Abstract
Abstract
Motor units serve both as the mechanical apparatus and the final stage of neural processing through which motor behaviours are enacted. Therefore, knowledge about the contractile properties and organization of the neural inputs to motor units supplying finger muscles is essential for understanding the control strategies underlying the diverse motor functions of the human hand. In this brief review, basic contractile properties of motor units residing in human hand muscles are described. Hand motor units are not readily categorized into the classical physiological types as established in the cat gastrocnemius muscle. In addition, the distribution of descending synaptic inputs to motor nuclei supplying different hand muscles is outlined. Motor neurons innervating intrinsic muscles appear to have relatively independent lines of input from supraspinal centres whereas substantial divergence of descending input is seen across motor nuclei supplying extrinsic hand muscles. The functional significance of such differential organizations of descending inputs for the control of hand movements is discussed.
Andrew Fuglevand is professor of Physiology and Neuroscience at the University of Arizona, USA. He did his doctoral studies at the University of Waterloo with David Winter and Aftab Patla and received post-doctoral training with Roger Enoka at the University of Arizona and with Brenda Bigland-Ritchie at the John Pierce Laboratory in New Haven. He has a long-standing interest in understanding how the CNS orchestrates the activities of multiple muscles in the elaboration of movement. Much of his work focuses at the level of the motor neuron and motor unit – the primary output elements through which the CNS controls skeletal muscle. He uses various approaches in this work, including computer simulation, in vivo recording of motor unit activity, and more recently, in-vitro electrophysiology to examine synaptic integration in motor neurons.
 |
Introduction
A great deal of work has been carried out in attempts to unravel the mysteries by which the brain controls the complex apparatus that constitutes the human hand. Much less attention, however, has been directed toward understanding the output machinery through which neural commands are transduced into the exquisite motor behaviours produced by the hand. The motor unit, namely a motoneurone and the many muscle fibres singularly innervated by branches of the motoneurone's axon, is the quantal element underlying this neuromechanical transduction. Therefore, this brief review focuses on the contractile properties and neural control of motor units in human hand muscles as a way to better understand the infrastructure and limits of hand motor control.
Contractile properties of hand motor units
Force capacity
Two main approaches have been developed to enable assessment of the mechanical properties of motor units in humans: spike-triggered averaging and intraneural microstimulation. Each approach has its own advantages and limitations (see Thomas, 1995; Bigland-Ritchie et al. 1998; Keen & Fuglevand, 2004). Spike-triggered averaging extracts the average force transient (akin to a twitch) associated with the discharge of individual motor units from the whole muscle force signal recorded during voluntary contraction (Milner-Brown et al. 1973a). Intraneural microstimulation involves activating single motor axons with microelectrodes inserted into peripheral nerves of human volunteers and recording the resulting isometric responses with sensitive force transducers (Westling et al. 1990). The predominant muscles studied with these two methods have been hand muscles. This is partly because the mechanical actions of muscles that insert into the fingers are relatively isolated from the contaminating actions of other muscles as would occur at more proximal joints. Also, for intraneural microstimulation, the peripheral nerves supplying hand muscles are much more accessible than those supplying more proximal muscles.
Regardless of the method used, experimental work characterizing motor unit properties in humans and other mammals has been consistent with regard to two findings (see Fuglevand et al. 1993). First, twitch or tetanic forces of motor units that constitute a muscle vary over an extremely wide range, usually 100-fold or greater. And second, the frequency distribution of motor units according to force capacity is markedly skewed toward motor units that produce small forces, with few units that generate large forces.
Recruitment
Control over this wide array of force-generating elements in muscle appears to be enacted in a highly stereotyped way. With few exceptions (e.g. Ter Haar Romeny, 1982; Butler et al. 1999), motor units tend to be activated in an orderly sequence, from those that exert the lowest forces towards those that produce the greatest (for a detailed review of this issue, see Duchateau & Enoka, 2011). As shown by Milner-Brown et al. (1973b) (Fig. 1), the twitch force of a motor unit (estimated with spike-triggered averaging) was directly related to the voluntary muscle force at which the unit was recruited (‘threshold force’). These data clearly show an orderly progression of recruitment – motor units with the lowest thresholds (earliest recruited) were the weakest, and those that had higher thresholds (later recruited) were progressively stronger. Furthermore, this figure illustrates the other two properties mentioned above (note the log–log axes), namely, the wide range of twitch forces (∼100-fold), and the skewed distribution, with >50% of the sampled units having twitch forces compressed within the range of 0.1–1 g, whereas the remaining units have their forces distributed over a 10-fold greater range (from 1–10 g).
Figure 1. Relationship between motor unit force (twitch tension) and muscle force (threshold force) at which motor units were recruited.
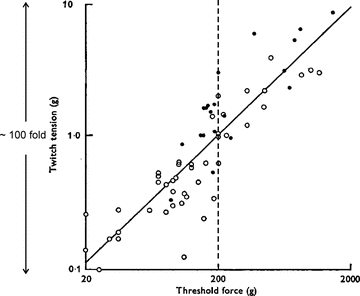
Data were obtained from the first dorsal interosseus muscle in one human subject. Spike-triggered averaging was used to estimate twitch force. Linear relationship between these two variables indicates that motor units are recruited in order from weakest to strongest. Note the 100-fold range in twitch forces with roughly half of the units producing forces of 1 g or less. Also, at a muscle force of ∼ 200 g (vertical dashed line) at least half of all units have been recruited yet this only represents about 10% of muscle force range tested. Adapted from Milner-Brown et al. (1973).
One consequence of this organization is that fine resolution of force is an in-built control feature, such that when performing delicate motor tasks involving weak muscle contractions, subtle adjustments in force can be accomplished by drawing upon a large population of weak motor units. As shown in Fig. 1, more than 50% of the motor units would likely be activated during a muscle contraction that exerts less than 10% (∼200 g in Fig. 1) of maximal muscle force. Furthermore, the presence of a relatively small population of very strong motor units endows muscle with a large dynamic range without the added necessity of dedicating a large number of neural elements for controlling powerful contractions. The neural mechanisms that underlie such an elegantly organized control system were largely revealed in the seminal work of Henneman and colleagues and are collectively referred to as the Size Principle (see Henneman & Mendell, 1981).
Motor unit types
In addition to force, contraction speed and fatigability are also important physiological characteristics of motor units. Indeed, these properties underlie the classical tripartite scheme (Burke et al. 1973) used to categorize motor units into type S (slowly contracting, low force, highly resistant to fatigue), FR (fast contracting, intermediate force, relatively resistant to fatigue), and FF (fast contracting, high force, very fatigable). These added dimensions, therefore, imply that orderly recruitment should progress from the weakest, slowest and most fatigue resistant motor units toward the strongest, fastest, and most fatigable units. It should be pointed out that this classification scheme was originally elaborated based on data obtained from motor units in cat gastrocnemius muscle. Also, designation as fast was not based on twitch contraction time, but rather on the presence of a modest decline in force during subtetanic stimuli referred to as ‘sag’. Furthermore, fatigability was characterized for all motor units using a single protocol involving repetitive, constant frequency (40 Hz) trains of stimuli. Indeed, in some other hindlimb muscles of the cat and rat, the majority of motor units possessed intermediate sensitivities to fatigue, and therefore could not be categorized as type FF, FR or S (e.g. Goslow et al. 1977; Lev-Tov et al. 1988; Tötösy de Zepetnek et al. 1992; Bakels & Kernell, 1993).
Attempts to categorize motor units of the human hand using the standard physiological properties have yielded unexpected results (Thomas et al. 1991; Fuglevand et al. 1999). These units did not readily cluster into the classical types (see Bigland-Ritchie et al. 1998; Duchateau & Enoka, 2011). For example, unlike in the cat gastrocnemius (Fig. 2A), no clear relation is seen between contractile speed and force in human hand motor units (Fig. 2B). Perhaps one reason for the species differences shown in Fig. 2 is that in most studies involving non-humans, the muscle tendons are directly secured to force-measuring devices. In human studies, motor unit forces are transmitted from tendon through bony articulations and compliant tissues (skin, fat pads) before being detected by an externally positioned transducer. Increased compliance can reduce the magnitude of detected twitches but does not appear to alter contraction time (Loring & Hershenson, 1992). Therefore, increased compliance in force measurements associated with human experiments might compress the magnitude of detected forces, but would seem unlikely to alter the general relation between force and contraction time.
Figure 2. Relation between twitch contraction time and motor unit force for cat gastrocnemius (A) and thenar muscles of the human hand (B).
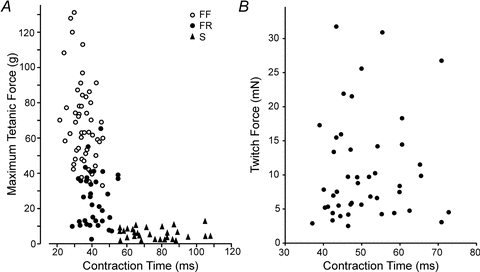
In the cat, units with the longest duration contraction times (i.e. the slowest) tend to be the weakest, while units that are the strongest tend to have the briefest contraction times. For human thenar units, no association is seen between contraction time and force. PanelAredrawn from Burke & Tsairis (1974) (with permission of John Wiley and Sons); PanelBredrawn from Thomas et al. (1990) (with permission of the American Physiological Society).
It should also be pointed out that ‘sag’ has not been observed in human motor units. While this finding might imply that human hand muscles do not possess type FR or FF units, a relatively wide range of fatigability argues against a homogeneous population of type S units. Furthermore, human muscle possesses a significant proportion of fast muscle fibre types based on biochemical and contractile analyses of single fibres (e.g. Hilber et al. 1999). Nevertheless, one consistent feature shared by cat and human data is that strong motor units tend to fatigue more rapidly than do weak units (Fuglevand et al. 1999). Therefore, recruitment of human hand motor units will likely progress from the weakest and most fatigue resistant toward the strongest and most fatigable. Designation of motor units as slow or fast, however, does not seem germane to categorization of human hand motor units. This verdict might also extend to other human muscles; for example, motor units in human lower limb muscles also fail to exhibit a relationship between contractile speed and force (Macefield et al. 1996; cf. Van Cutsem et al. 1997).
Number of motor units
What would seem to be one of the most straightforward and fundamental features of motor unit organization, namely the total number of motor units constituting a muscle, has been surprisingly elusive. In humans, there are no direct measures of the number of motoneurones innervating any muscle. While there are anatomical (Feinstein et al. 1955) and electrophysiological (McComas et al. 1971; Bromberg, 2007) means to estimate motor unit numbers in humans, both methods are susceptible to several sources of error. Perhaps the most reliable information at present available about relative numbers of motor units supplying different muscles comes from retrograde labelling of motoneurones in non-human primates. In such studies, intrinsic hand muscles have been shown to be innervated by ∼50–200 motoneurones, while more proximal muscles like biceps and triceps brachii are each supplied by more than 1000 motoneurones (Jenny & Inukai, 1983).
Motor unit number itself seems to play a critical role in determining precision of muscle force. When human subjects attempt to produce a constant force during isometric contractions, the force inadvertently fluctuates about the specified target level. Such force variability increases in roughly in proportion to the target force (Enoka et al. 2003). Unexpectedly, this noise in force control isgreaterfor hand muscles compared to more proximal muscles (Hamilton et al. 2004). Furthermore, based on available estimates of motor unit numbers and computer simulation, a key factor underlying greater noisiness in hand muscles was relatively low numbers of motor units (Jones et al. 2002; Hamilton et al. 2004). In addition, augmented force variability in hand muscles may be related to greater variability and common modulation in motor unit discharge rate compared to more proximal muscles (Negro et al. 2009). Therefore, the widely held view that hand muscles are optimally designed for fine control may require reconsideration.
Organization of descending pathways controlling the hand
Complexity of muscle coordination
One conspicuous feature of many hand muscles is that they operate across multiple joints. For example, the main finger extensor, extensor digitorum (ED), when active generates torque about the elbow, wrist, metacarpalphalanegeal, proximal interphalangeal and distal interphalangeal joints simultaneously (An et al. 1981). Moreover, ED, like its flexor counterparts, flexor digitorum superficialis and flexor digitorum profundus, gives rise to four distal tendons that insert into each of the fingers. Therefore, attempts to move a single finger in isolation require that other muscles be co-activated to counteract the unwanted actions produced by the agonist (Schieber, 1995; Valero-Cuevas, 2000). For example, Fig. 3 shows electromyographic (EMG) activity recorded in the author's laboratory from 24 muscles or muscle compartments in the human forearm and hand during unloaded flexion–extension movements of the metacarpophalangeal (MCP) joint of a single finger. Several muscles, including many not directly involved in moving the finger, exhibited significant activity during this basic motor task. Such studies indicate that ‘simple’ finger movements actually involve complex orchestration of activities of multiple muscles of the hand.
Figure 3. EMG recording from 24 muscle/muscle compartments during unloaded finger movements in a human subject.
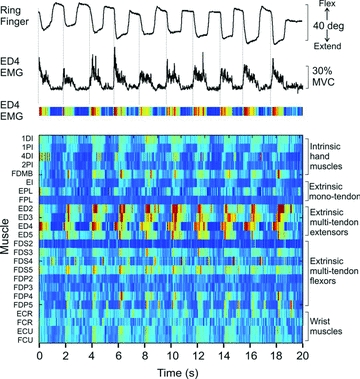
Subject was instructed to flex and extend the metacarpal phalangeal (MCP) joint of the ring finger paced by a metronome. Top trace shows angular displacement of the MCP joint of the ring finger (digit 4). Second trace from top shows rectified and filtered EMG signal recorded from the digit 4 compartment of extensor digitorum (ED4), one of the prime movers for this task. Third trace shows the ED4 EMG represented as a colour plot with warmer colours indicating higher levels of activity. Each row in the bottom panel shows the activity of one of the 24 muscles recorded over the 20 s of this trial involving 10 repeated extension flexion movements. All EMG signals were normalized to maximum voluntary contraction (MVC). The colour of each small bin within a row indicates the average normalized EMG over a 30 ms epoch (darkest blue = 0%, deep red = ∼30% MVC EMG). EMG signals were recorded from five intrinsic hand muscles (1DI – first dorsal interosseus, 1PI – first palmar interosseus, 4DI – fourth dorsal interosseous, 2PI – second palmar interosseus, FDMB – flexor digiti minimi brevis), three extrinsic hand muscle with a single tendon (EI – extensor indicis, EPL – extensor pollicis longus, FPL – flexor pollicis longus), four compartments of the multitendoned extensor digitorum (ED2–ED5), four compartments each of the multitendoned flexor muscles flexor digitorum superficialis (FDS2–5) and flexor digitorum profundus (FDP2–5), and four wrist muscles (ECR – extensor carpi radialis, FCR – flexor carpi radialis, ECU – extensor carpi ulnaris, FCU – flexor carpi ulnaris). All muscles except FDI and FDMB were recorded with intramuscular electrodes. Many muscles were involved in these unloaded movements of a single joint of an individual finger.
Two mutually non-exclusive scenarios can be envisioned as to how corticospinal (and reticulospinal – see Baker, 2011, this issue) pathways might be organized to coordinate the activities of multiple muscles needed to perform finger movements (Schieber, 1990). In one, separate pathways operate on each of the requisite motor nuclei. In the other, selection of the muscles into functional groups is determined in part by the pattern of divergence of individual descending pathways across different motor nuclei in the spinal cord. This latter type of organization, while less flexible, might underlie the assemblage of muscles into synergistic groups that serve as the building blocks of the behavioural repertoire of an animal.
Descending pathway organization underlying the precision grip
There is some evidence for both these scenarios from electrophysiological and anatomical studies carried out in non-human primates (Lemon, 2008). In humans, the extent of divergence of synaptic input can be estimated from the level of short-term synchrony in the discharge times of pairs of motor units recorded from two muscles (Sears & Stagg, 1976; Kirkwood, 1979; Nordstrom et al. 1992; Stephens et al. 1999). Because short-term synchrony appears to be minimally affected by lesions of the dorsal roots (Kirkwood et al. 1982) or by removal of normal tactile input (McIsaac & Fuglevand, 2006), yet is markedly diminished with section of descending pathways (Kirkwood et al. 1982; Datta et al. 1991), the source of such synchrony is thought to be central rather than peripheral.
We examined the degree of short-term synchrony across pairs of motor units residing in two extrinsic hand muscles whose activities must be tightly coordinated in the production of the precision grip, flexor pollicis longus (inserting into the thumb) and the index finger compartment of flexor digitorum profundus (Hockensmith et al. 2005). As illustrated in the example cross-correlation histograms in Fig. 4, the magnitude of short-term synchrony for pairs of motor units residing in thesedifferentmuscles (thumb-index, Fig. 4) was found to be just as large as that for pairs of motor units in the same muscle (thumb-thumb, Fig. 4). Furthermore, the widths of the synchronous peaks were relatively narrow (on average, less than 10 ms) and not different between the thumb–thumb and thumb–index finger combinations of motor-unit pairs (Fig. 4). Collectively, these results imply that descending pathways diverge extensively to operate on the two motor nuclei supplying thumb and index finger muscles as a unit and thereby compel them to operate in unison. Interestingly, such across-muscle synchrony was seen only in the dominant but not in the non-dominant hand (Fig. 4). Whether such lateralized differences are laid down early in development or represent plastic changes associated with chronic usage are questions currently under investigation.
Figure 4. Representative cross-correlation histograms of firing times for pairs of human motor units recorded during the precision grip.
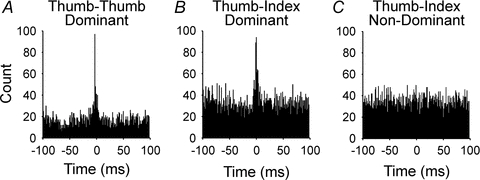
A, both units of the pair recorded within the extrinsic thumb muscle, flexor pollicis longus (Thumb–Thumb) of the dominant hand.B, one unit of pair recorded from the thumb muscle and other unit recorded from index finger compartment of flexor digitorum profundus (Thumb–Index) of the dominant hand.C, one unit recorded from the thumb muscle and other unit recorded from index finger muscle (Thumb–Index) of the non-dominant hand. Overall, synchrony for pairs of motor units residing in separate muscles (Thumb–Index) of the dominant hand was just as large as that for pairs of units both within the thumb muscle. The high level of synchrony seen across muscles in the dominant hand, however, was absent in the non-dominant hand. Redrawn from Hockensmith et al. (2005) (with permission of the Society for Neuroscience).
In contrast to the extrinsic muscles of the dominant hand described above, virtually no short-term synchrony was observed across intrinsic muscles participating in the precision grip (McIsaac & Fuglevand, 2008). This result suggests that the descending pathways that control the activities of intrinsic muscles provide more concentrated input to individual motor nuclei than those pathways destined for motor nuclei innervating extrinsic hand muscles. To a large extent, these observations parallel those based on more direct evaluation of cortical projections to spinal motor nuclei in the monkey (Fetz & Cheney, 1980; Buys et al. 1986). Furthermore, these results are consistent with focal activation of motor units in intrinsic but not in extrinsic hand muscles of human subjects in response to cortical stimulation (Gandevia & Rothwell, 1987). The contrasting organizations of the descending pathways targeting extrinsic and intrinsic muscles seem in harmony with postulated functions of these two groups of muscles (Long et al. 1970). Intrinsic muscles configure the digits to the unique dimensions of an object to be handled. Highly independent pathways, therefore, enable the fractionated actions of the digits needed for such a function. Extrinsic muscles provide the primary gripping forces during object manipulation. Because gripping necessitates the production of precisely counterbalanced forces between the thumb and one or more fingers, extrinsic muscles have their activities linked by divergent descending inputs.
Multitendoned muscles
In humans, there are three multi-tendon extrinsic finger muscles located in the forearm that give rise to four parallel tendons to insert on the four fingers. These muscles appear to comprise relatively distinct mechanical compartments (Kilbreath & Gandevia, 1994; Keen & Fuglevand, 2003, 2004a), each of which operates on a separate digit. A substantial degree of motor unit synchrony, however, is observed for pairs of units residing in different compartments of these multitendoned muscles (Keen & Fuglevand, 2004b; Reilly et al. 2004; Winges & Santello, 2004, McIsaac & Fuglevand, 2007). As shown in Fig. 5, the magnitude of synchrony for motor-unit pairs residing in adjacent muscle compartments, while smaller than that for pairs in the same compartment, is substantial for all three multi-tendoned muscles. These findings imply, therefore, that last-order inputs, while primarily destined to supply motoneurones innervating one compartment, also ramify to contact motoneurones innervating other compartments, particularly adjacent compartments. It is possible that such across compartment synchronization might help to coordinate grip forces across digits during grasping (Santello & Fuglevand, 2004). However, this divergence of synaptic input may also limit the ability to differentially activate separate muscular compartments and thereby underlie the inability to move the fingers independently (Schieber & Santello, 2004; see Van Duinen & Gandevia, 2011, this issue).
Figure 5. Mean (SD) common input strength (CIS – index representing magnitude of short-term synchrony; Nordstrom et al. 1992) for pairs of motor units residing in the same compartment or adjacent compartments of three human multi-tendoned hand muscles, extensor digitorum (ED), flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP).
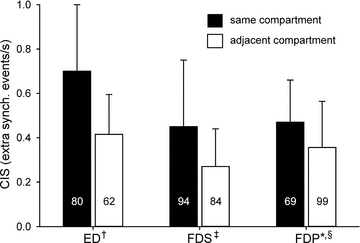
Mean (SD) CIS values: ED same = 0.70 (0.30), ED adjacent = 0.41 (0.18), FDS same = 0.45 (0.30), FDS adjacent = 0.27 (0.17), FDP same = 0.47 (0.19), FDP adjacent = 0.36 (0.21). Values inside of bars indicate number of motor unit pairs. Data compiled from: †Keen & Fuglevand (2004b); ‡McIsaac & Fuglevand (2007); *McIsaac & Fuglevand (unpublished data); §Winges & Santello (2004).
In summary, motor units in human hand muscles, like those in virtually every mammalian muscles examined, are activated in an orderly sequence from weakest and most fatigue resistant toward the strongest and most fatigable. Contractile speed, however, does not appear to vary systematically with other physiological properties in human hand motor units as it does in other mammalian muscles, such as in the cat gastrocnemius. Somewhat unexpectedly, hand muscles may be undersupplied in terms of numbers of motor units, and this may contribute to relatively greater noisiness in the control of hand muscles compared to more proximal muscles. In terms of organization of descending pathways controlling hand muscles, motor neurons supplying intrinsic muscles appear to have relatively independent lines of input from supraspinal centres. In contrast, descending inputs to motor nuclei innervating extrinsic hand muscles and muscle compartments appear to diverge extensively and will tend to cause more than one digit to move when activated. While such an organization may simplify control of multiple muscles needed for finger movements, it may also limit the ability to move fingers independently.
Acknowledgments
This work was supported by National Institutes of Health grants NS39489 and NS070897.
References
- An KN, Hui FC, Morrey BF, Linscheid RL, Chao EY. Muscles across the elbow joint: a biomechanical analysis. J Biomech. 1981;14:659–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Average but not continuous speed match between motoneurons and muscle units of rat tibialis anterior. J Neurophysiol. 1993;70:1300–1306. doi: 10.1152/jn.1993.70.4.1300. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Fuglevand AJ, Thomas CK. Contractile properties of human motor units: is man a cat? Neuroscientist. 1998;4:240–249. [Google Scholar]
- Bromberg MB. Updating motor unit number estimation (MUNE) Clin Neurophysiol. 2007;118:1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Tsairis P. The correlation of physiological properties with histochemical characteristics in single muscle units. Ann NY Academy Sci. 1974;228:145–159. doi: 10.1111/j.1749-6632.1974.tb20507.x. [DOI] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol. 1999;518:907–920. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Farmer SF, Stephens JA. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol. 1991;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM. Human motor unit recordings: origins and insight into the integrated motor system. Brain Res. 2011;1409:42–61. doi: 10.1016/j.brainres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J EMG Kines. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Feinstein B, Lindegard B, Nyman E, Wohlfart G. Morphologic studies of motor units in normal human muscles. Acta Anat (Basel) 1955;23:127–142. doi: 10.1159/000140989. [DOI] [PubMed] [Google Scholar]
- Fetz E, Cheney P. Postspike facilitation of forelimb muscle activity by primate cortico-motoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor unit pools. J Neurophysiol. 1993;70:2470–2488. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Cameron WE, Stuart DG. The fast twitch motor units of cat ankle flexors. 1. Tripartite classification on basis of fatigability. Brain Res. 1977;134:35–46. doi: 10.1016/0006-8993(77)90923-4. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Jones KE, Wolpert DM. The scaling of motor noise with muscle strength and motor unit number in humans. Exp Brain Res. 2004;157:417–430. doi: 10.1007/s00221-004-1856-7. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology section 1 The Nervous System. II. Bethesda: American Physiological Society; 1981. pp. 423–507. Motor Control part 1. [Google Scholar]
- Hilber K, Galler S, Gohlsch B, Pette D. Kinetic properties of myosin heavy chain isoforms in single fibers from human skeletal muscle. FEBS Lett. 1999;455:267–270. doi: 10.1016/s0014-5793(99)00903-5. [DOI] [PubMed] [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci. 2005;25:4560–4564. doi: 10.1523/JNEUROSCI.0046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Hamilton AF, Wolpert DM. Sources of signal-dependent noise during isometric force production. J Neurophysiol. 2002;88:1533–1544. doi: 10.1152/jn.2002.88.3.1533. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Role of inter-tendonous connections in distribution of force in human extensor digitorum. Muscle Nerve. 2003;28:614–622. doi: 10.1002/mus.10481. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Distribution of motor unit force in human extensor digitorum assessed by spike-triggered averaging and intraneural microstimulation. J Neurophysiol. 2004a;91:2515–2523. doi: 10.1152/jn.01178.2003. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum. J Neurophysiol. 2004b;91:57–62. doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. On the use and interpretation of cross-correlation measurements in the mammalian central nervous system. J Neurosci Methods. 1979;1:107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sear TA, Tuck DL, Westgaard RH. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Pratt CA, Burke RE. The motor-unit population of the cat tenuissimus muscle. J Neurophysiol. 1988;59:1128–1142. doi: 10.1152/jn.1988.59.4.1128. [DOI] [PubMed] [Google Scholar]
- Long C, Conrad PW, Hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. An electromyographic study. J Bone Joint Surg. 1970;A52:853–867. [PubMed] [Google Scholar]
- Loring SH, Hershenson MB. Effects of series compliance on twitches superimposed on voluntary contractions. J Appl Physiol. 1992;73:516–521. doi: 10.1152/jappl.1992.73.2.516. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AJ, Bigland-Ritchie B. Contractile properties of single motor units in human toe extensors assessed by intraneural motor-axon stimulation. J Neurophysiol. 1996;75:2509–2519. doi: 10.1152/jn.1996.75.6.2509. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–131. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Influence of tactile afferents on the coordination of muscles during a simulated precision grip. Exp Brain Res. 2006;174:769–774. doi: 10.1007/s00221-006-0643-z. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol. 2007;97:550–556. doi: 10.1152/jn.01071.2006. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Common synaptic input across motor nuclei supplying intrinsic muscles involved in the precision grip. Exp Brain Res. 2008;188:159–164. doi: 10.1007/s00221-008-1432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973a;228:285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973b;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol. 2009;587:5925–5938. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle. J Neurophysiol. 2004;92:734–742. doi: 10.1152/jn.00027.2004. [DOI] [PubMed] [Google Scholar]
- Santello M, Fuglevand AJ. Role of across-muscle motor unit synchrony for the coordination of forces. Exp Brain Res. 2004;159:501–508. doi: 10.1007/s00221-004-1975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. How might the motor cortex individuate movements? Trends Neurosci. 1990;13:440–445. doi: 10.1016/0166-2236(90)90093-p. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: the roles of extrinsic finger muscles. J Neurosci. 1995;15:284–297. doi: 10.1523/JNEUROSCI.15-01-00284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motorneuron activity. J Physiol. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Harrison LM, Mayston MJ, Carr LJ, Gibbs J. The sharing principle. Prog Brain Res. 1999;123:419–426. [PubMed] [Google Scholar]
- Ter Haar Romeny BM, Denier van der Gon JJ, Gielen CC. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol. 1982;78:360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Human motor units studied by spike-triggered averaging and intraneural motor axon stimulation. Adv Exp Med Biol. 1995;384:147–160. doi: 10.1007/978-1-4899-1016-5_12. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Westling G, Bigland-Ritchie B. Twitch properties of human thenar motor units measured in response to intraneural motor-axon stimulation. J Neurophysiol. 1990;64:1339–1346. doi: 10.1152/jn.1990.64.4.1339. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. Attempts to physiologically classify human thenar motor units. J Neurophysiol. 1991;65:1501–1508. doi: 10.1152/jn.1991.65.6.1501. [DOI] [PubMed] [Google Scholar]
- Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Motor-unit categorization based on contractile and histochemical properties: a glycogen depletion analysis of normal and reinnervated rat tibialis anterior muscle. J Neurophysiol. 1992;67:1404–1415. doi: 10.1152/jn.1992.67.5.1404. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Van Cutsem M, Feiereisen P, Duchateau J, Hainaut K. Mechanical properties and behaviour of motor units in the tibialis anterior during voluntary contractions. Can J Appl Physiol. 1997;22:585–597. doi: 10.1139/h97-038. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Gandevia SC. Constraints for control of the human hand. J Physiol. 2011;589:5583–5593. doi: 10.1113/jphysiol.2011.217810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling G, Johansson RS, Thomas CK, Bigland-Ritchie B. Measurement of contractile and electrical properties of single human thenar motor units in response to intraneural motor-axon stimulation. J Neurophysiol. 1990;64:1331–1338. doi: 10.1152/jn.1990.64.4.1331. [DOI] [PubMed] [Google Scholar]
- Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92:3210–3220. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]


