Non-technical summary
Lower urinary tract disorders including painful and overactive bladder conditions are very difficult to treat. Neuromodulation which is one of the successful therapies for lower urinary tract disorders, stimulates afferent nerves to modulate the neural pathway and achieve a therapeutic effect. We show that the metabotropic glutamate receptor 5 is activated in the central nervous system during pudendal neuromodulation. Understanding the neurotransmitter mechanisms involved in neuromodulation therapy may promote the development of new pharmacological treatments or improve the clinical outcome by combining neuromodulation with pharmacological therapy.
Abstract
Abstract
This study used MTEP, a metabotropic glutamate receptor 5 (mGluR5) antagonist, to examine the role of mGluR5 in the neural control of the urinary bladder and in the inhibition of the micturition reflex by pudendal nerve stimulation (PNS). Experiments were conducted in 11 female cats under α-chloralose anaesthesia when the bladder was infused with either saline or 0.25% acetic acid (AA). AA irritated the bladder, induced bladder overactivity and significantly (P< 0.001) reduced bladder capacity to 14.9 ± 10.3% of the saline control capacity. MTEP (0.1–50 mg kg−1, i.v.) significantly (P< 0.05) increased bladder capacity during saline distension but not during AA irritation. However, MTEP induced a transient inhibition of isovolumetric bladder contractions under both conditions. PNS (5 Hz), which was tested at the threshold (T) intensity for inducing a complete inhibition of isovolumetric bladder contractions and at an intensity of 3–4T, suppressed AA-induced bladder overactivity and significantly increased bladder capacity to 68.0 ± 31.3% at 1T(P< 0.05) and 98.5 ± 55.3% at 3–4T(P< 0.01) of the saline control capacity. MTEP dose dependently (0.1–50 mg kg−1, i.v.) suppressed PNS inhibition of bladder overactivity at low intensity (1T) but not at high intensity (3–4T). During saline infusion PNS significantly (P< 0.05) increased bladder capacity to 167.7 ± 27.1% at 1Tand 196.0 ± 37.4% at 3–4T. These inhibitory effects were not observed after MTEP (0.1–50 mg kg−1, i.v.) which also increased bladder capacity. These results indicate that glutamic acid has a transmitter function in bladder and somato-bladder reflex mechanisms and raise the possibility that mGluR5 may be a target for pharmacological treatment of lower urinary tract disorders.
Introduction
Glutamate activates two classes of receptors, ionotropic (iGluRs) and metabotropic glutamatergic receptors (mGluRs). Previous studies (Maggi et al. 1990; Matsumoto et al. 1995; Yoshiyama et al. 1993a,b, 1995; Kakizaki et al. 1998) revealed that iGluRs are involved in central nervous system (CNS) control of the lower urinary tract (LUT). However, iGluR antagonists are not used clinically to treat LUT symptoms due to their CNS side effects (Bruno et al. 2001; Andersson & Wein, 2004). The mGluRs are G-protein-coupled receptors consisting of eight subtypes (mGluR1–8) in three groups (groups I–III) (De Blasi et al. 2001). Group I receptors (mGluR1 and mGluR5) activate phospholipase C, release intracellular Ca2+ and activate protein kinase C. The mGluR5s are widely expressed in CNS and primarily function as postsynaptic receptors that increase neuronal excitability and potentiate the effect of glutamate on iGluR (Jia et al. 1999; Carlton et al. 2001; Kew, 2004).
The mGluRs play a critical role in inflammation-induced hyperexcitability of spinal cord neurons (Neugebauer et al. 1999; Kew, 2004). Pathological conditions such as nerve injury can increase mGluR5 expression and induce plasticity in glutamatergic synaptic transmission that may play a role in neuropathic pain (Hudson et al. 2002; Dolan et al. 2003). Intrathecal administration of group I mGluR agonists induces hyperalgesia and nociceptive behaviours (Fisher & Coderre, 1996, 1998). Conversely, mGluR5 antagonists attenuate peripheral thermal nociception and inflammatory pain (Bhave et al. 2001; Walker et al. 2001) as well as inhibit the irritation-induced bladder overactivity (Guarneri et al. 2008). However, mGluR5 antagonists also significantly reduce the antinociceptive/antihyperalgesic effects of cannabinoids or capsaicin injected into the periaqueductal grey matter or the spinal cord (Palazzo et al. 2001, 2002; Hama & Uran, 2004). Thus the involvement of mGluR5 in nociceptive mechanisms is complex and seems to vary at different sites in the CNS.
In this study we investigated the role of mGluR5 in nociceptive and antinociceptive mechanisms affecting urinary bladder reflexes in anaesthetized cats. Intravesical infusion of dilute acetic acid was used as the nociceptive stimulus to induce bladder overactivity. PNS was used as the antinociceptive stimulus to model the clinical use of neuromodulation in treating painful and overactive bladder conditions. In humans interstitial cystitis/bladder pain syndrome (IC/BPS) is a symptom complex of urinary urgency, frequency and bladder pain that can be treated effectively by either PNS or sacral root stimulation (Peters, 2002; van Kerrebroeck et al. 2007). Pudendal neuromodulation has been reported to be superior to sacral neuromodulation in patients with intractable LUT symptoms or IC/BPS (Peters et al. 2002, 2005). We hypothesized that pudendal inhibition of irritation-induced bladder overactivity could be due to activation of mGluR5s by the pudendal afferent firing at the synapses in the spinal cord or brain. MTEP (a potent and selective mGluR5 antagonist) was used to block the mGluR5s and test our hypothesis. Understanding the neurotransmitter mechanisms involved in pudendal neuromodulation may promote the development of new pharmacological treatments or improve the clinical outcome by combining neuromodulation with pharmacological therapy.
Methods
All protocols used in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh. Due to the physiological and anatomical similarities between cat and human in the lower urinary tract, cats were used in this study and humanely killed at the end of the experiments by intravenous injection of 5 ml (2 mmol ml−1) KCl under deep anaesthesia.
Experimental setup
Experiments were conducted in a total of 11 female cats (2.8–3.2 kg) anaesthetized initially with isoflurane (2–5% in oxygen) and maintained with α-chloralose (65 mg kg−1i.v. with supplementation as necessary). Heart rate and blood oxygen level were measured by a pulse oximeter (9847 V, NONIN Medical, Inc., Playmouth, MN, USA) with the sensor attached to the tongue. Systemic blood pressure was measured via a catheter in the carotid artery. Drug and fluid were administered via the ulnar vein, and airway access was secured with a tracheostomy tube.
The ureters were isolated via an abdominal incision, cut, and drained externally. The bladder was cannulated through the urethra with a double lumen catheter. One lumen was used to infuse saline or 0.25% acetic acid (AA) at a rate of 0.5–2 ml min−1, and the other lumen was attached to a pressure transducer to record the bladder pressure. A ligature was tied around the urethra to prevent leakage. The pudendal nerve was dissected from the left side via a 3–4 cm incision between the tail and the sciatic notch. A tripolar cuff electrode (NC223pt, MicroProbe, Inc., Gaithersburg, MD, USA) was applied around the nerve and connected to a stimulator (S88, Grass Medical Instruments, Quincy, MA, USA).
Stimulation protocol
Initially a cystometrogram (CMG) was performed with saline infusion to determine the bladder capacity that was defined as the bladder volume threshold to induce a large amplitude (>30 cm H2O) and long duration (>20 s) bladder contraction. Then, multiple saline CMGs were repeated to evaluate the reproducibility. Once the bladder capacity was determined during saline infusion, pharmacological studies were performed in two experimental groups. In the AA group (n = 6 cats) 0.25% AA was infused into the bladder during repeated CMGs in order to activate nociceptive bladder C-fibre afferents and induce overactive bladder reflexes. In the saline experimental group (n = 5 cats) repeated CMGs were performed to initiate reflex bladder activity by non-nociceptive bladder afferent Aδ-fibres. In both experimental groups the bladder was first infused to a volume about 100–110% of the bladder capacity to induce isovolumetric rhythmic bladder contractions. Based on our previous studies (Tai et al. 2006, 2008, 2011; Chen et al. 2010), 5 Hz PNS was used to determine the intensity threshold (T) for completely inhibiting the isovolumetric rhythmic bladder contractions. Then, multiples (1Tor 3–4T) of the threshold intensity were used in the pharmacological experiments. PNS at these intensities (1–4T) induced clearly observable contractions of anal sphincter.
Before administering MTEP (3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine), the bladder capacity was first determined in both experimental groups under the following three conditions: (1) control condition, no stimulation was applied during the CMG; (2) 1Tcondition, PNS at 1Tintensity was applied during the CMG; (3) 3–4Tcondition, PNS at 3–4Tintensity was applied during the CMG. Then, increasing cumulative doses of MTEP (0.1, 0.3, 1, 3, 10, 30, or 50 mg kg−1, i.v.) were administered to the animal at about 50–70 min intervals between each dose. After each dose of MTEP, three CMGs were performed under the three different conditions (i.e. control, 1Tand 3–4T) to determine the drug effect on bladder capacity. Before administering each dose of MTEP, an additional control CMG was performed to infuse the bladder to a volume about 100–110% of the micturition threshold volume to induce rhythmic bladder contractions. Individual doses of MTEP were administered during the isovolumetric rhythmic bladder contractions, and the acute MTEP effect on isovolumetric rhythmic bladder contractions was monitored in the subsequent 10–15 min period. The bladder was emptied after each CMG, and a 5–10 min rest period was inserted between successive CMGs to allow the distended detrusor to recover.
Data analysis
For the repeated CMG recordings, bladder capacities were measured and normalized to the measurement of the first saline control CMG in the same animal so that the results from different animals could be compared. The transient inhibitory effect of MTEP on the isovolumetric rhythmic bladder contractions was measured by the duration of the complete inhibition of the bladder activity. Repeated measurements under the same conditions in the same animal were averaged. The results from different animals were averaged and reported as mean ± standard error of the mean. Statistical significance (P< 0.05) was detected by Student'sttest or ANOVA followed by Bonferroni post tests.
Results
Intensity-dependent inhibitory effect of PNS on isovolumetric bladder activity
The inhibition of isovolumetric rhythmic bladder contractions during AA irritation was dependent on the intensity of PNS (see Fig. 1). The intensity threshold (T) to induce a complete inhibition was determined to be 6 V in the experiment shown in Fig. 1. The same protocol was used to determine the inhibition threshold during saline distension. Complete inhibition of isovolumetric rhythmic bladder contractions as shown in Fig. 1 was elicited in all animals. The inhibition thresholds (T) were not significantly different during AA irritation (3.2 ± 1.3 V) or during saline distension (2.3 ± 0.3 V).
Figure 1. Intensity-dependent inhibitory effects of PNS on isovolumetric bladder contractions induced by distension of the bladder by 0.25% AA.
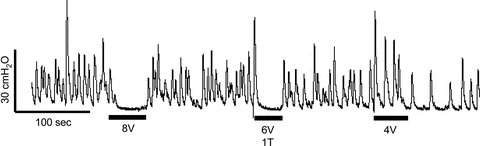
Threshold intensity (T) for eliciting inhibition was 6 V. The black bars under the bladder pressure trace indicate stimulation duration. Stimulation: 5 Hz frequency, 0.2 ms pulse width.
Dose-dependent effect of MTEP on pudendal inhibition of bladder overactivity
CMGs revealed that bladder capacity which averaged 7.9 ± 1.8 ml during saline infusion was reduced by more than 80% to 1.2 ± 0.5 ml during infusion of 0.25% AA (first 2 traces in Fig. 2A, and Fig. 3A). PNS at intensities of 1Tand 3–4Tsignificantly increased bladder capacity (third and fourth traces in Fig. 2A, and Fig. 3A).
Figure 2. Dose-dependent effect of MTEP on pudendal inhibition and micturition reflex during CMGs.
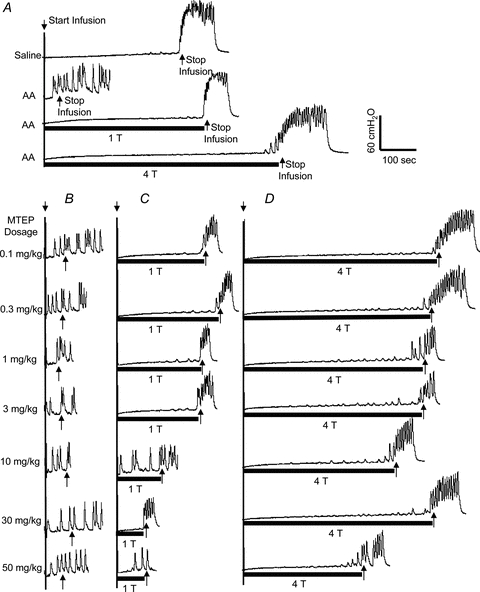
A, control CMGs without MTEP during saline or 0.25% AA infusion with/without stimulation.B–D, the MTEP dosage marked on the left is for panelsB, CandD. The CMGs at increasing cumulative doses of MTEP were performed in sequence from left to right and from top to bottom in each panel.B, the AA CMGs without stimulation.C, the AA CMGs during 1Tstimulation.D, the AA CMGs during 4Tstimulation. The black bars under the pressure trace indicate stimulation duration. Stimulation: 5 Hz, 0.2 ms, inhibition thresholdT = 1.5 V. Short arrows indicate the start and stop of bladder infusion.
Figure 3. Summarized results of the effects of MTEP on the micturition reflex and on inhibition of the micturition reflex during PNS after irritation of the bladder with 0.25% AA.
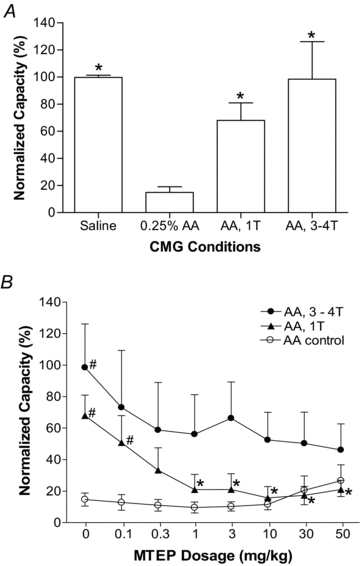
A, effect of 0.25% AA irritation and PNS inhibition on bladder capacity. *Significantly different from 0.25% AA data.B, dose-dependent effect of MTEP on bladder capacity with or without PNS during AA infusion. *Significantly different from the bladder capacity measured at 1Twithout MTEP treatment (i.e. at 1Tand 0 mg kg−1). #Significantly different from the AA control data. Stimulation: 5 Hz, 0.2 ms.T, threshold intensity for inducing complete inhibition of isovolumetric rhythmic bladder contractions.n = 6 cats.
The effect of MTEP on pudendal inhibition of bladder overactivity is dependent on the drug dosage and the intensity of PNS (Fig. 2CandD). Administering cumulative doses of MTEP (0.1, 0.3, 1, 3, 10, 30 and 50 mg kg−1, i.v.) did not significantly change the bladder capacity in the absence of stimulation (Fig. 2B), but progressively reduced the effect of PNS (Fig. 2CandD). At a stimulation intensity of 1T, the highest doses of MTEP completely blocked the pudendal inhibition (Fig. 2BandC). However, at the higher stimulation intensity (4T) even the highest doses of MTEP only partially reduced the inhibitory effect of PNS on the bladder capacity (Fig. 2D). The summarized data from six cats is shown in Fig. 3. Bladder irritation by infusing 0.25% AA significantly (P< 0.001) reduced bladder capacity to 14.9 ± 10.3% of saline control capacity (Fig. 3A). PNS at intensities of 1Tand 3–4Tsignificantly increased the capacity to 68.0 ± 31.3% (P< 0.05) and 98.5 ± 55.3% (P< 0.01), respectively, of the saline control capacity (Fig. 3A). Cumulative doses of MTEP did not significantly change the small bladder capacity caused by AA irritation (Fig. 3B). However, at doses greater than 1 mg kg−1 MTEP significantly (P< 0.05) reduced the increase in bladder capacity induced by PNS at 1T(Fig. 3B). Cumulative doses of MTEP only partially (P> 0.05) reduced the inhibitory effect of higher PNS intensities (3–4T) (Fig. 3B).
Transient effect of MTEP on isovolumetric rhythmic bladder contractions during AA irritation
Although cumulative doses of MTEP did not change the small bladder capacity induced by AA irritation (Fig. 3B), they did cause a transient complete inhibition of the isovolumetric rhythmic bladder contractions (Fig. 4A). This transient effect was observed in all animals during AA irritation (n = 6), but did not occur at every MTEP dose in each animal. The effect was usually detectable within 1–2 min of MTEP administration and lasted 1–10 min. The duration of the observed transient inhibition increased with increasing doses of MTEP as shown in Fig. 4B that excluded the data from tests where the MTEP-elicited transient inhibition was absent (i.e. duration = 0 min).
Figure 4. Transient inhibitory effect of MTEP on isovolumetric rhythmic bladder contractions during AA distension.
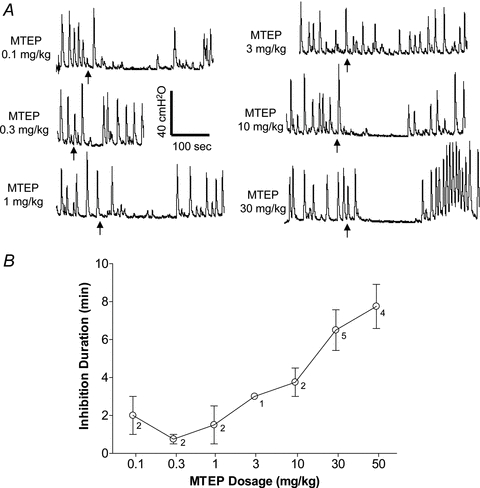
A, recordings showing examples of complete inhibition of reflex bladder contractions during a short period after administration of MTEP (indicated by arrows).B, average duration of the complete inhibition increases with cumulative MTEP doses.n = 6 cats. Note: results inBexclude the data from tests where the MTEP-elicited transient inhibition was absent (i.e. duration = 0 min, see 3 mg kg−1 dose inA). The number beside each data point indicates the number of animals that exhibited the transient inhibition.
Effect of MTEP on pudendal inhibition of bladder activity during saline distension
Before administration of MTEP the bladder capacity measured during saline distension was significantly (P< 0.05) increased by PNS to 167.7 ± 27.1% (1T) and 196.0 ± 37.4% (3–4T) of control capacity (Fig. 5AandF). Administering cumulative doses of MTEP (0.1, 1, 10 and 50 mg kg−1, i.v.) significantly (P< 0.05) increased the bladder capacity in the absence of stimulation to approximately 150% of control capacity (Fig. 5B–F). PNS at 1Tand 3–4Tafter treatment with MTEP did not increase bladder capacity further (Fig. 5B–F). Bladder capacities during PNS at 1Tand 3–4Tintensities were not significantly different before or after MTEP administration (Fig. 5F).
Figure 5. Dose-dependent effect of MTEP on pudendal inhibition and micturition reflex during saline infusion CMGs.
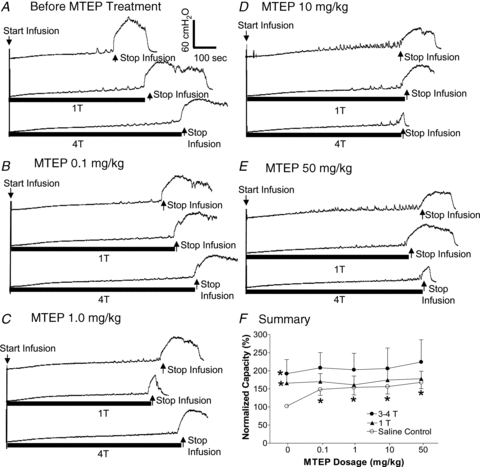
A–E, CMG traces with or without PNS at different dosage of MTEP. Stimulation: frequency 5 Hz, pulse width 0.2 ms, and inhibition thresholdT = 1.5 V. The black bars under bladder pressure traces mark the stimulation duration. Arrows indicate the start and stop of saline infusion.F, bladder capacity measured at different dosage of MTEP (n = 5 cats). *Significantly different from the bladder capacity measured during saline control before MTEP treatment (i.e. saline control at 0 mg kg−1).
Inhibitory effect of MTEP on isovolumetric rhythmic bladder contractions during saline distension
MTEP at every dosage completely inhibited isovolumetric rhythmic bladder contractions during saline distension in all animals (n = 5) (Fig. 6). The inhibition was only monitored for 10–15 min in order to follow the same experimental protocol as in the experiments using AA irritation. Since the inhibition lasted more than 10–15 min, the bladder was emptied and the recording was interrupted (see Fig. 6). However, in two animals at an MTEP dose of 1 mg kg−1 the recording was continued until spontaneous bladder contractions returned (second trace in Fig. 6) indicating a period of complete inhibition lasting about 30 min.
Figure 6. Transient inhibitory effect of MTEP on isovolumetric rhythmic bladder contractions during saline distension.
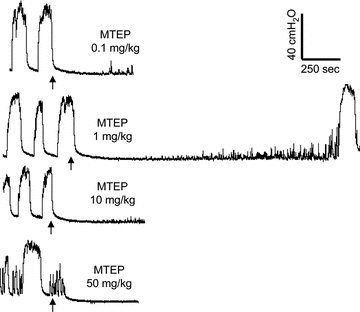
After the MTEP is given (indicated by arrow) a complete inhibition of bladder contractions is observed in all cats at different doses. The inhibition lasted longer than the 10–15 min observation period (n = 5). In two cats the inhibition was monitored until contractions returned indicating a recovery period of about 30 min (see the second trace).
Discussion
This study revealed that intravenous administration of MTEP, an mGluR5 antagonist, suppressed reflex bladder activity as well as the PNS-induced inhibition of bladder activity. The magnitude of the effects of MTEP depended on the conditions of the experiment (see Table 1). For example when the bladder was filled with saline the MTEP inhibition of isovolumetric bladder contractions and the increase in bladder capacity was prominent, while the MTEP suppression of PNS inhibition was modest. However, in AA-irritated bladders MTEP had a weaker effect on isovolumetric bladder contractions, did not change bladder capacity but significantly blunted the increase in bladder capacity induced by PNS. These results indicate that glutamic acid is an excitatory transmitter in the micturition reflex pathway and also a transmitter in the PNS-evoked central inhibitory mechanisms that modulate bladder capacity (Fig. 7).
Table 1.
Changes in bladder capacity under different experimental conditions
| PNS | |||
|---|---|---|---|
| Untreated | MTEP treated | MTEP | |
| Saline CMG | Increase | No effect | Increase |
| AA CMG | Increase | No effect | No effect |
Figure 7.
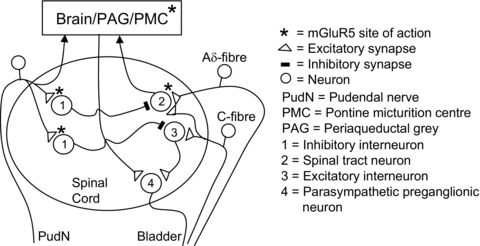
The possible locations (*) in the sacral spinal cord and brain stem of mGluR5s that participate in: (1) the spinobulbospinal and spinal micturition reflex pathways, and (2) the micturition inhibitory mechanism evoked by PNS. * also indicates possible sites of action of the mGluR5 antagonist.
MTEP could act at multiple sites to suppress micturition reflexes. Saline distension of bladder primarily activates non-nociceptive, mechano-sensitive, Aδ bladder afferents that trigger a spinobulbospinal micturition reflex transmitted through the spinal cord to synapses in the periaqueductal grey (PAG) and the pontine micturition centre (PMC) (Fig. 7) (Fowler et al. 2008). On the other hand AA irritation of the bladder activates nociceptive, C-fibre bladder afferents that facilitate the supraspinal micturition reflex and/or activate a spinal micturition reflex (Fig. 7) (Chancellor & de Groat, 1999; Fowler et al. 2008). Although glutamic acid and iGluRs, such as AMPA and NMDA receptors, are known to be important at spinal and supraspinal synapses in the micturition reflex pathways (Yoshiyama et al. 1993a,b; Yoshiyama & de Groat, 2005) the sites where mGluR5s play a role are still uncertain.
mGluRs are widely expressed in both the central and peripheral nervous systems and are known to modulate neuronal excitability and synaptic transmission (Shigemoto et al. 1993; Valerio et al. 1997; Hama & Urban, 2004). Neuroanatomical and immunocytochemical studies (Vidnyanszky et al. 1994; Alvarez et al. 2000; Cartmell & Schoepp, 2000) suggest that group I mGluRs are excitatory and primarily localized postsynaptically. Previous studies in rats (Yoshiyama & de Groat, 2007) showed that intrathecal injection of MCPG (a group I/II mGluR antagonist) did not alter the micturition reflex induced by saline distension of the bladder; while intrathecal injection of ACPD (a group I/II mGluR agonist) inhibited the micturition reflex induced by saline distension (Tanaka et al. 2003). More recent studies in rats demonstrated that intravenous administration of MPEP, a less selective and less potent mGluR5 antagonist than MTEP (Lea & Faden, 2006), dose dependently increased bladder capacity and inhibited the micturition reflex induced by saline distension of the bladder (Guarneri et al. 2008; Hu et al. 2009) and also increased bladder capacity during AA irritation (Guarneri et al. 2008). MPEP did not change bladder Aδ afferent firing in response to saline distension indicating that a central rather than a peripheral action must mediate the effect of the drug (Hu et al. 2009).
Our present study in cats showed that MTEP suppresses reflex bladder contractions recorded under isovolumetric conditions during both saline distension and AA irritation of the bladder, but significantly increases bladder capacity only during saline infusion. These data suggest that MTEP raises the volume threshold for triggering the spinobulbospinal micturition reflex but does not alter the volume threshold for the spinal C-fibre reflex and/or the facilitated spinobulbospinal reflex. The suppression of the spinobulbospinal micturition reflex by MTEP suggests that mGluR5s expressed on interneurons in the spinal cord or brain stem are essential for excitatory transmission in the reflex pathway (Fig. 7). Conversely, mGluR5s seem to be less important at spinal synapses involved in the micturition reflex activated by nociceptive C-fibre afferents.
Our recent study in cats (Chen et al. 2010) showed that enkephalins and opioid receptors can account for only part of the PNS inhibition of the spinobulbospinal micturition reflex activated by Aδ afferents, indicating that other neurotransmitters/receptors must be involved in the inhibition. The present study indicates that the mGluR5 mechanisms might play a role in the non-opioid PNS inhibition (Fig. 5) and in PNS inhibition of the bladder overactivity initiated by AA activation of nociceptive C-fibre bladder afferents (Figs 2 and 3). Whether opioid receptors play a role in PNS inhibition of nociceptive bladder activity still needs to be investigated. However, based on these findings it seems reasonable to hypothesize that PNS inhibition of bladder activity must involve multiple neurotransmitters/receptors and that these neurotransmitters might be different for the inhibitory mechanisms modulating the spinobulbospinal and spinal micturition reflex pathways.
The site at which MTEP affects pudendal nerve-mediated inhibition is uncertain. It is known that group I mGluRs are expressed on spinal inhibitory interneurons and that activation of these receptors enhances firing, and thereby, increases downstream inhibition (Vidnyanszky et al. 1994). Therefore, pudendal nerve inhibition of bladder activity may depend on glutamatergic excitatory transmission via mGluR5s on spinal interneurons that release inhibitory neurotransmitters (such as enkephalins, GABA and glycine) which have been shown to be involved in the inhibitory mechanisms controlling bladder reflexes (de Groat & Ryall, 1968; Booth et al. 1985; Mallory et al. 1991; Noto et al. 1991; de Groat et al. 1993; Miyazato et al. 2004). Because electrophysiological experiments have revealed that stimulation of pudendal afferents evokes short latency, presumably disynaptic, inhibitory responses in bladder preganglionic neurons (de Groat & Ryall, 1969; de Groat et al. 1982) it seems reasonable to hypothesize that pudendal primary afferent terminals make direct glutamatergic (mGluR5 mediated) excitatory synaptic connections with inhibitory interneurons that in turn suppress excitatory transmission in spinal and/or supraspinal components of micturition reflexes (Fig. 7). A suppression by MTEP of excitatory transmission at these sites would be expected to significantly reduce the pudendal inhibition of bladder activity induced by either Aδ- or C-fibre afferents. The dose-dependent decrease in PNS inhibition during AA irritation (Figs 2 and 3) supports this idea. The failure of PNS to increase bladder capacity during saline infusion following administration of a range of MTEP doses (0.1–50 mg kg−1, i.v.) is also consistent with this hypothesis. However, the experiments using saline distension are difficult to interpret because MTEP even in the lowest dose significantly increased bladder capacity to 150% of control. This inhibitory effect of MTEP may have shifted bladder capacity to a maximum level thereby occluding rather than blocking the inhibitory effect of PNS. This interpretation is consistent with the demonstration prior to MTEP treatment that increasing the PNS intensity from 1Tto 3–4Tdid not significantly increase the inhibition during saline infusion suggesting that it was maximal.
It is worth noting that immunohistochemical studies have indicated the localization of mGluR5 receptors in cat brain (Reid et al. 1995; Goodwin et al. 1996), but studies regarding the localization in cat spinal cord are currently not available. However, distribution of mGluR5 receptors in the spinal cord has been shown in many other mammalian species including mice, rats and sheep (Anwyl, 1999; Karim et al. 2001; Dolan et al. 2003; Pitcher et al. 2007; Li et al. 2010). Therefore, it is highly likely that mGluR5 receptors are also located in the cat spinal cord. However, an immunohistochemical study of the cat spinal cord is warranted to localize the mGluR5 receptors.
In this study PNS induced observable contractions of the anal sphincter. Although 5 Hz PNS was used, the frequency of sphincter afferent activity induced by contractions might be different from 5 Hz. Both the PNS-induced and the sphincter contraction-induced afferent activity in the pudendal nerve could contribute to the inhibitory effect of PNS on reflex bladder activity.
Understanding the neurotransmitter mechanisms involved in pudendal neuromodulation could identify pharmacological targets for development of new therapies to treat patients suffering from IC/BPS, pelvic pain or LUT symptoms. Furthermore, neuromodulation therapies currently used in clinical applications could also be improved to achieve better clinical outcomes when combined with pharmacological treatments that enhance synaptic transmission in the inhibitory reflex pathways.
Acknowledgments
This study is supported by the NIH under grants DK-068566, DK-090006, DK-077783, and by the Christopher and Dana Reeve Foundation.
Glossary
Abbreviations
- AA
acetic acid
- CMG
cystometrogram
- CNS
central nervous system
- IC/BPS
interstitial cystitis/bladder pain syndrome
- iGluR
ionotropic glutamatergic receptors
- LUT
lower urinary tract
- mGluR
metabotropic glutamatergic receptors
- PAG
periaqueductal grey
- PMC
pontine micturition centre
- PNS
pudendal nerve stimulation
Author contributions
Experiments were performed at the University of Pittsburgh. All authors contributed to the conception and design of the experiments, the collection, analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. All authors have approved the final version of the manuscript.
References
- Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Booth AM, Hisamitsu T, Kawatani M, de Groat WC. Regulation of urinary bladder capacity by endogenous opioid peptides. J Urol. 1985;133:339–342. doi: 10.1016/s0022-5347(17)48935-x. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Iorio PD, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol. 2010;224:282–291. doi: 10.1016/j.expneurol.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin JP, Nicoletti P. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System, Vol. 3, Nervous Control of the Urogenital System. London: Harwood Academic Publishers; 1993. pp. 227–289. [Google Scholar]
- de Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J Auto Nerv Syst. 1982;5:23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S, Kelly JG, Monteiro AM, Nolan AM. Up-regulation of metabotropic glutamate receptor subtypes 3 and 5 in spinal cord in a clinical model of persistent inflammation and hyperalgesia. Pain. 2003;106:501–512. doi: 10.1016/j.pain.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. Neuroreport. 1996;7:2743–2747. doi: 10.1097/00001756-199611040-00067. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. Hyperalgesia and allodynia induced by intrathecal (RS)-dihydroxyphenylglycine in rats. Neuroreport. 1998;9:1169–1172. doi: 10.1097/00001756-199804200-00038. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin DW, van Horn SC, Erisir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci. 1996;16:8181–8192. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri L, Poggesi E, Angelico P, Farina P, Leonardi A, Clarke DE, Testa R. Effect of selective antagonists of group I metabotropic glutamate receptors on the micturition reflex in rats. BJU Int. 2008;102:890–898. doi: 10.1111/j.1464-410X.2008.07748.x. [DOI] [PubMed] [Google Scholar]
- Hama AT, Urban MO. Antihyperalgesic effect of the cannabinoid agonist WIN55,212-2 is mediated through an interaction with spinal metabotropic glutamate-5 receptors in rats. Neurosci Lett. 2004;358:21–24. doi: 10.1016/j.neulet.2003.12.111. [DOI] [PubMed] [Google Scholar]
- Hu Y, Dong L, Sun B, Guillon MA, Burbach LR, Nunn PA, Liu X, Vilenski O, Ford APDW, Zhong Y, Rong W. The role of metabotropic glutamate receptor mGlu5 in control of micturition and bladder nociception. Neurosci Lett. 2009;450:12–17. doi: 10.1016/j.neulet.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, Winter J. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22:2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- Kakizaki H, Yoshiyama M, Roppolo JR, Booth AM, De Groat WC. Role of spinal glutamatergic transmission in the ascending limb of the micturition reflex pathway in the rat. J Pharmacol Exp Ther. 1998;285:22–27. [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RW. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN. Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther. 2004;104:233–244. doi: 10.1016/j.pharmthera.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 2010;112:162–172. doi: 10.1111/j.1471-4159.2009.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Giachetti A, Meli A. The effect of MK-801 on the micturition reflex in anesthetized rats. Eur J Pharmacol. 1990;181:105–109. doi: 10.1016/0014-2999(90)90250-a. [DOI] [PubMed] [Google Scholar]
- Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Hisamitsu T, de Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci Lett. 1995;183:58–61. doi: 10.1016/0304-3940(94)11114-x. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Ohyama C, Ogawa Y. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J Urol. 2004;171:1353–1356. doi: 10.1097/01.ju.0000099840.09816.22. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Role of metabotropic glutamate receptor subtype mGluR1 in brief nociception and central sensitization of primate STT cells. J Neurophysiol. 1999;82:272–282. doi: 10.1152/jn.1999.82.1.272. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int. 1991;47:19–22. doi: 10.1159/000282243. [DOI] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, Rossi F, Maione S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Marabese I, de Novellis V, Oliva P, Rossi F, Berrino L, Rossi F, Maione S. Metabotropic and NMDA glutamate receptors participate in the cannabinoid-induced antinociception. Neuropharmacology. 2001;40:319–326. doi: 10.1016/s0028-3908(00)00160-x. [DOI] [PubMed] [Google Scholar]
- Peters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Rev Urol. 2002;4(suppl. 1):S36–S43. [PMC free article] [PubMed] [Google Scholar]
- Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–764. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- Pitcher MH, Ribeiro-da-Silva A, Coderre TJ. Effects of inflammation on the ultrastructural localization of spinal dorsal horn group I metabotropic glutamate receptors. J Comp Neurol. 2007;505:312–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- Reid SN, Romano C, Hughes T, Daw NW. Immunohistochemical study of two phosphoinositide-linked metabotropic glutamate receptors (mGluR1 alpha and mGluR5) in the cat visual cortex before, during, and after the peak of the critical period for eye-specific connnections. J Comp Neurol. 1995;355:470–477. doi: 10.1002/cne.903550311. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Exp Neurol. 2011;228:109–117. doi: 10.1016/j.expneurol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am J Physiol Renal Physiol. 2008;294:F591–F602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kakizaki H, Shibata T, Ameda K, Koyanagi T. Effects of a selective metabotropic glutamate receptor agonist on the micturition reflex pathway in urethane-anesthetized rats. Neurourol Urodyn. 2003;22:611–616. doi: 10.1002/nau.10138. [DOI] [PubMed] [Google Scholar]
- Valerio A, Rizzonelli P, Paterlini M, Moretto G, Knöpfel T, Kuhn R, Memo M, Spano P. mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: a developmental study. Neurosci Res. 1997;28:49–57. doi: 10.1016/s0168-0102(97)01175-9. [DOI] [PubMed] [Google Scholar]
- van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Nijholt AABL, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Vidnyanszky Z, Hamori J, Negyessy L, Ruegg D, Knopfel T, Kuhn R, Gorcs TJ. Cellular and subcellular localization of the mGluR5a metabotropic glutamate receptor in rat spinal cord. Neuroreport. 1994;6:209–213. doi: 10.1097/00001756-199412300-00053. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini P, Kuhn R, Urban L. mGlu5 receptors and nociceptive function. II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC. Supraspinal and spinal α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid andN-methyl-d-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132:1017–1026. doi: 10.1016/j.neuroscience.2005.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC. Role of spinal metabotropic glutamate receptors in regulation of lower urinary tract function in the decerebrate unanesthetized rat. Neurosci Lett. 2007;420:18–22. doi: 10.1016/j.neulet.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat – possible sites of action. J Pharmacol Exp Ther. 1993a;265:844–850. [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Interactions between NMDA and AMPA/kainate receptors in the control of micturition in the rat. Eur J Pharmacol. 1995;287:73–78. doi: 10.1016/0014-2999(95)00615-7. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, Thor KB, de Groat WC. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br J Pharmacol. 1993b;110:77–86. doi: 10.1111/j.1476-5381.1993.tb13774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


