Non-technical summary
Limb movements are initiated and controlled by nerve cells in several brain regions. Of these, neurones in the red nucleus supplement actions of corticospinal neurones and may even substitute them after injuries of the motor cortex. We have investigated how commands sent from the red nucleus to spinal neurones, and via them to limb muscles, are monitored by a population of spinal neurones providing the cerebellum with feedback information on operation of spinal neuronal networks. The results show that actions expected to be evoked by neurones in the red nucleus on motoneurones may be monitored by the cerebellum, as actions of corticospinal neurones are, although they are monitored by spinal rather than reticulospinal neurones. These results may provide us with a better understanding of mechanisms contributing to the recovery of motor functions following brain injuries.
Abstract
Abstract
We recently demonstrated that feline ventral spinocerebellar tract (VSCT) neurones monitor descending commands for voluntary movements initiated by pyramidal tract (PT) neurones as well as locomotor movements relayed by reticulospinal (RS) neurones. The aim of the present study was to examine whether VSCT neurones likewise monitor descending commands from the red nucleus (RN). Extracellular records from the spinal border (SB) subpopulation of VSCT neurons revealed that a third (31%) of SB neurones may be discharged by trains of stimuli applied in the RN. Moreover, when RN stimuli failed to discharge SB neurones they facilitated activation of some of these neurones by RS and/or PT neurones, while activation of other SB neurones was depressed. We propose that the facilitation and depression of actions of RS neurones by RN neurones might serve to reflect a higher or lower excitability of motoneurones and therefore a likely higher or lower efficacy of the RS descending commands, prompting the cerebellum to adjust the activation of reticulospinal neurones. Activation of SB neurones by RN stimuli alone would also allow monitoring and adjusting the RN descending commands. Intracellular records from SB neurones revealed both monosynaptic and disynaptic EPSPs and disynaptic IPSPs evoked by RN stimuli. The disynaptic actions remained following transection of axons of reticulospinal neurones within the medullary longitudinal fascicle (MLF) and were therefore taken to be relayed primarily by spinal neurones, in contrast to EPSPs and IPSPs evoked by PT stimuli found to be relayed by reticulospinal rather than spinal neurones.
Introduction
Rubrospinal tract neurones act together with corticospinal neurones to initiate voluntary movements and may even substitute some functions of corticospinal neurones after injuries to the pyramidal tract (PT) system (Ghez, 1975; Gibson et al. 1985; Houk et al. 1988; Martin & Ghez, 1988; Cheney et al. 1991; Pettersson et al. 2000; van Kan & McCurdy, 2001). They may also contribute to centrally initiated locomotion (Orlovsky, 1972; Armstrong, 1986; Arshavsky et al. 1988; Rho et al. 1999; Muir & Whishaw, 2000; Lavoie & Drew, 2002). It would therefore be expected that feed-back information forwarded from the spinal cord to the cerebellum concerns descending commands relayed not only by corticospinal (Hammar et al. 2011; Jankowska et al. 2011) but also by rubrospinal neurones. However feed-back information on actions of rubrospinal neurones has so far only been demonstrated for those evoked on forelimb motoneurones. In addition, it was shown to be forwarded via cervical propriospinal neurones to neurones in the lateral reticular nucleus (Alstermark et al. 1981) and only via them to the cerebellum, rather than directly to cerebellar neurones.
Descending actions on hindlimb motoneurones relayed by feline PT and reticulospinal (RS) neurones have recently been found to be monitored more directly, by ventral spinocerebellar tract (VSCT) neurones including their spinal border (SB) subpopulation (Hammar et al. 2011; Jankowska et al. 2011). The present study therefore aimed at examining whether rubrospinal actions are monitored in the same way. Oscarsson (Magni & Oscarsson, 1961; Oscarsson, 1973) hypothesized that input to spinocerebellar neurones is provided not only by the corticospinal but also by the cortico-rubrospinal pathway and stimuli applied in the red nucleus (RN) were shown to evoke both monosynaptic and disynaptic EPSPs in VSCT neurones (Baldissera & Weight, 1969; Baldissera & Roberts, 1975; Baldissera & ten Bruggencate, 1976). However, it was not established whether stimuli applied in RN may evoke in SB neurones not only EPSPs but also spike potentials which would be required to forward information on commands relayed by RN neurones to the cerebellum. Furthermore, monosynaptic and/or disynaptic actions from the RN were only found in a relatively small proportion of VSCT neurones. Hence, it remained an open question whether feedback information on descending commands from the rubrospinal neurones is forwarded to the cerebellum in the same way as information on descending commands from the reticulospinal and corticospinal neurones. The main aim of the present study was to find the answers to this question.
Methods
Ethical approval
All experiments were approved by the Ethics Committee for Animal Research at the University of Gothenburg (Göteborgs Djurförsöksetiska Nämnd) and comply with NIH and EU guidelines for animal care and with the ethical policies and regulations ofThe Journal of Physiology(Drummond, 2009). The animals were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on six deeply anaesthetised cats weighing 3.4–4.8 kg, four of which were also used for experiments described by Jankowska et al. (2011). Anaesthesia was induced with sodium pentobarbital (Apoteksbolaget, Sweden; 40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–3 h, up to 55 mg kg−1, i.v.). Additional doses of α-chloralose were given when motor reactions were evoked during dissection or when increases in the continuously monitored blood pressure or heart rate were evoked by the experimental procedures. Atropin (0.05–0.2 mg kg−1i.m.) was sometimes administered during the preliminary surgical procedures to reduce tracheal secretion. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; 0.3 mg kg−1i.v.) and the animals were artificially ventilated. Neuromuscular relaxation was induced only after several hours of surgery and when the animal had reached a deep and stable level of anaesthesia and was thereafter maintained by adding pancuronium bromide to the buffer infusion (see below) at doses corresponding to about 0.2 mg kg−1 h−1). Mean blood pressure was kept at 100–130 mmHg and end-tidal concentration of CO2 at about 4–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml−1 h−1 kg−1). The core body temperature was kept at about 37.5°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of pentobarbital i.v. followed by formalin perfusion.
After the initial vein, artery and tracheal cannulation, a laminectomy exposed the third to fifth lumbar (L3–L5), low thoracic (Th11–Th13) and, in some experiments, the second to fourth cervical (C2–C4) segments of the spinal cord. The quadriceps (Q) and sartorius (Sart) muscle nerves were transected and mounted for stimulation in a subcutaneous cuff electrode while branches of the sciatic nerve were mounted on pairs of silver electrodes in paraffin oil pool.
A craniotomy was made over the frontal lobes to allow insertion of a tungsten electrode (impedance 30–150 kΩ) into the right RN, aiming at Horsley–Clarke co-ordinates A3.5, L2.5 and H–3. However, the positioning of the electrode was guided by records of antidromic field potentials following stimulation of the left lateral funiculus (Fig. 1B). It was left at the depth at which the largest of these potentials were recorded and from which the descending volleys were evoked at the lowest thresholds (≤20 μA) from the surface of the lateral funiculus at the Th11–Th13 and/or the C4–C5 segments (for details see Stecina et al. 2008). The caudal part of the cerebellum was exposed for placement of electrodes in the left medial longitudinal fascicle (MLF), in the right pyramid (PT) and in the region of the left n. interpositus in the cerebellum. These electrodes were inserted at an angle of 35–45 deg (with the tip directed rostrally). The initial targets were at Horsley–Clarke co-ordinates P9, L0.6, H–5 for MLF; P7, R1.2, H–10 for PTs and P7, L3, H0 for the cerebellum. The final positions of these electrodes were likewise adjusted on the basis of thresholds of descending volleys. At the end of the experiments the stimulation sites were marked with electrolytic lesions (0.2 mA constant current for 10 s). Their location was subsequently verified on 50 μm thick frontal sections of the brainstem, cut in the plane of insertion of the electrodes using cryostate, counterstained with cresyl violet and scanned. Location of the stimulation sites in the MLF, PTs and the cerebellum is summarized in Fig. 1 in the companion paper (Jankowska et al. 2011); only that of the stimulation sites in the contralateral RN is therefore shown in Fig. 1A.
Figure 1. Location of stimulation sites in the contralateral RN.
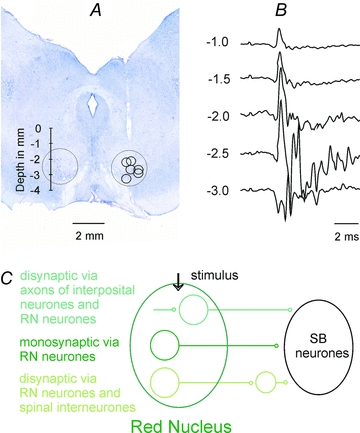
A, location of electrolytic lesions made at the end of the experiments to mark the stimulation sites overlaid on a transverse section of the midbrain of one of the preparations. The extent of the RN is indicated by the circles, the depths according to Horsley–Clarke's coordinates being indicated to the left.B, a series of antidromic field potentials evoked along the most lateral electrode track to the right recorded at the depths indicated to the left.C, diagram of monosynaptic and two disynaptic pathways via which synaptic actions could be evoked by stimuli applied in RN.
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli (0.2 ms duration, intensity expressed in multiples of threshold, T, for the most sensitive fibres in the nerve). For activation of the rubrospinal, corticospinal and reticulospinal tract fibres trains of four or five constant current cathodal stimuli of 0.2 ms duration at 250 or 330 Hz (within the train) were used. The trains were repeated at 1–3 Hz. The stimuli were at intensities of ≤100 μA for RN, 100–150 μA for PT and ≤100 μA for MLF. No particular measures were taken to avoid inadvertent activation of axons of ipsilateral RN neurones after they have crossed the midline, but the risk of current spread to these axons by stimuli applied in the ventral part of the contralateral RN was estimated to be negligible when the stimulus intensity did not exceed 100 μA (Stecina et al. 2008). As shown previously, no current spread to the opposite PT was found when PT stimuli were ≤100 μA (see e.g. Jankowska et al. 2006). No evidence for current spread from the PT electrode to the MLF fibres was found, as estimated by comparing descending volleys evoked from the MLF from within the PTs and from the areas dorsal to the PTs. No attempts were made to differentiate between effects of stimuli applied within the ipsilateral and contralateral MLF, as even submaximal stimuli would encroach upon fibres on the other side of the midline. Up to 150 μA MLF stimuli were expected to activate a large proportion of ponto- and medullary reticulospinal tract fibres (see Jankowska et al. 2003). These stimuli would also activate vestibulospinal tract fibres arising from the medial vestibular nucleus (which do not project caudally as far as the lumbar segments) but would not activate fibres from the lateral vestibular (Deiter's) nucleus (Nyberg-Hansen & Mascitti, 1964), so the effects in the lumbar segments can be attributed to reticulospinal fibres.
Stimuli applied in the cerebellum were used for identification of spinocerebellar neurones by antidromic activation. However, as both contralaterally ascending VSCT neurones and ipsilaterally ascending DSCT neurones could be activated by such stimuli; only neurones activated from both the cerebellum and the contralateral but not ipsilateral lateral funiculus at the Th11–13 were classified as VSCT neurones. The lateral funiculi were stimulated transdurally using pairs of silver ball electrodes.
Descending volleys were likewise recorded transdurally using one silver ball electrode in contact with the dura mater and the reference electrode inserted in one of the back muscles. During placement of the stimulating electrodes the descending volleys were recorded from the lateral border of the dorsal columns at the C3–C4 segments and from the left lateral funiculus at the Th11–12 segments. During recording, the volleys were recorded from the surface of the dorsal columns at the border between the L4 and L5 segments.
Glass micropipettes filled with 2 m solution of potassium citrate were used for recording. Pipettes with impedance 2–4 and 4–6 MΩ were selected for extracellular and intracellular recording, respectively.
Intracellular records were obtained from 54 neurones of the spinal border (SB) subpopulation of VSCT neurones (Lundberg, 1971; Lundberg & Weight, 1971) and extracellular records from 74 neurones. They were identified by their location within the most lateral part of the ventral horn, lateral to the location of motor nuclei in the L4 segment in addition to their antidromic activation from the cerebellum and the contralateral lateral funiculus.
Analysis
Both the original data and averages of 10–40 single postsynaptic potentials were stored on line. Changes in the recorded potentials were estimated by measuring either the peak amplitudes or the areas of these potentials within selected time windows. The timing of extracellularly evoked discharges was estimated using peristimulus time histograms and the number of discharges was determined using cumulative sums of data points from the histograms. The comparisons were made for discharges evoked by 20–50 trains of up to six stimuli (for details see Jankowska et al. 1997, 2011). A software sampling and analysis system designed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg) was used for these purposes. Differences between samples of neurons were assessed for statistical significance using Student'sttest for unpaired or paired data, assuming equal variance.
Criteria for evaluation of direct and indirect actions of rubrospinal tract neurones and the timing of these actions
Synaptic actions most reliably attributable to rubrospinal neurones are evoked by electrical stimuli applied within the red nucleus, after having verified that they are not replicated by similar stimuli applied outside this nucleus (Hongo et al. 1969; Baldissera et al. 1972). Nevertheless, electrical stimuli may activate neurones in the red nucleus in two ways, either directly or trans-synaptically via interposito-rubral fibres providing input to these neurones, as schematically indicated in Fig. 1C. The thresholds for the direct and trans-synaptic activation were found to be similarly low but the proportions of directly and trans-synaptically excited neurones differ depending on the electrode location within the red nucleus and the stimulus intensity. At stimulus intensities near maximal for activating rubrospinal neurones (about 100 μA) some neurones are usually activated directly and others trans-synaptically, but a given neurone is never activated twice (Baldissera et al. 1972). Therefore the two components of the descending volleys recorded from the spinal cord reflect activation of different proportions of rubrospinal neurones. The second component is as a rule delayed by 0.5–1.0 ms with respect to the first component, corresponding to one synaptic delay plus an additional delay for generation of spike potentials in the neurones, the exact timing between the two components depending on the relative effectiveness of the stimuli needed to evoke direct and indirect activation of RN neurones.
With this in mind, monosynaptic actions of rubrospinal neurones on spinal neurones should be evoked at synaptic delays of ≤1 ms from the first components of rubral descending volleys but could also be evoked at 0.5–1.0 ms longer delays if they were induced by the second components of the descending volleys. Disynaptically evoked postsynaptic potentials could thus not be differentiated from those evoked monosynaptically on the basis of their latencies. Differentiation based on temporal facilitation of disynaptic but not monosynaptic PSPs evoked by successive stimuli would be similarly less reliable than temporal facilitation of effects of directly stimulated axons, e.g. reticulospinal or corticospinal fibres, because monosynaptic actions evoked by trans-synaptically excited RN could be facilitated at the rubral level (with associated increases of the later components of the descending volleys, see Fig. 6AandB). Hence, although the lack of temporal facilitation would define PSPs as evoked monosynaptically, the occurrence of such facilitation could not be used to refute the monosynaptic coupling. The most reliable criterion for disynaptically evoked postsynaptic actions relayed by spinal neurones is therefore the combination of being evoked by double or triple but not single stimuli, at latencies exceeding 1 ms and displaying temporal facilitation.
Figure 6. Examples of disynaptic EPSPs evoked from the RN.
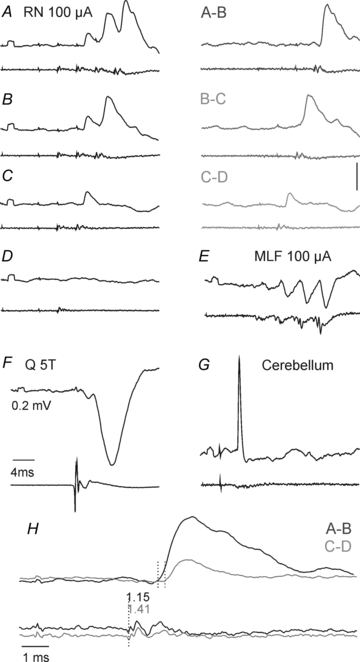
In each panel upper records are intracellular records from an SB neurone and lower records from the cord dorsum.A–D, EPSPs evoked by the 2nd, 3rd and 4th stimuli but not by single stimuli applied in the RN. EPSPs evoked by consecutive stimuli have been visualized by subtracting PSPs evoked by smaller numbers of stimuli inA–B, B–CandC–D.EandF, IPSPs evoked in the same neurone by stimulation of the MLF and of the Q nerve.G, antidromically evoked spike potential following cerebellar stimulation.H, superimposed expanded records of EPSPs evoked by the 2nd (light grey; second dotted line) and 4th (dark grey; first dotted line) RN stimuli. They are aligned with respect to the RN stimuli that evoked them and illustrate the considerably shorter latencies of EPSPs evoked by the 4th stimuli, whether they were measured from the first or the second components of the descending volleys. Both were consistent with disynaptic coupling from the first components (indicated by the dotted line on the cord dorsum records) or with monosynaptic coupling from the later components following trans-synaptic activation of RN neurones (Baldissera et al. 1972).
Results
Are discharges of SB neurones evoked by stimuli applied in the RN?
EPSPs evoked in VSCT neurones by single stimuli applied in the RN in deeply anaesthetized preparations as reported by Baldissera & ten Bruggencate (1976) would not be likely to give rise to action potentials. However, as most of these EPSPs were evoked di- or polysynaptically, with additional synaptic relays either within the RN (see the last section of the Methods) or at a spinal level, they might be expected to be larger in non-anaesthetized preparations. Synaptic actions of rubrospinal neurones on VSCT neurones might therefore be comparable to actions of corticospinal neurones and likewise discharge them. In order to counteract the depressive effects of anaesthesia, we enhanced synaptic transmission in pathways between RN and the spinocerebellar neurones by 4-AP (Sigma, USA; 0.1–0.2 mg kg−1, i.v.) as in previous studies (Hammar et al. 2011; Jankowska et al. 2011), and used relatively long trains of RN stimuli (5–6 stimuli at 300 or 400 Hz). We used the spinal border subpopulation of VSCT neurones located in the L4 segment, the excitatory input to a high proportion of which is derived from descending tract neurones rather than from peripheral afferents (Lundberg & Weight, 1971; Baldissera & Roberts, 1975; Baldissera & ten Bruggencate, 1976; Fu et al. 1977; Hammar et al. 2011).
Under these conditions, trains of stimuli applied in the co RN evoked discharges of 13 out of 29 extracellularly recorded SB neurones. However, in several neurones spike potentials only appeared occasionally, less than after every 10th stimulus, and could not be reliably differentiated from spontaneously occurring discharges. They consistently followed the 3rd–5th stimuli in only nine (31%) neurones. In the same sample the same proportion of neurones (31%) were discharged by stimuli applied in the co PT but a much higher proportion (85%) were discharged by MLF stimuli. RN and PT stimuli were also less effective than MLF stimuli in that they evoked responses of SB neurones three times less frequently than MLF stimuli (0.11 ± 0.03 and 0.09 ± 0.03 against 0.33 ± 0.04 responses per stimulus, respectively). In addition, responses evoked by RN and PT stimuli usually appeared only after the 4th–5th stimulus in the train while they appeared after the 2nd, 3rd and sometimes even 1st MLF stimuli, as illustrated in Fig. 2.
Figure 2. Comparison of effectiveness of RN, PT and MLF stimuli in discharging SB neurones.
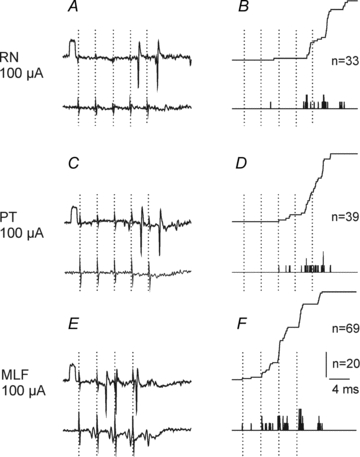
Left panels, single extracellular records from one of the neurones in which consistent discharges were evoked from the RN with the corresponding records from the cord dorsum below. The timing of 5 RN and PT stimuli and of 4 MLF stimuli is indicated by dotted lines. Note that descending volleys induced by these stimuli appeared at latencies of about 3 ms, i.e. just prior to the next stimulus. Right panels, peristimulus time histograms of responses evoked by 20 sequences of the stimuli (bottom traces) and cumulative sums of these responses (upper traces; each spike adding one step to the curve. The figures give the total number of responses evoked by the illustrated sequences of stimuli. In this and the following figures rectangular pulses at the beginning of records are calibration pulses (0.2 mV unless stated otherwise). The negativity is downwards in microelectrode records and upwards in records from the cord dorsum.
Are discharges of SB neurones evoked from the MLF facilitated or depressed by stimuli applied in the RN?
Despite infrequent activation of SB neurones by RN stimuli, RN neurones could provide subliminal excitatory input to SB neurones that failed to discharge in response to these stimuli and thereby contribute to activation of SB neurones by other stimuli. This was verified using two measures: changes in the proportions of neurones activated by joint and separate RN and MLF stimuli and changes in the number of responses evoked by these stimuli. Figure 3B illustrates the stronger combined effects of trains of stimuli applied in the RN and MLF than effects of stimulation of the MLF alone, with a greater number of responses evoked after the second and third stimulus in the cumulative sums. The facilitation was evoked in only 5/24 (21 %) of the neurones tested, and combined effects of trains of stimuli applied in the RN and MLF were more frequently weaker than effects of stimulation of the MLF alone, in 15/24 (63%) of the neurones. An example of a lower number of responses after each stimulus is given in Fig. 3F and the data for the whole sample are shown in Fig. 4. The data points above and below the grey horizontal line in Fig. 4 represent facilitation and depression of responses to MLF stimuli above their control values of 7–10 per sequence of 20 stimuli, with 5% changes in both directions considered as null, or as being within the errors of measurement. Despite the greater number of cells in which responses evoked from the MLF were depressed, or only negligibly affected, the dominating effect of RN stimuli was facilitation as the overall effect of these stimuli amounted to 126 ± 30%.
Figure 3. Interactions between effects of stimuli applied in the RN, the MLF and the PT on responses of SB neurones.
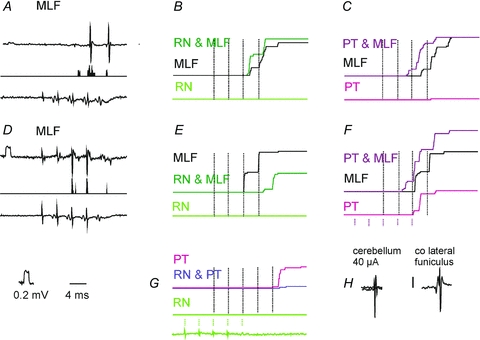
A, an example of single sweep extracellular records of responses of a SB neurone to 4 MLF stimuli (top record), peristimulus time histogram of responses to 20 sequences of these stimuli (as in Fig. 2F) and the corresponding records of descending volleys.B, cumulative sums of responses evoked when MLF stimuli were applied alone (as in Fig. 2F; middle trace) and when they were associated with a partly overlapping train of 5 stimuli applied in the RN (top trace) which failed to induce any discharges by itself (lower trace). Dotted vertical lines indicate the timing of the four MLF stimuli; the timing of RN stimuli is indicated at the bottom inG. Note the much greater number of responses evoked by the second MLF stimulus when preceded by RN stimuli, even though the total number was only moderately increased.C, as inB, but for effects of MLF and PT stimuli.D–F, as inA–C, but from another SB neurone in which the number of responses evoked by MLF stimuli preceded by RN stimuli was considerably decreased.G, effects of combining RN and PT stimuli on responses evoked by PT stimuli in the same SB neurone as inEandF.HandI, examples of antidromically evoked activation of the first neurone by stimuli applied within the cerebellum and to the contralateral lateral funiculus. Other indications are as in Fig. 2.
Figure 4. Interactions between effects of stimuli applied in the RN, the MLF and the PT on reponses of SB neurones.
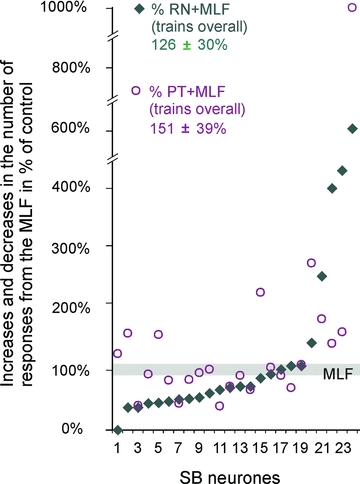
Plots of numbers of responses to 20 sequences of trains of stimuli applied in the MLF when they were preceded by and overlapped with trains of stimuli in the contralateral RN (filled symbols) or PT (open symbols). The data are expressed as a percentage of the total number of responses evoked by MLF stimuli during a 20 ms time windows after the first MLF stimulus in 24 SB neurones. The data are ranked from the lowest to the highest number of responses evoked by joint actions of MLF and RN stimuli represented by filled symbols. The grey horizontal bar indicates the number of MLF evoked responses expressed as 100 ± 5%. The differences between overall effects (including facilitatory as well as depressive effects) of RN and PT stimuli were not statistically significant (Student'sttest for paired samples).
However, stimulation of the RN sometimes resulted in mixed effects. Even when responses to the first MLF stimuli were enhanced, those to the later MLF stimuli were often weakened (as exemplified in Fig. 3E). The degree of facilitation of responses evoked by the first effective stimulus was therefore more marked. In the 19 neurones out of the total sample of 24 SB neurones in which such a comparison was made, it occurred in a larger proportion (52% after the first stimulus compared to 21% after a stimulus train). The depression of responses evoked by single MLF stimuli was accordingly found in a smaller proportion of SB neurones (48% compared to 63%).
In individual SB neurones the effects of PT and RN stimuli on discharges evoked from the MLF were either in the same or in the opposite direction, being either facilitated from both, or facilitated from PT but depressed from NR, or vice versa. Facilitation from both PT and RN is illustrated in Fig. 3BandC, depression evoked by RN stimuli but facilitation by PT stimuli is illustrated in Fig. 3EandF and the data for the whole sample of extracellularly recorded SB neurones are summarized in Fig. 4. The proportions of SB neurones in which the total number of responses was increased following RN stimuli (21%) was smaller than that observed following PT stimuli (37%), but facilitation after the first effective stimuli occurred in similar proportions (52 and 57% of the neurones respectively).
When PT stimulation evoked neuronal discharges by itself, effects of RN stimulation were examined not only on responses evoked from the MLF but also on responses following PT stimulation. In all five neurones in which this was investigated the total number of these responses was reduced when they were combined with RN stimulation. In two of these SB neurones none or only occasional discharges were evoked by RN stimuli alone but when these stimuli preceded PT stimulation the responses were depressed to 26% and 10% respectively. In the remaining three SB neurones in which weak discharges were evoked from RN the depression was estimated to amount to 33%, 24% and 72%, respectively. In neurones which were subsequently penetrated, a good correspondence was found between facilitation or inhibition evoked from the RN and the dominating EPSPs or IPSPs evoked by the same stimuli.
Distribution of monosynaptic and disynaptic EPSPs and disynaptic IPSPs from the RN
Monosynaptic EPSPs evoked from RN were found in a much smaller proportion of neurones than from the MLF (Table 1). As described in Methods they were identified by their appearance after the first stimulus as well as after the subsequent stimuli in a train, at segmental latencies of <1 ms from the first component of the descending volley, with examples in Fig. 5AandB, andEandF and Fig. 6. However, as rubrospinal neurones may be activated both directly and trans-synaptically and as the second components of the descending volleys reflect the indirect trans-synaptic activation, monosynaptically evoked EPSPs were expected to follow not only the first but also the second component of the volley. As illustrated in Fig. 5AandE, the first and second components of EPSPs evoked by single stimuli were evoked at about 0.5 and 1.0 ms, being compatible with monosynaptic actions following both components of the descending volleys. Such EPSPs were often evoked in neurones in which the dominating input from the PT was inhibitory, as in the SB neurones illustrated in Fig. 5A–DandE–H.
Table 1.
Comparison of synaptic actions from the contralateral RN, contralateral PT and ipsilateral MLF in spinal border neurones and in lumbar motoneurones
| SB neurones present study | VSCT and SB neurones* | α motoneurones** | |||
|---|---|---|---|---|---|
| co RN n = 42 | co PT n = 38 | i MLF n = 42 | co RN n = 82 | co RN n = 12 | |
| Proportions of neurones with: | |||||
| Monosynaptic EPSPs | 17% | 49% | 17% | ||
| Disynaptic EPSPs | 43% | 39% | 49% | 19% | 53% |
| Mono and/or disynaptic EPSPs | 57% | 83% | |||
| Disynaptic IPSPs | 81% | 55% | 54% | 41% | 42% |
| Mean latencies (ms) of: | |||||
| Monosynaptic EPSPs (0.5–0.9 ms segmental) | 4.33 ± 0.11 | 3.28 ± 0.05 | |||
| Disynaptic EPSPs (1.1–1.4 ms segmental) | 4.66 ± 0.20 | 5.86 ± 0.76 (trisyn) | 4.02 ± 0.15 | 4.79 ± 0.09 | |
| Disynaptic IPSPs (1.1–2.2 ms segmental) | 5.16 ± 0.06 | 5.95 ± 0.24 (trisyn) | 4.24 ± 0.11 | 5.17 ± 0.18 | |
Data from Fig. 1 in Baldissera & ten Bruggencate, 1976.
Data from Fig. 7 in Stecina et al. 2008.
Note that latencies of disynaptic EPSPs from RN were 1.2 ms shorter than of most likely trisynaptic EPSPs from co PT. The difference between latencies of disynaptic IPSPs from RN and from PT was smaller (0.79 ms) but it was also compatible with a more direct coupling. Note also similar latencies of disynaptic EPSPs and IPSPs of RN origin in SB neurones and in hindlimb alpha-motoneurones.
Figure 5. Examples of monosynaptic EPSPs from RN in two SB neurones.
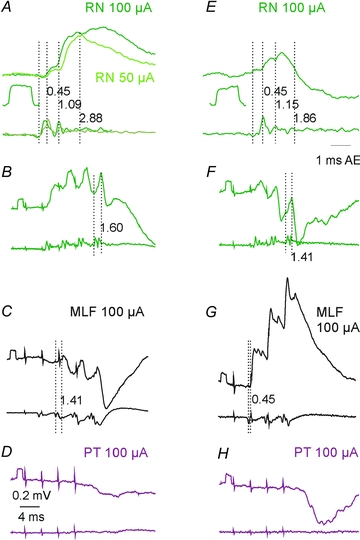
In all panels, upper traces are intracellular records and lower traces are records from the cord dorsum. Left panels, records from one of the two SB neurones illustrating monosynaptic EPSPs evoked by single 50 and 100 μA stimuli (A), EPSPs followed by disynaptic IPSPs after the 2nd, 3rd and 4th stimuli (B), disynaptic IPSPs from the MLF, possibly preceded by small EPSPs (C), and IPSPs from the PT (D). Right panels, similar records from the second SB neurone penetrated in the same experiment but with a different pattern of input, with smaller EPSPs (E), but with larger IPSPs from RN (F), monosynaptic EPSPs followed by disynaptic EPSPs rather than IPSPs from the MLF (G), and larger IPSPs from the PT (H). Note that records inAandEare expanded 2× vertically and 4× horizontally. The first vertical dotted lines in each panel indicate the positive inflection of the first descending volley induced by the stimuli in the same segment as the cells recorded from. The following dotted lines indicate the onset of various components of postsynaptic potentials following this volley with the latencies indicated to the right of these lines. Rectangular pulses at the beginning of records as well as those below records inAandEare 0.2 mV calibration pulses.
Disynaptic EPSPs were evoked by only the second or successive stimuli and showed distinct temporal facilitation (Fig. 5A–D). The temporal facilitation is best seen when comparing the net effects of successive stimuli after having subtracted effects of the earlier stimuli, as in the right panels in Fig. 6A–C and in the superimposed expanded records in Fig. 6H. They were evoked at longer segmental latencies from the first components of the descending volleys than those classified as evoked monosynaptically. Disynaptic EPSPs were found in similar proportions of SB neurones from RN, PT and MLF. However, not only monosynaptic but also disynaptic EPSPs from RN were often evoked in neurones in which the dominating input from the MLF and PT was inhibitory, as in that illustrated in Fig. 6E.
Disynaptic IPSPs from RN (Fig. 5F) were evoked in a larger proportion of SB neurones than IPSPs from either PT or MLF (Table 1) and they often cut short the monosynaptic EPSPs preceding them, as in the neurone illustrated in Fig. 5F. A more potent inhibitory input from RN may thus explain why effects of stimuli applied in RN were less effective in discharging SB neurones than those applied in PT despite the more direct excitatory input. Proportions of neurones with monosynaptic EPSPs were the same in the present sample of SB neurones and in the sample of neurones of Baldissera & Bruggencate (1976). The proportion of neurones with disynaptic EPSPs of RN origin were either smaller than in α-motoneurones or similar (Stecina et al. 2008) but that with disynaptic IPSPs was larger.
Are synaptic actions of rubrospinal and pyramidal tract neurones on SB neurones relayed in a similar way?
The differences in distribution of disynaptic EPSPs and IPSPs from the coRN, coPT and MLF raised the question whether disynaptic actions of RN neurones could be mediated in a similar way as disynaptic actions of PT neurones, i.e. by being relayed by reticulospinal neurones. A closer inspection of EPSPs evoked by the second or third stimulus in the train (see the expanded records in Fig. 6H) revealed that while the latencies from the first component of the descending volley exceeded 1 ms (being compatible with disynaptic coupling), the latencies from the second components of descending volleys were <1 ms. These later components could therefore represent monosynaptic actions of trans-synaptically activated RN neurones (Baldissera et al. 1972) with direct actions on SB neurones but could also be relayed by spinal interneurones. In order to verify whether disynaptic rubral actions could be relayed at a spinal level, we compared effects of stimuli applied in the contralateral RN before and after transection of axons of reticulospinal neurones descending in the MLF. The extent of the MLF lesion was monitored by recording descending volleys evoked by MLF stimuli applied a few millimetres rostral to the transection by using records from the ipsilateral lateral funiculus at a Th12 level and L4 levels. The lesion was gradually extended until the volleys disappeared (cf. Fig. 7LandM). The histological reconstruction of the lesion (Fig. 7K) showed that it covered an area corresponding to the whole MLF and the neighbouring parts of the reticular formation bilaterally but spared both pyramids.
Figure 7. Comparison of effects of stimuli applied in the contralateral RN and the contralateral PT before and after the MLF lesion.
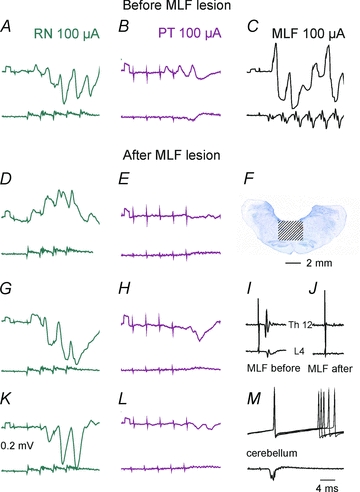
A–C, DandE, GandHandK–M, records from four SB neurones, the first recorded prior the MLF lesion and the three remaining neurones after the lesion. Upper traces, intracellular records from the neurones. Lower traces, records from the cord dorsum.F, the extent of the lesion.IandJ, records from the lateral funiculus at the Th12 and L4 levels showing that the volleys evoked by MLF stimulation disappeared after the lesion. Note that both EPSPs and IPSPs were evoked from the RN after the lesion but only IPSPs followed PT stimuli.
In eight SB neurones intracellularly recorded after such a lesion both monosynaptic (n = 2) and disynaptic EPSPs (n = 6) and disynaptic IPSPs (n = 8) were evoked by RN stimuli with examples in Fig. 7GandH. The latencies and the amplitudes of these PSPs both fell within the same ranges as before the lesion. In contrast, no EPSPs from the PT were evoked in any of these neurones and IPSPs (Fig. 7HandJ) tended to be smaller and appeared after later stimuli in the train and at longer latencies.
Extracellular records from 34 SB neurones obtained after the MLF transection revealed that 41% of the neurones were discharged by RN stimuli (as compared to 31% in preparations with the MLF intact) and that the rates of discharges in the two preparations were similar (0.14 ± 0.04 and 0.11 ± 0.03 responses per stimulus for the whole sample, or 0.33 ± 0.07 and 0.34 ± 0.06 responses for those effectively activated). Records from one of the most effectively activated SB neurones, after the 3rd, 4th or 5th stimulus, are illustrated in Fig. 8. Figure 8AandB shows also that stimuli applied within the nucleus were more effective than those applied close to its dorsal or ventral borders. When latencies of descending volleys evoked from these locations (Fig. 8FandG) were compared to the latencies of antidromic field potentials (Fig. 8E) they showed in addition that they met the requirements for volleys in directly and trans-synaptically (i.e. after an additional delay) activated rubrospinal neurones (Baldissera et al. 1972) as in preparations with intact MLF.
Figure 8. Examples of activation of SB neurones by stimuli applied in the contralateral RN after the MLF lesion.
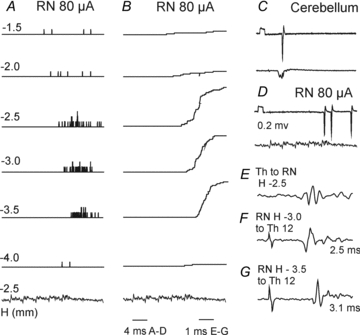
AandB, peristimulus time histograms and cumulative sums of responses of an SB neurone by a train of 5 stimuli applied in the RN at different depths in millimetres below the inter-aural level (H level 0) according to Horsley–Clarke's coordinates (see Fig. 1A) together with cord dorsum potentials following NR stimuli at a depth H–2.5.C, five superimposed records of antidromic responses evoked from the cerebellum.D, single record of responses evoked by a train of 5 stimuli at a depth H–2.5 and descending volleys recorded from the cord dorsum at L4.E, antidromic field potential recorded in the RN by stimuli applied at Th12.FandG, descending volleys recorded from the cord dorsum at Th12 when stimuli were applied at the indicated depths in the RN. Note that the descending volleys evoked from the more dorsal location were evoked at a shorter latency than those from more ventral location, directly and trans-synaptically, respectively, and that the latencies of the earliest discharges of the illustrated SB neurone were also shorter when the stimuli were applied more dorsally.
Discussion
The results of a previous study (Hammar et al. 2011) led to the conclusion that SB neurones forward information on the likely output of descending commands of reticulospinal neurones on spinal neurones and thereby reflect the probability of activating motoneurones. This proposed function of SB neurones may therefore be added to the functions of VSCT neurones discussed previously, such as comparing input and output from spinal interneurones (Lundberg, 1971; Lindström, 1973; Baldissera & Roberts, 1975). The other recent study (Jankowska et al. 2011) revealed that SB neurones may reflect the probability of activating motoneurones not only by reticulospinal but also by corticospinal neurones and neurones or fibres stimulated within the mesencephalic locomotor region relayed by reticulospinal neurones. The results of the present study extend the number of descending commands monitored in this way by SB neurones to those originating from rubrospinal neurones.
As shown in the results RN neurones may discharge SB neurones albeit both the proportion of SB neurones and the degree to which they are activated are about three times weaker than following stimulation of axons of RS neurones within the MLF. Discharges evoked by RN neurones might thus be a much less reliable source of information on descending commands than those evoked by RS neurones and the weak excitatory input from RN neurones to SB neurones would make them even less likely to monitor the descending commands initiated by RS neurones. However, RN neurones were found to facilitate responses of SB neurones evoked by reticulospinal neurones to the same extent as PT neurones and in this way may contribute to the monitoring of descending commands relayed by RS neurones, as discussed by Hammar et al. (2011).
Previously analysed actions of RN neurones on motoneurones revealed that the most direct coupling between these neurones is disynaptic (Hongo et al. 1969) in contrast to both monosynaptically and disynaptically evoked synaptic actions found in SB neurones, both by Baldissera et al. (1969, 1975, 1976) and in the present study. The amplitudes of disynaptic EPSPs evoked in motoneurones appeared to be larger than in SB neurones and the motoneurones might be more easily discharged than SB neurones, at least as judged by limb movements evoked by RN stimulation. We nevertheless propose that the excitatory actions of RN neurones on SB neurones reflect the excitatory actions of RN neurones on motoneurones; together with the facilitation of activation of SB neurones by RS neurones they could be used to signal to the cerebellum that RS neurones may be relatively more effective in activating motoneurones. Vice versa, the reflection of inhibitory actions of RN neurones on motoneurones and depression of activation of SB neurones by RS neurones, could be used to signal that actions of RS neurones are likely to be relatively less effective. If so, the results of this study would indicate that input from RN neurones to SB neurones more likely signals a probability of activation of motoneurones by rubrospinal neurones than serves to monitor the descending rubral commands.
In view of the previously reported projections from the RN to the medial part of the ipsilateral reticular formation in the rat (Yasui et al. 2001) and the contralateral parvicellular reticular nucleus in the cat (Robinson et al. 1987) we expected a coupling between RN and RS neurones similar to that between PT and RS neurones, even though it was not established whether neurones targeted by RN neurones in these regions of the reticular formation project to the spinal cord and could relay RN actions to SB neurones. Latencies of disynaptic EPSPs and IPSPs evoked in SB neurones from the RN would be compatible with such a possibility as they exceeded the latencies of monosynaptically evoked EPSPs from the MLF by 1.1–1.8 ms, compatible with synaptic actions evoked with one additional synaptic delay. These disynaptic EPSPs and IPSPs could, however, be mediated by spinal interneurones as well as by RS neurones. In motoneurones disynaptic EPSPs and IPSPs evoked from the RN were frequently found not to be associated with similar actions from the MLF, or to be associated with opposite actions (Stecina et al. 2008), showing that spinal neurones may relay RN actions in their own right. In the present study we found even stronger evidence to this end by finding that disynaptic actions from RN remain after transection of axons of reticulospinal neurones in the MLF. Even if some rubrospinal actions were relayed by reticulospinal neurones, the relative contribution of such actions would thus be much weaker than actions evoked by PT stimuli which were abolished by the MLF lesions. A further difference found between disynaptic actions of RN and PT neurones on SB neurones was that disynaptic EPSPs and IPSPs of RN origin are evoked at shorter latencies than those from the PT (see Table 1), while the same latencies would be expected if they were both relayed by reticulospinal neurones.
The evidence that premotor interneurones mediating inhibition of motoneurones mediate at least some di- and/or trisynaptic IPSPs evoked from the RN (Baldissera & ten Bruggencate, 1976) could apply to SB neurones as well as to other subpopulations of VSCT neurones. Firstly, because the same categories of spinal interneurones were shown to inhibit VSCT with dominating excitatory input from Ib afferents (the Ib subpopulation of VSCT neurones), or from Ia afferents (SB neurones), or with dominating inhibitory input from peripheral afferents (for references see Jankowska et al. 2010). Secondly, because the sample of Baldissera & ten Bruggencate (1976) might have included some SB neurones, as judged by very low threshold of EPSPs evoked in some of those illustrated in their Figs 4 and 6. Baldissera and ten Bruggencate (1976) demonstrated the involvement of inhibitory premotor interneurones by a mutual facilitation of disynaptic or trisynaptic IPSPs evoked from the RN and by group Ia, as well as by group Ib afferents. EPSPs evoked from the NR were on the other hand attributed to interneurones in polysynaptic pathways, primarily from skin afferents but also from group II and III muscle afferents. Our observations on input from primary afferents to SB neurones corroborate this conclusion with respect to SB neurones as we only found evidence for actions of inhibitory premotor interneurones upon them (Jankowska et al. 2010). We would therefore postulate that excitatory actions of RN neurones on SB neurones are mediated by either non-premotor interneurones, or by propriospinal neurones. Propriospinal neurones co-excited by rubro-spinal as well as by cortico- and reticulo-spinal neurones, e.g. those located in the 3rd and 4th cervical segments (Lundberg, 1979; Alstermark et al. 2007), would seem the most likely candidates, but those co-ordinating activity of neurones within the cervical and lumbo-sacral enlargements might also be involved.
Monosynaptic EPSPs evoked in SB neurones by RN neurones would not replicate rubral actions on feline motoneurones because of lack of evidence for direct coupling between them (Hongo et al. 1969). However, these monosynaptic EPSPs together with disynaptic and polysynaptic EPSPs could provide an indication on the likely excitatory input to motoneurones, whether direct or indirect. Provided that monosynaptic EPSPs gave rise to discharges of SB neurones they could also be used to compare input to spinal interneurones with their output, as proposed by Lundberg (1971). However, the monosynaptic input from RN neurones might also be considered to reflect the parallel direct coupling to SB neurones and motoneurones present in some species, in particular in primates in which it has been firmly demonstrated (Holstege et al. 1988; Cheney et al. 1991). Provided a relationship between monosynaptic input from RN neurones to motoneurones and SB neurones exists, SB neurones in primates might be activated by RN neurones to a much greater extent than they are in the cat. They might also be more involved in predicting actions of RN neurones on motoneurones, despite differences in the size and cytoarchitecture of the RN in primates (for references see Yamaguchi, 2006), which may be coupled to the increasing role of the corticospinal and corticobulbar tract for skilled digit movements (for review see Pettersson et al. 2007). Such a role might be in keeping with the importance of the red nucleus for the initiation and control of centrally initiated voluntary movements and for replacing functions of damaged corticospinal neurones, demonstrated by a reorganization of rubrospinal actions following a pyramidal tract lesion and contributing to recovery of lost motor function in primates (see e.g. Cheney et al. 1991; Belhaj-Saif & Cheney, 2000) and by activity of rubral neurones during voluntary movements in humans (see e.g. Habas et al. 2010). Predicting the actions of RN neurones on motoneurones by their actions on SB neurones the cerebellum may thus be as important in primates as in the cat to allow adjustments of the descending commands sent by RN neurones.
Acknowledgments
We wish to thank Jytte Grännsjö for excellent assistance during the experiments and for histological analysis. The study was supported by grants from NINDS/NIH (R01 NS040863) and the Swedish Research Council (15393-01 for E.J.; 522-2005-7255 for I.H.).
Glossary
Abbreviations
- CC DSCT
Clarke's column dorsal spinocerebellar tract
- co
contralateral
- dh DSCT
dorsal horn dorsal spinocerebellar tract
- DSCT
dorsal spinocerebellar tract
- i
ipsilateral
- L
lumbar
- MLF
medial longitudinal fascicle
- MLR
mesencephalic locomotor region
- PT
pyramidal tract
- Q
quadriceps
- RN
red nucleus
- RS
reticulospinal
- SB
spinal border neurones
- Th
thoracic
- VSCT
ventral spinocerebellar tract neurones
Author contributions
I.H. and E.J.: conception and design of the experiments; collection, analysis and interpretation of the data; drafting the article. E.N.: collection, analysis and interpretation of the data. All authors approved the final version to be published.
References
- Alstermark B, Isa T, Pettersson LG, Sasaki S. The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol (Oxf) 2007;189:123–140. doi: 10.1111/j.1748-1716.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lindstrom S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal also projecting to forelimb motoneurones. Exp Brain Res. 1981;42:282–298. doi: 10.1007/BF00237495. [DOI] [PubMed] [Google Scholar]
- Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26:273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behav Brain Res. 1988;28:193–199. doi: 10.1016/0166-4328(88)90096-4. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Roberts WJ. Effects on the ventral spinocerebellar tract neurones from Deiters' nucleus and the medial longitudinal fascicle in the cat. Acta Physiol Scand. 1975;93:228–249. doi: 10.1111/j.1748-1716.1975.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, ten Bruggencate G. Rubrospinal effects on ventral spinocerebellar tract neurones. Acta Physiol Scand. 1976;96:233–249. doi: 10.1111/j.1748-1716.1976.tb10192.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Weight F. Descending monosynaptic connexions to spinal border cells. Acta Physiol Scand. 1969;76:28A–29A. [PubMed] [Google Scholar]
- Belhaj-Saif A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol. 2000;83:3147–3153. doi: 10.1152/jn.2000.83.5.3147. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters inThe Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TC, Jankowska E, Tanaka R. Effects of volleys in cortico-spinal tract fibres on ventral spino-cerebellar tract cells in the cat. Acta Physiol Scand. 1977;100:1–13. doi: 10.1111/j.1748-1716.1977.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Ghez C. Input-output relations of the red nucleus in the cat. Brain Res. 1975;98:93–308. doi: 10.1016/0006-8993(75)90511-9. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol. 1985;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Guillevin R, Abanou A. In vivo structural and functional imaging of the human rubral and inferior olivary nuclei: A mini-review. Cerebellum. 2010;9:167–173. doi: 10.1007/s12311-009-0145-1. [DOI] [PubMed] [Google Scholar]
- Hammar I, Krutki P, Drzymala-Celichowska H, Nilsson E, Jankowska E. A trans-spinal loop between neurones in the reticular formation and in the cerebellum. J Physiol. 2011;589:653–665. doi: 10.1113/jphysiol.2010.201178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Blok BF, Ralston DD. Anatomical evidence for red nucleus projections to motoneuronal cell groups in the spinal cord of the monkey. Neurosci Lett. 1988;95:97–101. doi: 10.1016/0304-3940(88)90639-8. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7:344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Houk JC, Gibson AR, Harvey CF, Kennedy PR, van Kan PL. Activity of primate magnocellular red nucleus related to hand and finger movements. Behav Brain Res. 1988;28:201–206. doi: 10.1016/0166-4328(88)90097-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat. J Neurophysiol. 2010;104:1872–1883. doi: 10.1152/jn.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Nilsson E, Hammar I. Processing information related to centrally initiated locomotor and voluntary movements by feline spinocerebellar neurones. J Physiol. 2011;589:5709–5725. doi: 10.1113/jphysiol.2011.213678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson L-G, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- Lindström S. Recurrent control from motor axon collaterals of Ia inhibitory pathways in the spinal cord of the cat. Acta Physiol Scand. 1973;89(Suppl. 392):1–43. [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12:317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Integration in propiospinal motor centre controlling the forelimb in the cat. In: Asanuma H, Wilson VS, editors. Integration in the Nervous System. Tokyo, New York: Igaru-Shoin; 1979. pp. 47–65. [Google Scholar]
- Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12:295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Magni F, Oscarsson O. Cerebral control of transmission to the ventral spinocerebellar tract. Arch Ital Biol. 1961;99:369–396. [Google Scholar]
- Martin JH, Ghez C. Red nucleus and motor cortex: parallel motor systems for the initiation and control of skilled movement. Behav Brain Res. 1988;28:217–223. doi: 10.1016/0166-4328(88)90099-x. [DOI] [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. Eur J Neurosci. 2000;12:1113–1122. doi: 10.1046/j.1460-9568.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Mascitti TA. Sites and mode of termination of fibers of the vestibulospinal tract in the cat. J Comp Neurol. 1964;122:369–387. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of rubrospinal neurons during locomotion. Brain Res. 1972;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of spinocerebellar paths. In: Iggo I, editor. Handbook of Sensory Physiology. II. Berlin: Springer Verlag; 1973. pp. 339–380. [Google Scholar]
- Pettersson LG, Blagovechtchenski E, Perfiliev S, Krasnochokova E, Lundberg A. Recovery of food-taking in cats after lesions of the corticospinal (complete) and rubrospinal (complete and incomplete) tracts. Neurosci Res. 2000;38:109–112. doi: 10.1016/s0168-0102(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate – recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol. 1999;81:2297–2315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- Stecina K, Slawinska U, Jankowska E. Ipsilateral actions from the feline red nucleus on hindlimb motoneurones. J Physiol. 2008;586:5865–5884. doi: 10.1113/jphysiol.2008.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan PL, McCurdy ML. Role of primate magnocellular red nucleus neurons in controlling hand preshaping during reaching to grasp. J Neurophysiol. 2001;85:1461–1478. doi: 10.1152/jn.2001.85.4.1461. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Goto N. Development of the human magnocellular red nucleus: a morphological study. Brain Dev. 2006;28:431–435. doi: 10.1016/j.braindev.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Yokota S, Ono K, Tsumori T. Projections from the red nucleus to the parvicellular reticular formation and the cervical spinal cord in the rat, with special reference to innervation by branching axons. Brain Res. 2001;923:187–192. doi: 10.1016/s0006-8993(01)03196-1. [DOI] [PubMed] [Google Scholar]


