Abstract
Abstract
More than 30 muscles drive the hand to perform a multitude of essential dextrous tasks. Here we consider new views on the evolution of hand structure and on peripheral and central constraints for independent control of the digits of the hand. The human hand is widely assumed to have evolved from hands like those of African apes, yet recent studies have shown that our hands and those of the earliest hominids are very similar and unlike those of living apes. Understanding the limits of hand function may come from investigation of our last common ancestor with the great apes, rather than the great apes themselves. In the periphery, movement across the full range of joint space can be limited by mechanical linkages among the extrinsic muscles. Further, peripheral limits occur when the hand adopts some positions in which the contraction of muscles fails to move the joints on which they usually act; there is muscle ‘disengagement’ and functional paralysis for some actions. Surprisingly, the central nervous system drives the hand seamlessly through this landscape of mechanical limits. Central constraints on control of the individual digits include the spillover of neural drive to neighbouring muscles and their ‘compartments’, and the inability to make maximal muscle forces when multiple digits contract strongly which produces a force deficit. The pattern of these latter constraints correlates with amounts of daily use of each digit and favours enslaved extension to lift fingers from an object but selective flexion of fingers to contact it.
Hiske van Duinen (left) obtained her PhD in Groningen (the Netherlands) and studied the interaction between physical fatigue and cognitive performance, using hand tasks. She worked on the control of the human hand and its individual digits during her postdoctoral studies in Sydney (Australia). She now focuses on how the brain integrates the various signals that play a role in proprioception, again with a particular focus on the hand, during a postdoc in Stockholm (Sweden). Furthermore, she is interested in neurophysiological changes that occur to the subject's own hand when they have the illusion that they ‘own’ a rubber hand. Simon Gandevia (right) obtained his PhD in 1978 and medical degree in 1980 in Sydney. Early work focused on proprioceptive mechanisms involved in force and joint movement perception. He has continued to work on neural mechanisms controlling hand muscles but established research into neural control of human respiratory muscles in health and disease. Additional studies probe changes at spinal and central levels that accompany human muscle fatigue. He helped to establish the Prince of Wales Medical Research Institute, now Neuroscience Research Australia, in Sydney in 1993 and has long had editorial roles with several physiology journals.
 |
Background
Superficially, the structure of the human arm resembles that of the living African apes, especially the chimpanzee although the size and mobility of its thumb have long been recognized (Napier, 1972). Since the ancient Greeks, it has been tempting to regard the hand as a pinnacle of efficient design, as an indicator of a creator's intervention. Sir Charles Bell (1833), who wrote one of the earliest accounts of the upper limb in the animal kingdom, recognised the human hand as ‘this more perfect instrument’, but one which ‘corresponds with man's superior mental capacities’ such that it should be ‘capable of executing whatever man's ingenuity suggests’. He realised that the hand was not a mere appendage grafted onto the limb but that it required the brain to put it into action. He did not speculate how this might occur but deduced, as did Galen, that man had ‘hands given to him because he was the wisest creature’ (pp. 207–208). There has been frequent discovery of new fossil hominids (e.g. Brown et al. 2004; Kivell et al. 2011). Despite the problems of dealing with ‘scrappy remains’ (Mayr, 2001), major new insight has come from the near-complete skeleton ofArdipithecus ramidus(Lovejoy et al. 2009), an early hominid (from 4.4 million years ago). This early primate's hand clearly resembles that of humans and not that of the African apes. What previously had been considered unique human specialisations for the hand actually arose much earlier in the hominid lineage thanHomo sapiens, whose origin is put at 200,000 years ago.
The human thumb confers great scope for dexterity and its long length relative to the index gives it the highest ‘opposability index’ among primates (Napier, 1972), while its rotated first metacarpal and unique carpometacarpal joint enhance its range of movement for grasping and manipulation (Wood-Jones, 1949). Furthermore, the thumb is moved by a muscle in the forearm, flexor pollicis longus (FPL), which provides the only way to flex its distal joint and is rudimentary in apes. This evolutionary development of the long flexor muscle, often thought to be a specialisation in humans, is now known to be present inArdipithecus ramidus, i.e. much earlier (by ∼2 million years) than previously appreciated (Lovejoy et al. 2009). The presence of FPL in humans is associated with a high capacity to sense thumb voluntary forces at remarkably low levels compared even to intrinsic hand muscles (muscles with their origin and insertion in the hand; Kilbreath & Gandevia, 1993) and to detect length changes at its distal joint (Refshauge et al. 1998). In evolutionary terms, some elements of hand structure have developed surprisingly early and the hominid thumb is well adapted for a range of grasping and manipulation tasks.
What limits hand function?
One way to approach human hand function is to consider how it is constrained by the various structures that generate its myriad movements. Here one can proceed from the hand muscles themselves right back to the cortical structures that drive their contraction (see Fig. 1). There are 31 muscles of different and often complex architecture involved in hand movement with 19 residing in the hand (the intrinsic hand muscles). The hand has 19 articulations, 18 tendons crossing the wrist, and at least 25 degrees of freedom. Not unexpectedly, the problem of controlling the hand seems complex at a physiological and computational level, if one considers each component separately. Four levels of limits to individual control of the digits are shown diagrammatically in Fig. 1.
Figure 1. Summary of possible links in schematic form.
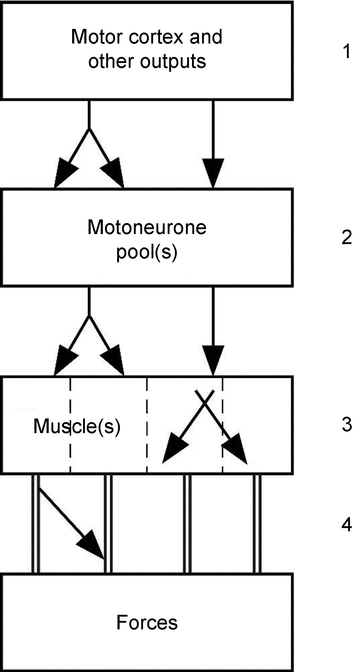
The distribution of output to individual muscles is intermingled at the level of the primary motor cortex and probably also at other supraspinal sites (1). Links can also occur at the level of the spinal cord, with some motoneurone pools receiving divergent inputs from corticofugal axons (2). Three major forearm muscles consist of multiple muscle bellies with tendons to each finger so that the muscles have four ‘compartments’ (3); there can be transfer of force between and within the different compartments of one muscle, but also between muscles (i.e. inter- and intramuscular force transfer). Furthermore, there are connections between the tendons for muscles on both the dorsal and ventral side of the hand (4).
In the next section we mention some of the more peripheral limitations to the control of the individual digits and then discuss some studies that expose some functional central limitations to performance. First, we will illustrate some constraints which must be dealt with continuously by the central nervous system (CNS). By positioning the hand voluntarily with all fingers extended except the middle one which is flexed (Fig. 2A), it is not possible to further flex the distal joint of the middle finger, nor to extend it if the middle phalanx is restrained. Here all the flexor and extensor actions on the distal joint are mechanically impossible; its muscles are ‘disengaged’. The full picture of disengaged paralysis involves the tendons of flexor digitorum profundus and the digital extensor apparatus and is not restricted to one simple anomaly. This posture has been used to study proprioceptive mechanisms (e.g. Gandevia & McCloskey, 1976; Smith et al. 2009), but it is easy to overlook the paralysis that ensues whenever it is reached. Similarly, with all the fingers flexed, extension at the distal joints remains impossible (Fig. 2BandC). The CNS can seamlessly drive the hand through postures in which its degrees of freedom for movement are unexpectedly restricted by the simple mechanics of the motors which move its digits.
Figure 2. Photographs of the hand to illustrate limits which occur in particular postures.
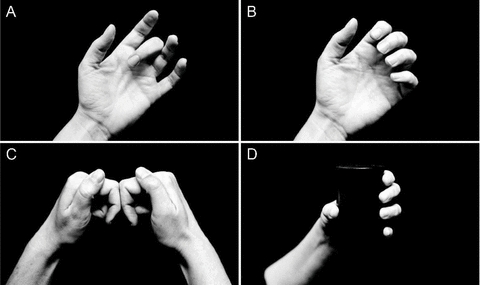
A, when the hand is comfortably positioned with the fingers extended except that (say) the middle one is flexed, it is impossible to flex or extend its distal interphalangeal joint. It is functionally paralysed.B, when the other fingers are partially flexed, flexion but not extension is possible at the distal interphalangeal joint.C, the two hands are positioned with the middle phalanx of the index finger touching. In this position, the extensors of the index are functionally paralysed.D, grasping a test cylinder. This object can be instrumented to measure the forces under the pads of the digits. Note that the thumb must oppose the forces generated by the four fingers.
Tendon, inter-muscle and intramuscular connections
Voluntary movement of the fingers and thumb can reach a vast array of positions across the hand's joint space. This space can be increased by applying additional force near the end of an angular range, as occurs when a large object is manipulated. The presence of direct connections among the tendons and muscles which work on the hand would restrict the hand's movement repertoire. These range from links within and between the muscle fascicles and fibres to large tendons connecting to the tendon of an adjacent muscle. The best example of connections between the digital flexors is the anomalous tendon that courses distally from FPL to join the index tendon of flexor digitorum profundus (FDP) proximal to the wrist (Linburg & Comstock, 1979). Here voluntary flexion of the tip of the thumb flexes the distal joint of the index finger. This is observed clinically in about one in five hands. The anomaly appears less frequently in musicians (Karalezli et al. 2006), but rarely requires surgical excision. Additional interconnections between the long flexor tendons occur in the distal forearm (Kilbreath & Gandevia, 1994), in the carpal tunnel (Leijnse et al. 1997), and even in the palm. By contrast, in the macaque monkey there is no separate long thumb flexor. The muscle fascicles of FDP insert onto an extensive distal aponeurosis and this distributes forces generated proximally broadly into the digits (Schieber et al. 2001).
For the extensor muscles, overt intertendinous links (juncturae tendinum) and intertendinous fascia between the extensor tendons on the dorsum of the hand have been more studied anatomically, perhaps because of the restriction they place on selective extension movements (von Schroeder & Botte, 1993). The juncturae tendinum interconnect the four digital tendons of extensor digitorum. There are usually three, with the largest interconnecting the ring and little finger, and with none for the extensor indicis (von Schroeder et al. 1990; Celik et al. 2008). They are also likely to limit full independent flexion at the metacarpophalangeal joints (S. Gandevia, unpublished observations).
At more proximal levels, force and displacement interactions can occur within muscles, an issue of particular concern given that flexor digitorum profundus (FDP), flexor digitorum superficialis (FDS) and extensor digitorum (ED) are muscles with tendons to each of the fingers. These interactions may occur, for example, because a motor unit's territory is such that force is ‘injected’ into more than one distal tendon. This sort of ‘lateral’ force transmission exerted by individual muscle fibres and motor units can be significant in some animal preparations (e.g. Street, 1983; see also Young et al. 2000). The topic of lateral force transmission is controversial but it appears that such an effect can even result in inter-muscle force transfer (e.g. for review Patel & Lieber, 1997; Huijing, 1999, 2009). This process has been most studied in the lower limb for gastrocnemius and soleus in animals (for details see Maas & Sandercock, 2008) but some evidence exists for it in humans (e.g. Bojsen-Moller et al. 2010). However, the unresolved issues are the magnitude of these effects and the conditions under which they are functionally significant.
In the human hand, some data suggest that within the functional range of forces and joint spaces the limits imposed by inter-muscle interactions might be relatively modest. First, during a steady weak grasp in flexion an active motor unit pulls largely on the intended digit with little lateral distribution of force to adjacent digits (Fig. 3) (Kilbreath et al. 2002; Yu et al. 2007), and this is likely to apply to extensor digitorum (Keen & Fuglevand, 2004b). Second, again during a steady weak contraction, intraneural or intramuscular electrical stimulation produces more focal pull on one digit than does a voluntary contraction (Keen & Fuglevand, 2003; Yu et al. 2007). These data are far from conclusive and the issue needs examination under a wider range of conditions because there are circumstances (at least for the deep finger flexors) where inadvertent force or movement is generated at fingers which are not instructed to contract and where there is minimal electromyographic activity (e.g. Kilbreath & Gandevia, 1994; see also Aoki et al. 2003). If hand muscles do not always behave as simple in-line motors, then the spread of their mechanical effects must depend on the links of force–length curve, viscoelastic properties, and the changes induced by muscle contractions. Such detailed biomechanical information is largely lacking.
Figure 3. Data from a single subject to show the forces produced by single motor units in the flexor digitorum profundus on the finger pads during a weak static grasp of an instrumented cylinder.
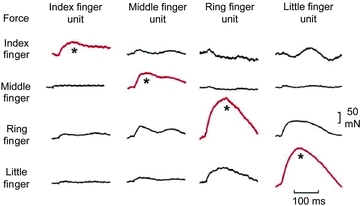
Each column represents data from a single unit. The spike-triggered average change in force under each finger produced by that unit is shown. The change in force under the test finger (red traces) was consistently greater than under the non-test fingers. However, both the ring-finger and little-finger motor units also produced relatively large forces under the adjacent finger (i.e. the little and ring fingers, respectively). (Modified from Kilbreath et al. 2002.)
Central limits and linkages
In terms of central control of hand movement, the primary motor cortex has long been recognised to have a crucial role (for review see Lemon, 2008; Scott, 2008; Petersen et al. 2010). Given the peripheral interconnections described above, it is naive to believe that there might be a formal muscle ‘map’ in the cortex from which labelled-lines descend to individual motoneurone pools. However, this emphasis, which probably derived from early cortical stimulation studies in non-human primates and humans, has been prevalent (Penfield & Boldrey, 1937; Penfield & Rasmussen, 1952; cf. Leyton & Sherrington, 1917; Baker, 2011). It is being replaced by views which recognise first the transformations that must take place between a command for movement reaching motor cortical areas and generating corticofugal projections which ultimately produce force, and second the cortical malleability required to learn and reproduce everyday hand movements (e.g. Davidson et al. 2007; Graziano & Aflalo, 2007; for review see Schieber, 2011). The corticospinal outputs that can drive a particular arm muscle, even an intrinsic hand muscle, are located within overlapping regions of the motor cortex. This view is reinforced by several lines of evidence derived from studies in non-human primates including intracortical electrical stimulation (e.g. Andersen et al. 1975), neuroanatomical tracing (e.g. Rathelot & Strick, 2006), cortical cell recording (e.g. Schieber, 2002), as well as neuroimaging and lesion studies in humans (e.g. Schieber, 1999; Beisteiner et al. 2001). Motor cortical cells (many of which have corticospinal projections) discharge with movement of more than one digit and those associated with a particular movement are not tightly clustered. The corticospinal projection provides a further complication because the axons branch to supply more than one motor nucleus (Shinoda et al. 1981). These overlapping areas in the motor cortex may be optimal for daily usage of the hand, but also constrain the ability to control the digits independently.
The problem of producing the wide repertoire of hand actions has been seemingly simplified by some restrictions on the output to the various hand muscles. This involves the use of synergies in which the number of patterns of muscle activation is reduced because everyday hand activities involve many postures in which the joint angles are correlated. For example, when objects are grasped, the changes in positions of many interphalangeal joints are correlated (e.g. Santello et al. 1998, 2002). Thakur and colleagues (2008) showed that fewer than 10 synergies could underlie many motions within hand joint space for the haptic exploration of a wide range of everyday objects. Furthermore, when force at the digit tip needs to be varied, the relative activity among the contracting muscles can be scaled linearly based on the pattern of muscle activity (i.e. synergy) which produces the largest force (Valero-Cuevas, 2000). However, this view of synergies may be simplistic given, for example, that the CNS may continuously modify output to muscles dependent on their mechanical effectiveness (Hudson et al. 2009).
Superimposed on this type of control can be other sensorimotor ‘rules’ which simplify the ongoing problem of controlling the hand. Here, a major principle is the use of a high safety margin in the grasping force when lifting an object which can have variable mass and frictional properties (Johansson & Westling, 1984; Westling & Johansson, 1984).
In attempts to study the independence of digit control, one approach has revealed short-term synchronisation between the firing of motor units acting on different digits. This short-term synchronisation is the synchronous firing of two motor units, which occurs more often than expected by chance (based on their firing frequencies), and this is likely to signify a common descending drive. This phenomenon is seen in the long flexors and the long extensors of the human hand, such as the flexor digitorum superficialis (FDS) and profundus (FDP), and extensor digitorum (ED) (e.g. Keen & Fuglevand, 2004a; Reilly & Hammond, 2004; Winges & Santello, 2004; McIsaac & Fuglevand, 2007). Furthermore, it occurs between the long flexor of the thumb (flexor pollicis longus, FPL) and especially the index finger compartment of the FDP (Winges & Santello, 2004; Hockensmith et al. 2005). This common drive is usually strongest for neighbouring digits, and diminishes between digits further apart. There is stronger synchronisation on the ulnar than the radial side (e.g. McIsaac & Fuglevand, 2007, 2008). As there were only limited pairs of motor units in extensor compartments that were further than one compartment apart, it is difficult to compare the amount of common drive between flexors and extensors (see Fig. 4 in McIsaac & Fuglevand, 2007). However, also for the extensor the linkage seems to be lower between the index and middle finger, and higher between the middle and ring finger. The lower value for the index–middle combination could be due to the presence of an extra index finger extensor (extensor indicis). It may also reflect more discrete control on the radial side of the hand, as the index and thumb are more often used individually (Ingram et al. 2008). This is likely to be functionally useful. An indirect argument in favour of functionality is that motor units of the index finger compartment of FDP and the long flexor of the thumb show short-term synchronisation (Winges & Santello, 2004; Hockensmith et al. 2005), while there is no such synchronisation between the intrinsic hand muscles of the index finger and thumb (McIsaac & Fuglevand, 2008; for review see Fuglevand, 2011).
Figure 4. Comparison of motor unit recruitment of the long multi-tendoned flexors and extensor of the human hand.
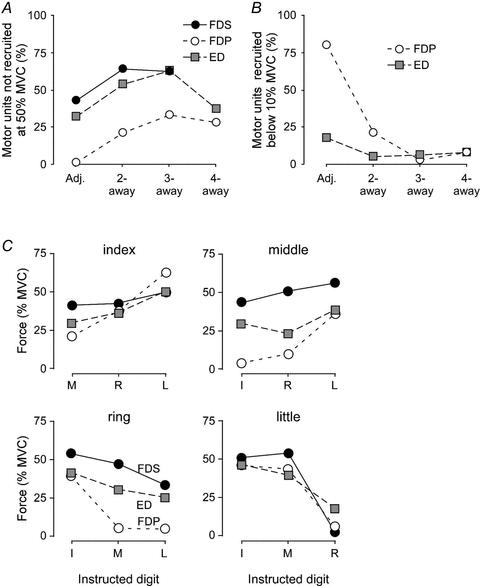
A, the percentage of motor units that were not recruited at 50% of the maximal force (MVC) of the contracting digit for flexor digitorum profundus (FDP, open circles), flexor digitorum superficialis (FDS, filled circles), and extensor digitorum (ED, grey squares), depending on the distance between the test finger and the contracting digit.B, the percentage of motor units that were recruited below 10% MVC of the contracting digit for FDP (open circles) and ED (grey squares; unfortunately, these data are missing for FDS).C, the mean motor unit recruitment thresholds during voluntary contractions of non-test fingers are plotted for all index, middle, ring and little finger units of the FDS (filled circles), FDP (open circles) and ED (grey squares). (For original sources for panelCand further details see van Duinen et al. 2009.)
The common drive, seen as short-term synchronisation, is present when two (or more) digits contract together. However, there is also a spillover of neural drive when one digit is supposed to move by itself. This spillover has been shown in experiments studying the ‘recruitment thresholds’ (defined below) of motor units acting on other digits during single digit contractions (Kilbreath & Gandevia, 1994; Butler et al. 2005; van Duinen et al. 2009). In these experiments, motor units were recorded from one (test) compartment of the respective muscles, while subjects were asked to contract the compartment of the other digits up to 50% of their maximal force. When the subjects contracted these other digits (one by one), motor units of the test compartment were often recruited. The amount of force produced by the other digits at the time of recruitment of the motor unit of the test compartment is termed the recruitment threshold. The general finding for all three muscles was that, the closer the contracting compartment to the test finger, the more motor units were recruited (Fig. 4A). This panel shows the number of motor units that were not recruited, so it shows a higher percentage with digits further away. Furthermore, the closer the other digit, the lower the force at which the motor units were recruited (i.e. a lower recruitment threshold; Fig. 4B). This panel shows the number of motor units that were recruited below 10% MVC. This percentage was much higher for adjacent digits, especially for FDP. Recruitment thresholds were lower (i.e. neural spillover was more evident) on the ulnar than radial side (Fig. 4C). Besides the general pattern for the three muscles, there were also differences between the muscles: the spillover was highest in FDP and lowest in FDS, especially for the middle and ring fingers. Thus, the control of the compartments of FDS was more ‘focal’. The recruitment thresholds for ED are in between these for the two flexor muscles (Fig. 4C), but in terms of number of motor units that are (not) recruited, the behaviour of ED motor units is more similar to FDS. Finally, this spillover was remarkably similar between FPL and the index part of FDP compared with that between less adjacent digital components of FDP (Kilbreath & Gandevia, 1994).
The long extensors for the thumb (extensor pollicis longus and brevis) are separate from ED. Nevertheless, when the thumb extends, motor units of ED are recruited. Hardly any motor units acting on the middle finger are recruited (<10%), but almost 40% of the units acting on index and ring fingers are recruited, and more than 60% of the units acting on the little finger. When the thumb flexors are activated, there is also spillover of neural drive between the FPL and the compartments of FDP (see Fig. 2 in Kilbreath & Gandevia, 1993, and Figs 3 and 5 in Kilbreath & Gandevia, 1994). Again, one has to ask whether this spillover is functional. Is the frequent recruitment of motor units acting on the little finger when we extend the thumb part of a fixed pattern of muscle activation, perhaps to balance forces around the wrist?
Figure 5. Force enslavement (red) and force deficits (blue) in flexion (left) and extension (right).
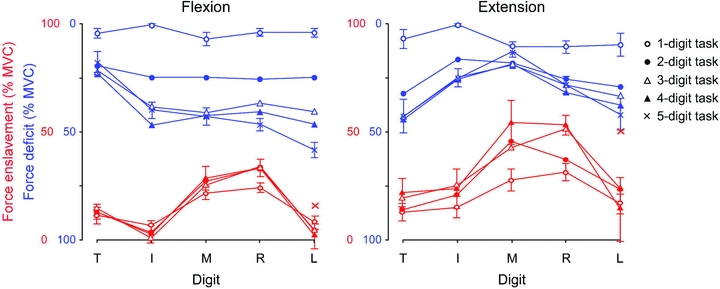
These two panels show both enslavement forces (in red) and force deficits (in blue) for all digits (T thumb; I index; M middle; R ring; L little finger) in single- and multi-digit tasks. A single-digit task is a task in which a subject is instructed to contract only one digit; however, the other digits show involuntarily produced force. In multi-digit tasks, two, three, four or five digits are instructed to move. In the five-digit tasks there is no enslavement. The enslaved force per digit is the average force produced by that digit when it was not a task digit. The ‘x’ for the little finger is the enslaved force for this finger when force produced in the opposite direction is not taken into account (so not part of a five-digit task). The force deficits (blue) are show on a scale from 100 down to 0% MVC. In this way low force deficits can be seen as high forces (higher lines in the figure). The force deficit in the different tasks for the separate digits is 100% MVC minus the average force of each digit when it is instructed to produce 100% MVC. Note the relatively low force deficits for the thumb in flexion and the relatively high force deficits of the thumb in extension. (Modified from Yu et al. 2010.)
The spillover in neural drive to neighbouring digits also results in force production by these digits. Several studies have shown such spillover of force, which has been termed force enslavement (e.g. Zatsiorsky et al. 1998, 2000; Olafsdottir et al. 2005; Yu et al. 2010). Most of these studies looked at force enslavement during maximal voluntary contractions (MVC), while the previous studies on recruitment thresholds often did not exceed 50% MVC. However, there is also enslavement at lower force levels (e.g. Slobounov et al. 2002; Reilly & Hammond, 2004). Until recently, most studies looked at either flexion or extension, but when we compare the amount of enslavement in flexion and extension, the enslaved forces in extension are higher than in flexion, when recorded in the same apparatus (see the red lines in Fig. 5) (Oliveira et al. 2008; Yu et al. 2010). We hypothesise that the level of enslavement might depend on the amount of individual daily usage (for data on usage see Ingram et al. 2008). This might indeed be the case (Fig. 6).
Figure 6. The relation between individually daily use and force enslavement.

This represents the percentage of individual daily use of the five digits (data from Ingram et al. 2008). The thumb is the digit used most frequently on its own, followed by the index finger. Daily use is plotted against the amount of enslavement in flexion (filled diamonds) and extension (open diamonds) of the individual digits in the single-digit tasks (data from Yu et al. 2010).
These experiments on both motor unit recruitment thresholds (the spillover of neural drive from one digit to another) and enslavement (the spillover of force from one digit to another) show some central limitations in driving muscles of different digits independently. However, the studies of enslavement force also showed another central limitation: the inability to drive multiple muscles maximally at the same time. That is, when multiple digits had to contract, the subjects were not able to reach their maximal force, thus showing a force ‘deficit’. The force deficits of the fingers were lower in extension than flexion (i.e. opposite to the enslavement values), but this was the other way around for the thumb (see the blue lines in Fig. 5). In flexion, the thumb usually opposes the forces of the fingers. If a finger is added to a contraction, this will increase the total sum of the force of the active fingers (even though the force deficits of the individual fingers might increase). The thumb will have to oppose this higher force and shows no increase in its force deficit. Indeed, Olafsdottir and colleagues (2005) showed that the thumb produced a higher maximal force and lower force deficits when it was opposing the fingers compared to when it was parallel to the fingers. In contrast, in extension, when all fingers extend, the extension of the thumb is not in the opposite direction and in this situation, the thumb shows even a higher force deficit than most fingers. When looking at the force deficit, why is it not possible to drive muscles maximally when the drive has to be divided between multiple muscles? These deficits may be comparable to those when trying to produce force with two hands or arms, a phenomenon known as the bilateral deficit (Gandevia, 2001). Neuroimaging data suggest that bilateral deficit in force and muscle activity results from a decline in input to (and thus output from) the primary motor cortex, as activity in the precentral gyrus was lower during bilateral than during unilateral contractions. The activity in the (ventral) premotor area was also lower during bi- than unimanual contractions, which might explain the lower input to the primary motor cortex. However, this also suggests that the main cause of the bilateral deficit is ‘upstream’ of the premotor cortex (Post et al. 2007). The premotor cortex may have an important role in the recovery following stroke (for review Ward, 2011). The supplementary motor area, which also plays a role in bimanual coordination, did not show this difference in the level of activity between bimanual and unimanual contractions. We suggest that part of the deficit might thus be caused by the output from this area, but this needs to be tested. There could be a limit in the amount of drive that can be sent to two limbs or to multiple compartments at the same time. There is evidence that the decrease in maximal voluntary force during bilateral contractions (compared to unilateral ones) is accompanied by a drop in voluntary drive to the muscles (Van Dieen et al. 2003) but other studies did not find this (Jakobi & Cafarelli, 1998), or found only a marginal change (Herbert & Gandevia, 1996).
Voluntary drive (or voluntary activation) can be measured using twitch interpolation in which the superimposed twitch produced by a motor nerve stimulus during a maximal voluntary contraction is compared during the maximal twitch in rest (Merton, 1954), or the superimposed twitch is evoked by cortical stimulation (Todd et al. 2003). Little was known about the maximal voluntary activation of extrinsic hand muscles until a recent study using the new method of cortical stimulation (Todd et al. 2003). This revealed that subjects were also able to activate the individual compartments of the FDP to ∼92%, even when the test finger was in a biomechanically disadvantaged position (for details see van Duinen et al. 2010). The intrinsic hand muscles can also be activated almost maximally (e.g. Merton, 1954; Herbert & Gandevia, 1996), but they are special in that they can be ‘controlled’ at very low levels, even below the recruitment threshold for the earliest recruited units (Gandevia & Rothwell, 1987).
Conclusions
The human hand is not a ‘perfect instrument’, but one which can be limited in how it operates, particularly when focusing on the precise individual control of its components. This brief survey of hand function has tried to cover some well known, some lesser known and some newly appreciated constraints on its performance. These constraints begin with high-level signals which select and drive motor cortical outputs and end up with force transmission laterally within and between muscle compartments. Recognition of the hand's versatility of function despite some of the biomechanical constraints at the level of the muscle–tendon unit exposes the remarkable capacity of the CNS to operate such a complex organ. Proprioceptive input is critical for this capacity and its importance is often under-appreciated. For the most part, when undertaking ‘usual’ activities, with modest forces in the middle of joint angular ranges, we propose that the peripheral constraints identified here are only minor impediments to function. However, this is not the case when the interconnections between muscles are loaded or undergoing very large length changes. As yet we do not have quantitative biomechanical descriptions of these links and the local factors which affect them. Finally, it is salutary to know that some specialisations of the human hand, such as a long robust thumb and opposable digits, have developed earlier during evolution than formerly thought. Whatever the constraints to the hand's mechanical function, we can still marvel at the capacity of the central controller which drives it to perform myriad tasks.
Acknowledgments
S.G. and his work on the hand are supported by the National Health and Medical Research Council.
Glossary
Abbreviations
- CNS
central nervous system
- ED
extensor digitorum
- FDP
flexor digitorum profundus
- FDS
flexor digitorum superficialis
- FPL
flexor pollicis longus
References
- Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proc R Soc Lond B Biol Sci. 1975;188:31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Aoki T, Francis PR, Kinoshita H. Differences in the abilities of individual fingers during the performance of fast, repetitive tapping movements. Exp Brain Res. 2003;152:270–280. doi: 10.1007/s00221-003-1552-z. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B, Moser E, Deecke L. Finger somatotopy in human motor cortex. Neuroimage. 2001;13:1016–1026. doi: 10.1006/nimg.2000.0737. [DOI] [PubMed] [Google Scholar]
- Bell C. The Hand, Its Mechanism and Vital Endowments as Evincing Design. London: W. Pickering; 1833. [Google Scholar]
- Bojsen-Moller J, Schwartz S, Kalliokoski KK, Finni T, Magnusson SP. Intermuscular force transmission between human plantarflexor muscles in vivo. J Appl Physiol. 2010;109:1608–1618. doi: 10.1152/japplphysiol.01381.2009. [DOI] [PubMed] [Google Scholar]
- Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Saptomo EW, Due RA. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- Butler TJ, Kilbreath SL, Gorman RB, Gandevia SC. Selective recruitment of single motor units in human flexor digitorum superficialis muscle during flexion of individual fingers. J Physiol. 2005;567:301–309. doi: 10.1113/jphysiol.2005.089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik S, Bilge O, Pinar Y, Govsa F. The anatomical variations of the extensor tendons to the dorsum of the hand. Clin Anat. 2008;21:652–659. doi: 10.1002/ca.20710. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Chan V, O'Dell R, Schieber MH. Rapid changes in throughput from single motor cortex neurons to muscle activity. Science. 2007;318:1934–1937. doi: 10.1126/science.1149774. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ. Mechanical properties and neural control of human hand motor units. J Physiol. 2011;589:5595–5602. doi: 10.1113/jphysiol.2011.215236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:239–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Muscle activation in unilateral and bilateral efforts assessed by motor nerve and cortical stimulation. J Appl Physiol. 1996;80:1351–1356. doi: 10.1152/jappl.1996.80.4.1351. [DOI] [PubMed] [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci. 2005;25:4560–4564. doi: 10.1523/JNEUROSCI.0046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Taylor JL, Gandevia SC, Butler JE. Coupling between mechanical and neural behaviour in the human first dorsal interosseous muscle. J Physiol. 2009;587:917–925. doi: 10.1113/jphysiol.2008.165043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. J Biomech. 2009;42:9–21. doi: 10.1016/j.jbiomech.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Ingram JN, Kording KP, Howard IS, Wolpert DM. The statistics of natural hand movements. Exp Brain Res. 2008;188:223–236. doi: 10.1007/s00221-008-1355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobi JM, Cafarelli E. Neuromuscular drive and force production are not altered during bilateral contractions. J Appl Physiol. 1998;84:200–206. doi: 10.1152/jappl.1998.84.1.200. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Karalezli N, Karakose S, Haykir R, Yagisan N, Kacira B, Tuncay I. Linburg-Comstock anomaly in musicians. J Plast Reconstr Aesthet Surg. 2006;59:768–771. doi: 10.1016/j.bjps.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Role of intertendinous connections in distribution of force in the human extensor digitorum muscle. Muscle Nerve. 2003;28:614–622. doi: 10.1002/mus.10481. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol. 2004a;91:57–62. doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Distribution of motor unit force in human extensor digitorum assessed by spike-triggered averaging and intraneural microstimulation. J Neurophysiol. 2004b;91:2515–2523. doi: 10.1152/jn.01178.2003. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Neural and biomechanical specializations of human thumb muscles revealed by matching weights and grasping objects. J Physiol. 1993;472:537–556. doi: 10.1113/jphysiol.1993.sp019961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gorman RB, Raymond J, Gandevia SC. Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J Physiol. 2002;543:289–296. doi: 10.1113/jphysiol.2002.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell TL, Kibii JM, Churchill SE, Schmid P, Berger LR. Australopithecus sedibahand demonstrates mosaic evolution of locomotor and manipulative abilities. Science. 2011;333:1411–1417. doi: 10.1126/science.1202625. [DOI] [PubMed] [Google Scholar]
- Leijnse JN, Walbeehm ET, Sonneveld GJ, Hovius SE, Kauer JM. Connections between the tendons of the musculus flexor digitorum profundus involving the synovial sheaths in the carpal tunnel. Acta Anat. 1997;160:112–122. doi: 10.1159/000148003. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan and gorilla. Quart J Exp Physiol. 1917;11:135–222. [Google Scholar]
- Linburg RM, Comstock BE. Anomalous tendon slips from the flexor pollicis longus to the flexor digitorum profundus. J Hand Surg Am. 1979;4:79–83. doi: 10.1016/s0363-5023(79)80110-0. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO, Simpson SW, White TD, Asfaw B, Suwa G. Careful climbing in the Miocene: the forelimbs ofArdipithecus ramidusand humans are primitive. Science. 2009;326:70e1–8. [PubMed] [Google Scholar]
- Maas H, Sandercock TG. Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat. J Appl Physiol. 2008;104:1557–1567. doi: 10.1152/japplphysiol.01208.2007. [DOI] [PubMed] [Google Scholar]
- Mayr E. What Evolution Is. New York: Basic books; 2001. [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol. 2007;97:550–556. doi: 10.1152/jn.01071.2006. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Common synaptic input across motor nuclei supplying intrinsic muscles involved in the precision grip. Exp Brain Res. 2008;188:159–164. doi: 10.1007/s00221-008-1432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Interaction between muscle fibres in a twitch. J Physiol. 1954;124:311–324. doi: 10.1113/jphysiol.1954.sp005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier J. Primates and their Adaptations. London: Oxford University Press; 1972. [Google Scholar]
- Olafsdottir H, Zatsiorsky VM, Latash ML. Is the thumb a fifth finger? A study of digit interaction during force production tasks. Exp Brain Res. 2005;160:203–213. doi: 10.1007/s00221-004-2004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MA, Hsu J, Park J, Clark JE, Shim JK. Age-related changes in multi-finger interactions in adults during maximum voluntary finger force production tasks. Hum Mov Sci. 2008;27:714–727. doi: 10.1016/j.humov.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: Macmillan; 1952. [Google Scholar]
- Petersen NC, Butler JE, Taylor JL, Gandevia SC. Probing the corticospinal link between the motor cortex and motoneurones: some neglected aspects of human motor cortical function. Acta Physiol. 2010;198:403–416. doi: 10.1111/j.1748-1716.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- Post M, van Duinen H, Steens A, Renken R, Kuipers B, Maurits N, Zijdewind I. Reduced cortical activity during maximal bilateral contractions of the index finger. Neuroimage. 2007;35:16–27. doi: 10.1016/j.neuroimage.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci U S A. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Kilbreath SL, Gandevia SC. Movement detection at the distal joint of the human thumb and fingers. Exp Brain Res. 1998;122:85–92. doi: 10.1007/s002210050494. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Hammond GR. Human handedness: is there a difference in the independence of the digits on the preferred and non-preferred hands? Exp Brain Res. 2004;156:255–262. doi: 10.1007/s00221-003-1783-z. [DOI] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. J Neurosci. 1998;18:10105–10115. doi: 10.1523/JNEUROSCI.18-23-10105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Patterns of hand motion during grasping and the influence of sensory guidance. J Neurosci. 2002;22:1426–1435. doi: 10.1523/JNEUROSCI.22-04-01426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Somatotopic gradients in the distributed organization of the human primary motor cortex hand area: evidence from small infarcts. Exp Brain Res. 1999;128:139–148. doi: 10.1007/s002210050829. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Motor cortex and the distributed anatomy of finger movements. Adv Exp Med Biol. 2002;508:411–416. doi: 10.1007/978-1-4615-0713-0_46. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Dissociating motor cortex from the motor. J Physiol. 2011;589:5613–5624. doi: 10.1113/jphysiol.2011.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Gardinier J, Liu J. Tension distribution to the five digits of the hand by neuromuscular compartments in the macaque flexor digitorum profundus. J Neurosci. 2001;21:2150–2158. doi: 10.1523/JNEUROSCI.21-06-02150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586:1217–1224. doi: 10.1113/jphysiol.2007.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Johnston J, Chiang H, Ray W. The role of sub-maximal force production in the enslaving phenomenon. Brain Res. 2002;954:212–219. doi: 10.1016/s0006-8993(02)03288-2. [DOI] [PubMed] [Google Scholar]
- Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J Appl Physiol. 2009;106:950–958. doi: 10.1152/japplphysiol.91365.2008. [DOI] [PubMed] [Google Scholar]
- Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- Thakur PH, Bastian AJ, Hsiao SS. Multidigit movement synergies of the human hand in an unconstrained haptic exploration task. J Neurosci. 2008;28:1271–1281. doi: 10.1523/JNEUROSCI.4512-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Van Dieen JH, Ogita F, De Haan A. Reduced neural drive in bilateral exertions: a performance-limiting factor? Med Sci Sports Exerc. 2003;35:111–118. doi: 10.1097/00005768-200301000-00018. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Gandevia SC, Taylor JL. Voluntary activation of the different compartments of the flexor digitorum profundus. J Neurophysiol. 2010;104:3213–3221. doi: 10.1152/jn.00470.2010. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Yu WS, Gandevia SC. Limited ability to extend the digits of the human hand independently with extensor digitorum. J Physiol. 2009;587:4799–4810. doi: 10.1113/jphysiol.2009.177964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schroeder HP, Botte MJ. The functional significance of the long extensors and juncturae tendinum in finger extension. J Hand Surg Am. 1993;18:641–647. doi: 10.1016/0363-5023(93)90309-Q. [DOI] [PubMed] [Google Scholar]
- von Schroeder HP, Botte MJ, Gellman H. Anatomy of the juncturae tendinum of the hand. J Hand Surg Am. 1990;15:595–602. doi: 10.1016/s0363-5023(09)90021-1. [DOI] [PubMed] [Google Scholar]
- Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol. 2011;589:5625–5632. doi: 10.1113/jphysiol.2011.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53:277–284. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]
- Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92:3210–3220. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]
- Wood-Jones F. The Principles of Anatomy as seen in the Hand. London: Balliere, Tindall and Cox; 1949. [Google Scholar]
- Young M, Paul A, Rodda J, Duxson M, Sheard P. Examination of intrafascicular muscle fiber terminations: implications for tension delivery in series-fibered muscles. J Morphol. 2000;245:130–145. doi: 10.1002/1097-4687(200008)245:2<130::AID-JMOR4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Yu WS, Kilbreath SL, Fitzpatrick RC, Gandevia SC. Thumb and finger forces produced by motor units in the long flexor of the human thumb. J Physiol. 2007;583:1145–1154. doi: 10.1113/jphysiol.2007.135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WS, van Duinen H, Gandevia SC. Limits to the control of the human thumb and fingers in flexion and extension. J Neurophysiol. 2010;103:278–289. doi: 10.1152/jn.00797.2009. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biol Cybern. 1998;79:139–150. doi: 10.1007/s004220050466. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]


