Non-technical summary
Sodium channels are obligatory for the conduction of action potentials along axons. There are several different sodium channel subtypes expressed in vagal sensory neurons, and it is difficult to pharmacologically block these subtypes selectively. We used virally delivered shRNA to selectively block the production of one of the sodium channel subtypes expressed in vagal sensory neurons, namely NaV1.7, and found that by selectively inhibiting the expression of this channel the conduction of action potentials was blocked in the majority of vagal sensory neurons. This study also shows that NaV1.7 is required for the elicitation of classical vagal reflexes such as cough.
Abstract
Abstract
There has been much information learned in recent years about voltage gated sodium channel (NaV) subtypes in somatosensory pain signalling, but much less is known about the role of specific sodium channel subtypes in the vagal sensory system. In this study, we developed a technique using adeno-associated viruses (AAVs) to directly introduce shRNA against NaV1.7 subtype gene into the vagal sensory ganglia of guinea pigsin vivo. NaV1.7 gene expression in nodose ganglia was effectively and selectively reduced without influencing the expression of other sodium channel subtype genes including NaV1.1, 1.2, 1.3 1.6, 1.8, or 1.9. Using a whole cell patch-clamp technique, this effect on NaV1.7 gene expression coincided with a reduction in tetrodotoxin-sensitive sodium current, a requirement for much larger depolarizing stimulus to initiate action potentials, and reduction in repetitive action potential discharge. Extracellular recordings in the isolated vagus nerve revealed that the conduction of action potentials in sensory A- and C-fibres in many neurons was effectively abolished after NaV1.7 shRNA introduction. Moreover, bilateral NaV1.7 shRNA injected animals survived for several months and the vagal reflex behaviour, exemplified by citric acid-induced coughing, was significantly suppressed. These data indicate that selectively silencing NaV1.7 ion channel expression leads to a substantial decrease in neural excitability and conduction block in vagal afferent nerves.
Introduction
Molecular studies of sodium channels have identified nine α-subunit isoforms, referred as NaV1.1 to NaV1.9 (Catterall et al. 2005), and most of those except the NaV1.4 subtype have been found in sensory neurons (Cummins et al. 2007; Kwong et al. 2008; Dib-Hajj et al. 2010). Each of the NaV channel subtypes has unique kinetic properties that differentiate their effects on electrogenesis and propagation of action potentials. The overall voltage-gated sodium current in many sensory neurons is biphasic, with a rapidly activating and inactivating current followed by a slowly activating and inactivating current (Caffrey et al. 1992) (Roy & Narahashi, 1992). The kinetics of the currents produced and their sensitivity to tetrodotoxin (TTX) help differentiate the relative roles of sodium channel subtypes. Thus, the fast component of the voltage-gated sodium current is blocked by TTX, whereas a slow component is TTX resistant. This is explained by the fact that the slow current is due to the TTX-resistant channels, NaV1.8 and 1.9.
The concept that certain NaV channels make rational therapeutic targets for various types of pathological pain has motivated extensive investigation of NaV in somatosensory neurons. Various channelopathies have led to the conclusion that TTX-sensitive NaV1.7 and the TTX-resistant NaV1.8 and 1.9 are important for the peripheral control of pain (Dib-Hajj et al. 2010). This conclusion is based in part on the observation that nociceptive dorsal root ganglion (DRG) neurons commonly express NaV1.7, 1.8, and 1.9 (Dib-Hajj et al. 2010) whereas the non-nociceptor, large diameter neurons in DRG do not express NaV1.8 and 1.9 (Caffrey et al. 1992; Tate et al. 1998; Catterall et al. 2005).
There has been much less attention given to the study of NaV expression and function in vagal sensory neurons. Unlike the somatosensory system, NaV1.8 and 1.9 are not limited to nociceptors but are also expressed in vagal low threshold-mechanosensors innervating the respiratory tract (Kwong et al. 2008). Although TTX is slightly more potent at blocking the A-wave than the C-wave of the vagus nerve, the conduction in all vagal nerves is virtually abolished by 1 μm TTX (Farrag et al. 2002). This indicates that TTX-sensitive NaV ion channels are obligatory for action potential conduction in vagal axons. At the mRNA level, NaV1.7 is the most abundantly expressed TTX-sensitive channel in nodose ganglia, but our preliminary data showed that vagal afferent neurons also consistently express other TTX-sensitive channels, such as NaV1.2 and 1.3 (Kwong et al. 2008).
To critically evaluate the role specifically of NaV1.7 in vagal afferent neurons, we have taken a shRNA approach to selectively knockdown the expression of NaV1.7 in sensory neurons of the adult guinea pig nodose ganglia. Our data demonstrate that NaV1.7 strongly regulates vagal afferent nerve excitability and is essential for conduction of action potentials in virtually all vagal afferent nerves. We also show that silencing NaV1 7in vivoimpairs vagal reflexes such as cough, presumably by preventing communication between visceral tissue and the brainstem.
Methods
In vivotransfection of vagal primary afferent neurons
Male Hartley guinea pigs weighting ∼180–300 g (Hilltop Laboratory Animals, Inc., Scottsdale, PA, USA) were used in this study. This method has been described in detail (Kollarik et al. 2010). In brief, animals were anaesthetized with a mixture of ketamine/xylazine (50 mg kg−1/10 mg kg−1). Approximately 3 cm of incision was made over a shaved superficial portion of the masseter muscle area. The skin was retracted from the exposed muscle area using clamps. Vagal nodose ganglia were carefully exposed by scraping the muscle and membrane tissue using blunt-tipped instruments. Sterile cotton-tipped applicators and cotton pads were used to control bleeding throughout the surgery. The virus microinjection assembly consisted of a pulled glass micropipette (∼20 μm tip diameter) attached to a 1 ml syringe via plastic tubing. Micropipettes were filled with solution of virus using capillary force. The tip of the micropipette was gently inserted into the nodose ganglia. Virus (∼2–3 μl with a titre of (1–3) × 1012 PFU, pre-introduced into the pipette) was then injected by depressing the plunger (∼0.5 p.s.i.). The left nodose ganglia received an injection of AAV 2/8 vector encoding eGFP – a control (scrambled) shRNA, while right nodose ganglia received the virus encoding eGFP-NaV1.7 shRNA, in exception of coughing behaviour experiments, where both nodose ganglia of each animal received eGFP-control or -NaV1.7 shRNA. The animals were cared for post-operatively in the vivarium of the Johns Hopkins Asthma Centre. All procedures are approved by the Johns Hopkins Animal Care and Use Committee.
Generation of AAV8-based shRNA vector against guinea pig NaV1.7
Four shRNAs were designed to target the guinea pig NaV1.7 sequence using a commercial siRNA design programme (Dharmacon Inc., Boulder, CO, USA). Oligonucleotides were synthesized (Invitrogen, Paisley, Scotland), annealed and cloned downstream of the human U6 promoter in a derivative of the pENTR1A plasmid (Invitrogen, modified by GlaxoSmithKline). A negative control shRNA, based upon sequence of a commercially available negative control siRNA (Dharmacon Inc., Boulder, CO, USA) was also generated. The NaV1.7-targeting shRNA plasmids were then co-transfected with a guinea pig NaV1.7-expressing plasmid (gpNaV1.7/pCDNA3.1D/His-Topo) into HEK293 cells using Lipofectamine 2000 (Invitrogen). Efficiency of gene suppression was determined by TaqMan analysis using the primer and probe sequences for guinea pig NaV1.7 (Table 1). One of the shRNA clones exhibiting the highest degree of NaV1.7 knockdown was selected for further study.
Table 1.
Sequences of primers and probes used for quantitative RT-PCR analysis
| Gene | Primer sequence (5′ → 3′) | Probe sequence |
|---|---|---|
| NaV1.1 | Forward: TGAATGCCCTTTTAGGAGCAAT | FAM-CCATCCATCATGAATGTGCTTCTGGTTTG-MGB |
| Reverse: TGATGCTGAAAATTAGCCAGAATATAAG | ||
| NaV1.2 | Forward: GGACCTGACAGCTTCCGCTT | FAM-CCCTTGCTGCTATTGAACAACGCATTGC-MGB |
| Reverse: GCGCTCCTGTTTGGGTCTCT | ||
| NaV1.3 | Forward: AGCTTCCAGGGTGGATTGC | FAM-TGCACCACCAGACTGCGACCCT-MGB |
| Reverse: CTGCTTGCTCCCATTCTCAAC | ||
| NaV1.6 | Forward: TGGGCAGGTGGTTACTGCAT | FAM-TCCACATTCAGTCAATGCAACTTAGAACA-MGB |
| Reverse: CGGCAGCCTCTCCTCTGTTT | ||
| NaV1.7 | Forward: TTTTGCGGCTGCCCTAGA | FAM-CCCCCTCTTCTCATAGCAAAACCTAA-MGB |
| Reverse: CATGGCAATGAGCTGGACTTT | ||
| NaV1.8 | Forward: CTCTTCCGAGTCATCCGCCT | FAM-CACGCTGCTCTTTGCCCTCATGATGTC-MGB |
| Reverse: GCCCGATGTTGAAGAGAGCA | ||
| NaV1.9 | Forward: CACCAGGGTCCTCGGAGAGT | FAM-CCAGTGGCCTGGACACTATGAAAGCAA-MGB |
| Reverse: TTGGTGGTGGTGACTATGGG | ||
| GAPDH | Forward: CCACACCTAATGTGTCGGTTGT | FAM-ATCTGACCTGCCGCCTGGAGAAACC-MGB |
| Reverse: CCTTCTTGATGTCATCGTATTTGG |
The gpNaV1.7-targeting shRNA was as follows:
5′-GGGCATAGATTACGTGAAATTCAAGAGATTTCACGTAATCTATGCCC-3′
The sequence of the negative control shRNA was as follows:
5′-GTAGCGACTAAACACATCAATTCAAGAGATTGATGTGTTTAGTCGCTA-3′
Underlining denotes the sense and antisense sequences of the shRNA, respectively, separated by a ‘loop’ sequence. The U6 promoter–shRNA expression cassettes from this shRNA plasmid were subcloned into the AAV2-based shuttle plasmid pZAC2.1eGFP3 (kind gift of Dr Jim Wilson, Gene Therapy Program, University of Pennsylvania) and AAV8-based vectors were generated by the triple plasmid co-transfection method (Gao et al. 2002) and titrated by TaqMan analysis at the Vector Core, Gene Therapy Program, University of Pennsylvania).
Cell dissociation
Four to five weeks after AAV virus injection, animals were killed with CO2 and nodose ganglia were harvested and cleared of connective tissue with continuous wash with Krebs solution (in mm: 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 11.1 dextrose (pH 7.4). Nodose ganglia were then incubated in the solution containing dispase and collagenase (2 mg ml−1 each) dissolved in HBSS without calcium or magnesium at 37°C. Cells were washed three times with L-15 medium supplemented with 10% fetal bovine serum and then plated on laminin-treated glass cover slips.
Cell picking and quantitative real-time PCR
The nodose ganglia neurons adhered to coverslips were placed in a 37°C incubator and fed with the fresh L-15 medium 2 h later. Cell picking was performed within an hour after cell flooding. The coverslips were perfused continuously with Krebs solution and neurons expressing GFP were picked using pulled glass electrode (∼10 neurons in each electrode), collected in RNAse inhibitor (Invitrogen)-containing microcentrifuge tubes, immediately frozen and stored over dry ice. Pooled neurons were lysed and reverse transcribed using the Cells-to-Ct kit (Ambion) following the manufacturer's protocol. Neuron samples were lysed by the addition of 50 μ of lysis buffer. The lysis was stopped after 5 min and 22.5 μl of cell lysate was reverse transcribed with an RT- negative control (37°C for 60 min, 95°C for 5 min). Samples were pre-amplified using a multiplex mix of all the gene primer/MGB probe sets (Table 1: 45 μm each primer and 12.5 μm each probe final concentration) and TaqMan PreAmp Master Mix (Applied Biosystems) following the manufacturer's protocol (95°C for 10 min, 10 cycles of 95°C for 15 s and 60°C for 4 min). The pre-amplification reactions were diluted 1:5 with 1× TE buffer (Tris:EDTA buffer) and 4 μl was used for each individual analysis. Gene expression analysis was carried out using Taqman Fast Universal Mater Mix, No AmpErase UNG (Applied Biosystems), the appropriate single gene primers (final concentration of 900 μm each) and MGB probe (final concentration of 250 μm). Plates were cycled on an AB7900HT TaqMan Instrument (95°C for 10 min, 50 cycles of 95°C for 15 s and 60°C for 1 min). The relative abundance of each sodium channel subunit gene was determined and normalized to GAPDH reference gene expression. The expression of evaluated sodium channel gene in different treatment groups was compared by using Student's unpairedttest.
Vagus nerve extracellular recording
The vagus nerve with nodose ganglia was isolated from control animals and from animals approximately 4 weeks after the virus injection. GFP expression levels in the nodose ganglia were examined under an upright microscope illuminated with mercury arc to confirm the virus transduction. Adhering connective tissue of the nodose ganglia was then pinned to one side of a two-compartment recording chamber which contained silicone elastomer at the bottom, to be tightly exposed to a recording electrode. The vagus nerve was gently pulled into the adjacent compartment of the chamber through a small hole, suctioned tightly into a Krebs solution containing stimulus electrode. The chamber hole was sealed with Vaseline to prevent solution flow into adjacent compartment. Both compartments of the chamber were separately perfused with Krebs solution (37 ± 2°C), passing through a water-jacketed heating coil. The recording electrodes were filled with 3 m KCl. The vagus nerve was stimulated with a large supramaximal square pulse (60 V, 0.8 ms pulse duration), and compound action potential were measured at six to nine positions equally spaced within the nodose ganglion. At each position, the depth of the recording electrode into the ganglion was adjusted to obtain maximum signal amplitude. The measured action potentials were amplified (Microelectrode AC amplifier 1800; A-M Systems, Everett, WA, USA), filtered (0.3 kHz of low cut-off and 1 kHz of high cut-off), monitored on an oscilloscope (TDS340; Tektronix, Beaverton, OR, USA), recorded and analysed by NerveOflt software (Phocic, Baltimore, MD, USA). For the TTX experiments, the vagus was desheathed under a dissecting microscope to improve drug delivery. The tissue was treated with TTX for at least 30 min at each concentration. Time control experiments revealed no diminution in the amount of recorded action potential during the time course of the experiment.
Patch clamp electrophysiology
Standard whole cell patch clamp was performed to record sodium current and action potentials. Nodose ganglia were dissociated in a similar manner as described above and the recordings were performed within 24 h after plating. All experiments were conducted at room temperature. Patch electrode pipettes were fabricated from borosilicate glass (Warner Instruments, LLC, Hamden, CT, USA) to obtain tip resistance of 1.0–3.4 MΩ in the external solution. Series resistance was compensated at ∼70%. Coverslips with neurons were mounted in a chamber with an upright microscope illuminated with mercury arc and equipped with a Zeiss ×40 water immersion objective (Zeiss, Germany). Recordings were carried out using an Axopatch-800B patch-clamp amplifier (Axon Instruments/Molecular Devices, Union City, CA, USA), interfaced to a PC. Data acquisition, voltage control, and analysis were carried out with Axopatch and Axograph software. The cells were superfused with external solution by gravity feed at approximately 2 ml min−1.
External solution for recording sodium current contained (in mm): 0.1 CdCl2, 1 CaCl, 10 Hepes, 1 MgCl2, 14 NaCl, 126 choline-Cl, 20 TEA-Cl and 3 KCl titrated to pH 7.3 with CsOH (∼320 mosmol l−1, adjusted with d-glucose). The pipette solution consisted of (in mm): 140 CsF, 1.1 EGTA, 10 Hepes, 0.1 CaCl2, 2 MgCl2 and 10 NaCl titrated with CsOH to pH 7.3 (∼310 mosmol l−1, adjusted with d-glucose) (Cummins et al. 2009). Sodium currents were measured while holding the neurons at –80 mV, then prepulse potential of –120 mV (10 s) followed by a 50 ms depolarizing pulse from –100 to 35 mV in 5 mV increment. –P/6 subtraction was applied. For the measurements in the presence of TTX, 1 μm TTX dissolved in the recording solution was applied to the chamber 7 min prior to recording. In four experiments we found no difference in the current amplitude when vehicle rather than TTX was added for 7 min (106 ± 4% in respect to the original current without TTX). For the capsaicin sensitive current measurements, 10 mm capsaicin stock solution (in ethanol) was diluted into 1 μm in the recording solution and applied for 50 s. For action potential measurements, cells were clamped through the whole cell configuration and the external solution was composed of (in mm): 2 CaCl2, 10 Hepes, 2 MgCl2, 140 NaCl, 2 KCl titrated to pH 7.3 with NaOH (∼315 mosmol l−1) (Cummins et al. 2009); and pipette solution (in mm): 140 KCl, 11 EGTA, 5 Hepes, 1 CaCl2 and 2 MgCl2 titrated with KOH to pH 7.3 (∼310 mosmol l−1). Action potentials were measured by injecting 10 to 170 pA in increments of 10 pA. The conductance–voltage relationships were fit to a single Boltzmann function:
wherezis the valence, Vm is the half-maximal voltage, Kis the Boltzmann constant, Tis the temperature andeis the electronic charge. Input membrane resistance was measured by taking the slope of a straight line fit to the voltage–current plots of five hyperpolarizing steps. Membrane threshold was obtained by measuring the potential at the beginning of the sharp upward rise of action potential traces. The data are presented as means ± SEM and compared using ANOVA.
Inhalation challenges
Coughing in conscious guinea pigs was studied as previously described (Canning et al. 2004). Animals were placed in a chamber continuously filled with room air. Respiratory efforts were monitored by sound and by pressure changes within the chamber. An ultrasonic nebulizer (∼5 μm particle size) delivered citric acid (0.01, 0.1 and 0.3 m) dissolved in distilled water for 5 min at each dose, followed by an additional 5 min with the nebulizer turned off. Respiratory activity was recorded using an EMKA data acquisition system (Falls Church, VA, USA). Coughing was counted using the sound and pressure recordings and by visual confirmation. Animals were killed with CO2 after the experiments. The data are presented as means ± SEM and compared using ANOVA.
Statistics
All data are presented as mean ± SEM. Statistical analysis was assessed with Prism software (GraphPad). Each analysis method is noted in the figure and table legends. Statistical significance of differences was accepted atP< 0.05.
Results
TTX sensitivity of action potential conduction in nodose A- and C-fibres
The compound action potential measurement is a convenient method for evaluating action potential conduction along the vagus nerve, but the vagus nerve is a mixed nerve comprising jugular and nodose afferent nerve fibres and preganglionic parasympathetic efferent nerve fibres. To selectively evaluate nodose afferent nerves we placed an extracellular recording electrode near the cell bodies of the nodose ganglion (Fig. 1). The vagus nerve was stimulated at submaximal (10 V), maximal (30 V) and supramaximal (60 V) intensities, and the multiunit potentials were recorded in the nodose ganglion. The experiment was performed in the total of six vagi. At all stimulus intensities, a small group of action potentials were observed in the A-wave and a much larger group with conduction velocities in the C-wave. TTX at 0.1 μm abolished the response to 10 V stimulation (n = 6) in all six vagi. It abolished the response to the suparmaximal stimulus (60 V) in three preparations and reduced the response ∼50% in the other three experiments. Both the A- and C-wave were similarly inhibited in those three experiments. At a concentration of 1 μm, TTX abolished all responses at all stimulus intensities in each of the six experiments. The fidelity of the recording electrode is such that an action potential in a single fibre can be detected (Undem et al. 2003). These data therefore show that in virtually 100% of nodose axons, a TTX-sensitive sodium channel is required for action potential conduction.
Figure 1. Representative traces showing multiunit potentials recorded from the nodose ganglion elicited by a supramaximal stimulus (60 V) applied via a concentric stimulating electrode placed on the vagus nerve (∼50 mm) caudal of the ganglion.
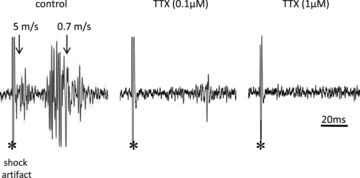
The initial deflection denoted by * is the shock artefact, followed by a group of multiunit potentials conducting in the A-wave and a larger group conducting in the C-wave. The arrows denote the conduction velocity of the responses arising at 5 m s−1 in the A-wave and 0.7 m s−1 in the C-wave. The same nerve was used to obtain the control potentials, the response after 30 min incubation with 0.1 μm TTX; and 30 min with 1 μm TTX. In 6 experiments the response to both the A-wave and C-wave was abolished at all stimulus intensities with 1 μm TTX, and the response to 0.1 μm TTX was largely reduced.
AAV vector encoding NaV1.7 shRNA reduced the NaV1.7 gene expression
Adeno-associated virus (AAV) encoding enhanced green fluorescence protein (eGFP) gene was directly injected into the left and right nodose ganglia. One side received the AAV encoding NaV1.7 shRNA gene and the other received AAV encoding control (scrambled) shRNA gene. Consistent with our previous report (Kollarik et al. 2010), the AAV efficiently transduced nodose neurons. Approximately 50–60% of the guinea pig nodose neurons were brightly expressing GFP in both right and left ganglia; we have previously noted that ∼80% of the neurons express GFP as detected immunohistochemically (Kollarik et al. 2010).
Nodose ganglion neurons strongly expressing GFP, based on visual inspection, were collected for quantitative real-time PCR analysis for identification of voltage gated sodium channel subtype gene expression. Previously, PCR analysis of adult guinea pig nodose tissue was performed and seven voltage gated sodium channel α-subunit isoform genes were identified: NaV1.1, 1.2, 1.3, 1.6, 1.7, 1.8 and 1.9 (Kwong et al. 2008). For our study, 10–15 GFP expressing neurons were pooled for each sample and all samples showed some level of sodium channel α-subunit isoform gene expressions (Fig. 2A). When the data from 10 samples were averaged it was clear that NaV1.7 gene was most dominantly expressed in the control shRNA transfected neurons (22.7 ± 5.7% of GAPDH reference gene). The order of NaV mRNA expression was 1.7 > 1.9 > 1.8 = 1.2 = 1.3 > 1.6 = 1.1 (Fig. 2B). The data also show that NaV1.7 gene expression was selectively and significantly (P< 0.01) reduced in nodose neurons isolated from the NaV1.7 shRNA treated ganglia (n = 11 samples). In 11 experiments with NaV1.7 shRNA treated ganglia, the expression of NaV1.7 was reduced by ∼90% (P< 0.01), whereas there was no significant reduction in the expression of the other sodium channels (Fig. 2B).
Figure 2. Quantitative real-time PCR for NaV1.1, 1.2, 1.3, 1.6, 1.7, 1.8 and 1.9 expressions.
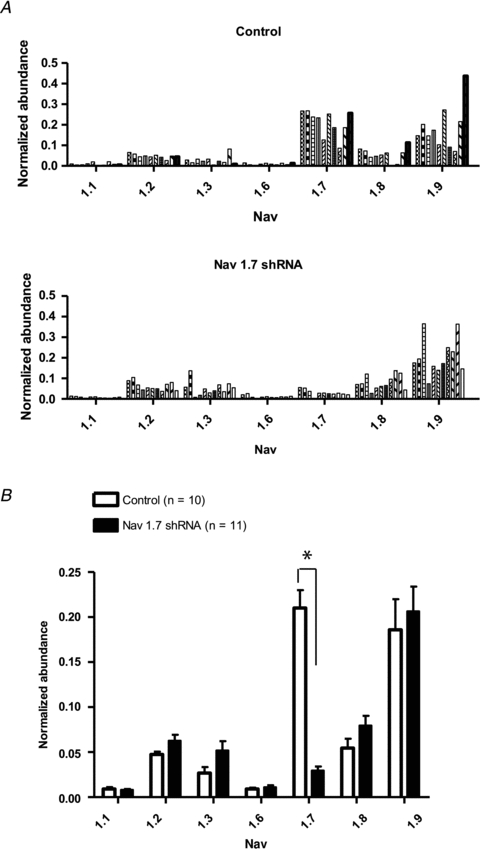
A, each bar represents mRNA expression in a sample, containing 10–15 neurons, isolated from nodose ganglia. The top panel represents neurons isolated from a nodose ganglion previously treated with AAV-GFP with control (scrambled) shRNA. The bottom panel represents neurons from ganglia treated with AAV-GFP with NaV1.7 shRNA. All neurons analysed are GFP positive.B, each bar represents the mean ± SEM of mRNA expression represented inA. Open bars represent neurons from control shRNA ganglia and dark bars are neurons from NaV1.7 shRNA treated ganglia. Statistical comparisons were made using unpairedttests between two shRNA treatments. An astrisk (*) denotes a significant difference (P< 0.01) between treated and control values.
Decreased sodium channel current in nodose ganglion neurons
Whole cell patch clamp was used to measure voltage-gated sodium currents in dissociated AAV virus transduced (i.e. GFP expressing) nodose ganglia neurons. The sodium current was carried mainly by TTX-sensitive channels; the TTX-resistant component averaged 20.1 ± 3.8% (range 5–52%) of the current. We observed no neurons in which TTX abolished all current, nor did we observe neurons where the majority of the current was TTX-resistant. The results are comparable with what we found with non-transfected neurons where the TTX-resistant component was 35 ± 12% of the total peak current (P> 0.05 to control shRNA treated neurons).
In neurons transduced with NaV1.7 shRNA, the fast TTX-sensitive component of the sodium current was strongly inhibited by >60% (P< 0.01; Fig. 3A and B). We also performed sodium current measurements in the presence and absence of 1.0 μm TTX (Fig. 3B). The mean data presented in the Fig. 3B show that on average there is a TTX-sensitive current, albeit very small, remaining in the NaV1.7 shRNA treated neurons. In the majority (12/17) of NaV1.7 shRNA treated neurons, there was virtually no TTX-sensitive current present, and the current density was the same as that observed with control neurons after TTX treatment. In 5 of 17 NaV1.7 shRNA treated neurons there was a sizeable TTX-sensitive current that persisted. Thus, in 12 of 17 nodose neurons NaV1.7 appeared to account for virtually all the TTX-sensitive sodium current. TheVm (half-maximum voltage) of the sodium current in the neurons transduced with NaV1.7 shRNA (∼–23 mV) was significantly more depolarized compared to the neurons transduced with control shRNA (–32 mV) (P< 0.01). TTX application caused an even larger change inVm leading to an averageVm value of about –15 mV (Table 2).
Figure 3. Sodium currents measured in nodose ganglion neurons.
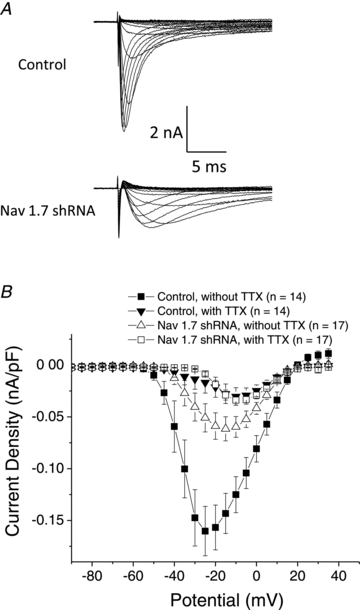
A, families of sodium current in neurons isolated from ganglia treated with control shRNA (top) and with NaV1.7 shRNA (bottom). Ionic currents were measured by pulsing to various potentials (–100 to 40 mV with 5 mV increments) for 50 ms. Traces obtained at –100 through 5 mV are shown.B, current density–voltage relationships of control and NaV1.7 shRNA treated neurons in the absence and presence of 1 μm TTX. Each point is the mean ± SEM (n = 14–17).
Table 2.
Boltzmann parameters of the conductance–voltage relationships
| n | Vm | z | |
|---|---|---|---|
| Before TTX (control) | 14 | −32.17 ± 1.53 | 7.0 ± 0.89 |
| After TTX (control) | 14 | −16.4 ± 1.92* | 5.8 ± 0.24 |
| Before TTX (NaV1.7 shRNA) | 17 | −23.2 ± 1.87† | 5.2 ± 0.36 |
| After TTX (NaV1.7 shRNA) | 17 | −14.8 ± 1.04* | 5.8 ± 0.39 |
Vm andzvalues are mean ± SEM. Statistical comparisons were made using two-way ANOVAs (shRNA treatment and TTX application factors) followed by Bonferroni'spost hoctests. An asterisk denotes a significant difference caused by TTX treatment (*P< 0.01). A † denotes a difference (P< 0.01) inVm between control and NaV1.7 shRNA treated neurons.
Capsaicin (1 μm) was applied at the end of each experiment. We observed 19 out of 31 neurons studied were capsaicin sensitive. There were seven capsaicin-sensitive neurons among 17 NaV1.7 shRNA treated neurons. There was no statistically significant difference in the amplitude of the sodium current between capsaicin-sensitive and insensitive neurons.
NaV gene expression in individual (non-pooled) neurons
There was a TTX-sensitive current remaining in 5 of 17 NaV1.7 shRNA treated neurons even though NaV1.7 expression was consistently reduced ∼90% in the pooled neurons. We therefore wanted to determine whether or not NaV1.7 expression reduction was consistent at an individual neuronal level. The NaV1.7 was silenced >90% in 8 of 8 individual GFP expressing neurons we evaluated (0% in 5 neurons and 3.4%, 0.1%, and 1.2% in the other 3 neurons in respect to normalized GAPDH reference gene expression; data not shown). It therefore appears that a failure in silencing of NaV1.7 expression is unlikely to accounts for the TTX-sensitive current in the 5 of 17 neurons.
Although the TTX-sensitive sodium ion channels other than NaV1.7 are poorly expressed in nodose neurons (as revealed in our pooled neuron experiments) we asked whether there was appreciable expression of NaV1.1, 1.2, 1.3 or 1.6 in individual neurons (e.g. 10% more in respect to normalized GAPDH expression). We found that in 4 of 22 neurons a non-NaV1.7 TTX-sensitive channel was expressed above this arbitrary cut-off: NaV1.6 (16% expression, one neuron); NaV1.1 (101% expression, one neuron) and NaV1.2 (14% and 12%, in 2 neurons). These data must be cautiously interpreted but provide some support for the conclusion that in a small subset of neurons there may be sufficient expression of TTX-sensitive channels other than NaV1.7 to account for the a sizeable component of fast inward current.
Action potential activity in individual neurons
To further investigate the effect of NaV1.7 shRNA transfection using AAV virus at the cellular level, action potential measurements were performed on shRNA treated neurons. The amount of current required to evoke action potentials for control shRNA transfected neurons averaged 18 ± 4 pA (n = 12), which was not significantly different from naïve neurons (24 ± 3 pA, n = 12). However, in NaV1.7 shRNA treated neurons, rheobase was significantly increased to 105 ± 13 pA (Fig. 4) (n = 9, P< 0.01). The action potential membrane threshold was also significantly different, –46 ± 1 mV (n = 12) for control and –29 ± 2 (n = 9) (P< 0.01) for NaV1.7 shRNA treated neurons (Table 3). The treated and control neurons had similar resting membrane potentials and spike width at 0 mV (Table 3) and their input resistance was not significantly different to each other (Naïve: 711.5 ± 107 MΩ, control shRNA: 855.1 ± 128 MΩ and NaV1.7 shRNA: 676 ± 185 MΩ, n = 6, 7, and 6 respectively).
Figure 4. Examples of current-clamp recording of nodose neurons isolated from naïve (wild-type), control shRNA treated, and NaV1.7 shRNA treated ganglia.
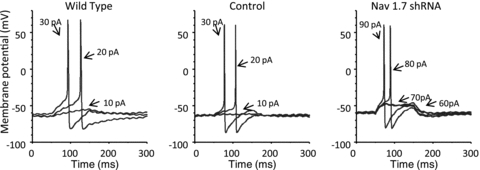
The neurons were stimulated with a 100 ms depolarizing current pulse of the stated pA intensity.
Table 3.
Resting membrane potential, action potential threshold, rheobase and spike width at 0 mV of (n) number of experiments (Mean ± SEM)
| Naïve | Control | NaV1.7 shRNA | |
|---|---|---|---|
| Resting membrane potential (mV) | −50.7 ± 3.07 (7) | −50.4 ± 1.45 (12) | −48.3 ± 3.61 (8) |
| Threshold (mV) | −41.6 ± 1.13 (12) | −46.1 ± 0.70 (12) | −29.3 ± 2.42 (9)* |
| Rheobase (pA) | 24.2 ± 2.88 (12) | 17.8 ± 3.64 (12) | 105 ± 13.23 (9)* |
| Width at 0 mV (ms) | 3.7 ± 0.61 (12) | 2.3 ± 0.29 (12) | 2.8 ± 0.22 (9) |
Statistical comparisons were made using one-way ANOVAs followed by Tukey'spost hoctest. An asterisk denotes a significant difference between naïve and control neurons (*P< 0.01 compared to naïve and control groups).
The action potential discharge pattern to a prolonged suprathrehsold stimulus pulse was also strongly influenced by the NaV1.7 shRNA (Fig. 5). A 1 s pulse of 400 pA led to repetitive discharge of action potentials throughout the stimulus at a frequency that was consistently above 25 Hz for control shRNA treated neurons. In NaV1.7 shRNA transfected neurons, the peak frequency ranged from 1 to 34 Hz with an average of about 10 Hz (n = 8), significantly different from that of control (P< 0.05). Current clamp experiments were also performed in naïve (wild-type) guinea pig nodose ganglia neurons and the results were very similar to those obtained with control shRNA treated neurons (>25 Hz, n = 9), indicating that transduction with AAV carrying control shRNA with eGFP genes does not influence the active or passive electrophysiological properties of the neurons.
Figure 5. Recordings of repetitive firing of action potentials in isolated nodose neurons.
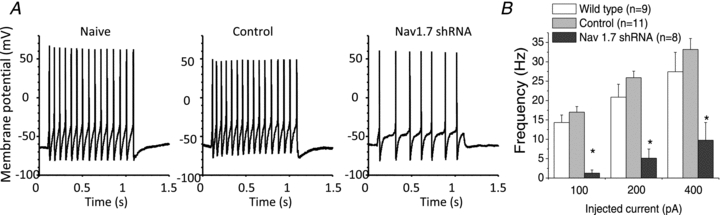
A, examples of current-clamp recording of nodose neurons isolated from naïve, control shRNA treated, and NaV1.7 shRNA treated ganglia. Each neuron was stimulated with 100 pA current pulse for 1 s.B, the mean ± SEM of the number of spikes during a 1 s current pulse of the stated intensity (n≥ 8 neurons). Statistical comparisons were made using two-way mixed model (repeated measures test) ANOVAs (factors of injected current and shRNA treatment) followed by Bonferroni'spost hoctest. An asterisk denotes a significant differences from naïve and control groups (P< 0.05).
Action potential conductance in the vagus nerves following NaV1.7 shRNA transfection
The functional effect of NaV1.7 silencing on action potential conduction was next evaluated with the extracellular electrophysiological recording preparation discussed above (Fig. 1). This experimental design evaluates the activity of all vagal nodose sensory neurons (both transduced and uninfected), while the gene expression and electrophysiological analysis described above were carried out specifically on transduced (confirmed by GFP) neurons.
Multiunit potentials were recorded from nine positions within most nodose ganglia (Fig. 6A). In some ganglia, due to the morphology of the ganglion, only six positions were studied. The recorded positions were predetermined based on past extensive experience with this technique; the positions were approximately equally spaced within the ganglia, away from the through fibres carried by the vagus nerve. The multi-unit potential was induced by applying an electrical square pulse of maximum intensity to the peripheral cut end of the vagus nerves. In total, right and left vagi from 18 animals were studied, with recordings made from 157 positions in NaV1.7 shRNA treated ganglia and 156 positions in control shRNA treated ganglia (each animal had shRNA injected into only one ganglion, allowing the contralateral ganglion/vagus nerve to serve as control).
Figure 6. Recordings of conducted action potentials in the vagus nerves.
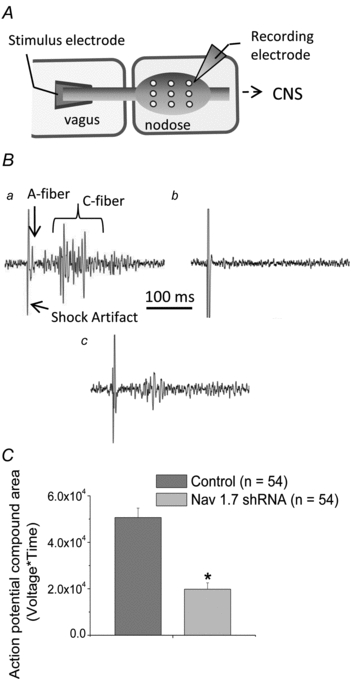
A, a schematic diagram of the extracellular recording technique used to evaluate action potential conduction of nodose axons in vagus nerves. All axons in the vagus nerve were stimulated with a 0.8 ms, 65 V square pulse, and the action potentials arriving at the ganglia were evaluated with an extracellular recording electrode positioned in nine positions in a given ganglion. In each animal one ganglion was treated with NaV1.7 shRNA and the contralateral ganglion was treated with control shRNA.Ba, representative large multiunit recording typically observed in 100% (156 of 156) of the electrode positions in ganglia isolated from control shRNA treated nodose ganglia.b, representative recording from a position in which no action potentials were recorded by the extracellular electrode. This occurred in 56/157 recording positions in NaV1.7 shRNA treated ganglia.c, representative trace of a recording at a position in which an obvious, yet small, multiunit recording, occurred in 101/157 positions in NaV1.7 shRNA treated ganglia.C, the area under the multiunit action potential curve obtained from extracellular recordings in control shRNA treated and NaV1.7 shRNA treated ganglia. From each nodose ganglion, compound action potentials from three positions were chosen and used for area calculation (n = 54 for each group, measured from 18 animals total). Statistic comparisons were made using one-tailedttests. An asterisk denotes a significant difference (P< 0.01) between the values.
A single square pulse stimulation of the vagus nerve led to a barrage of action potentials in each of the 156 positions of the control shRNA treated ganglia (100%) (Fig. 6B). It should be noted that the observable ‘spikes’ represent a multiunit recording comprising single action potentials in each of multiple afferent neurons. The earliest arising action potentials correspond to A-fibres, whereas the bulk of activity arrives later and corresponds to C-fibres (Fig. 6Ba). By contrast, in vagi treated with the NaV1.7 shRNA, no action potentials were recorded from 56 of 157 positions (Fig. 6Bc). In other words, action potential conduction in the A- and C-fibres was completely blocked in the large numbers of neurons being sampled at nearly 40% of the sites evaluated. In the remaining 101 sites, action potential activity was observed, but the number and size of the multi-units observed were smaller than the ones in the control ganglia (Fig. 6Bc). To provide some sort of quantification of this activity, an area under the curve analysis was carried out (3 random positions from each ganglion), revealing that NaV1.7 shRNA injected the nodose ganglia produced >60% reduction in multiunit recordings (Fig. 6C) (P< 0.01).
Effect of NaV1.7 silencing on the cough reflex in awake guinea pigs
We subsequently studied the effects of silencing NaV1.7 gene expressionin vivoon evoked cough responses in awake animals. In these experiments, both the right and left nodose ganglia in each animal received NaV1.7 shRNA. The animals were evaluated 1–4 months later. All animals survived this treatment, gained normal weight and did not display any abnormal behaviours. Naïve guinea pigs reproducibly cough upon inhalation of citric acid, which evoked 11 ± 3 coughs in these guinea pigs (n = 18). In contrast, citric acid-induced cough was nearly abolished in animals bilaterally treated with NaV1.7 shRNA (Fig. 7). Two animals that were treated bilaterally with control (scrambled) shRNA responded to citric acid similar to naïve guinea pigs, coughing 6 and 23 times.
Figure 7. The number of coughs, expressed as means ± SEM, evoked by exposing guinea pigs to an aerosol containing 0.1 m citric acid.
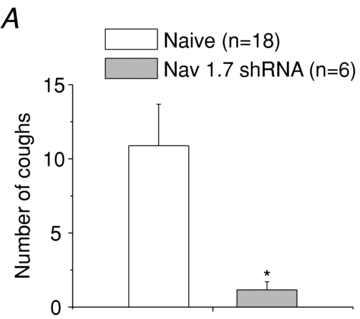
Guinea pigs were naïve (n = 18) or treated bilaterally with NaV1.7 shRNA 1–4 months prior to the experiment (n = 6). Statistic comparisons were made using one-tailedttests. An asterisk denotes a significant difference (P< 0.05), between the values. Two animals treated bilaterally with control shRNA 5 weeks prior to the experiment coughed an average of 15 times in response to 0.1 m citric acid (not shown).
In contrast to the cough reflex, silencing NaV1.7 expression bilaterally in nodose neurons had no consistent effect on respiration rate. In naïve animals the respiration rate was 118 ± 6 breaths min−1 (n = 18). The respiration rate in the two control shRNA treated animals averaged 127 breaths min−1 (109 and 145 breaths min−1) while the four NaV1.7 shRNA treated animals produced an average of 108 ± 12 breaths min−1 (in the range of 86–144 breaths min−1).
Discussion
The results demonstrate that AAV vectors encoding specific shRNA can be effectively used to silence NaV1.7 channel gene expression in sensory neuronsin vivowithout inhibiting expression of other NaV subunit genes. The data support the conclusion that in vagal sensory neurons NaV1.7 plays a critical role in regulating neuronal excitability, and an obligatory role in action potential conduction in the majority of vagal afferent nerves. The results also reveal that animals survive for several months with no overt symptoms of ill health with NaV1.7 expression silenced in the majority of nodose neurons. This could potentially provide a useful research model for those interested in investigating the contribution of vagal reflex activity to physiological and pathophysiological processes.
The qPCR analyses show that in neurons transduced with NaV1.7 shRNA, as indicated by the presence of GFP, the expression of NaV1.7 mRNA expression was strongly and selectively inhibited. This allowed for an evaluation of the role of NaV1.7 in nodose nerve function. Overall the sodium current was inhibited by about 70% in NaV1.7 shRNA treated neurons. In 70% of nodose neurons, NaV1.7 appeared to account for virtually all of the TTX-sensitive sodium current. It is not clear why an appreciable TTX-sensitive current persisted in 5 of 17 NaV1.7 mRNA treated neurons. We did not find other unique electrophysiological characteristic in these neurons. It should be noted that that the presence of GFP indicates a successful transfection, but since the expression of GFP and shRNA are by necessity driven by different promoters, the levels of expression of GFP and shRNA may vary in individual neurons. Therefore, there may have been incomplete silencing of NaV1.7 gene expression in five of the GFP expressing neurons. However when we evaluated the gene expression in randomly selected individual GFP expressing neurons, the NaV1.7 shRNA was universally effective at silencing the gene. In a minority of vagal afferent neurons, TTX-sensitive channels other than NaV1.7 may have carried much of the TTX-sensitive current. This hypothesis finds support in our analysis that showed in 4 of 22 neurons 1.2, 1.3 or 1.6 was expressed at levels appreciably higher than the pooled average.
NaV1.6 is thought to be involved in action potential conduction in some sensory nerves. In the peripheral nervous system, NaV 1.6 is mainly, but not exclusively, expressed in large diameter neurons in the DRG. Anin situhybridization analysis of NaV1.6 expression in rat DRG shows that this channel is most highly expressed in a subset of larger diameter neurons that do not express NaV1.8 or 1.9 (Fukuoka et al. 2008; Fukuoka & Noguchi, 2011). Consistent with these studies, NaV1.6 has been observed mainly in myelinated peripheral axons, particularly at the nodes of Ranvier (Caldwell et al. 2000), although others have noted NaV1.6 immunoraectivy in C-fibre axons in the mouse sciatic nerve (Black et al. 2002). The nodose ganglion comprises both C-fibre and Aδ-fibres. It is devoid of the very large diameter (NaV1.8 and 1.9 lacking) neurons seen in the DRG. In guinea pigs at least, even the non-nociceptive stretch sensitive A-fibre neurons express NaV1.8 and 1.9 (Kwong et al. 2008). It is therefore not surprising that NaV1.6 was found to be relatively poorly expressed in the vagal sensory neurons.
Our results using vagal sensory neurons are different from those of Nassar et al. (2004) who studied NaV1.7 silencing in murine DRG neurons. In their study, NaV1.7 was conditionally knocked out in NaV1.8 expressing neurons using a cre-recombinase-loxP system. This produced only a marginal reduction in fast TTX-sensitive inward current (Nassar et al. 2004). These results were interpreted as evidence that NaV1.1 and 1.6 carry the TTX-sensitive conductance in these DRG neurons.
The vast majority of fast inward sodium current in the adult guinea pig neurons was consistently TTX sensitive. We did not observe the large heterogeneity in TTX-sensitivity seen in rat nodose neurons (Li & Schild, 2007). In rats, the sodium current in faster conducting A-fibre neurons is exclusively TTX sensitive while nearly all the current is TTX resistant in slower conducting A-fibre (12% of the total population). In the C-fibre population the current is ∼50% TTX sensitive. We have previously reported that there is a TTX-resistant current in all nodose neurons innervating the guinea pig airways, including the fast-conducting A-fibre population (Kwong et al. 2008).
Based on elegant modelling studies of neonatal rat nodose neurons, the fast TTX-sensitive sodium current strongly regulates neuronal excitability (Schild & Kunze, 1997). Since NaV1.7 is responsible for the majority of the fast inward current, its absence should profoundly affect neuronal excitability. This prediction was satisfied in our current clamp studies showing a large change in rheobase. The observation that the action potential discharge frequency was substantially reduced in neurons lacking NaV1.7 is also consistent with models of the contribution of the fast inward current to repetitive action potential firing (Schild & Kunze, 1997). The resting membrane potential was not affected by silencing NaV1.7. This was not unexpected, as it has been suggested in DRG nociceptors that NaV1.9 is likely to play a more important role in this regard (Herzog et al. 2001).
Our extracellular recordings of action potentials conducted along the vagus nerves supports the hypothesis that NaV1.7 is essential for nerve conduction in vagal afferent axons. The findings that the NaV channel isoform expression is relatively homogenous in the nodose ganglia is consistent with the observation that action potential conduction was blocked in both the fast and slow conducting axons (A- and C-fibres). The data supporting this conclusion were the complete absence of any multiunit action potentials being observed in ∼40% of some 157 recording positions in 18 nodose ganglia treated with NaV1.7 shRNA, upon electrical stimulation of the peripheral cut end of the vagus nerve. By contrast, strong multiunit recordings were obtained in 100% of the 156 electrode positions in 18 ganglia treated with the control shRNA. The simplest interpretation of these data is that if NaV1.7 gene expression was effectively silenced in a nodose neuron, then it was incapable of conducting an action potential from the stimulated end of the nerve to the cell bodies in the ganglion. Alternatively NaV1.7 may be required for the initiation of action potentials, even upon very strong electrical stimulation (supramaximal voltage of a 0.8 ms square-pulse). There were multi-unit potentials recorded in 60% of the sites, but even at these sites the number of axons contributing to the multiunit potential was largely inhibited. This is consistent with our finding that strong GFP intensity was noted in only about 50% of the neurons isolated from the ganglia injected with NaV1.7 shRNA, with weak expression of GFP in another 10–20% of neurons and no expression at all in the remaining neurons. Therefore, NaV1.7 mRNA was likely to be normal or near normal in a minority of the ganglion neurons, which likely accounts for the small activity seen at many recording sites. Nevertheless, the data cannot rule out the possibility that in a small subset of vagal axons, sodium channels other than NaV1.7 are responsible for action potential conduction. Indeed, our patch clamp electrophysiology analysis and qPCR of isolated single neurons revealed that a sodium channel other than NaV1.7 may account for the TTX-sensitive current in a small minority of nodose neurons.
Our findings are consistent with the observation that the potent and selective NaV1.7 antagonist proTX-II blocks nerve conduction in anex vivosaphenous nerve preparation (Schmalhofer et al. 2008). In this preparation, proTX-II inhibited propagation of action potentials in C-fibres more potently and effectively than the Aβ fibres. However, it should be noted that nearly 1 μm of the drug was required for 50% inhibition of the compound potential even in the C-fibres, and the selectivity for NaV1.7versusother NaV channels was found to be minimal at that concentration (Schmalhofer et al. 2008). Our finding that TTX abolished conduction of action potentials in virtually all nodose axons is consistent with compound action potential analysis of the vagus nerveper se(Keynes et al. 1971; Farrag et al. 2002). Therefore, the blockade of action potential conduction in the vagal sensory axons we observed in NaV1.7 shRNA treated ganglia was predicted by the observation that NaV1.7 carries the majority of TTX-sensitive sodium current in vagal sensory neuronal cell bodies. Olfactory neurons may share with vagal neurons a dependency on NaV1.7 for action potential conduction. Recent studies in mutant mice in which NaV1.7 was conditionally knocked out of olfactory sensory neurons have led to the conclusion that although this channel only contributes to a minority of the TTX-sensitive current in the cell soma, in the axons it is ‘an essential and non-redundant requirement to initiate information transfer from OSN terminals to neurons in the olfactory bulb’ (Weiss et al. 2011).
Our results indicate that blocking NaV1.7 expression in vagal sensory nerves will inhibit conduction of action potentials by vagal A- and C-fibre axons. The NaV1.7 shRNA therefore provides a tool with which one can silence the communication between visceral tissues and the nucleus tractus solitarii, without cutting the vagus nerve (which also interrupts efferent parasympathetic transmission). Acute bilateral vagotomy of laboratory birds and mammals results in a substantial reduction in respiration rate (Richards, 1968). Guinea pigs are particularly susceptible to this, and in fact they rarely survive the treatment in the absence of artificial respiration. We found here that the animals with bilateral treatment with NaV1.7 shRNA survived for several months, with no apparent behavioural problems. Moreover, the respiration rate in these animals was normal or near normal. This may be explained by central adaptation mechanisms or by a large ‘safety factor’ in the vagal regulation of respiration. Unlike acute vagotomy, NaV1.7 silencing using AAV virus probably occurred in a gradual fashion over the course of days to weeks, thereby allowing time for central adaptation. Also unlike acute vagotomy, the shRNA treatment did not abolish conduction in 100% of the vagal axons. The axons from neurons situated in the jugular vagal ganglion were likely to be largely spared, as well as at least 20% of the nodose neurons (as discussed above). If there is a large safety factor in the vagal regulation of respiration, then the activity in a minority of neurons may have been sufficient for the maintenance of normal respiration. Indeed, we have reported previously that unilateral vagotomy (which would reduce overall afferent drive by ∼50%) has only marginal effects of respiratory rate (Canning et al. 2004).
The animals with bilateral silencing of NaV1.7 in the nodose ganglia showed a defect in vagal reflex behaviour. Coughing is a well-recognized reflex that requires intact vagal afferent nerves (Canning & Chou, 2009). The observation that the cough reflex was largely inhibited in NaV1.7 silenced animals, even upon a strong stimulus (inhalation of 0.1 m citric acid) indicates that a substantial amount of vagal afferent activity was blocked. This method of selective silencing of vagal afferent, and not efferent, nerve function may be useful in studies of other putative vagal reflex behaviours. Although these animals kept under carefully controlled conditions of the vivarium appeared relatively healthy, it is likely that they will exhibit informative defects in studies that severely challenge homeostasis (changes in blood volume, tonicity, temperature, caloric restricton, visceral inflammation, tilt-table studies, etc.).
Individuals carrying a loss of function mutation in NaV1.7 (SCN9A) have been described. This is a rare mutation, and thus not readily amenable for investigation. Nevertheless, these individuals have been found to present with a severe defect in the ability to detect painful stimuli (Cox et al. 2006). The results presented here predict that these people may breathe normally (vagal afferent activity is not a critical regulator of respiration rate in humans; subjects with bilateral lung transplants have no afferent communication to the brainstem, yet breathe relatively normally), but have alterations in their cough reflex physiology. One might also predict that they will have some defects in their ability to reflexively control certain vagal autonomic functions.
The results presented here suggest a novel therapeutic strategy for treating cough. Dry irritable cough is a common symptom of respiratory disease. To date, there is little evidence that available anti-tussive drugs inhibit this pathological ‘neuropathic’ cough any better than placebo. The present data point to the possibility that NaV1.7 may be a rational target for the development of peripherally acting anti-tussive agents. Cough, however, is also an important defensive reflex. In this respect the present data may serve as a caveat for those developing strong blockers of NaV1.7 as novel analgesic drugs. The complete cessation of reflex cough would be an undesirable side-effect. NaV1.7 has recently been found to be an obligatory sodium channel for the sensation of smell (Weiss et al. 2011), and thus anosmia also needs to be considered as a potential unwanted side effect of NaV1.7 channel antagonists.
Acknowledgments
This work was supported in part by funding from the National Institutes of Health, Bethesda, MD, and from GlaxoSmithKline Pharmaceuticals, USA. M.K. is supported in part by CEVYPET (EU) and VEGA 1/0276/10.
Glossary
Abbreviations
- AAV
adeno-associated virus
- DRG
dorsal root ganglion
- eGFP
enhanced green fluorescence protein
- NaV
voltage gated sodium channel
- TTX
tetrodotoxin
Author contributions
All authors contributed to conception and design of the experiments, data collection, analysis and interpretation. Y.M. and B.J.U. contributed to the initial drafting of the article, and all authors revised the article for important intellectual content and approved the final version of the manuscript. Generation and validation of AAV8-based shRNA vector against guinea pig NaV1.7 was performed at GlaxoSmithKline Pharmaceuticals, Stevenage, UK and Upper Providence, PA, USA. All neuronal experiments were performed at Johns Hopkins University.
References
- Black JA, Renganathan M, Waxman SG. Sodium channel Nav1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res. 2002;105:19–28. doi: 10.1016/s0169-328x(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Rush AM, Estacion M, Dib-Hajj SD, Waxman SG. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat Protoc. 2009;4:1103–1112. doi: 10.1038/nprot.2009.91. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- Farrag KJ, Costa SK, Docherty RJ. Differential sensitivity to tetrodotoxin and lack of effect of prostaglandin E2 on the pharmacology and physiology of propagated action potentials. Br J Pharmacol. 2002;135:1449–1456. doi: 10.1038/sj.bjp.0704607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Kobayashi K, Yamanaka H, Obata K, Dai Y, Noguchi K. Comparative study of the distribution of the α-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J Comp Neurol. 2008;510:188–206. doi: 10.1002/cne.21786. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Noguchi K. Comparative study of voltage-gated sodium channel α-subunits in non-overlapping four neuronal populations in the rat dorsal root ganglion. Neurosci Res. 2011;70:164–171. doi: 10.1016/j.neures.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol. 2001;86:1351–1364. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- Keynes RD, Ritchie JM, Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971;213:235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Carr MJ, Ru F, Ring CJ, Hart VJ, Murdock P, Myers AC, Muroi Y, Undem BJ. Transgene expression and effective gene silencing in vagal afferent neuronsin vivousing recombinant adeno-associated virus vectors. J Physiol. 2010;588:4303–4315. doi: 10.1113/jphysiol.2010.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing JP, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol. 2008;586:1321–1336. doi: 10.1113/jphysiol.2007.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods. 2007;164:75–85. doi: 10.1016/j.jneumeth.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SA. Vagal control of thermal panting in mammals and birds. J Physiol. 1968;199:89–101. doi: 10.1113/jphysiol.1968.sp008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74:1476–1484. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Oh EJ, Lancaster E, Weinreich D. Effect of extracellular calcium on excitability of guinea pig airway vagal afferent nerves. J Neurophysiol. 2003;89:1196–1204. doi: 10.1152/jn.00553.2002. [DOI] [PubMed] [Google Scholar]
- Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, Zizzari P, Gossage SJ, Greer CA, Leinders-Zufall T, Woods CG, Wood JN, Zufall F. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–190. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]


