ethnicity analysis is that 15% of the “European” subjects were African-Americans, and 5% of Asian ancestry.
DISCUSSION
Meta-analysis is a useful technique for summarizing the data from a number of studies. However, it is only valid if the data in each study are comparable.34–35 Determination of meaningful myopia progression rates is dependent on many factors, including refraction techniques that reliably detect small changes in refraction over time, and implementation of appropriate exclusion criteria. The studies included in this meta-analysis presented progression rates in a variety of ways. Most reported group means over a wide age range. However, some studies report age-specific rates, necessitating the use of the mid range of the age groups in our analysis.
Previous studies have shown that the mean rate of myopia progression in Asian children is greater than in age-matched European children.14, 24, 36–37 This is in agreement with our meta-analysis which determined the mean rate of myopia progression in Asian children to be approximately 0.20 D per year faster than their age-matched European counterparts. Figure 2 plots the estimated myopia progression for average 9.4 year-old European and Asian children during each of three years of follow-up, and demonstrates a consistent difference in progression rates between the two ethnicities with the Asian children exhibiting faster progression at each time point.
The prevalence of myopia in Asian populations has been found to be significantly higher than that in Europeans,38, 5, 39–41 and is increasing at a rapid rate. 42, 6 As yet, it has not been established whether the higher prevalence is a result of an inherent increased susceptibility to myopiagenic environmental factors, or merely that Asian children generally have a lifestyle that is more likely to lead to the development of myopia. What has been established is the positive association between myopia progression and the number of myopic parents for both Asian43 and European44 children. Whether this association is related to genetic commonalities or shared environments is again unclear. Perhaps of even greater concern is that the higher progression rate and earlier onset of myopia in Asian children45 is consistent with the observation that there has been a rise in the prevalence of high myopia46 (worse than −6 D) in Asians, with its associated risk of severe visual impairment. These findings raise the question of whether earlier screening for the onset of myopia should be implemented for Asian children, along with more regular examinations.
In contrast to the 0.87 dioptres per year annualized progression rate for Asians found in this meta-analysis, a large, longitudinal survey47 estimated the mean annual progression rate of myopic Chinese school children aged 5 to 13 years old at baseline to be 0.35 dioptres per year. However this study differed from those used in our meta-analysis as the sample was population-based rather than recruited from schools or brought into a study by parents, and the participants were from a semi-rural rather than an urban area of China
Summary
Approximately 11% of the articles identified by the literature search satisfied our inclusion criteria. Meta-analysis revealed higher myopia progression rates in urban Asians compared with urban Europeans. Younger children demonstrated greater annual rates of progression of myopia and females had slightly greater mean progression rates than males.
ACKNOWLEDGMENTS
The authors thank Kevin Frick, PhD and Judith Flanagan, PhD, for assistance with preparation of the manuscript.
Funded by grants from the Brien Holden Vision Institute and the Australian Government’s CRC scheme through the Vision CRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lim R, Mitchell P, Cumming RG. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:3021–3026. [PubMed] [Google Scholar]
- 2.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 3.Fan DS, Lam DS, Li KK. Retinal complications after cataract extraction in patients with high myopia. Ophthalmology. 1999;106:688–691. doi: 10.1016/S0161-6420(99)90152-5. [DOI] [PubMed] [Google Scholar]
- 4.Hochman MA, Seery CM, Zarbin MA. Pathophysiology and management of subretinal hemorrhage. Surv Ophthalmol. 1997;42:195–213. doi: 10.1016/s0039-6257(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 5.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, Hou PK. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999;76:275–281. doi: 10.1097/00006324-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hosaka A. Population studies—myopia experience in Japan. Acta Ophthalmol Suppl. 1988;185:37–40. doi: 10.1111/j.1755-3768.1988.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 8.Goh WS, Lam CS. Changes in refractive trends and optical components of Hong Kong Chinese aged 19–39 years. Ophthalmic Physiol Opt. 1994;14:378–382. [PubMed] [Google Scholar]
- 9.Seet B, Wong TY, Tan DT, Saw SM, Balakrishnan V, Lee LK, Lim AS. Myopia in Singapore: taking a public health approach. Br J Ophthalmol. 2001;85:521–526. doi: 10.1136/bjo.85.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. 1997;38:334–340. [PubMed] [Google Scholar]
- 12.Saw SM, Goh PP, Cheng A, Shankar A, Tan DT, Ellwein LB. Ethnicity-specific prevalences of refractive errors vary in Asian children in neighbouring Malaysia and Singapore. Br J Ophthalmol. 2006;90:1230–1235. doi: 10.1136/bjo.2006.093450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pararajasegaram R. VISION 2020-the right to sight: from strategies to action. Am J Ophthalmol. 1999;128:359–360. doi: 10.1016/s0002-9394(99)00251-2. [DOI] [PubMed] [Google Scholar]
- 14.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 15.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858. [PubMed] [Google Scholar]
- 17.Fan DS, Lam DS, Lam RF, Lau JT, Chong KS, Cheung EY, Lai RY, Chew SJ. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004;45:1071–1075. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- 18.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, Kimura S. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–2789. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- 20.Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, Everett D. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–987. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- 21.Khoo CY, Chong J, Rajan U. A 3-year study on the effect of RGP contact lenses on myopic children. Singapore Med J. 1999;40:230–237. [PubMed] [Google Scholar]
- 22.Saw SM, Chua WH, Gazzard G, Koh D, Tan DT, Stone RA. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005;89:1489–1494. doi: 10.1136/bjo.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000;77:549–554. doi: 10.1097/00006324-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, Katz J. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46:51–57. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 25.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79:233–236. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- 26.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122:1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 27.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J J AAPOS. 2008;12:332–339. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Tan NW, Saw SM, Lam DS, Cheng HM, Rajan U, Chew SJ. Temporal variations in myopia progression in Singaporean children within an academic year. Optom Vis Sci. 2000;77:465–472. doi: 10.1097/00006324-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Walline JJ, Jones LA, Sinnott L, Manny RE, Gaume A, Rah MJ, Chitkara M, Lyons S. A randomized trial of the effect of soft contact lenses on myopia progression in children. Invest Ophthalmol Vis Sci. 2008;49:4702–4706. doi: 10.1167/iovs.08-2067. [DOI] [PubMed] [Google Scholar]
- 32.Weizhong L, Zhikuan Y, Wen L, Xiang C, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthalmic Physiol Opt. 2008;28:57–61. doi: 10.1111/j.1475-1313.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Lan W, Ge J, Liu W, Chen X, Chen L, Yu M. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt. 2009;29:41–48. doi: 10.1111/j.1475-1313.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 34.Eysenck HJ. Meta-analysis and its problems. BMJ. 1994;309:789–792. doi: 10.1136/bmj.309.6957.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein DF. Flawed meta-analyses comparing psychotherapy with pharmacotherapy. Am J Psychiatry. 2000;157:1204–1211. doi: 10.1176/appi.ajp.157.8.1204. [DOI] [PubMed] [Google Scholar]
- 36.Lam CS, Edwards M, Millodot M, Goh WS. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci. 1999;76:370–380. doi: 10.1097/00006324-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: a randomized clinical trial. Part II. Final report by the patient care team. Am J Optom Physiol Opt. 1987;64:482–498. [PubMed] [Google Scholar]
- 38.Lam CS. The incidence of refractive errors among school children in Hong Kong and its relationship with the optical components. Clin Exper Optom. 1991;74:97–103. [Google Scholar]
- 39.He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 40.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101:405–407. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- 41.Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiol Rev. 1996;18:175–187. doi: 10.1093/oxfordjournals.epirev.a017924. [DOI] [PubMed] [Google Scholar]
- 42.Hosaka A. The growth of the eye and its components. Japanese studies. Acta Ophthalmol Suppl. 1988;185:65–68. doi: 10.1111/j.1755-3768.1988.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 43.Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Familial clustering and myopia progression in Singapore school children. Ophthalmic Epidemiol. 2001;8:227–236. doi: 10.1076/opep.8.4.227.1609. [DOI] [PubMed] [Google Scholar]
- 44.Kurtz D, Hyman L, Gwiazda JE, Manny R, Dong LM, Wang Y, Scheiman M. Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2007;48:562–570. doi: 10.1167/iovs.06-0408. [DOI] [PubMed] [Google Scholar]
- 45.Lin LL, Chen CJ, Hung PT, Ko LS. Nation-wide survey of myopia among schoolchildren in Taiwan, 1986. Acta Ophthalmol Suppl. 1988;185:29–33. doi: 10.1111/j.1755-3768.1988.tb02657.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- 47.Zhao J, Mao J, Luo R, Li F, Munoz SR, Ellwein LB. The progression of refractive error in school-age children: Shunyi district, China. Am J Ophthalmol. 2002;134:735–743. doi: 10.1016/s0002-9394(02)01689-6. [DOI] [PubMed] [Google Scholar]