Non-technical summary
For the correct execution of centrally initiated movements, it is vital that the cerebellum receives continuous feedback from the spinal cord. We recently demonstrated that a population of spinocerebellar neurones provides the cerebellum with feedback on the likely outcome of descending commands relayed via reticulospinal neurones. We now demonstrate that the same spinocerebellar neurones provide information on the likely outcome of commands from the corticospinal tract (pyramidal tract) neurones as well as from the mesencephalic locomotor region. The results indicate that both voluntary motor actions and those related to locomotion are relayed by reticulospinal neurones and are monitored by the same population of spinocerebellar neurons, which may thereby provide the brain with information necessary for avoiding errors in issuing movements.
Abstract
Abstract
Feed-back information on centrally initiated movements is processed at both supraspinal and spinal levels and is forwarded by a variety of neurones. The aim of the present study was to examine how descending commands relayed by reticulospinal neurones are monitored by a population of spinocerebellar tract neurones. Our main question was whether a spinal border (SB) subpopulation of ventral spinocerebellar tract (VSCT) neurones monitor actions of reticulospinal neurones with input from the mesencephalic locomotor region (MLR) as well as from pyramidal tract (PT) neurones. In the majority of intracellularly recorded SB neurons, stimuli applied in the MLR and in the medullary pyramids evoked EPSPs in parallel with EPSPs evoked by stimulation of axons of reticulospinal neurones in the medial longitudinal fascicle (MLF). In extracellularly recorded neurones short trains of stimuli applied in the ipsilateral and contralateral pyramids potently facilitated discharges evoked from the MLF, as well as EPSPs recorded intracellularly. In both cases the facilitation involved the disynaptic but not the monosynaptic actions. These results indicate that reticulospinal neurones activating SB neurones (or more generally VSCT neurones) are co-excited by axon-collaterals of other reticulospinal neurones and by fibres stimulated within the MLR and PTs. The study leads to the conclusion that these spinocerebellar neurones monitor descending commands for centrally initiated voluntary as well as locomotor movements relayed by reticulospinal neurones. Thereby they may provide the cerebellum with feed-back information on the likely outcome of these commands and any corrections needed to avoid errors in the issuing movements.
Introduction
We recently demonstrated that spinocerebellar tract neurons may provide feed-back information on potential actions of reticulospinal (RS) neurones on motoneurones (Hammar et al. 2011). We also showed that both ventral spinocerebellar tract (VSCT) neurones and dorsal spinocerebellar tract (DSCT) neurones located in Clarke's column process such information but are apparently concerned with its different aspects since reticulospinal neurones provide both excitatory and inhibitory input to VSCT neurones but only inhibitory input to DSCT neurones (Hammar et al. 2011).
Information on the potential actions of reticulospinal neurones might likewise concern different kinds of centrally initiated movements because reticulospinal neurones may relay descending commands of various origins. With respect to the centrally initiated locomotion, reticulospinal neurones were shown to excite neurons of the swimming circuits in the brainstem and spinal cord inXenopustadpoles and to initiate locomotion in tadpoles (Soffe et al. 2009) as well as in zebrafish (Fetcho et al. 2008) or lamprey (Dubuc et al. 2008; Le Ray et al. 2011). They were also found to be of critical importance for the initiation of locomotion induced by stimuli applied in the n. cuneiformis (most often referred to as the mesencephalic locomotor region, MLR; for the latest reviews see Dubuc et al. 2008; Jordan et al. 2008). The relationships between neurones, or fibres, stimulated within the MLR and neurones in the reticular formation were only investigated in a handful of studies. However, stimulation within the MLR was consistently found to evoke short latency excitation in a high proportion of reticulospinal neurones in the nuclei giganto- and magnocellularis (Orlovsky, 1970; Iwakiri et al. 1995). The shortest latencies indicate that at least some reticulospinal neurones are excited monosynaptically while somewhat longer latencies are compatible with disynaptic actions that might be mediated by other reticular neurones activated monosynaptically. Such neurones may e.g. include neurones in the medial pontine reticular formation, which in turn activate neurones in the nucleus reticularis gigantocellularis (Iwakiri et al. 1995).
Reticulospinal neurones were likewise shown to co-relay voluntary movements (for review see Alstermark & Lundberg, 1992; Jankowska & Edgley, 2006; Schepens et al. 2008; Riddle & Baker, 2010) and to be involved in the control of primate hand and finger movements as well as of movements of proximal muscles (Riddle et al. 2009; Riddle & Baker, 2010). As pyramidal tract (PT) neurones activate, or at least contact, neurones in several of the reticular nuclei (Peterson et al. 1974; He & Wu, 1985; Keizer & Kuypers, 1989; Canedo & Lamas, 1993; Matsuyama & Drew, 1997), cortical commands relayed via reticulospinal neurones may indeed require only one more synapse than the monosynaptic and disynaptic reticulospinal actions on motoneurones (Grillner & Lund, 1968; Floeter et al. 1993; Gossard et al. 1996; Jankowska et al. 2003). Collateral information about these commands forwarded by spinocerebellar neurones should thus have great impact on the cerebellar control of voluntary movements. In studies of descending input to VSCT neurones, the most direct actions of PT neurones were found to be evoked disynaptically (Magni & Oscarsson, 1961; Fu et al. 1977). However, it has not been established whether these were secondary to activation of reticulospinal or spinal neurones, and whether the reticulospinal neurones are co-activated by PT neurones and neurones, or fibres, stimulated within the MLR. The main aim of the present study has been to address these questions with respect to the spinal border (SB) subpopulation of VSCT neurones (Burke et al. 1971). SB neurones were chosen for two reasons. Firstly, because the selective inhibitory input from peripheral afferents to these neurones may allow them to monitor the degree to which motoneurones are inhibited, which is of critical importance for the probability of activation of these neurones (see discussion in Hammar et al. 2011). Secondly, because of their specific terminal projection area within the sub-lobules C 1 and 2 in the paramedian lobule of the cerebellum (Matsushita & Ikeda, 1980; Matsushita & Yaginuma, 1989), in addition to lobules II, III and IV of the anterior lobe. These specific projection areas suggest that paramedian cerebellar neurones may be specialized in monitoring information on descending commands of reticulospinal neurones provided by SB neurones and may therefore be of particular interest in future studies on mechanisms of the use of this information by cerebellar neurones. The results as described in this paper show that SB neurones should be able to monitor descending commands for centrally initiated voluntary movements evoked via PT neurones as well as locomotion.
Methods
Ethical approval
All experiments were approved by the Ethics Committee for Animal Research at the University of Gothenburg (Göteborgs Djurförsöksetiska Nämnd) and comply with NIH and EU guidelines for animal care and with the ethical policies and regulations ofThe Journal of Physiology(Drummond, 2009). The animals were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on 10 deeply anaesthetised cats weighing 3.4–4.8 kg, four of which were also used for experiments described in the companion paper (Jankowska et al. 2011). Anaesthesia was induced with sodium pentobarbital (Apoteksbolaget, Sweden; 40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–3 h, up to 55 mg kg−1, i.v.). Additional doses of α-chloralose were given when motor reactions were evoked during dissection or when increases in the continuously monitored blood pressure or heart rate were evoked by the experimental procedures. Atropin (0.05–0.2 mg kg−1i.m.) was sometimes administered during the preliminary surgical procedures to reduce tracheal secretion. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; 0.3 mg kg−1i.v.) and the animals were artificially ventilated. Neuromuscular relaxation was induced only after several hours of surgery and when the animal had reached a deep and stable level of anaesthesia and was thereafter maintained by adding pancuronium bromide to the buffer infusion (see below) at doses corresponding to about 0.2 mg kg−1h−1. Mean blood pressure was kept at 100–130 mmHg and end-tidal concentration of CO2 at about 4–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). The core body temperature was kept at about 37.5°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of pentobarbital i.v. followed by formalin perfusion.
In order to increase the probability of activation of SB neurones, as well as to enhance any indirect actions of the tested stimuli, 0.1–0.2 mg kg−1 doses of the potassium (K+) channel blocker 4-aminopyridine (4-AP) were applied intravenously during recording. These doses were expected to result in a plasma concentration of 4-AP of about 1 μm and could be compared to clinically used doses of 10 mg, corresponding to 0.14 mg kg−1 in a 70 kg patient, with minimal side effects (for latest references see Alvina & Khodakhah, 2010).
Following the initial vein, artery and tracheal canulation, a laminectomy exposed the third to fifth lumbar (L3–L5), low thoracic (Th11–Th13) and in three experiments also second to fourth cervical (C2–C4) segments of the spinal cord. The quadriceps (Q) and sartorius (Sart) muscle nerves were transected and mounted in subcutaneous cuff electrodes for stimulation.
The caudal part of the cerebellum was exposed by a craniotomy and tungsten electrodes (impedance 30–150 kΩ) were positioned in the left medial longitudinal fascicle (MLF), in the left and/or right pyramids (PT) in the low medulla and in the region of the right n. interpositus in the cerebellum. The electrodes were inserted at an angle of 35–45 deg (with the tip directed rostrally). A craniotomy was also made over the frontal lobes to allow insertion of a tungsten electrode into the left n. cuneiformis (at an angle of 20 deg, with the tip directed caudally). The initial targets were at Horsley–Clarke co-ordinates P9, R0.6, H–5 for MLF; P7, L1.2, H–10 for PTs; P7, R3, H0 for the cerebellum; and P0–2, L3 and H0 for n. cuneiformis. However, the final positions of the MLF, PT and cerebellar electrodes were adjusted on the basis of records of descending volleys evoked by single stimuli from the surface of the lateral funiculus at the Th11–Th13 and/or the C4–C5 segments (for details see Results). The electrodes were positioned at sites from which distinct descending volleys were evoked at stimulus intensities of 20 μA or less. Stimuli applied in the n. cuneiformis were not followed by any distinct descending volleys; the location of the electrode was therefore adjusted using records of field potentials evoked by a short train (n = 3–5) of stimuli within the area of the ventral horn in which the largest monosynaptic field potentials were evoked from the MLF. The rationale behind this test was that stimuli applied within MLR sites identified as inducing rhythmic locomotor-like activity in decerebrate animals were reported to evoke short latency field potentials (Noga et al. 1995; Degtyarenko et al. 1998; Jordan et al. 2008) and that stimuli applied within the MLR should activate reticulospinal neurones (Orlovsky, 1970; Iwakiri et al. 1995). We verified that such field potentials were evoked from an area extending about 2 mm rostro-caudally but only about 1 mm dorso-ventrally; trains of four stimuli at 100 or 80 μA evoked similar field potentials from the centre of this area as well as from locations 0.5 mm more dorsal or ventral, but at 50 μA practically only from its centre. The stimulating electrodes were left at locations at which they were evoked at 50–80 μA. At the end of the experiments the stimulation sites were marked with electrolytic lesions (0.2 mA constant current for 10 s). Locations of these stimulation sites were subsequently verified on 50 or 60 μm thick frontal sections of the brainstem, cut using a cryostat in the plane of electrode insertion, mounted on slides, counterstained with cresyl violet and scanned (see Fig. 1). In four experiments an additional electrode was introduced into the contralateral red nucleus (see Jankowska et al. 2011).
Figure 1. Location of electrolytic lesions marking stimulation sites in the brain.
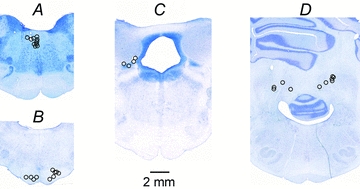
A, stimulation sites in the ipsilateral MLF.B, four stimulation sites in the ipsilateral PT and six in or just outside the contralateral PT.C, four stimulation sites in the region of the ipsilateral n. cuneiformis (MLR).D, stimulation sites in the region of the ipsilateral n. interpositus in the cerebellum in four experiments and of the contralateral n. interpositus in six experiments.
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli (0.2 ms duration, intensity expressed in multiples of threshold, T, for the most sensitive fibres in the nerve). For activation of fibres stimulated within the brain we applied constant current cathodal stimuli (0.2 ms, 20–150 μA in the MLF and the cerebellum (in all experiments), 50–100 μA in the PTs (in the ipsilateral PT in 4 experiments and the contralateral PT in 4 experiments) and in the MLR (in 6 experiments). Stimuli of up to 150 μA applied in the MLF were expected to activate a large proportion of ipsilaterally descending ponto- and medullary reticulospinal tract fibres although encroaching on some decending contralaterally (see Jankowska et al. 2003). These stimuli would also activate vestibulospinal tract fibres arising from the medial vestibular nucleus (which, however, do not project as far caudally as the lumbar segments) but would not activate fibres from the lateral vestibular (Deiter's) nucleus (Nyberg-Hansen & Mascitti, 1964), so that any effects observed in lumbar segments can be attributed to reticulospinal fibres. Up to 100 μA stimuli would be expected to be near maximal for fibres within the stimulated PT with minimal, if any, spread of current to the other PT (Jankowska et al. 2006). Stimuli applied in the cerebellum were used for identification of the spinocerebellar neurones by antidromic activation. However, as both contralaterally ascending VSCT neurones and ipsilaterally ascending DSCT neurones could be activated by such stimuli, effects of cerebellar stimulation were compared with effects of stimulation of the lateral funiculi at the level of the Th12–13 segments; only neurones activated from the cerebellum and from the contralateral but not ipsilateral lateral funiculus were thus classified as VSCT neurones.
Descending volleys were recorded transdurally from the cord dorsum. During placement of the stimulating electrodes they were recorded monopolarly from the lateral border of the dorsal columns at C3–C4 and from the surface of the left lateral funiculus at Th11–12. During recording the cord dorsum electrodes were placed in contact with the surface of the dorsal columns at the border between the L4 and L5 segments.
Glass micropipettes filled with 2 m solution of potassium citrate were used for recording. Micropipettes with impedance 2–4 and 4–6 MΩ (with tips broken to about 1.5 and 1.0 μm, respectively) were selected for extracellular and intracellular recording.
Intracellular records were obtained from 50 SB neurones and extracellular records from 52 neurones. They were identified by their location within the most lateral part of the ventral horn, lateral to the location of motor nuclei in the L4 segment, and by antidromic activation from the cerebellum and the contralateral lateral funiculus. However, we cannot exclude that some of these neurones belonged to the more medially located subpopulations of VSCT neurones, especially when only recorded from extracellularly, and when input from group Ia and Ib afferents could not be used to differentiate between them. Nor is a mainly inhibitory input from peripheral afferents an exclusive feature of SB neurones, albeit more frequently found in SB than in other VSCT neurones (Eccles et al. 1961; Burke et al. 1971).
Analysis
The records were digitalised using the analog to digital converter Digidata (Axon Instruments, Union City, CA, USA) at a resolution of 30 μs per address. Both the original data and averages of 10–40 single postsynaptic potentials were stored on line. Changes in the recorded potentials were estimated by measuring either peak amplitudes or areas within selected time windows (using a software sampling and analysis system designed by E. Eide, T. Holmström and N. Pihlgren, University of Gothenburg). The time windows within which the areas were measured were selected to include the most likely monosynaptic components of the PSPs evoked by presynaptic neurones, generally between their onset and the first 1/3 of their declining phase, but avoiding the inclusion of any later components. Peak amplitudes were compared only in those cases in which there were problems with estimating either the onset or the reference baseline of potentials evoked by the second stimulus. The differences between potentials evoked by different combinations of stimuli were assessed by comparing averages of 20 or 40 individual consecutive records. These differences were considered genuine in a given neurone if they exceeded 10% of control records and were consistently found in at least two to three stimulus combinations. The timing of extracellularly evoked discharges was estimated using peri-stimulus time histograms and the number of discharges using cumulative sums of data points from the histograms, both created on-line. These were compared for discharges evoked by 20–50 trains of up to six stimuli using the same software (see Jankowska et al. 1997). Differences between samples of neurons were assessed for statistical significance using Student'sttest for unpaired or paired data, assuming equal variance at the 5% confidence level.
Results
Postsynaptic potentials evoked by stimuli applied in the mesencephalic locomotor region (MLR) and in the medullary pyramids (PT)
Intracellular records obtained from 26 SB neurones revealed that MLR stimuli evoked EPSPs and/or IPSPs in the majority (84%) of the investigated neurones, with predominant EPSPs found in 73% and predominant IPSPs in 27% of these neurones. As illustrated in Fig. 2BandD, these PSPs appeared after the second, or third and successive stimuli but never after the first stimulus, and showed distinct temporal facilitation. They were often associated with small descending volleys (indicated by the first dotted lines in Fig. 2BandD) and with monosynaptic EPSPs or disynaptic IPSPs evoked by stimuli applied in the MLF (Fig. 2AandC). Latencies of the earliest EPSPs evoked from the MLR and the MLF generally fell within the same narrow range (0.5–0.9 ms) when measured with respect to the first components of descending volleys. The same temporal relationships were found between latencies of monosynaptic field potentials evoked from the MLF and those evoked from the MLR (Fig. 3GandH) or of IPSPs (Fig. 2CandD; Table 1B). For potentials following the second or the successive stimuli, the latencies were measured after having subtracted any potentials evoked by the preceding stimuli.
Figure 2. Comparison of timing of the most direct synaptic actions of stimuli applied in the MLF, MLR and PTs.
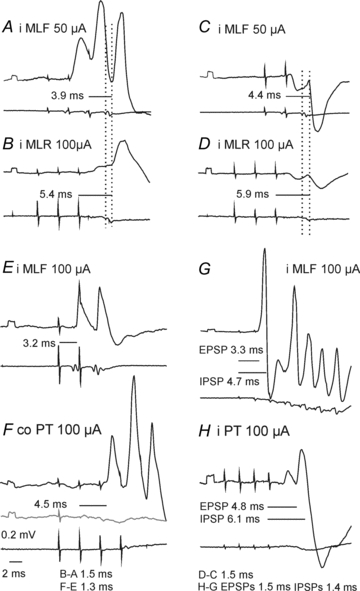
Upper traces, microelectrode records (with negativity downwards); lower traces, records from the surface of the cord dorsum (with negativity upwards).AB, EFandGH, pairs of intracellular records from three SB neurones in the L4 segment, the first one comparing synaptic actions evoked from the MLR and the MLF and the latter two from the PTs and the MLF.CD, a similar comparison of extracellular field potentials evoked from the MLF and from the MLR. Averages of 20 successive responses. iMLF, ipsilateral medial longitudinal fascicle; iMLR, ipsilateral mesencephalic locomor region. iPT and coPT, ipsilateral and contralateral pyramidal tract. Rectangular potentials at the beginning of all microelectrode records are calibration pulses (0.2 mV). Dotted vertical lines indicate the first component of the descending volleys and the onset of potentials following them. Horizontal lines indicate latencies of potentials from the stimuli which evoked them. For example inFthe latency of the first EPSP was measured from the second stimulus as no EPSPs were evoked by single stimuli (grey trace). Latencies from the descending volleys inABandCDwere 0.8–0.9 and 1.1–1.2 ms. The figures belowFandHindicate differences in the latencies from the stimuli of potentials evoked by the MLR and MLF or PT and MLF stimuli.
Figure 3. Examples of occlusion between synaptic actions evoked in SB neurones from the MLF and the MLR.
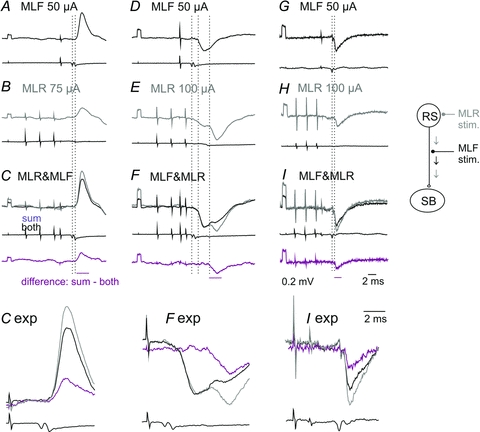
Upper and lower traces in each panel are microelectrode records and records from the cord dorsum.A–C, D–FandG–Iare intracellular records from two SB neurones and records of field potentials at the same location as in Fig. 2CandD respectively, all averaged (A–F n = 40, G–I n = 20). InC, FandIare superimposed potentials evoked by combined actions of MLF and MLR stimuli, sums of separate actions of these stimuli (A+B, D+EandG+Hrespectively) and computer generated differences between them, as indicated. The time windows within which the areas of the potentials were measured are indicated by horizontal lines at the bottom of each column. In the bottom row middle sections of records inC, FandIare 3 times expanded. Note that the sums (light grey) are larger (130%, 125% and 132%) than the effects of jointly applied stimuli (black). The differences represented by sum – both traces were 91%, 35% and 70% of potentials evoked by MLR stimuli alone inB, EandH. Trains of MLR stimuli were applied in advance of single MLF stimuli expecting that axons of RS neurones activated by MLR stimuli will be refractory during MLF stimulation, so that effects of the two stimuli closely following each other, as indicated in the diagram, would not summate linearly.
Table 1.
Latencies of EPSPs and IPSPs evoked from the MLF, MLR and PTs
| EPSPs | IPSPs | |||
|---|---|---|---|---|
| A. Latencies | mean ± SEM | n | mean ± SEM | n |
| MLF monosynaptic | 3.43 ± 0.07 | 14 | ||
| MLF disynaptic | 4.15 ± 0.08 | 11 | 4.63 ± 0.12 | 21 |
| MLR disynaptic | 5.41 ± 0.14 | 19 | 5.70 ± 0.14 | 10 |
| Ipsi PT disynaptic | 5.81 ± 0.36 | 4 | 6.31 ± 0.25 | 9 |
| Contra PT disynaptic | 5.18 ± 0.31 | 9 | 6.20 ± 0.38 | 11 |
| B. Differences | EPSPs | IPSPs | ||
|---|---|---|---|---|
| Monosyn. | Disyn. | Monosyn. | Disyn. | |
| MLF dis-MLF | 0.72 | 1.20 | 0.48 | |
| MLR-MLF | 1.98 | 1.26 | 2.27 | 1.55 |
| ipsi PT-MLF | 2.39 | 1.67 | 2.88 | 2.16 |
| contra PT-MLF | 1.75 | 1.03 | 2.77 | 2.05 |
| ipsi PT-MLR | 0.41 | 0.61 | ||
| contra PTMLR | −0.23 | 0.50 | ||
A, latencies measured from the effective stimuli (means and standard errors of means in ms). B, differences between mean latencies in ms of PSPS evoked from different sources.
Latencies of EPSPs from the MLR measured from the stimuli exceeded latencies of monosynaptic EPSPs evoked from the MLF by 1.5–2.0 ms (Fig. 2AandB; Table 1B). These differences would be compatible with disynaptic MLR actions mediated by monosynaptically activated RS neurones, because latencies of responses of RS neurones evoked from the MLR by suprathreshold stimuli amounted to 1–2 ms and to 2–5 ms when evoked by near-threshold stimuli (Orlovsky, 1970; Iwakiri et al. 1995). Nevertheless we will conservatively set the upper limit of disynaptically evoked PSPs at 1.8 ms.
Intracellular records from the same 26 SB neurones revealed that stimuli applied in the ipsilateral PT evoked EPSPs in a smaller proportion (15%) than stimuli applied in the MLR, while IPSPs were evoked in a similar proportion of these neurones (35%). EPSPs and IPSPs from the contralateral PT were evoked in larger proportions of these neurones (38% and 46%, respectively). Similar to postsynaptic potentials evoked from the MLR, those evoked by PT stimuli appeared only after the second or third stimulus in a train but consistently failed to appear after the first stimulus and, as illustrated in Fig. 2FandH, showed distinct temporal facilitation. They were evoked at similar latencies as postsynaptic potentials evoked by MLR stimuli or exceeded latencies of those evoked by MLR stimuli by about 0.4–0.6 ms (Table 1B), which suggests a similar coupling.
In order to assess whether disynaptic PSPs evoked by stimuli applied within the MLF, MLR and PT might be relayed by common neurones two measures were used, occlusion and spatial facilitation of actions of these stimuli. Occlusion was concluded to occur when joint application of two supramaximal stimuli resulted in smaller PSPs than the sum of PSPs evoked separately, under conditions when effects evoked by the second of these stimuli on the same relay neurones would coincide with the refractory period following the first stimulus. Spatial facilitation was indicated by the opposite effect, when joint application of two submaximal stimuli evoked PSPs that were larger than the sum of PSPs evoked by these stimuli applied separately, under conditions when subthreshold EPSPs evoked on common relay neurones were added, thereby facilitating activation of relay neurones which the two stimuli failed to activate when applied separately (see Lundberg (1975) and Burke (1999) for the theoretical basis of such analysis). The comparisons were made off-line using averages of 20–40 successive records of PSPs evoked by two stimuli applied either jointly or separately, as described in Methods. The comparisons were made at different stimulus intensities and inter-stimulus intervals to identify the optimal parameters in each individual SB neurone and only repeatable and reproducible differences exceeding 10% were considered as due to occlusion or facilitation.
Occlusion between near maximal effects evoked from the MLF and MLR
Records in Fig. 3A–F show examples of occlusion between EPSPs and IPSPs evoked from the MLF and MLR. As shown, the sums of the PSPs (grey records) were distinctly larger than PSPs evoked by jointly applied MLF and MLR stimuli (Fig. 3CandF black traces). The computer created areas of differences between them (bottom traces) were almost as large as the areas of potentials evoked from the MLR. This indicates that the illustrated MLR effects were to a great extent evoked by RS neurones with axons stimulated within the MLF which were refractory at the time when they should have discharged following their synaptic activation by MLR stimuli. Similar indications for occlusion were found in 7 out of 20 tests (between 3 EPSPs and 4 IPSPs evoked from the MLF and MLR; see Table 2). As PSPs recorded intracellularly were often distorted by spontaneously occurring EPSPs and/or IPSPs, those extracted by averaging might have been underestimated. This analysis was therefore supplemented by tests for occlusion on more stable albeit smaller extracellular field potentials. The occlusion was found to occur between monosynaptic field potentials evoked from the MLF and disynaptic field potentials from the MLR (Fig. 3G–I) in support of conclusions based on comparisons of intracellular records.
Table 2.
Proportions of SB neurones in which facilitation, occlusion or no effects were found between synaptic actions evoked from the MLF, MLR and ipsilateral PTs
| MLR-MLF | ipsi PT-MLF | ipsi PT-MLR | |||
|---|---|---|---|---|---|
| EPSPsn = 20 | EPSPsn = 13 | EPSPsn = 12 | |||
| facilitated | 13 | facilitated | 6 | facilitated | 6 |
| occluded | 3 | occluded | 1 | occluded | 2 |
| no effect | 7 | no effect | 7 | no effect | 6 |
| IPSPsn = 20 | IPSPsn = 13 | IPSPsn = 12 | |||
|---|---|---|---|---|---|
| facilitated | 15 | facilitated | 8 | facilitated | 8 |
| occluded | 4 | occluded | 3 | occluded | 1 |
| no effect | 3 | no effect | 3 | no effect | 4 |
As both facilitation and occlusion occurred in some of the neurones the numbers of these neurones add to more than 100%. Facilitation was concluded to occur when the area of potentials evoked by joint application of the indicated stimuli exceeded 10% of the sum of areas of potentials evoked separately. Maximal differences found for EPSPs ranged between 12% and 67% and for IPSPs between 11% and 25%. Occlusion was concluded to occur when the area of potentials evoked by the joint application of the indicated stimuli was at least 10% smaller than the sum of areas of potentials evoked separately. For EPSPs the differences ranged between 12% and 32% and for IPSPs between 15% and 40%.
Mutual facilitation of submaximal EPSPs following stimulation of the MLF, MLR and PTs
Under behavioural, or more natural experimental conditions RS neurones may be activated by nerve impulses from several converging sources of input. One of the aims of this study has been to establish whether RS neurones forwarding collateral information on their descending actions to SB neurones, and via them to neurones in the cerebellum, relay descending commands initiated by both MLR and PT stimuli. In order to address this question we took advantage of the neuronal network depicted in the diagram in Fig. 4H. As shown in this diagram, axons of RS neurones may provide input to other RS neurones via axon collaterals given off before the stem axons reach the MLF (see e.g. Ito & McCarley, 1987; Matsuyama et al. 1993). Stimuli applied to RS axons running in the MLF would thus give rise to not only descending volleys (indicated by black arrows) but also ascending volleys (grey arrows) resulting in synaptic activation of RS neurones and subsequently in descending volleys (grey arrows) following those directly evoked by MLF stimuli. Such synaptically induced volleys are delayed by about 0.7–0.8 ms, are generally much smaller, depend to a great extent on the excitability of RS neurones and are subject to temporal facilitation (see Jankowska et al. 2003; Edgley et al. 2004). The number of synaptically activated RS neurones reflected in the amplitude of the indirect volleys may be enhanced by EPSPs evoked from other sources (see records below the diagram in Fig. 4H). An increase of synaptic volleys and hence of their actions on SB neurones by stimuli applied within the MLR, PTs and the MLF could thus be used as a measure of co-excitation of the same RS neurones by axon collaterals of RS neurones and by MLR and PT stimulation matching other measures of such co-activation.
Figure 4. Examples of facilitation of excitatory actions of stimuli applied in the MLF, MLR and PTs on SB neurones.
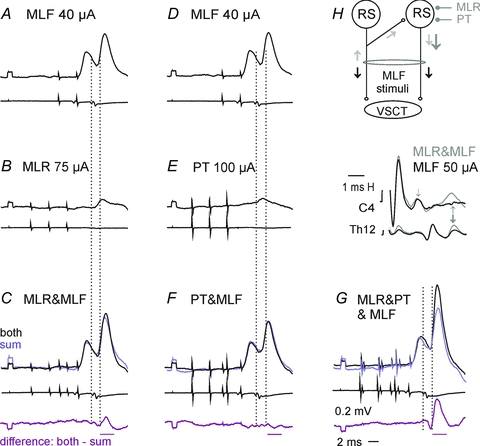
Upper and lower traces inA–Gare intracellular records from a SB neurone and records from the cord dorsum (averages of 40 single traces).H, records of descending volleys from the C4 and Th12 segments following the last of the indicated stimuli (with shock artefacts truncated). InC, FandGblack traces are potentials evoked by combined actions of MLR and MLF, PT and MLF or MLR and MLF as well as PT stimuli, while other traces are sums of separate actions of these stimuli, as indicated; bottom row records are computer generated differences between them. InH, black traces are descending volleys evoked by MLF stimuli while grey traces are volleys evoked by the same MLF stimuli but preceded by MLR stimuli. The diagram indicates the hypothesized mediation of disynaptic actions of the MLF, MLR and PT stimuli via the same RS neurones. Black arrows indicate descending nerve volleys induced by stimulation of axons of RS neurones in the MLF. Small grey arrows indicate the subsequently evoked second components of these volleys nerve volleys attributable to synaptic activation of RS neurones by axon collaterals of MLF fibres and large grey arrows those facilitated by fibres stimulated within the MLR and PTs. The time windows within which the areas of the potentials were measured are indicated by horizontal lines at the bottom of each column. Note that the sums are smaller than the effects of jointly applied stimuli inCandG(85% and 67%) but not inF. Time calibration inGis for all of the records except those inH.
Figure 4C shows that EPSPs evoked under conditions when two weak MLF stimuli were applied jointly with three MLR stimuli (black trace) were larger than the sum of EPSPs evoked by the same stimuli applied separately. The facilitation was distinct, albeit small, but was increased considerably when PT stimuli were added to MLF and MLR stimuli. PT stimuli hardly elicited any facilitatory effect by themselves in this series of records (Fig. 4F), but potently increased EPSPs evoked by MLF stimuli (compare the difference traces in Fig. 4G).
As shown in Table 2, mutual facilitation similar to the one described above was found in a high proportion of neurones. It was found between disynaptic EPSPs from the MLF and MLR as well as between those evoked from the MLF and PTs or MLR and PTs.
The facilitated components of EPSPs evoked by MLF stimuli were delayed by 0.5–0.7 ms with respect to the monosynaptic EPSPs (differences statistically significant atP< 0.001; Student'sttest for two samples assuming equal variance) and by 0.2–0.3 ms with respect to the disynaptic EPSPs (not statistically significant; pairedttest). They were thus compatible with disynaptic EPSPs mediated by synaptically activated RS neurones. EPSPs facilitated by PT stimuli were evoked at practically the same latencies as those facilitated by MLR stimuli (not statistically significant; pairedttest). Provided that the MLR actions on RS neurones were evoked monosynaptically, as argued above, the same should apply to actions of fibres stimulated within the PTs.
Mutual facilitation of submaximal IPSPs following stimulation of the MLF, MLR and PTs
Reticulospinal neurones evoke not only excitation but also inhibition of SB neurones (see Hammar et al. 2011) and hence inhibitory modulation of SB neuronal activity could likewise be used as feedback information forwarded to the cerebellum, even if we can at present only speculate on how this information is decoded at a cerebellar level. It was therefore of interest to examine whether IPSPs evoked from the MLF can be attributed to RS neurones co-excited by fibres stimulated within the MLR and PTs or to distinct RS neurones. In order to address this question we used the same experimental approach as in experiments described in the preceding sections, analysing mutual interactions between inhibitory effects of MLF, MLR and PT stimuli.
The results showed that IPSPs evoked from the MLF are facilitated by a preceding stimulation of the MLR (Fig. 5A–C) as well as of the PT (Fig. 5D–F). They also revealed mutual facilitation of IPSPs evoked from the MLR and PTs (Fig. 5G–I); the proportions of neurones in which it was found are given in Table 2. However, the facilitation frequently appeared combined with occlusion of some components of these IPSPs. As shown in Fig. 5FandI, while some parts of the records following combined actions of MLF and PT or MLR and PT stimuli (black traces) were larger than the sums of IPSPs evoked by the separate applied stimuli others were smaller. However, as EPSPs are often evoked conjointly with IPSPs, an occlusion between IPSPs could not be fully reliably differentiated from a possible facilitation of EPSPs preceding or following the IPSPs.
Figure 5. Examples of facilitation of inhibitory actions of stimuli applied in the MLF, MLR and PTs on SB neurones.
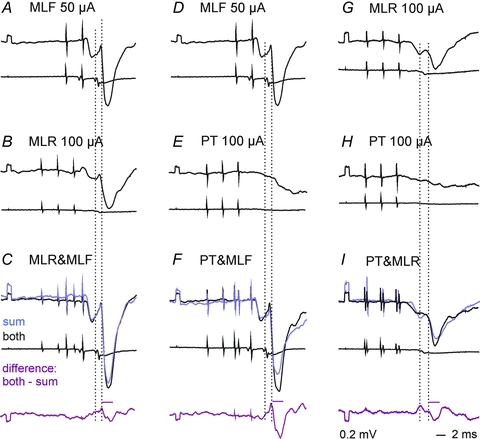
Upper and lower traces inA–FandG–Iare intracellular records from two SB neurones and records from the cord dorsum. InC, FandIblack traces are potentials evoked by combined actions of MLR and MLF, PT and MLF or PT and MLR, the remaining traces are sums of separate actions of these stimuli (A+B, D+EandG+Hrespectively) and of computer generated differences between them, as indicated. The time windows within which the areas of the potentials were measured are indicated by horizontal lines above the bottom records. Note that the sums are smaller than the effects of jointly applied stimuli (87%, 76%, 75%).
Mutual facilitation of activation of extracellularly recorded SB neurones following stimulation of the MLR and MLF
In order to allow the SB neurones to forward relevant feed-back information to the cerebellum the effects of interactions between synaptic actions from the MLF, MLR and PTs on these neurones should be reflected in changes in their firing pattern. We therefore analysed these interactions not only intracellularly but also extracellularly. Stimuli applied within the MLR were found to facilitate activation of SB neurones in three ways. Firstly, by inducing disynaptically evoked responses in neurones in which MLF stimuli evoked only monosynaptic responses. Secondly, by increasing the proportion of stimuli to which the neurones responded disynaptically, e.g. from two to 10 of the 20 subsequent stimuli. Thirdly, by making the neurones respond to earlier stimuli, e.g. to the second rather than fourth stimulus in a train of five stimuli. Stimulation within the MLR by itself did either not (Fig. 6DandI) or only rarely evoked neuronal discharges when tested under our experimental conditions unless following much longer trains of stimuli. Effects of joint actions of MLF, PT and MLR stimuli were therefore analysed during time windows within which we did not have to subtract responses evoked from the MLR.
Figure 6. Examples of facilitation of disynaptically evoked discharges by MLF stimuli.
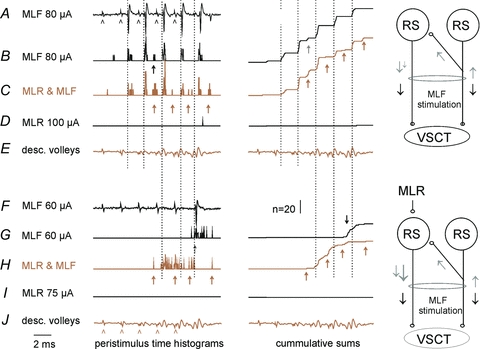
A–DandF–I, records from two SB neurones in the same experiment with cord dorsum potentials inEandJ.A, extracellularly recorded responses of the first neurone.B–D, peristimulus time histograms of responses evoked by 20 trains of five stimuli applied to MLF, both MLF and MLR, and MLR only.E–I, as inA–Dbut for another neurone. Records to the right ofB, C, GandHare cumulative sums of the histograms. Five stimuli applied in MLR coincided with the MLF stimuli. Arrowheads inAandJindicate timing of these stimuli. Dotted lines indicate the onset of monosynaptically evoked responses (at a latency 3.3–3.5 ms from the stimulus, 0.7–0.9 from the first component of the descending volleys). Arrows indicate discharges delayed with respect to the earliest ones by 1.2–2.2 ms; these were classified as evoked disynaptically. Note that the first neurone was activated from the MLF predominantly monosynaptically, while the second one predominantly disynaptically. Note also the overall much more marked facilitation of the disynaptic activation that was not preceded by monosynaptically evoked discharges, which might have prevented re-excitation of SB neurones at too short intervals. The diagrams indicate the most plausible explanation of the more effective indirect effects (grey arrows) of joint stimulation of MLR and MLF than of MLF only (see Discussion).
The series of records in Fig. 6A–D illustrate the common finding that the earliest discharges evoked from the MLF (the onset of which is indicated by vertical dotted lines) were only negligibly affected by MLR stimuli. In the illustrated neurone these discharges followed each MLF stimulus by 3.4–3.6 ms, being delayed with respect to the first component of the descending volleys by 0.6–0.8 ms, and thereby fulfilling the criteria for monosynaptically evoked responses.
In the sample of 14 SB neurones monosynaptically activated by MLF stimuli, the probability of activation was only marginally increased by stimuli applied in the MLR. MLF stimuli alone and MLF stimuli preceded by MLR stimuli evoked on average the same number of responses per stimuli (0.40 ± 0.07 and 0.42 ± 0.007) and at practically the same latencies (3.51 ± 0.04 and 3.5 ± 0.02 ms from the stimuli, respectively; differences not statistically significant, paired Student'sttest).
In contrast to these negligible effects on monosynaptic responses, those appearing at longer latencies were considerably facilitated, thereby replicating the interactions between effects of MLF and MLR stimuli on hindlimb motoneurones (Floeter et al. 1993). In the neurone illustrated in Fig. 6 longer latency responses (arrows) only appeared after the second MLF stimulus when it was applied alone (Fig. 6B) but were evoked after both this and all successive stimuli when associated with MLR stimuli (Fig. 6C). Overall, in 24 SB neurones in which occasional late responses were evoked by MLF stimuli alone, their number increased from 0.10 ± 0.02 to 0.28 ± 0.03 responses per stimulus, i.e. more than doubled, even though they were evoked at practically the same latencies (4.57 ± 0.07 and 4.53 ± 0.07 ms respectively; not significantly different). In 4 of 8 neurones in which MLF stimuli failed to evoke late responses, such responses did, however, appear following joint application of MLR and MLF stimuli, as in Fig. 6F–I, at a rate of 0.44 ± 0.17 per stimulus and at the same latency of 4.53 ± 0.18 ms. The minimal latencies of these later discharges were only about 1 ms longer than those evoked monosynaptically and are thus generally compatible with latencies of disynaptically evoked responses. The facilitation of synaptic activation of RS neurones by axon collaterals stimulated in the MLF and by axons stimulated in the MLR as illustrated in Fig. 4 may thus be potent enough to evoke disynaptic activation of SB neurones following their monosynaptic activation by MLF stimuli.
The facilitatory effects varied between preparations and depended on the number, intensity and timing of stimuli applied in the MLF and in the MLR. Activation of SB neurones by the MLF alone usually required three to five stimuli at 400 Hz and was only occasionally evoked by double or single stimuli. Facilitatory effects evoked from the MLR likewise required a train of at least three to five stimuli. Partly overlapping trains of MLF and MLR stimuli were therefore routinely used. The timing between these stimuli appeared to be less critical and a potent facilitation was found within a fairly wide range of stimulus intervals. This is illustrated in Fig. 7 with the time course of the facilitatory effects in a neurone that responded to double MLF stimuli when they were applied alone but was activated by single MLF stimuli following a short train of MLR stimuli.
Figure 7. Effectiveness of MLR stimuli as a function of intervals between the MLR and MLF stimuli.
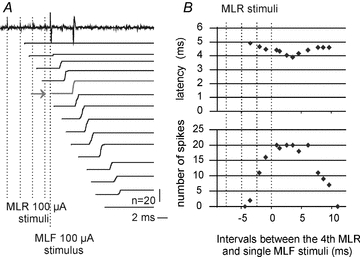
A, an example of responses of a SB neuron evoked by single stimuli applied in the MLF preceded by MLR stimuli and cumulative sums of responses evoked by 20 such stimuli at increasing intervals from the MLR stimuli. The four MLR stimuli remained stationary, as indicated by vertical dotted lines, while MLF stimuli were moved; they were applied at times corresponding to the beginning of the successive cumulative sums. The horizontal arrow indicates effects evoked at the optimal conditioning testing interval. When not preceded by MLR stimuli and at the shortest and longest intervals, the responses appeared only when double MLF stimuli were used. Note that not only the number of the stimuli, indicated by the amplitude of the cumulative sums, but also their synchronization, indicated by the slope of the rising phases of the cumulative sums were changing.B, plots of latencies of the responses and of their number as a function of intervals between the MLF stimulus and the fourth of the MLR stimuli. Note that the facilitation to at least 50% of effective stimuli occurred when the MLF stimuli were applied between the third and fourth MLF stimulus and within the time window of about 5 ms following the 4th MLR stimulus.
Mutual facilitation of activation of extracellularly recorded SB neurones following stimulation of the PTs and MLF
Stimuli applied in the PTs only had negligible effects on monosynaptic activation of SB neurones from the MLF but increased the incidence of disynaptic discharges to 0.39 ± 0.06 responses per stimulus as compared to 0.11 ± 0.04 responses for MLF stimuli alone (P< 0.001). The degree of facilitation by PT and MLR stimuli in the same neurones was comparable, e.g. by decreasing the latency of discharges to the same degree (to 4.21 ± 0.08 ms and 4.27 ± 0.06 ms as compared to 4.64 ± 0.09), not statistically significant. However, as the degree of facilitation critically depended on the parameters of PT and MLF stimuli, the above values are given for the purpose of providing evidence that PT and MLF activation of SB neurones may be evoked by the same reticulospinal neurones and not to quantify the facilitation.
Facilitation following PT stimuli was found both when these stimuli failed to evoke discharges by themselves, as illustrated in Fig. 8A–C, and when they did evoke them. However, while stimulation of the ipsilateral PT was always followed by facilitation, stimulation of the contralateral PT evoked facilitation in some SB neurones but had an inhibitory effect on the other ones (Fig. 8D–F), which was time related to IPSPs recorded intracellularly (Fig. 8GandH).
Figure 8. Facilitation or inhibition of discharges of a SB neurone evoked from the MLF following stimulation of medullary pyramids.
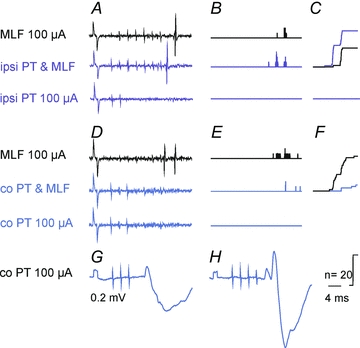
A–C, facilitation of MLF evoked responses of a SB neurone by stimulation of ipsilateral PT fibres.D–F, inhibiton of similarly evoked responses of the same neurone by stimulation of contralateral PT fibres.GandH, intracellular records of PSPs evoked by the same stimuli as inD–Fin a subsequently penetrated SB neurone.AandD, extracellular records from SB neurones (single traces).BandE, on-line generated peristimulus time histograms of responses evoked by successive 20 trains of MLF stimuli and of the same stimuli preceded by PT stimuli.CandF, cumulative sums of data points inBandE. Note larger effects of conditioned stimuli inBand smaller effects inF. Other indications as in previous figures.
Interactions between effects of MLR and PT stimuli attributable to their actions on the same RS neurones
Intracellular records illustrated in Fig. 4G demonstrated that joint actions of PT and MLR stimuli have stronger facilitatory effect on disynaptic EPSPs evoked from the MLF than separate actions of these stimuli. They thereby provide evidence that these effects are mediated by at least some shared reticular neurones. In extracellular records we likewise found that jointly applied MLR and PT stimuli facilitated discharges evoked from the MLF stimuli more potently than either MLR or PT stimuli alone (Fig. 9C–F). Mutual facilitation of effects of stimuli applied in the MLR and in the PTs was found in 9/14 neurones. The facilitation occurred when both MLR and PT stimuli were subthreshold for discharging the neurones (Fig. 9AandB) as well as when PT stimuli were suprathreshold and only MLR stimuli were subthreshold. Responses evoked by joint PT and MLR stimuli were evoked at latencies 5.25 ± 0.25 ms, i.e. about 0.8 ms longer than the latencies of disynaptic discharges evoked by MLF stimuli, suggesting either a longer conduction time from the MLR or PTs than from the MLF, or longer time between the EPSPs evoked by MLR and PT stimuli and the subsequent action potentials generated by them. The number of responses evoked by MLR and PT stimuli (0.60 ± 0.11) was comparable to the number of responses evoked by MLR and PT stimuli applied jointly with MLF stimuli (0.66 ± 0.09). Even though PT stimuli might, theoretically, activate SB neurones via spinal neurones, there are no indications that this might be the case for MLR stimuli and the only common known site at which spatial facilitation of actions from the MLR and from the ipsilateral and contralateral PTs might occur would be reticulospinal neurones (see Discussion).
Figure 9. Shared relay neurones of MLF, MLR and PT actions on SB neurones.
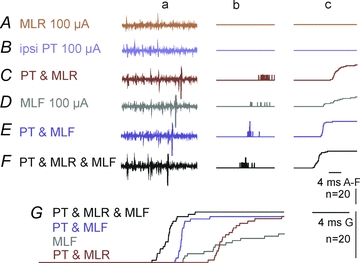
Records from a SB neurone weakly activated by stimulation of the MLF in which effects of either separate or joint stimulation of the MLR and the ipsilateral PT were examined. Column ‘a’, examples of single sweep records from this neurone. Column ‘b’, peristimulus time histograms of 20 sequences of trains of 5 stimuli. Column ‘c’, cumulative sums of responses making up the histograms, as in Figs 6 and 8.A–C, effects of stimuli applied in the ipsilateral MLR, PT and MLF alone.D–F, effects of combinations of these stimuli.G, superimposed 3 times enlarged cumulative sums fromC–F.
Discussion
Reticulospinal neurones as relay neurones of centrally initiated locomotion as well as voluntary movements
The reticulospinal neuronal system has been long recognized as an important system integrating commands from several central structures including pyramidal and extrapyramidal cortical systems, basal ganglia, and neuronal systems involved in visual, labyrinthine and other postural adjustment (for references see e.g. Peterson et al. 1974; Mori et al. 2001; Prentice & Drew, 2001; Jankowska & Edgley, 2006; Deliagina et al. 2008; Martin et al. 2010). Particular attention has been paid to the role of RS neurones in locomotion initiated by stimuli applied in the MLR (for references see e.g. Armstrong, 1988; Dubuc et al. 2008; Jordan et al. 2008; Le Ray et al. 2011). However, a recent study on theXenopustadpole led to the conclusion that some reticulospinal neurones are in fact the source of rhythmic excitation that drives spinal cord neurones to fire during swimming and that they constitute an integral part of the rhythm generating circuitry (Soffe et al. 2009).
The issue to what extent locomotion evoked by MLR stimulation and voluntary movements of PT origin are relayed by the same reticulospinal neurones has not yet been resolved. Shared brainstem relays of centrally initiated locomotion and of voluntary movements would be compatible with the reported mutual facilitation of effects of stimulation of the MLR and pyramids at locations rostral to the transection at a medullary level (Shik et al. 1968) and in view of locomotion related changes in activity of PT neurones (Marple-Horvat et al. 1993; Beloozerova et al. 2003; Drew et al. 2004). However, as the initiation of locomotion involves delays of the order of seconds (Shik et al. 1969), the timing of such interactions cannot be used to elucidate whether they occur at the level of reticulospinal neurones, and in particular whether they occur at the level of the same neurones. One approach might be to record from reticulospinal neurones, as in preliminary studies of Orlovsky (1970), Iwakiri et al. (1995) and Garcia-Rill & Skinner (1987) and to examine whether individual neurones are co-excited by stimuli applied in the MLR and in the PTs. However, this would leave the spinal target cells of reticulospinal neurones investigated in this way undefined and would not resolve the issue of possible direct interactions with spinocerebellar neurones.
By recording from SB neurones we could examine whether the RS neurones that excite them are co-activated by stimuli applied in the PTs as well as in the MLR, using occlusion and spatial facilitation of effects of MLF stimuli when preceded by properly timed MLR and/or PT stimuli as a measure of such co-activation. As previously indicated (Jankowska et al. 2003; Edgley et al. 2004) stimuli applied in the MLF give rise to descending volleys but also to nerve impulses in axon collaterals of reticulospinal neurones that in turn provide input to other reticulospinal neurones, or perhaps even back to the same neurones. The indirect activation of reticulospinal neurones could thus be facilitated by an additional input from fibres stimulated within the MLR or within the PTs. Monosynaptically evoked actions of reticulospinal neurones might on the other hand remain unaffected, unless at the level of the spinocerebellar neurones. The results in this study did in fact only reveal negligible effects on monosynaptic actions but marked facilitation of disynaptic components of PSPs evoked by reticulospinal neurones and of disynaptic actions evoked by all of the combinations of the tested stimuli: MLR–MLF, PT–MLF, MLR–PT–MLF and MLR–PT.
In the rare cases of SB neurones in which combinations of MLR, PT and MLF stimuli evoked weaker effects than when these stimuli were applied separately (see examples in Figs 5 and 8) inhibitory interactions within either supraspinal or spinal neuronal networks were involved. It was e.g. reported that stimuli applied in the medial part of the pontine reticular formation inhibited neurones in the n. reticularis magnocellularis (Iwakiri et al. 1995) and it would thus be conceivable that inhibitory neurones located in the pontine nuclei could be preferentially activated by PT or MLR stimuli, thereby preventing the activation of reticulospinal neurones in n. reticularis magnocellularis. Inhibitory interactions between reticular neurones were also found in other studies, e.g. by Ito & McCarley (1987). Inhibition of reticulospinal neurones could also be evoked via cerebellar networks activated via the reticulo-spino-cerebellar loop investigated in this study. Alternatively, inhibition could be evoked at a spinal level, with MLR stimuli activating RS neurones which in turn might inhibit SB neurones via spinal inhibitory interneurones. We consider this possibility because MLF, MLR and/or PT stimuli evoked predominant IPSPs in some of intracellularly recorded neurones of our sample, with examples in Figs 5 and 8.
Mutual facilitation between synaptic actions from the MLF, MLR and PTs could similarly occur not only at the brainstem level but also at the level of spinal interneurones, as postulated for mutual facilitation of effects of stimulation of the MLR and MLF on motoneurones (Shefchyk & Jordan, 1985; Floeter et al. 1993; Gossard et al. 1996; Jankowska & Stecina, 2007). However, even though spinal interneurones might be co-excited by monosynaptic actions of MLF and PT fibres, fibres or neurones stimulated within the MLR lack direct descending projections and could therefore only provide disynaptic input to these interneurones. The earliest synaptic actions of the MLR on SB neurones would thus be trisynaptic, in contrast to the timing of MLR actions reported in Results. Furthermore, no excitatory premotor interneurones have been found to have collateral actions on SB neurones (Jankowska et al. 2010). Therefore, any disynaptic excitatory actions of reticulospinal neurones on motoneurones mediated by premotor interneurones (Floeter et al. 1993; Jankowska et al. 2003) could hardly be paralleled by disynaptic EPSPs in SB neurones.
A few comments might be added on the methodological aspects of our study. As the degree of occlusion and spatial facilitation was dependent on stimulus parameters, it greatly varied in individual neurones, especially in neurones in which both occlusion and facilitation (of different components of the PSPs) could occur at different intervals. Considering these variations, the study focused on optimizing effects seen in individual cells, rather than using standard experimental conditions. However, we set the lower limit of differences between effects of separately and jointly applied stimuli most likely due to occlusion or facilitation at 10% of the compared areas. Such differences were repeatedly found when averages of 20–40 single records were used to extract potentials evoked by the various combinations of stimuli from the synaptic noise and could be exactly quantified (see e.g. Fetz & Cheney, 1980; Munson et al. 1980; Lemon et al. 1986 for similarly used criterion of reproducibility and analysis of averages of small signals). We therefore considered such differences as another test for the null hypothesis under conditions when no reliable measurements needed for statistical comparisons could be made from individual records. However, in order to compensate for the limitations of quantification of our results we increased the number of tests on which our conclusions were based, including effects of both supramaximal (occlusion) and submaximal (facilitation) stimuli and changes in both intracellularly and extracellularly (field potentials) evoked PSPs, the number of spike potentials evoked by various stimulus combinations and descending volleys.
Information on descending commands of reticulospinal neurones forwarded to the cerebellum
Considering that reticulospinal neuronal systems utilize several channels to supply the cerebellum with information on their state of activation, the content of information forwarded by SB neurones could be weighed against information provided via other reticulo-cerebellar connections, via climbing fibres systems as well as via other spinocerebellar neurones. For the interpretation of the meaning of information relayed by SB neurones it may therefore be of particular interest that one of the terminal projection areas of SB neurones, in the sublobule C of the paramedian lobule, was found to be distinct from projection areas of other spinocerebellar neurones (Matsushita & Ikeda, 1980). In contrast, the terminal projection areas of VSCT neurones located within laminae VII–VIII are in the anterior lobe and the posterior vermis where they greatly overlap with those of dorsal horn and Clarke's column DSCT neurones as well as with other less selective projection areas of SB neurones (Xu & Grant, 1988; Matsushita & Yaginuma, 1989). It would thus be conceivable that neurones in different parts of the cerebellum are used to extract different aspects of information forwarded via spinocerebellar neurones as a whole and that the projection of SB neurones to sublobule C may provide for a particular aspect of such feedback information.
How cerebellar neurones decipher whether information forwarded by reticulospinal and spinocerebellar neurones concerns locomotion, voluntary movements of a limb, or any other movements, and how this information is used is a separate issue. Many studies on corrections of mismatched movements concerned predictions and adjustments of visuo-motor reactions (reaching movements directed towards moving objects) and in particular updating the direction and adjusting the speed of the movements to the predicted location of the moving targets (see e.g. Alstermark & Lundberg, 1992; Pettersson & Perfiliev, 2002; Cavina-Pratesi et al. 2010) or eye movements (see Wong & Shelhamer, 2011). As such mismatches may be corrected with minimal delays of about 80–90 ms, they were concluded to be correcteden routeby subcortical neuronal networks (Pettersson & Perfiliev, 2002; see their Fig. 7). Corrections were also analysed during locomotion (for references see e.g. Duysens et al. 2000; Marigold & Misiaszek, 2009) with the attention focused mainly on proprioceptive information used for these corrections (see e.g. Dietz & Duysens, 2000; Zuur et al. 2010). They were reported to occur with even shorter latencies, of about 50–70 ms from the mismatch between the expected and the actual sensory feedback in humans (van der Linden et al. 2007) and 30–40 ms in the cat (Gorassini et al. 1994), likewise suggesting involvement of subcortical, possibly cerebellar pathways (van der Linden et al. 2007).
Predictions and adjustments considered in the present study are of a much more elementary character. They concern adjustments taking into account the degree of excitability of only one class of spinal neuron targeted by descending commands, the motoneurones, rather than the state of complex neuronal networks of either reaching or locomotor movements. Furthermore, they concern changes in motoneuronal excitability in relation to only one particular factor, the inhibition evoked by premotor interneurones activated by muscle spindle and tendon organ afferents. In addition, they involve corrections via the simplest possible neuronal coupling: direct between RS neurones and SB neurones and likewise direct between SB neurones and their cerebellar target neurones. They may serve to adjust descending commands to the degree of receptiveness of motoneurones and to prevent errors of the issuing movements as fast as possible so that these movements are neither too strong nor too weak (Hammar et al. 2011). The substrates of feedback subserved by SB neurones might thus be considered as basic building blocks of other more complex feed-back systems and serve as their model.
Acknowledgments
We wish to thank Jytte Grännsjö for excellent assistance during the experiments and for histological analysis. The study was supported by grants from NINDS/NIH (R01 NS040863) and the Swedish Research Council (VR 15393-01 for E.J.; 522-2005-7255 for I.H.).
Glossary
Abbreviations
- Co
contralateral
- DSCT
dorsal spinocerebellar tract
- I
ipsilateral
- L
lumbar
- MLF
medial longitudinal fascicle
- MLR
mesencephalic locomotor region
- PT
pyramidal tract
- Q
quadriceps
- RS
reticulospinal
- S
sartorius
- SB
spinal border neurones
- Th
thoracic
- VSCT
ventral spinocerebellar tract
Author contributions
I.H. and E.J.: conception and design of the experiments; collection, analysis and interpretation of data; drafting the article. E.N.: collection, analysis and interpretation of data. All authors approved the final version to be published
References
- Alstermark B, Lundberg A. The C3-C4 propriospinal system: target-reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 327–354. [Google Scholar]
- Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci. 2010;30:7258–7268. doi: 10.1523/JNEUROSCI.3582-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Burke R, Lundberg A, Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12:283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- Canedo A, Lamas JA. Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol. 1993;463:475–489. doi: 10.1113/jphysiol.1993.sp019606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, Goodale MA, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci. 2010;30:10306–10323. doi: 10.1523/JNEUROSCI.2023-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden Krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol. 1998;79:447–463. doi: 10.1152/jn.1998.79.1.447. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev. 2008;57:212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters inThe Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fenelon K, Gariepy JF, Smetana R, Menard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Hubbard JI, Oscarsson O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard JP, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res. 1993;92:407–419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Fu TC, Jankowska E, Tanaka R. Effects of volleys in cortico-spinal tract fibres on ventral spino-cerebellar tract cells in the cat. Acta Physiol Scand. 1977;100:1–13. doi: 10.1111/j.1748-1716.1977.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol. 1994;71:603–610. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Floeter MK, Degtyarenko AM, Simon ES, Burke RE. Disynaptic vestibulospinal and reticulospinal excitation in cat lumbosacral motoneurons: modulation during fictive locomotion. Exp Brain Res. 1996;109:277–288. doi: 10.1007/BF00231787. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. The origin of a descending pathway with monosynaptic action on flexor motoneurones. Acta Physiol Scand. 1968;74:274–284. doi: 10.1111/j.1748-1716.1968.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Hammar I, Krutki P, Drzymala-Celichowska H, Nilsson E, Jankowska E. A trans-spinal loop between neurones in the reticular formation and in the cerebellum. J Physiol. 2011;589:653–665. doi: 10.1113/jphysiol.2010.201178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XW, Wu CP. Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res. 1985;61:109–116. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Ito K, McCarley RW. Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res. 1987;409:97–110. doi: 10.1016/0006-8993(87)90745-1. [DOI] [PubMed] [Google Scholar]
- Iwakiri H, Oka T, Takakusaki K, Mori S. Stimulus effects of the medial pontine reticular formation and the mesencephalic locomotor region upon medullary reticulospinal neurons in acute decerebrate cats. Neurosci Res. 1995;23:47–53. [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K. Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:133–147. doi: 10.1113/jphysiol.2006.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson L-G, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat. J Neurophysiol. 2010;104:1872–1883. doi: 10.1152/jn.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Nilsson E, Hammar I. Do spinocerebellar neurones forward information on spinal actions of neurones in the feline red nucleus? J Physiol. 2011;589:5727–5739. doi: 10.1113/jphysiol.2011.213694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan L, Liu J, Hedlund P, Akay T, Pearson K. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Exp Brain Res. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Le Ray D, Juvin L, Ryczko D, Dubuc R. Supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res. 2011;188:51–70. doi: 10.1016/B978-0-444-53825-3.00009-7. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol. 1986;381:497–527. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. Vol. 1. New York: Raven Press; 1975. pp. 253–265. [Google Scholar]
- Magni F, Oscarsson D. Cerebral control of transmission to the ventral spinocerebellar tract. Arch Ital Biol. 1961;99:369–396. [Google Scholar]
- Marigold DS, Misiaszek JE. Whole-body responses: neural control and implications for rehabilitation and fall prevention. Neuroscientist. 2009;15:36–46. doi: 10.1177/1073858408322674. [DOI] [PubMed] [Google Scholar]
- Marple-Horvat DE, Amos AJ, Armstrong DM, Criado JM. Changes in the discharge patterns of cat motor cortex neurones during unexpected perturbations of on-going locomotion. J Physiol. 1993;462:87–113. doi: 10.1113/jphysiol.1993.sp019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Pavlides C, Pfaff D. Multimodal sensory responses of nucleus reticularis gigantocellularis and the responses' relation to cortical and motor activation. J Neurophysiol. 2010;103:2326–2338. doi: 10.1152/jn.01122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinocerebellar projections to the vermis of the posterior lobe and the paramedian lobule in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1980;192:143–162. doi: 10.1002/cne.901920110. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yaginuma H. Spinocerebellar projections from spinal border cells in the cat as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1989;288:19–38. doi: 10.1002/cne.902880103. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracerPhaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;389:617–641. doi: 10.1002/(sici)1096-9861(19971229)389:4<617::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, Kimura H. Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1993;17:9–21. doi: 10.1016/0168-0102(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25–40. [PubMed] [Google Scholar]
- Munson JB, Fleshman JW, Sypert GW. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980;44:713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Noga BR, Fortier PA, Kriellaars DJ, Dai X, Detillieux GR, Jordan LM. Field potential mapping of neurons in the lumbar spinal cord activated following stimulation of the mesencephalic locomotor region. J Neurosci. 1995;15:2203–2217. doi: 10.1523/JNEUROSCI.15-03-02203.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Mascitti TA. Sites and mode of termination of fibers of the vestibulospinal tract in the cat. J Comp Neurol. 1964;122:369–387. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. On relations between reticulo-spinal neurons and 'locomotor regions' of the brain stem (in Russian) Biofizika. 1970;15:171–177. [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M. Responses of ponto-medullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res. 1974;21:19–44. doi: 10.1007/BF00234256. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Perfiliev S. Descending pathways controlling visually guided updating of reaching in cats. Eur J Neurosci. 2002;16:1349–1360. doi: 10.1046/j.1460-9568.2002.02203.x. [DOI] [PubMed] [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85:679–698. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–2832. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–2253. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovskii GN, Severin FV. [Locomotion of the mesencephalic cat evoked by pyramidal stimulation] Biofizika. 1968;13:127–135. [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroenceph Clin Neurophysiol. 1969;26:549. [PubMed] [Google Scholar]
- Soffe SR, Roberts A, Li WC. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. J Physiol. 2009;587:4829–4844. doi: 10.1113/jphysiol.2009.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden MH, Marigold DS, Gabreels FJ, Duysens J. Muscle reflexes and synergies triggered by an unexpected support surface height during walking. J Neurophysiol. 2007;97:3639–3650. doi: 10.1152/jn.01272.2006. [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol. 2011;105:1130–1140. doi: 10.1152/jn.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Grant G. Collateral projections of neurons from the lower part of the spinal cord to anterior and posterior cerebellar termination areas. A retrograde fluorescent double labeling study in the cat. Exp Brain Res. 1988;72:562–576. doi: 10.1007/BF00250601. [DOI] [PubMed] [Google Scholar]
- Zuur AT, Lundbye-Jensen J, Leukel C, Taube W, Grey MJ, Gollhofer A, Nielsen JB, Gruber M. Contribution of afferent feedback and descending drive to human hopping. J Physiol. 2010;588:799–807. doi: 10.1113/jphysiol.2009.182709. [DOI] [PMC free article] [PubMed] [Google Scholar]


