Abstract
Abstract
Stroke often leads to impairment of hand function. Over the following months a variable amount of recovery can be seen. The evidence from animal and human studies suggests that reorganization rather than repair is the key. Surviving neural networks are important for recovery of function and non-invasive techniques such as functional magnetic resonance imaging allow us to study them in humans. For example, initial attempts to move a paretic limb following stroke are associated with widespread activity within the distributed motor system in both cerebral hemispheres, more so in patients with greater impairment. Disruption of activity in premotor areas using transcranial magnetic stimulation prior to movement can impair motor performance in stroke patients but not in controls suggesting that these new patterns of brain activity can support recovered function. In other words, this reorganisation is functionally relevant. More recently, research has been directed at understanding how surviving brain regions influence one another during movement. This opens the way for functional brain imaging to become a clinically useful tool in rehabilitation. Understanding the dynamic process of systems level reorganization will allow greater understanding of the mechanisms of recovery and potentially improve our ability to deliver effective restorative therapy.
Neurological damage, and in particular stroke, accounts for nearly half of all severely disabled adults. Upper limb impairment is a major contributory factor towards this disability. One of the commonest and most devastating consequences of stroke is the loss of both power and dexterity in the limbs contralateral to the injury. Treatment of these patients relies on rehabilitation. Strategies aimed at helping patients adapt to impairment are the cornerstone of this approach, but treatments aimed at reducing impairment are less well developed. This will require a greater understanding of the mechanisms of impairment and more particularly recovery. Animal models of focal cortical damage in adult brains demonstrate that widespread surviving cortical regions are able to change structure and function in response to afferent signals to a degree that is only normally seen in the developing brain (Schallert et al. 2000; Bury & Jones, 2002). The investigation of similar changes in humans is less well advanced and there are clearly greater limitations in studying the human brain. Functional brain imaging techniques, however, do allow measurement of task-related brain activation and so provide an opportunity for this. It is worth considering that the findings from animals apply almost exclusively to cortical damage, whereas much of the work in humans to date has been performed in patients with deep subcortical infarcts. Nevertheless, both approaches have yielded important results from which a clearer picture of functional cerebral reorganisation is beginning to emerge. Studies in various primate models have emphasised both the neural and peripheral limitations to hand function (van Duinen & Gandevia, 2011) as well as the underlying flexibility in the linkage between the primary motor cortex and motoneurones (Schieber, 2011).
Cerebral reorganisation in chronic stroke
Functional imaging studies in human stroke patients demonstrate changes in task-related (usually whole hand grasp or finger movements) brain activation, in particular (i) overactivations in secondary motor areas and (ii) shifts in somatotopic representation in primary and possibly secondary motor areas. In general it seems that more impaired patients have greater task-related activity in secondary motor areas such as dorsal and ventral premotor cortices (PMd and PMv), supplementary and cingulate motor areas in both affected and unaffected hemispheres and contralesional M1. Patients with the best outcome had a ‘normal’ activation pattern when compared to healthy controls (Ward et al. 2003a). One interpretation of these results is that patients with poorer outcome have greater damage to the monosynaptic projections from M1 to spinal cord motor neurons, and thus utilise secondary motor areas to generate a motor output. Indeed if one examines for a correlation between magnitude of task-related activity and a measure of corticospinal system integrity, as measured with transcranial magnetic stimulation (TMS), a very similar result is found (Ward et al. 2006) (Fig. 1). In other words, greater damage to corticospinal systems is associated with greater recruitment of secondary motor areas during hand squeezing. For chronic stroke patients with preserved ipsilesional M1, shifts in the peak ipsilesional sensorimotor activations have been found in comparison to control subjects (Weiller et al. 1993; Pineiro et al. 2001). Overall, these data suggest that there is a certain amount of remapping of hand representation in M1, even though undamaged, which may result from functionally relevant changes in both its afferent and efferent connections. Changes in somatotopic representation in secondary motor regions might result in stronger connections with different (e.g. more ventral or caudal) regions of M1 in order to facilitate access to intact portions of the direct corticospinal pathway. Shifts in somatotopic representation in secondary motor regions might also facilitate recruitment of surviving ischaemia-resistant small diameter myelinated corticospinal fibres, such as those arising from premotor cortex, to compensate for loss of large diameter fibres making up the monosynaptic projections.
Figure 1. Brain regions in which brain activity during affected hand grip increases with greater impairment and/or damage to descending corticospinal system in chronic subcortical stroke patients.
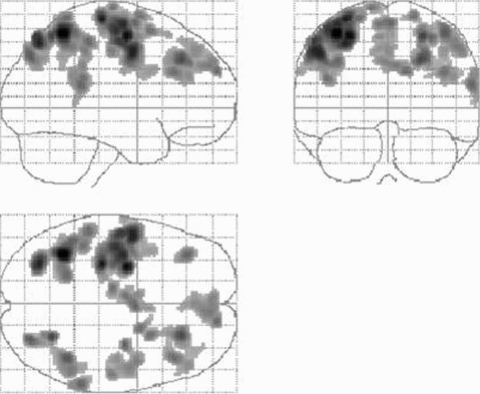
The figure consists of brain regions in which there is a negative correlation between corticospinal system integrity (assessed as the linear slope of the TMS recruitment curve) and task related BOLD signal. These are predominantly bilateral premotor (dorsal and ventral), supplementary motor area, parietal cortices together with contralesional M1. Results are displayed on a ‘glass brain’ shown from the right side (top left image), from behind (top right image), and from above (bottom left image). Here it is assumed that all patients have right hemisphere damage (subcortical). (From Ward et al. 2006.)
Is reorganisation functionally relevant?
The finding of premotor overactivation in patients with greater impairment does not immediately suggest that this is a mechanism for recovery. However, disruption of ipsilesional PMd (Fridman et al. 2004) or contralesional PMd (Johansen-Berg et al. 2002) using TMS impairs performance of a simple motor task in chronic stroke patients but not controls. This suggests that these regions are contributing to the performance of the disrupted task in a way that they do not in healthy subjects. Furthermore, TMS to contralesional PMd is more disruptive in patients with greater impairment (Johansen-Berg et al. 2002), demonstrating that PMd in the unaffected hemisphere is used more so by those with poorer outcome, possibly because the connections of ipsilesional PM are disrupted in such patients.
Another approach is to measure how task-related activity co-varies with modulation of task parameters. One study examined for regional changes in the control of force modulation after stroke, and specifically, how these changes were altered by variations in corticospinal system damage (Ward et al. 2007). In healthy humans increasing force production is associated with linear increases in BOLD signal in contralateral M1 and medial motor regions, implying that they have a functional role in force production (Ward & Frackowiak, 2003). In patients with minimal corticospinal system damage and excellent recovery, the cortical motor system behaved in a way that was similar to younger healthy controls. However, in patients with greater corticospinal system damage, force-related signal changes were seen mainly in contralesional dorsolateral premotor cortex, bilateral ventrolateral premotor cortices and contralesional cerebellum, but not ipsilesional primary motor cortex. Interestingly a qualitatively similar result was found in healthy volunteers with increasing age suggesting that this ‘reorganisation’ might be a generic property of the cortical motor system in response to a variety of insults (Ward et al. 2008). Thus not only do premotor cortices become increasingly active as corticospinal system integrity diminishes (Ward et al. 2008), but they can take on a new ‘M1-like’ role during modulation of force output, which implies a new and functionally relevant role in motor control.
Thus ‘secondary’ cortical motor regions appear to be recruited after focal damage to the motor system in those patients with greatest need. In addition, there is evidence that ipsilesional PM takes on an executive motor role, such that task-related signal increases linearly as a function of hand grip force in chronic stroke patients with significant impairment, but not in good recoverers or in controls (Ward et al. 2007). It appears then that the response to focal injury involves more than the redistribution of brain activity, as nodes within a remaining motor network may take on new roles not seen in healthy individuals.
The dorsal premotor cortex seems to play one of the central roles in post-stroke brain reconfiguration. At rest, it seems that the influence of contralesional premotor cortex on ipsilesional motor cortex is inhibitory in well recovered patients, but becomes less inhibitory/more excitatory in those with greater clinical impairment (Bestmann et al. 2010) (Fig. 2A). By using concurrent TMS-fMRI, it was also possible to see that during affected hand movement greater clinical impairment was associated with a stronger influence of contralesional premotor cortex on posterior parts of the ipsilesional sensorimotor cortex – parts of the ipsilesional hemisphere most likely to be able to generate descending motor signals to the spinal cord (Fig. 2B). This work points to the possible mechanism by which contralesional premotor cortex might exert its state-dependent influence over the surviving cortical motor system in a way that might support recovered motor function.
Figure 2.
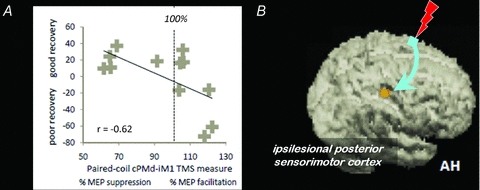
A, scatterplot showing the correlation, with regression line, between the combined clinical score and the interhemispheric cPMd-iM1 influence measured with paired-coil TMS (conditioned MEP/unconditioned MEP as a %) in each patient. For the combined clinical score (along they-axis) a higher value indicates better residual motor function. This measure correlated with the value of the interhemispheric cPMd-iM1 influence shown along thex-axis: a better motor recovery was associated with a physiological ‘inhibitory’ effect whereas poorer recovery was associated with less interhemispheric inhibition or even facilitation (i.e. paired-coil effects of >100%, as for the rightmost cases).B, statistical parametric map (SPM) for the interaction term TMShigh (GRIP − REST) > TMSlow (GRIP − REST) during concurrent TMS-fMRI overlaid on the rendered mean structural scan from all patients. In other words, the only brain region that contralesional PMd had an effect on during hand grip was ipsilesional sensorimotor cortex. The facilitatory influence of contralesional PMd on ipsilesional sensorimotor cortex during affected hand grip was found in all chronic subcortical stroke patients, but was greater in patients with more impairment. AH: affected hemisphere. (From Bestman et al. 2010.)
The increasingly facilitatory role of contralesional PMd on ipsilesional sensorimotor cortex (in those with greater impairment) appears to be in distinct contrast to another set of results. Others have suggested that contralesional M1 may exert an abnormally high degree of interhemispheric inhibitory drive towards ipsilesional M1 during attempted voluntary movement of the affected hand (Murase et al. 2004; Grefkes et al. 2008). Others have used this concept to try to transiently improve motor function after stroke by suppressing excitability in contralesional M1 (Fregni et al. 2006; Liepert et al. 2007; Nowak et al. 2008). Proof-of-principle studies in chronic mildly impaired subcortical stroke patients are encouraging (Talelli & Rothwell, 2006), but a critical question remains whether this normalization is appropriate for all patients.
There are two possibly contradictory sets of results here. On the one hand, the influence of premotor cortex becomes increasingly facilitatory towards ipsilesional sensorimotor cortex in patients with greater impairment (Bestmann et al. 2010). On the other, the influence of contralesional M1 becomes increasingly inhibitory in the same types of patients (Murase et al. 2004). One possibility is that control of inhibition from contralesional M1 is normally managed by circuits in ipsilesional M1 that suppress inputs prior to movement. If these are damaged by stroke, then the influence of contralesional M1 will appear negative. Conversely inputs from contralesional premotor cortex may normally assist production of certain types of movement and this facilitation may increase after damage to the lesioned hemisphere. Although the physiological signatures for the interhemispheric influences of contralesional M1 and premotor cortex appear different, the commonality for both sets of observations is that interhemispheric influences from contralesional to ipsilesional motor regions are systematically more abnormal in patients with more impaired clinical motor function.
Anatomical substrate supporting recovered motor function
The changes described above are manifest more strongly in patients with greatest deficit and presumably with the most significant damage to corticomotoneuronal pathways. It is worth considering the possible anatomical substrates supporting recovered motor function. Significant increases in task-related activity are often seen in the contralesional hemisphere but there appears to be little evidence to support the idea of ipsilateral projections from motor cortex to forelimbs, at least in primates (Soteropoulos et al. 2011). In humans, we have discussed how contralesional PMd appears to exert some cortico-cortical influence over the ipsilesional sensorimotor cortex (Bestmann et al. 2010). What about the possibility of direct contralateral pathways from secondary motor areas? In primates, contralateral projections from secondary motor areas to spinal cord motor neurons are usually less numerous and less efficient at exciting spinal cord motoneurons than those from M1 (Maier et al. 2002; Boudrias et al. 2006). Further insights have been gained by studies in primates in which layer V cortical neurons were stimulated and stimulus-triggered averages of electromyographic activity measured from forelimb muscles during a reach-to-grasp task (Boudrias et al. 2010a,b;). The onset latency and magnitude of facilitation effects from both lateral (PMd and PMv) and medial (SMA and dorsal cingulate motor area, CMAd) premotor regions was significantly longer and approximately 10 times weaker than those from M1 suggesting that the vast majority are likely to have a more indirect influence on motoneurons through corticocortical connections with M1 and/or interneurons in the spinal cord. However, there was evidence of a small number of projections to motoneurons at least as fast as those from M1, from each of the secondary motor areas. The target areas for these fast, presumably monosynaptic, pathways from the secondary motor areas were different. Proximal muscles were predominantly represented in PMd and PMv but for both SMA and CMAd, facilitation effects were more common in distal compared to proximal muscles.
Another possibility is that signals descend via alternative pathways such as reticulospinal projections to cervical propriospinal premotoneurons (Mazevet et al. 2003; Stinear & Byblow, 2004; Baker, 2011). These pathways have divergent projections to muscle groups operating at multiple joints (Mazevet & Pierrot-Deseilligny, 1994; Pierrot-Deseilligny, 1996). This solution might account for the multijoint ‘associated’ movements such as the synergistic flexion (together with weak extension) seen when patients with only poor and moderate recovery attempt isolated hand movements (Baker, 2011). Overall, it is feasible that a number of motor related circuits acting in parallel could generate an output to the spinal cord necessary for movement, and that damage in one of these circuits could be at least partially compensated for by activity in another (Dum & Strick, 1991; Rouiller et al. 1996).
The evolution of cerebral reorganisation after stroke
The studies in chronic stroke patients described above do not tell us how the reorganised state evolved. Longitudinal fMRI studies involving similar patients indicate an initial overactivation in many primary and secondary motor regions (Ward et al. 2003b). Thereafter functional recovery is associated with a focusing of task-related brain activation pattern, in a way that is seen during motor skill learning in normal subjects. It is clear from the results of chronic stroke patients that brain activation patterns will not return to normal in all cases. It is certainly plausible that a highly preserved neural system such as that subserving motor skill learning in the brain will be employed after stroke in order to maximise functional motor recovery. However, the degree to which this is successful will depend on the integrity of such networks in the post-stroke functional anatomy. Thus focusing will tend towards the most efficient system available. The degree to which this is achieved could be taken as a measure of the effectiveness of the therapy that has preceded that point in time.
Results of the analysis of brain activity in order to assess connectivity between brain regions after stroke are just starting to be seen (Grefkes & Fink, 2011). One example demonstrates reduced positive coupling from ipsilesional SMA and PMd to ipsilesional M1 very early (less than 72 h) after stroke. In patients who improve the most, these coupling parameters return towards normal over the first few weeks (Rehme et al. 2011). Other forms of analysis have used graph theory to show dynamic changes in network organisation after stroke, possibly reflecting adaptive reorganisation (Wang et al. 2010). Cerebral reorganisation undoubtedly contributes to functional recovery after stroke, but it is clear that a more detailed understanding of the natural history of these processes is required, together with the factors that influence them, before they can be utilised to rationalise therapeutic strategies in individual patients or groups.
The post-stoke brain: a new functional architecture
Thus in the chronic stroke brain, there is a new functional architecture, one which is not as effective as that in the intact brain, but which nevertheless will attempt to generate some form of motor signal to spinal cord motor neurons in the most efficient way. The exact configuration of this new functional anatomy will be determined most obviously by the extent of the anatomical damage as described above. However, there are a number of other factors which will contribute to how the new anatomical configuration of each patient works, not least the type and extent of previous treatment regimes, the premorbid state of their brain, current drug treatments and probably even their genetic status. In considering the capacity to change, biological age will be an important factor. Motor system reorganisation can also be seen after normal ageing, with older subjects tending to recruit more of the motor system than young subjects when performing a motor task (Talelli et al. 2008). It is still not clear whether this alteration is compensatory, but there is clearly residual capacity within the motor system which allows it to respond to an insult in a way which attempts to maintain performance. If we use techniques to assess connectivity, i.e. the influence that one region exerts over another, we can see that ipsilateral hemisphere pays an increasingly influential role with ageing, inviting the question whether ‘plasticity’ or the capacity to change is used up over time.
How will this help us to understand how best to treat the impairment suffered by patients after stroke? On one level, treatments can be considered as inputs that interact with a system, in this case the damaged post-stroke brain. The aim of this input is generally to optimise the functional organisation of the damaged system. What seems crucial is that an input will succeed in driving functionally useful change only to the extent that the brain regions and networks with which it interacts are intact and are able to influence motor output. Another category of ‘treatments’ are better considered as ways of conditioning the brain to make it more likely that activity driven change will occur in response to afferent input. For example, rTMS or drugs such as amphetamine may enhance the effect of physiotherapy if delivered just prior to the treatment session. Once again, the likelihood of their success is likely to depend on a number of factors relating to each patient's post-stroke residual functional anatomy. As yet, the mechanisms of action of these interventions are not well understood. However, the concept of residual functional anatomy provides a framework with which to explore whether and how interventions of different types work in all types of patients.
Predicting recovery with neuroimaging
There is still a long way to go before these studies influence how best to treat the impairment suffered by patients after stroke. The question is whether imaging and/or neurophysiological data can contribute to predictive models, not of outcome, but of the potential for therapy driven improvements in function. For example, a recent study demonstrated that the beneficial effects of facilitatory repetitive TMS over ipsilesional M1 on motor function of the affected hand were seen in patients with subcortical stroke but not in those with extension of the infarct into ipsilesional M1 (Ameli et al. 2009). Furthermore, task-related activity in ipsilesional M1 measured with fMRI at baseline correlated with improvement of motor performance induced by repetitive TMS. Although this seems an obvious result, this kind of stratification based on residual functional and structural anatomy is rarely considered, although clearly has the potential to improve trial design (Ward, 2008).
Stinear & colleagues (2007) also set out to determine whether characterising the state of the motor system of a series of chronic stroke patients would help in predicting an individual's capacity for further functional improvement at least 6 months following stroke made in a subsequent motor practice programme. A variety of tools were used, including TMS, structural MRI and functional MRI. The presence or absence of MEPs to TMS in the affected upper limb, and fractional anisotropy values were both used to assess the structural integrity of the descending white matter pathways in the posterior limb of the internal capsules. Not surprisingly, in patients with MEPs, meaningful gains with motor practice were still possible 3 years after stroke. The situation in patients without MEPs has always been more difficult to predict in the clinical setting but is often taken as a poor prognostic sign (Heald et al. 1993). Here, the functional potential in patients without MEPs was predicted by corticospinal pathway disruption as assessed with fractional anisotropy values acquired with diffusion tensor imaging. Specifically, it was stated that below a certain threshold, little therapy induced functional improvement was possible. Conversely, in some patients without MEPs, the DTI data suggested that functional improvement was possible. Interestingly, the patients also performed a simple motor task during fMRI, but the results as assessed by the degree of lateralisation to one hemisphere or the other did not contribute to the predictive model. Nevertheless, this kind of study illustrates how multi-modal imaging and neurophysiological data could be used to assess the state of the motor system and predict the potential for therapy driven functional improvements. Such information could be very valuable in the process of goal setting during rehabilitation.
In two similar approaches, thirteen baseline clinical/radiological measures were made and then assessed to see whether each was able to predict subsequent gains made during 6 weeks of rehabilitation therapy. Only two baseline measures were significant and independent predictors of clinical improvement. The first was a lower level of impairment and the second was lower motor cortex activation as measured with fMRI (Cramer et al. 2007). In a subsequent analysis on the same data set, integrity of corticospinal fibres from M1 was also found to correlate with gains made during treatment (Riley et al. 2011) (Fig. 3). This is an interesting finding, because in general, patients with greater impairment are more likely to have less task related ipsilesional M1 activity, although this is an inconsistent finding. Despite this, the result tells us that there is something in the imaging data which is independent of baseline clinical impairment which predicts improvements. Lower baseline motor cortex activation was also associated with larger increases in motor cortex activation after treatment, and so it was suggested that low baseline cortical activity represents underuse of surviving cortical resources. When used carefully, it appears that measures of brain function as well as structure can be important for optimal clinical decision making in the context of a restorative intervention.
Figure 3. Examples of stroke injury to the tract descending from M1.
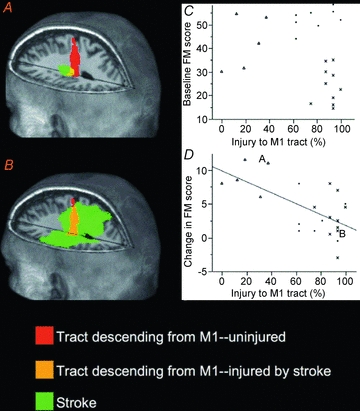
A, this subject had 37.5% of the M1 tract injured by stroke and had a gain of 11 points on the FM scale across the period of therapy.B, this subject had 93.4% of the M1 tract injured by stroke and had a gain of 1 point on the FM scale across the period of therapy.C, injury to the tract descending from M1 in relation to baseline FM score. A significant linear correlation was not present (P> 0.25). However, 3 subject clusters are apparent on inspection of the data: a subgroup of subjects with mild tract injury has mild to moderate motor deficits (marked as triangles); subjects with moderate to severe injury have either mild to moderate (marked as circles) or severe (marked as ‘x’) deficits. This injury/behaviour subgrouping was also apparent for the other 3 tracts.D, injury to the tract descending from M1 correlates (r = −0.65, P< 0.002) with the treatment-induced change in FM score. Subjects with mild tract injury had greater gains from treatment. A and B indicate the 2 subjects whose images appear on the left of the figure.
Functional MRI data acquired in the first few days after stroke has also been used to try to predict a subsequent change in motor performance (Marshall et al. 2009; Zarahn et al. 2011). A particular pattern of brain activation was highly predictive of clinical change over the next 3 months, a finding that was independent of initial stroke severity and lesion volume. Although the multivariate analysis used did not allow anatomical inference to be made, it is clear that there is something about the way the function of the brain responds to injury, over and above the anatomy of the damage, that holds clues about future clinical progression. The pattern was distributed and certainly not confined to the motor system, even though clinical improvement was measured in the motor domain. The idea that motor improvement may not be solely related to the integrity of the corticospinal system but also with other characteristics of the post-stroke brain is supported by the finding that motor performance at 3 months correlated only weakly with a measure of corticospinal tract integrity (using TMS) but strongly with a measure of intracortical excitability (Swayne et al. 2008). These findings suggest that the anatomy of the damage may set a limit on the extent of recovery, but that other parameters, perhaps preserved cortico-cortical connectivity, might be important when considering whether a patient has the capacity or potential to improve.
It is in this context that modern neuroimaging techniques may be able to shed light on post-stroke functional organisation in single subjects. This will require a shift away from approaches that look for correlations towards those that attempt to predict outcomes for individuals, something which is not currently done. Baseline data for these predictive models might include simple demographics, together with imaging and neurophysiological data. Techniques such as diffusion tensor imaging tractography and voxel based morphometry will allow some objective assessment of residual anatomy, and functional MRI can provide insights into the functional organisation of the residual systems. TMS and perhaps MR spectroscopy might be used to determine the state of populations of inhibitory and excitatory neurons. Ultimately this should facilitate an understanding of the mechanisms of interventions designed to reduce impairments, and also allow the stratification of patients based on the likely response to an intervention.
References
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, Fink GR, Nowak DA. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne OBC, Blankenburg F, Ruff C, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30:11926–11937. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrias MH, Belhaj-Saif A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex. 2006;16:632–638. doi: 10.1093/cercor/bhj009. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010a;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex. 2010b;20:169–186. doi: 10.1093/cercor/bhp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the unaffected forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, Trouard TP, Squire SW, Weinand ME, Savage CR, Wilkinson SB, Juranek J, Leu SY, Himes DM. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Küst J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134:1264–1276. doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993;116:1371–1385. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: a double-blind placebo-controlled crossover trial. Restor Neurol Neurosci. 2007;25:461–465. [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009;65:596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in humans. Acta Physiol Scand. 1994;150:27–38. doi: 10.1111/j.1748-1716.1994.tb09656.x. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Küst J, Karbe H, Fink GR. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke. 2001;32:1134–1139. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55:1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian, See J, Newton JM, Ward NS, Cramer SC. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Tanne J, Boussaoud D. Evidence for direct connections between the hand region of the supplementary motor area and cervical motoneurons in the macaque monkey. Eur J Neurosci. 1996;8:1055–1059. doi: 10.1111/j.1460-9568.1996.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–1528. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Dissociating motor cortex from the motor. J Physiol. 2011;589:5613–5624. doi: 10.1113/jphysiol.2011.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteropoulos DS, Edgley SA, Baker SN. Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey. J Neurosci. 2011;31:11208–11219. doi: 10.1523/JNEUROSCI.0257-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–434. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Swayne OBC, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganisation after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cerebral Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40:1772–1781. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Ward NS. Getting lost in translation. Curr Opin Neurol. 2008;21:625–627. doi: 10.1097/WCO.0b013e32831997af. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003a;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen H, Gandevia SC. Constraints for control of the human hand. J Physiol. 2011;589:5583–5593. doi: 10.1113/jphysiol.2011.217810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003b;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OBC, Lee L, Frackowiak RSJ, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OBC, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RSJ. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Swayne OBC, Newton JM. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging. 2008;29:1434–46. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Alon L, Ryan SL, Lazar RM, Vry MS, Weiller C, Marshall RS, Krakauer JW. Prediction of motor recovery using initial impairment and fMRI 48 h poststroke. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr047. 21, 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]


