Non-technical summary
Brain orexin/hypocretin neurons stimulate wakefulness, feeding, reward-seeking and healthy glucose balance. The activity of orexin neurons is tightly regulated by several hormones, neurotransmitters and nutrients. Intriguingly, elevated glucose concentration can block or silence the activity of orexin neurons. We identified an unexpected way to control these effects of glucose on orexin neurons. We found that supplying orexin neurons with other energy-related molecules, such as pyruvate and lactate, can stop glucose from blocking orexin neurons. We hypothesize that orexin neurons only ‘see’ glucose changes when the levels of other energy molecules are low, whereas high energy levels can stop glucose from regulating orexin cells. This may shed new light on understanding how the brain is influenced by changes in glucose levels during different metabolic situations, such as fasting, eating different diets, or in disease states such as diabetes and obesity.
Abstract
Abstract
Central orexin/hypocretin neurons promote wakefulness, feeding and reward-seeking, and control blood glucose levels by regulating sympathetic outflow to the periphery. Glucose itself directly suppresses the electrical activity and cytosolic calcium levels of orexin cells. Recentin vitrostudies suggested that glucose inhibition of orexin cells may be mechanistically unusual, because it persists under conditions where glucose metabolism is unlikely. To investigate this further, and to clarify whether background metabolic state regulates orexin cell glucosensing, here we analysed glucose responses of orexin cells in mouse brain slices, in the presence and absence of metabolic inhibitors and physiological energy substrates. Consistent with their documented insensitivity to glucokinase inhibitors, the glucose responses of orexin cells persisted in the presence of the mitochondrial poison oligomycin or the glial toxin fluoroacetate. Unexpectedly, in the presence of oligomycin, the magnitude of the glucose response was significantly enhanced. In turn, 2-deoxyglucose, a non-metabolizable glucose analogue, elicited larger responses than glucose. Conversely, intracellular pyruvate dose-dependently suppressed the glucose responses, an effect that was blocked by oligomycin. The glucose responses were also suppressed by intracellular lactate and ATP. Our new data suggest that other energy substrates not only fail to mimic the orexin glucose response, but paradoxically suppress it in a metabolism-dependent manner. We propose that this unexpected intrinsic property of orexin cells allows them to act as ‘conditional glucosensors’ that preferentially respond to glucose during reduced background energy levels.
Introduction
Orexin/hypocretin-containing neurons (‘orexin cells’) of the lateral hypothalamus recently emerged as key regulators of states of consciousness and energy balance (de Lecea et al. 2006; Sakurai, 2007). Their firing promotes wakefulness (Adamantidis et al. 2007), and their loss causes narcolepsy and obesity due to reduced energy expenditure (Hara et al. 2001). Independently of their consciousness-stabilizing role, orexin neurons also control glucose homeostasis by stimulating the sympathetic nervous system to increase the basal metabolic rate, hepatic glucose production, and glucose uptake by skeletal muscle (reviewed in Karnani & Burdakov, 2011). Whole-cell patch-clamp recordings from mouse neurons indicate that a key inhibitory signal regulating orexin cells, but not neighbouring neurons containing melanin-concentrating hormone, is glucose itself (Yamanaka et al. 2003; Burdakovet al, 2005). At physiological concentrations, glucose directly acts on orexin cells to stimulate membrane K+ currents, whose molecular identity is unclear (Gonzalez et al. 2009), but which cause membrane hyperpolarization and a suppression of cell firing rate (Burdakov et al. 2006). Glucose-induced inhibition of orexin cells has also been demonstrated using cytosolic calcium imaging in intact orexin cells isolated from rats (Muroya et al. 2001). Because the inhibitory response of orexin cells to glucose would be expected to alter both cognitive arousal and glucose homeostasis, it is important to understand how this response can be regulated, but little is known about this at present. Recently, we found that the orexin cell glucose response persists in the presence of glucokinase inhibitors and is mimicked by the non-metabolizable glucose analogue 2-deoxyglucose (Gonzalez et al. 2008). This suggests that, unlike mechanisms proposed to underlie glucose-induced inhibition of other cells (Dunn-Meynell et al. 2002; Mountjoy et al. 2007; Rorsman et al. 2008), orexin cell glucosensing may not require conventional glucose metabolism. On the other hand, measurements of orexin mRNA levels in response to acute insulin-induced hypoglycaemia suggest that a hypoglycaemic stimulus activates orexin cells when food is withheld but not when food is available (Cai et al. 1999). This suggests that background metabolic state may have a modulatory role on hypothalamic glucosensing, but it is unknown whether this occurs acutely at the level of physiological output (firing rate) of orexin cells.
To clarify the role of energy metabolism in orexin glucosensing, here we quantified orexin cell glucose responses in the presence of (1) metabolic inhibitors that are mechanistically different from those examined previously, and (2) different levels of common intracellular fuels such as pyruvate, lactate and ATP.
Methods
Preparation of brain slices from orexin-eGFP transgenic mice
Animal procedures were carried out according to the Animals (Scientific Procedures) Act 1986 (UK), and to guidelines in Drummond, 2009. Transgenic ‘orexin-eGFP’ mice were used to identify orexin neurons, as in our previous studies (Gonzalez et al. 2008). These mice express enhanced green fluorescent protein (eGFP) under the control ofprepro-orexinpromoter, resulting in very specific targeting of eGFP only to orexin neurons (Yamanaka et al. 2003; Burdakov et al. 2006). Mice were maintained on a 12 h light–dark cycle (lights on 08.00 h) and had free access to food and water. Animals (14–21 days postnatal) were killed during the light phase and coronal slices (250–300 μm thick) containing the lateral hypothalamus were prepared as previously described (Gonzalez et al. 2008).
Chemicals and solutions
ACSF was continuously gassed with 95% O2 and 5% CO2 and contained (in mm): 125 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.2 NaH2PO4, 21 NaHCO3, and 1 or 5 d-(+)-glucose, and other drugs as indicated. Basic pipette solution contained (in mm): 120 potassium gluconate, 10 KCl, 0.1 EGTA, 10 Hepes, 5 K2ATP (except in Fig. 4CandD), 1 NaCl, 2 MgCl2; pH adjusted to 7.3 with KOH. All chemicals were obtained from Sigma (UK). Stocks of oligomycin (a mixture of A, B and C) were made up in ethanol. The final concentration of ethanol in ACSF was ∼0.01%. The oligomycin experiments were carried out in extracellular oligomycin, and slices were incubated in oligomycin for at least 20 min prior to experiments, and for the duration of the experiment. Multiple cells were sometimes recorded from the same slice (without washing out oligomycin), provided the slice was healthy. Slices were discarded after a maximum of 2 h in oligomycin-containing solution.
Figure 4. Pyruvate suppression of glucose response is reversed by oligomycin and mimicked by intracellular ATP.
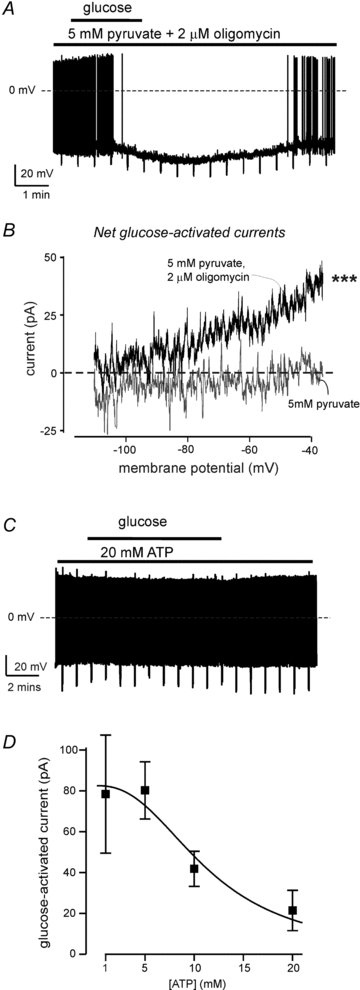
A, representative membrane potential response of an orexin cell to 1→5 mm switch in glucose in the presence of 5 mm pyruvate and 2 μm oligomycin (similar responses were observed in 5 cells).B, net currents induced by 1→5 mm glucose in 5 mm pyruvate (grey), and 5 mm pyruvate + 2 μm oligomycin. Each trace shows mean current (5 cells in each group). Repeated measures ANOVA showed that there is a significant difference between the two datasets (F(1,56) = 39.6, ***P< 0.001). Current at –40 mV was significantly greater in the presence of oligomycin (P< 0.01 by unpairedttest).C, representative membrane potential response of an orexin cell to 1→5 mm switch in glucose in the presence of 20 mm intracellular ATP (similar responses were observed in 5 cells).D, dose–response of ATP-induced inhibition of the current (at –40 mV) induced by 1→5 mm switch in extracellular glucose (n = 4–7 cells per point, IC50 = 11.44 mm, fit details are given in Methods).
Glucose concentrations
Extracellular glucose levels in the brain are generally considered to be about 10–30% of plasma glucose levels (Routh, 2002), but the extent of their fluctuations in different brain regions is still debated. In this study, we chose the 1→5 mm switch in bath [glucose] as the stimulus, for the following reasons. First, this is consistent with classicalin vivomeasurements of physiological levels of glucose in the brain (Silver & Erecinska, 1994, 1998). Second, analysis is more robust with large responses and hence we used the maximum glucose stimulus that is physiologically plausible. Third, this creates comparable conditions with previousin vitrostudies of glucosensing in the lateral hypothalamus (e.g. Kong et al. 2010; Gonzalez et al. 2008, Guyon et al. 2009). Finally, although some data suggest that physiological variations may be smaller in some brain regions (e.g. 1→2.5 mm; Routh, 2002), we reasoned that if a modulator (pyruvate, lactate and ATP in our study) blocks responses to a large glucose stimulus, it would also block responses to a small stimulus. Indeed, our control experiments indicated that 5 mm pyruvate blocks hyperpolarization induced by 1→2.5 mm glucose (n = 3), in the same way it blocks the response to 1→5 mm glucose (Fig. 3).
Figure 3. Responses of orexin cells to glucose in the presence of different cytosolic concentrations of pyruvate and lactate.

A, representative membrane potential response of an orexin cell to 1→5 mm switch in glucose in the presence of 5 mm pipette pyruvate (typical response ofn = 4 cells).B, representative membrane potential response of an orexin cell to 1→5 mm switch in glucose in the presence of 20 mm pipette lactate (typical response ofn = 6 cells).C, dose–response of pyruvate or lactate-induced inhibition of the current (at –40 mV) induced by 1→5 mm switch in extracellular glucose (n = 4–7 cells per point, IC50 values are 0.47 mm and 17.4 mm respectively, fit details are given in Methods).
Data acquisition and analysis
Conventional brain slice whole-cell patch-clamp recordings and analysis were performed at 37°C, as in our previous studies (described in detail in Burdakov et al. 2006). Membrane current–voltage relationships were obtained using voltage-clamp ramps that hyperpolarized the membrane at a rate of 0.1 mV ms-1. Glucose-activated current shown in the figures is net glucose-activated current obtained by subtracting currents in 1 mm glucose from those recorded at corresponding membrane potentials after a switch to 5 mm glucose (as described in Burdakov et al. 2006). Membrane potential was recorded using zero holding current, or by applying a fixed holding current to keep the cells at the same pre-stimulation potential (where indicated). Voltage-clamp data were corrected for an experimentally determined liquid junction potential of 10.1 mV. For intracellular infusion of drugs, the cell was left in standard whole-cell configuration for at least 5 min before changing extracellular [glucose], which reliably fills orexin cell cytosol with pipette solution (based on control experiments with pipette dyes). Statistical significance was evaluated using Student'sttest or ANOVA, as indicated.
Dose–response data in Figs 3C and 4D were fitted with the following general equation:
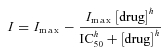 |
whereImax is the maximal current induced by 1→5 mm glucose, drug is pyruvate lactate or ATP, IC50 is the concentration of drug that produces half-maximal inhibition, andhis the Hill coefficient. Pyruvate data fit (Fig. 3C) was obtained withImax = 80.2 pA, h = 0.67, and IC50 = 0.47 mm. Lactate data fit (Fig 3C) was obtained withImax = 79.5 pA, h = 1.75, and IC50 = 17.36 mm. ATP data fit (Fig. 4D) was obtained withImax = 82.5 pA, h = 2.43, and IC50 = 11.44 mm.
Results
Orexin cell glucose response persists in the presence of metabolic poisons
To test whether an increase in mitochondrial ATP production is required for glucose-induced inhibition of orexin neurons, we first examined orexin cell glucose responses in the presence of the ATP synthase blocker oligomycin. As expected from decreased production of ATP and consequent opening of KATP channels, oligomycin (2 μm, based on Doolette 1997; >20 min pre-incubation, see Methods) significantly hyperpolarized orexin cells (membrane potential in oligomycin = –61.0 ± 4.0 mV, control = –42.2 ± 3.5 mV, n = 4 and 6, respectively, P< 0.01 by unpairedttest). To facilitate comparisons with oligomycin-untreated orexin cells, we returned the cells to –40 mV by sustained current injection (maintained throughout the recording). This revealed that oligomycin did not prevent glucose-induced hyperpolarization of orexin cells (Fig. 1A; glucose produced 15.1 ± 1.6 mV hyperpolarization, n = 4; see next section for comparison with controls), and did not reduce glucose-induced currents (see Fig. 2A, described below).
Figure 1. Membrane potential responses of orexin cells to glucose in the presence of metabolic poisons.
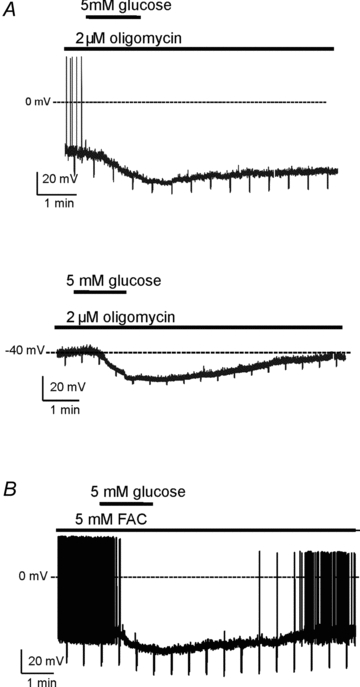
A, responses in the presence of 2 μm oligomycin. The cells were held at the same pre-stimulation potential of –40 mV by sustained current injection. Note that oligomycin compromised action potential firing in most cells. Top, an example response from a neuron that fired action potentials. Bottom, an example response from a neuron that no longer fired action potential. Quantification is given in Results.B, an example of glucose response in the presence of 5 mm fluoroacetate (FAC); the cell was held at the same pre-stimulation potential of –40 mV by sustained current injection. See Results for details.
Figure 2. Membrane current responses of orexin cells to sugars in the presence and absence of metabolic inhibitors.
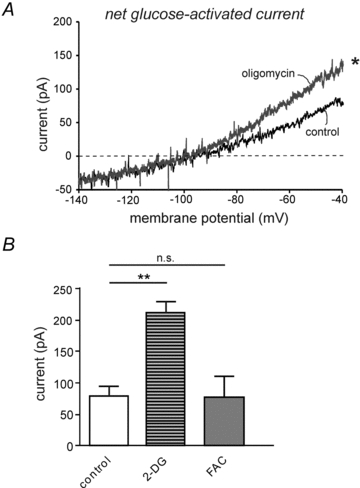
A, net current induced by a 1→5 mm switch in extracellular glucose in the presence and absence of 2 μm oligomycin. Each trace shows mean current obtained from 4 and 6 cells, respectively. Repeated measures ANOVA showed that there is a significant difference between the two datasets (F(1,90) = 6.17, *P< 0.02).B, sugar-activated currents compared at the same potential (–40 mV), under different conditions: ‘control’ (from black trace inA, n = 6), ‘2-DG’ (current induced by 5 mm 2-deoxyglucose, n = 6), ‘FAC’ (current induced by glucose in experiments shown in Fig. 1B, n = 5). Values are means ± SEM; **P< 0.01; n.s., P> 0.1, by unpairedttest).
Since glial glucose metabolism has been proposed to modulate glucose responses in some (neurochemically undefined) hypothalamic neurons (Ainscow et al. 2002), we also tested the effects of the glial poison fluoroacetate (Parsons & Hirasawa, 2010). We used 5 mm fluoroacetate (50 min pre-incubation and present throughout recording), which prevents glial metabolism of glucose in hypothalamic slice preparations (Parsons & Hirasawa, 2010). Consistent with previous data (Parsons & Hirasawa, 2010), fluoroacetate itself significantly hyperpolarized orexin neurons (membrane potential in fluoroacetate was –58.8 ± 2.0 mV, control value = –42.2 ± 3.5 mV, n = 5 and 6, respectively, P< 0.01 by unpairedttest). To facilitate comparisons with fluoroacetate-untreated orexin cells, we returned the cells to –40 mV by sustained current injection (maintained throughout the recording). Fluoroacetate did not prevent glucose-induced hyperpolarization of orexin cells (Fig. 1B, 11.0 ± 1.9 mV hyperpolarization, n = 5; see next section for comparison with controls), and also had no effect on glucose-induced current (Fig. 2B, described below).
Although the above data show that oligomycin and fluoroactetate do not abolish glucose responses, note that quantitative comparisons with control responses cannot be drawn from this current-clamp data. This is because the reduction in membrane conductance caused by these drugs would reduce the effects of any currents on membrane potential (Ohm's law). For this reason, we next examined glucose responses in voltage-clamp at the level of membrane currents.
2-Deoxyglucose or glucose co-applied with oligomycin produce larger responses than glucose
To further investigate the role of glucose metabolism in orexin cell glucose responses, we compared glucose-induced currents with those elicited by its non-metabolizable analogue 2-deoxyglucose, which we previously found to mimic the glucose response (Gonzalez et al. 2008). At a holding potential of –40 mV, currents induced by 5 mm 2-deoxyglucose were significantly larger than those produced at the same holding potential by 5 mm glucose (Fig. 2B, statistics are given in the figure legend;n = 6; for examples of 2-deoxyglucose-induced currents, see Gonzalez et al. 2008).
A possible explanation for the unexpectedly larger effects of 2-deoxyglucose is that metabolic breakdown of glucose may oppose, rather than drive, the orexin cell glucose response. We reasoned that if this is true, then block of glucose metabolism in orexin cells should enhance their glucose responses. Consistent with this idea, we found that in the presence of 2 μm oligomycin, glucose produced greater membrane currents than in the absence of the drug (Fig. 2A). On the other hand, fluoroacetate, a weaker metabolic poison because it only affects glial cells, did not significantly augment glucose-induced currents in orexin neurons during analysis either of currents at –40 mV (Fig. 2B), or of current–voltage ramps such as those shown in Fig. 2A (n = 5vs.6 controls;F(1,90) = 0.12, P> 0.5 by repeated-measures ANOVA, data not shown).
Mitochondrial substrates suppress glucose responses
The above data suggest that increased mitochondrial energy metabolism does not promote glucose-inhibition of orexin cells, but may in fact suppress it. To examine the latter possibility directly, we analysed orexin cell glucose responses in the presence of mitochondrial energy substrates pyruvate and lactate. To isolate the effects on orexin cells, we exploited the ability of the whole-cell recording mode to infuse different concentrations of drugs directly to cell cytosol by including them in the pipette solution. Increased cytosolic concentration of pyruvate suppressed the orexin cell membrane potential responses to glucose (Fig. 3A; 5 mm pyruvate reduced the response by ∼95%; glucose-induced hyperpolarization was 0.8 ± 0.6 mV with 5 mm pyruvate, and 14.3 ± 1.5 mV without, n = 4, P< 0.001 by unpairedttest). Similar results were obtained with extracellular pyruvate (M. M. Karnani & D. Burdakov, manuscript in preparation). Intracellular lactate also suppressed the glucose response, but was less potent (Fig. 3B, 5 mm lactate reduced the response by ∼26%; glucose-induced hyperpolarization was 10.6 ± 2.0 mV with 5 mm lactate, and 14.3 ± 1.5 mV without, n = 4, P = 0.194 by unpairedttest). Dose–response analysis of membrane currents elicited by glucose at –40 mV indicated that both pyruvate and lactate dose-dependently depressed the glucose response with IC50 values of 0.47 mm and 17.4 mm, respectively (Fig. 3C).
Pyruvate suppression of glucose response is reversed by oligomycin and mimicked by ATP
To test whether the inhibitory actions of pyruvate on orexin cell glucosensing involved its mitochondrial conversion to ATP, we compared the effects of pyruvate in the presence and absence of oligomycin. Oligomycin (2 μm) prevented pyruvate from abolishing glucose-induced hyperpolarization (Fig. 4A; glucose hyperpolarization in 5 mm pyruvate was 0.6 ± 0.5 mV without oligomycin and 13.2 ± 1.7 mV with oligomycin, n = 5 for both groups, P< 0.001 by unpairedttest). This was also observed at the level of membrane currents: glucose triggered no detectable currents in the presence of 5 mm pyruvate, but produced significant currents in the presence of pyruvate+oligomycin (Fig. 4B; statistics are given in the figure legend). The simplest interpretation of these data is that mitochondrial metabolism of pyruvate is at least partly responsible for the inhibitory influence of pyruvate on the glucose response. Since mitochondrial metabolism of pyruvate produces ATP, we also looked at how cytosolic ATP levels affect the glucose response. Increasing cytosolic (pipette) ATP levels suppressed the glucose effects on the membrane potential (Fig. 4C; glucose-induced hyperpolarization was 14.3 ± 1.5 mV in 5 mm ATP, and 3.2 ± 3.9 mV in 20 mm ATP, n = 5, P< 0.05), and induced a dose-dependent reduction in the glucose-induced current (Fig. 4D), although the estimated IC50 (11.44 mm) was an order of magnitude higher than cytosolic ATP levels measured so far in hypothalamic neurons (see Discussion).
Discussion
Our newin situdata suggest that the electrical activity of orexin neurons is more potently inhibited by glucose when intracellular energy levels are low, and that these cells progressively cease to sense glucose as intracellular energy levels increase. This is supported by two convergent lines of evidence: (1) increasing energy levels (in the form of cytosolic levels of pyruvate, lactate, or ATP) progressively block glucose responses; (2) when background energy levels are reduced with oligomycin, or by the use of the non-metabolizable glucose analogue 2-deoxyglucose, orexin cells generate greater sugar responses. The suppression of sensing responses by supplying cells with more energy is unusual since generally neuronal functions are enhanced by increased fuel availability. However, this paradoxical modulation is in line with the emerging view of orexin neurons as specialized metabolic sensors that respond to energy-related compounds differently from most other cells. Interestingly, our data on orexin cells are consistent with results obtained in other glucose-inhibited neurons, which show that keeping the cells in hyperglycaemic conditions can reduce their subsequent ability to respond to glucose (Canabal et al. 2007).
Our data further support the hypothesis that, unlike glucose-induced depolarization of pancreatic β-cells, glucose-induced hyperpolarization of orexin neurons does not require glucose metabolism. This hypothesis originated from the observations that glucose-induced hyperpolarization of orexin cells persists in the presence of high concentrations of different glucokinase inhibitors, and can be mimicked by 2-deoxyglucose (Gonzalez et al. 2008). Additional support for this hypothesis is now provided by observations that glucose-induced hyperpolarization is not blocked by the mitochondrial poison oligomycin or by the glial metabolic toxin fluoroacetate (Figs 1 and 2). In fact, oligomycin increased the size of the glucose-induced inhibitory currents in orexin cells (Fig. 2). We did not observe a similar increase in the presence of fluoroacetate, which could be because it mostly affects glial cells (Parsons & Hirasawa, 2010) and thus presumably elicits a smaller metabolic depression (compared to oligomycin) in our slice preparation. Although fluoroacetate did not affect the glucose response in our slice preparations, it cannot be ruled out that glial glucose metabolism may modulate the orexin cell glucose responsein vivo, where the coupling between neurons and glia is likely to be tighter.
Although our results identify novel functional properties of orexin cells and further support the ‘non-metabolic’ nature of glucose-induced inhibition in these neurons, the further molecular mechanisms are difficult to unravel at this stage because the molecular nature of the orexin glucosensor is unknown. Voltage-clamp data on orexin cells suggest that, throughout the physiological concentration range, glucose activates a background (voltage-independent) K+ current (Burdakov et al. 2006; Williams et al. 2008). A similar glucose-activated background K+ current was reported in crab neurosecretory neurons and in mouse ventromedial hypothalamic cells, although in other neurons, a Cl− current may contribute (reviewed in Burdakov & Lesage, 2009). The molecular nature of the glucose-activated K+ current is unclear: although it displays some biophysical hallmarks of KCNK channel family (which contains ∼15 subunits that can heterodimerize), we and others so far found that five of these subunits (TASK1/3, TREK1/2 and TRAAK) can be genetically deleted without abolishing glucose responses (Gonzalez et al. 2009; Guyon et al. 2009). Interestingly, in orexin cells, glucose-induced activation of K+ currents displays an unusual sugar selectivity and is caused by extracellular, but not intracellular, application of glucose, suggesting that an extracellular ‘glucose receptor’ might be involved (Burdakov et al. 2006; Gonzalez et al. 2008).
The physiological role of the modulation of orexin glucosensing by intracellular energy levels would depend upon the variability in the native levels of lactate, pyruvate and ATP in the cytosol of orexin cells. Although these values have not been directly measured in orexin neurons (but see Liu et al. 2011 for estimates of ATP levels), cytosolic lactate concentration has been calculated to be ∼23 mm in cultured mouse cortical neurons (Walz & Mukerji, 1988), cytosolic pyruvate concentration to be ∼100 μm in cultured mouse striatal neurons (Desagher et al. 1997), and cytosolic ATP concentration to be ∼1 mm in unidentified hypothalamic neurons (Ainscow et al. 2002). We estimated that pyruvate and lactate inhibit the orexin glucose response with IC50 values of 0.47 mm and 17.36 mm, respectively, which is in a similar range to the published concentrations of intracellular pyruvate and lactate. In contrast, our measurements suggest that intracellular ATP inhibited the glucose response with an IC50 of 11.44 mm, which is an order of magnitude higher than that reported in (Ainscow et al. 2002). However, a very recent study suggests that cytosolic ATP levels in orexin cells could be higher, around 5 mm, and may be further increased by sleep deprivation (Liu et al. 2011), which could potentially bring ATP to high enough levels to suppress the glucose response. Overall, we speculate that orexin activity, which is net-negative in terms of whole-body energy balance (Hara et al. 2001), is not always inhibited by glucose, but is instead flexibly tuned in a way that is optimal for survival. For example, under low-energy conditions such as starvation or anorexia, it could be advantageous for ingested glucose to suppress the orexin-driven net energy expenditure, thereby ensuring that more fundamental processes (such as keeping the brain alive) receive enough glucose. Conversely, when the brain has plenty of fuel (perhaps signalled by high levels of pyruvate and/or lactate), there may be little advantage in coupling glucose fluctuations to orexin activity, since under these conditions, acute changes in glucose levels would be compensated by other energy substrates.
In summary, our new data suggest that increased levels of other energy substrates not only fail to mimic the orexin glucose response, but paradoxically suppress it in a metabolism-dependent manner. We propose that this unexpected intrinsic property of orexin cells allows them to act as ‘conditional glucosensors’, which preferentially respond to glucose during reduced background energy levels.
Acknowledgments
This work was supported by the European Research Council (FP7 grant to D.B.) and Diabetes UK (PhD studentship to A.V.).
Author contributions
A.V., M.M.K. and J.A.G. performed experiments, L.T.J. and L.F. created and maintained orexin-eGFP mice, and D.B. designed the study, obtained funding, and wrote the paper. All authors approved the final version for publication.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K+ channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Lesage F. Glucose-induced inhibition: how many ionic mechanisms? Acta Physiol (Oxf) 2009;198:295–301. doi: 10.1111/j.1748-1716.2009.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolette DJ. Mechanism of adenosine accumulation in the hippocampal slice during energy deprivation. Neurochem Int. 1997;30:211–223. doi: 10.1016/s0197-0186(96)00055-1. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters inThe Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci. 2009;29:2528–2533. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol. 2011;300:R47–55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Bruning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Gan G, Suyama S, Gao XB. Intracellular energy status regulates activity in hypocretin/orexin neurones: a link between energy and behavioral states. J Physiol. 2011;589:4157–4166. doi: 10.1113/jphysiol.2011.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- Muroya S, Uramura K, Sakurai T, Takigawa M, Yada T. Lowering glucose concentrations increases cytosolic Ca2+ in orexin neurons of the rat lateral hypothalamus. Neurosci Lett. 2001;309:165–168. doi: 10.1016/s0304-3940(01)02053-5. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci. 2010;30:8061–8070. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. KATP-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, ichi Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]


