Background: Functional screening of malaria (P. falciparum) genes is a problem because of A + T content.
Results: Use of a P. knowlesi cDNA library and a yeast mutant identifies an important parasite cDNA/gene and reveals new regulation of lipid synthesis.
Conclusion: The P. knowlesi library will enable extrapolation to P. falciparum.
Significance: Functional screening of malaria genes is now greatly enhanced.
Keywords: Lipids, Lipid Synthesis, Membrane, Parasite, Phosphatidylethanolamine, Phosphatidylserine, Phospholipid Metabolism, Protein Processing, Yeast
Abstract
The 23-megabase genome of Plasmodium falciparum, the causative agent of severe human malaria, contains ∼5300 genes, most of unknown function or lacking homologs in other organisms. Identification of these gene functions will help in the discovery of novel targets for the development of antimalarial drugs and vaccines. The P. falciparum genome is unusually A + T-rich, which hampers cloning and expressing these genes in heterologous systems for functional analysis. The large repertoire of genetic tools available for Saccharomyces cerevisiae makes this yeast an ideal system for large scale functional complementation analyses of parasite genes. Here, we report the construction of a cDNA library from P. knowlesi, which has a lower A + T content compared with P. falciparum. This library was applied in a yeast complementation assay to identify malaria genes involved in the decarboxylation of phosphatidylserine. Transformation of a psd1Δpsd2Δdpl1Δ yeast strain, defective in phosphatidylethanolamine synthesis, with the P. knowlesi library led to identification of a new parasite phosphatidylserine decarboxylase (PkPSD). Unlike phosphatidylserine decarboxylase enzymes from other eukaryotes that are tightly associated with membranes, the PkPSD enzyme expressed in yeast was equally distributed between membrane and soluble fractions. In vitro studies reveal that truncated forms of PkPSD are soluble and undergo auto-endoproteolytic maturation in a phosphatidylserine-dependent reaction that is inhibited by other anionic phospholipids. This study defines a new system for probing the function of Plasmodium genes by library-based genetic complementation and its usefulness in revealing new biochemical properties of encoded proteins.
Introduction
Malaria, a serious infectious disease responsible for over 800,000 deaths annually, is caused by intraerythrocytic protozoan parasites of the genus Plasmodium. Plasmodium falciparum is the major cause of human parasitic fatalities. The worldwide emergence of drug-resistant Plasmodium strains has made treatment of malaria increasingly difficult, thus emphasizing the need for new chemotherapeutic strategies to combat this disease (1). Following invasion of red blood cells, malarial parasites must increase lipid synthesis for membrane biogenesis and cell division. Inhibition of membrane lipid synthesis provides an attractive target for antimalarial chemotherapy (2, 3). Lipid analysis of P. falciparum demonstrates a relatively high content (up to 35% of total phospholipid) of phosphatidylethanolamine (PtdEtn)2 (4), which often functions as a nonbilayer hexagonal phase lipid (5, 6).
PtdEtn synthesis in Plasmodium occurs through two major pathways, the serine decarboxylase-CDP-ethanolamine pathway and the phosphatidylserine (PtdSer) decarboxylation (PSD) pathway (Fig. 1). In the serine decarboxylase-CDP-ethanolamine pathway, serine is decarboxylated by a parasite-specific serine decarboxylase (PfSD) to form ethanolamine (Etn) (7, 8). Serine decarboxylation is unique to malarial parasites and plants (9). Ethanolamine, either formed through this reaction or directly taken up from the host erythrocyte (8), is then sequentially converted into phosphoethanolamine (P-Etn), CDP-ethanolamine, and PtdEtn by three parasite-specific enzymes, catalyzing an ethanolamine kinase (PfEK), an ethanolamine cytidylyltransferase (PfECT), and a CDP-choline/ethanolamine phosphotransferase (PfCEPT) reaction. P-Etn can also be methylated to form phosphocholine (P-Cho), which can be further metabolized to phosphatidylcholine (PtdCho) via a CDP-Cho intermediate. In the PtdSer decarboxylation pathway, serine is incorporated into phospholipid by a parasite PtdSer synthase and is subsequently decarboxylated to form PtdEtn by PtdSer decarboxylase (PfPSD) (10). Unlike yeast, Plasmodium lacks lipid methyltransferases for conversion of PtdEtn to PtdCho.
FIGURE 1.
PtdEtn synthesis pathways in Plasmodium and yeast. PtdEtn synthesis in Plasmodium occurs through two major pathways, the serine decarboxylase-CDP-ethanolamine pathway and the PtdSer decarboxylation pathway. The major enzymes executing reactions within these pathways are shown in boxes. In the serine decarboxylase-CDP-ethanolamine pathway, serine is decarboxylated by an unidentified serine decarboxylase (SD) to form ethanolamine. Serine decarboxylation is unique to malarial parasites and plants. Ethanolamine formed through this reaction is sequentially converted into phosphoethanolamine (P-Etn), CDP-ethanolamine (CDP-Etn), and PtdEtn by ethanolamine kinase (EK), ethanolamine-phosphate cytidylyltransferase (ECT), and ethanolamine phosphotransferase (EPT). In Plasmodium, P-Etn can be sequentially methylated to form phosphomonomethylethanolamine (P-MMe), phosphodimethylethanolamine (P-DMe), and phosphocholine (P-Cho) by a single phosphoethanolamine methyltransferase (PMT). The resultant phosphocholine is further metabolized by a choline-phosphate cytidylytransferase (CCT) and choline phosphotransferase (CPT) to produce CDP-Cho and PtdCho, respectively. In the PtdSer decarboxylation pathway, serine is incorporated into phosphatidylserine (PtdSer) by PtdSer synthase (PSS) and subsequently decarboxylated to form PtdEtn by PtdSer decarboxylase (PSD). In contrast to Plasmodium, yeast sequentially methylate PtdEtn via the action of the phospholipid methyltransferases, PEM1 and PEM2, to produce PtdCho. The black arrows show the parasite pathways, and the gray arrows show the yeast pathways. The enzymes catalyzing each step of the pathway are enclosed by boxes.
The PtdEtn synthetic pathways in Saccharomyces cerevisiae share some similarity with those of the parasite (see Fig. 1). In the absence of exogenous Etn, the major route for the synthesis of PtdEtn originates with the synthesis of PtdSer in the endoplasmic reticulum or closely related membranes (mitochondrion-associated membrane) by PtdSer synthase (11–13). After its synthesis, PtdSer is decarboxylated to form PtdEtn by PtdSer decarboxylase 1 (PSD1) at the inner mitochondrial membrane (14) or PtdSer decarboxylase 2 (PSD2) at the Golgi (14, 15). In the presence of Etn, PtdEtn is synthesized through the CDP-ethanolamine pathway. Unlike Plasmodium species, S. cerevisiae does not decarboxylate serine to form Etn. Previously, we have successfully used S. cerevisiae as a surrogate system to characterize P. falciparum genes and cDNAs involved in the synthesis and transport of lipid precursors (3, 16, 17). However, these studies required optimization of cDNAs because of the high A + T content of P. falciparum genes (∼81%) (18). In contrast to P. falciparum, yeast have an A + T content of 62%. To bypass the need for codon optimization and enable analysis of Plasmodium genes of unknown function, we constructed a cDNA library from P. knowlesi, which has an A + T content of 63% (19). P. knowlesi causes malaria in both monkeys and humans (19). The goals of this study were to use the P. knowlesi cDNA library to complement well defined genetic defects in yeast lipid synthesis and characterize the parasite gene product. This report demonstrates successful library-based complementation of yeast using the cDNA library and reveals new details about the properties and maturation of PkPSD.
EXPERIMENTAL PROCEDURES
Materials
All chemicals, including amino acids for yeast media, were purchased from either Sigma or Fisher. Other components for yeast growth media were purchased from Difco. Phospholipids were obtained from Avanti Polar Lipids. Silica gel H plates and Silica gel 60 plates were purchased from Analtech Corp. and EMD, respectively. Radioactive l-[G-3H]serine was from PerkinElmer Life Sciences. Reagents for protein determination were from Bio-Rad. Pre-cast SDS-polyacrylamide gels were purchased from Invitrogen. Mouse monoclonal antibodies against the V5 and His6 epitope tags of the PkPSD fusion protein were obtained from Invitrogen and Clontech, respectively. Other reagents used for ligand blotting were obtained from Bio-Rad and Sigma.
cDNA Library
The cDNAs were generated from P. knowlesi mRNAs and inserted by directional cloning into a modified pBEVY vector (20), pBEVY-DS, containing a single SfiI site. The vector is a URA3-based multicopy Escherichia coli/yeast shuttle vector in which the cloned Pk-cDNAs are under the regulation of ADH1 promoter.
Pk-cDNA Library Screening
Pk-cDNA library plasmids were transformed into an Etn auxotrophic strain, HKY44 (MATα psd1-Δ1::TRP1 psd2-Δ1::HIS3 dpl1Δ::KanMX trp1 ura3 his3 lys2 leu2) (21). Transformants that stably acquired a plasmid from the library were isolated based on the growth on minimal glucose, uracil dropout (SC-U) medium, supplemented with 2 mm Etn. Etn prototrophic strains were isolated by replica plating transformants onto SC-U medium with no Etn supplementation.
Cell Growth
psd1Δpsd2Δdpl1Δ strains harboring the PkPSD gene were cultured in SC-U or synthetic lactate uracil dropout (SL-U) medium (22) supplemented with 2 mm Etn. After washing twice with water, serial 5-fold dilutions of cells were plated onto the medium with or without 2 mm Etn. Plates were incubated at 30 °C for 2–4 days.
Whole Cell Radiolabeling and Phospholipid Analysis
psd1Δpsd2Δdpl1Δ strains harboring the predicted PkPSD sequence and psd1Δpsd2Δdpl1Δ strains with empty vector were grown in synthetic complete medium plus 2 mm Etn with glucose as a carbon source (SC). Cells in mid-log phase were harvested by centrifugation and washed twice by resuspension in water and recentrifugation. The cells were suspended in SC medium at an A600 of 0.35 in a volume of 2 ml. Radiolabeling was initiated by adding 10 μCi/ml l-[G-3H]serine, and growth was continued at 30 °C for 2 h with vigorous shaking. Labeled phospholipids were extracted as described previously (14, 23), and the lipid classes were resolved by thin layer chromatography, and radioactivity was quantified by liquid scintillation spectrometry.
Measurement of PSD Activities
Cell-free extracts, membrane fractions, and soluble fractions were isolated from psd1Δpsd2Δdpl1Δ strains harboring the predicted PkPSD cDNA or empty vector. Endogenous yeast PSD1 activity was measured using MSY30 (PSD1DPL1psd2Δ) (24). Strains were homogenized by glass bead beating, and subcellular fractions were prepared by differential centrifugation. The assay for PSD activity utilized Ptd[1′-14C]Ser as the substrate, and the reaction product was trapped as 14CO2 on 2 m KOH-impregnated filter paper, as described previously (25). PSD activities in psd1Δpsd2Δdpl1Δ strains harboring the predicted PkPSD cDNA are presented as enzyme-specific activity (nmol/mg·protein/45 min), or as the percentage of activity relative to the activity of yeast PSD1 wild type strains (100%), or as volume-normalized activity (nmol/ml/45 min) in tnt reactions. All enzyme reactions were performed at substrate (0.2 mm) and protein concentrations that produced a linear response with the amount of enzyme added to the reaction (usually 4–20 μg). In the most active preparations, the maximum substrate conversion to product did not exceed 10%.
Lipid Phosphorus Measurement
Yeast strains were cultured in SC-U medium and grown to mid-log phase at 30 °C. The cells were harvested by centrifugation and washed twice with water. The lipids were extracted, as described previously (26). The phospholipids were separated by two-dimensional thin layer chromatography (TLC) on Silica 60 plates using chloroform/methanol/ammonium hydroxide (65:35:5 v/v) followed by chloroform/acetic acid/methanol/water (75:25:5:2.2 v/v). Lipids were visualized with iodine vapor and quantified by measuring phosphorus (27). The results are shown as the percentage of total lipid phosphorus in each phospholipid fraction. Data are means ± S.D. for three or four independent experiments.
In Vitro Expression of PkPSD
A TnT quick-coupled transcription/translation system (Promega Corp.) was used to express PkPSD proteins in vitro. Reactions were initiated with 0.5 μl of 1 mm methionine and 2 μl of plasmid (0.5 μg/μl) harboring PkPSD with an N- or C-terminal His6 tag, added to 20 μl of TnT quick master mix kit, which contained rabbit reticulocyte lysate, an amino acid mixture without methionine, T7 RNA polymerase, nucleotides, salts, and RNasin ribonucleotide inhibitor. To study the effect of phospholipids on the expression and processing of the PkPSD protein, 2 μl of liposomes composed of dioleoyl phosphatidylserine (DOPS), dioleoyl phosphatidylethanolamine (DOPE), dioleoyl phosphatidylcholine (DOPC), dioleoyl phosphatidylglycerol (DOPG), dioleoyl phosphatidic acid (DOPA), or phosphatidylinositol (PI) from bovine liver or soybean were added to the TnT reaction and incubated for the indicated times at 30 °C. Liposomes were prepared fresh for each experiment. To prepare liposomes, phospholipid in chloroform was transferred into an Eppendorf tube and dried under nitrogen gas and chased with methanol. The lipid pellets were resuspended in 20 μl of methanol and dried using centrifugation under vacuum for 30 min to completely remove all organic solvents. The lipid pellets were resuspended in 0.1 m KCl, 10 mm Tris-Cl, pH 7.5, at 1 mg/ml, hydrated at 37 °C for 30 min, and then mixed with a vortex mixer to create multilamellar liposomes. To create unilamellar liposomes, the suspension was bath-sonicated for 20 min. Defined size unilamellar liposomes were made by passing multilamellar liposomes through an Avanti LiposoFast with 50-, 200-, or 1000-nm filter sets. When the in vitro transcription/translation step was temporally separated from processing steps, 0.2 mm cycloheximide was added to the TnT reaction to arrest translation, and the reactions were further incubated with liposomes. The expression and processing of PkPSD were monitored by Western blot analysis using anti-His6 antibody and by PSD enzyme assay as described above.
Construction of Vectors to Express PkPSD in E. coli or Yeast
E. coli vectors harboring plasmids for PkPSD-His6 or N-terminal 34-amino acid deleted PkPSD-His6 (PkPSDΔ34-His6) were created using a pET-45b(+) vector. Briefly, specific primers for the individual constructs were generated and used to amplify DNAs from a template cDNA harboring PkPSD using PCR with Pfu-DNA polymerase (Stratagene). The PkPSD and PkPSDΔ34 constructs containing a 5′ SpeI site and a 3′ and XhoI site were purified by agarose gel electrophoresis. The pET-45b(+) E. coli expression vector was digested with XbaI (compatible to SpeI ligation) and XhoI restriction enzymes, and the resultant DNA fragment was purified by electrophoresis. Appropriate ligation reactions yielded pET45-PkPSD-His6 and pET45-PkPSDΔ34-His6. The plasmids were introduced in BL21 and Rosetta strain to express the constructs. The pET-45b(+) vector was also utilized to create pET45-His6-PkPSDΔ34, which encodes an N-terminal His6 epitope linked to PkPSDΔ34. The PCR construct, PkPSDΔ34, was digested with KpnI at the 5′ end and XhoI at the 3′ end and subsequently ligated into the linearized pET-45b(+) vector, also cut with KpnI and XhoI restriction enzymes. The construct was introduced into BL21 and Rosetta strains to express the enzyme. The pET45-His6-PkPSDΔ34 construct was also used in vitro transcription/translation using a TnT kit.
A pYES2.1-V5 vector was used to express the proteins PkPSD, PkPSDΔ34, and PkPSD with an N-terminal 55-amino acid deletion (PkPSDΔ55) in yeast. Specific primers were generated so that proteins could be expressed as untagged or the C-terminal V5 fusion forms. The PCR constructs were amplified from a template cDNA harboring PkPSD. The constructs were ligated into a linearized pYES2.1-V5 TOPO vector (Invitrogen). The plasmids were transformed into an Etn auxotrophic strain, PTY44 (MATα psd1-Δ1::TRP1 psd2-Δ1::HIS3 trp1 ura3 his3 lys2 leu2).
RESULTS
Identification of P. knowlesi Phosphatidylserine Decarboxylase
An Etn auxotrophic mutant yeast strain devoid of PSD activity and incapable of forming P-Etn from sphingolipids, HKY44 (psd1Δpsd2Δdpl1Δ), was utilized to screen a multicopy Pk-cDNA library inserted into the vector pBEVY-DS. Approximately 650,000 uracil prototrophic pBEVY-DS library transformants were screened for Etn prototrophy, and 127 transformants were identified. Plasmids from nine uracil/ethanolamine yeast prototrophs were recovered using antibiotic resistance in E. coli. Sequencing of these plasmids revealed the presence of the ORF PKH_072580. The sequence was previously annotated as a putative phosphatidylserine decarboxylase. The remaining Etn prototrophs were also positive for PkPSD sequences when screened by PCR.
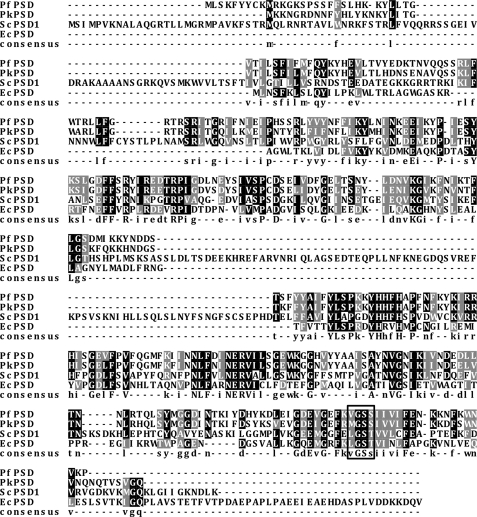
The putative PkPSD cDNA encodes a polypeptide of 354 amino acid residues with a deduced molecular weight of 41,524. The PkPSD protein shares strong sequence homology with the previously characterized PfPSD from P. falciparum with amino acid sequence identity of 72.5% and similarity of 88.2%. An MGSS sequence located at positions 306–309 in the C terminus corresponds to a VGSS sequence located at positions 314–317 of the PfPSD and appears related to the LGST motif, which is the endoproteolytic cleavage site of the E. coli and the S. cerevisiae PSD1 proenzymes (Fig. 2) (14, 28). The cleavage converts the inactive proenzyme to an active form containing a small α subunit with a pyruvoyl prosthetic group essential for catalysis and a large β subunit (29).
FIGURE 2.
Sequence alignment of PtdSer decarboxylase. The PkPSD sequence (XP_002258555) is aligned to PSD sequences from P. falciparum (AAN34609), E. coli (AP_004663), and S. cerevisiae (AAA34918). Identical and similar amino acids are shaded in black and gray, respectively. The box highlights the LGST sequence present in E. coli and yeast that is the site of proteolytic processing and pyruvoyl prosthetic group attachment. The corresponding sequence and its variants in PkPSD and PfPSD are also shown in the box.
Fig. 3a shows the growth of the psd1Δpsd2Δdpl1Δ strains harboring the PkPSD cDNA in minimal medium containing glucose. In the absence of Etn supplementation, the psd1Δpsd2Δdpl1Δ strain harboring the PkPSD cDNA grew with a pronounced lag but reached nearly the same saturation level after 2 days. Fig. 3b shows the growth on minimal glucose plates with or without Etn supplementation. The psd1Δpsd2Δdpl1Δ strains harboring the PkPSD cDNA grew robustly in the absence of Etn, whereas strains with empty plasmid vector failed to grow.
FIGURE 3.
PkPSD cDNA complements the Etn auxotrophy of the psd1psd2dpl1 strain. a, wild type, psd1Δpsd2Δdpl1Δ mutant, and psd1Δpsd2Δdpl1Δ mutant strains harboring pBEVY-PkPSD were inoculated into synthetic glucose medium supplemented with 2 mm Etn and grown overnight. Cells were harvested and reinoculated at an A600 = 0.005 in synthetic glucose medium plus or minus 2 mm Etn as indicated. Cell growth at 30 °C was monitored by A600. b and c, psd1Δpsd2Δdpl1Δ mutant strains harboring an empty vector, YEp352-ScPSD1, or pBEVY-PkPSD were cultured in SC-U (b) or SL-U (c) medium with or without 2 mm Etn as indicated. 5-Fold serial dilutions of cells were plated onto the medium and incubated at 30 °C for 2 days (b) and 3.5 days (c).
Next, we tested whether the presence of the PkPSD cDNA could support growth of the mutant strain under respiratory conditions in minimal medium with lactate as the carbon source, where PtdEtn synthesis is required for normal mitochondrial function. Fig. 3c shows that psd1Δpsd2Δdpl1Δ strains harboring PkPSD grew well under respiratory conditions in the absence of Etn. The ability of the PkPSD enzyme to support yeast growth under respiratory conditions indicates that the enzyme has access to pools of PtdSer located at the outer mitochondrial membrane. Collectively, the above data demonstrate that the Plasmodium PSD cDNA suppresses the growth defect of psd1Δpsd2Δdpl1Δ strains under both fermentative and respiratory conditions.
Characterization of PkPSD Expressed in Yeast
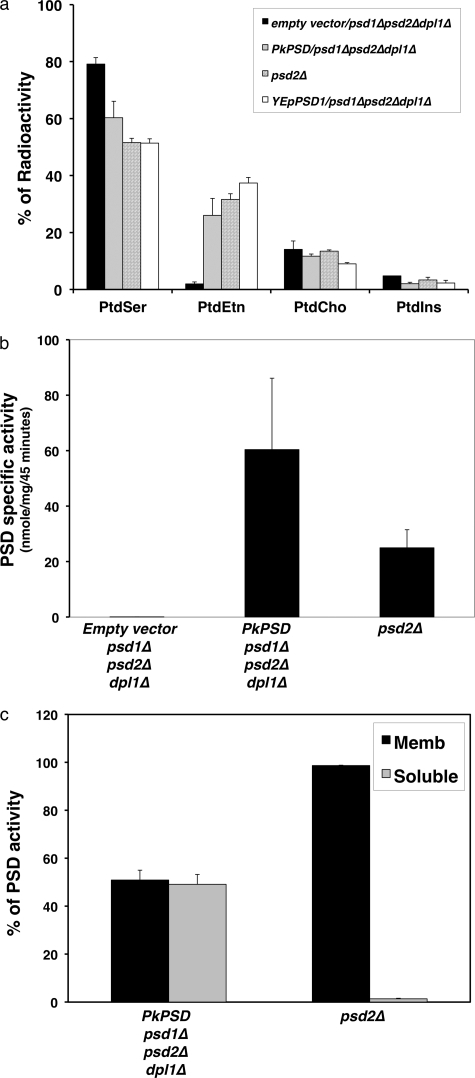
To characterize the PkPSD enzyme, catalytic activities were assayed both in vivo and in vitro. Yeast strains were labeled with [3H]serine in minimal glucose medium, and the incorporation of the radiolabel into aminophospholipids was analyzed by thin layer chromatography (TLC) of the lipid extracts prepared from the cells (Fig. 4a). The mutant psd1Δpsd2Δdpl1Δ strain harboring an empty vector failed to generate significant levels of radiolabeled PtdEtn because of the lack of PtdSer decarboxylases. The mutant psd1Δpsd2Δdpl1Δ strain expressing the PkPSD, and yeast strains lacking PSD2 but containing the PSD1 gene, expressed from either single copy or multicopy plasmids, readily produced radiolabeled PtdEtn at normal levels. This indicates that nascent PtdSer is efficiently converted to PtdEtn by the PkPSD enzyme at levels comparable with that produced by the chromosomal copy of yeast PSD1.
FIGURE 4.
PkPSD is a functional PtdSer decarboxylase enzyme. a, incorporation of radioactive serine into amino glycerophospholipids. Labeled phospholipids were extracted from log phase cells grown for 2 h in synthetic medium containing 10 μCi/ml l-[G-3H]serine. The lipid classes were resolved by thin layer chromatography, and radioactivity was quantified by liquid scintillation spectrometry. Data are the mean ± S.D. from 2 experiments performed in duplicate. Results are the percentage of total radiolabel incorporated into each phospholipid. The trace labeling in PtdIns is found primarily in fatty acids and co-migrating sphingolipids. b, PSD enzyme assays were performed with cell-free extracts from a parental strain (psd2Δ), a psd1Δpsd2Δdpl1Δ mutant strain harboring either an empty vector, or pBEVY-PkPSD. c, PSD enzyme assays were performed with membrane (black bar) and soluble (gray bar) fractions from a parental strain (psd2Δ) and a psd1Δpsd2Δdpl1Δ mutant strain harboring pBEVY-PkPSD. Assay for PSD utilized Ptd[1′-14C]Ser as the substrate, and the reaction product was trapped as 14CO2 on 2 m KOH impregnated filter paper. Data are means ± S.D. for two experiments each performed in duplicate.
Next, the enzyme activity was measured in cell extracts. Whole cell extracts, membrane fractions, and soluble fractions were incubated with Ptd[1′-14C]serine substrate, and the decarboxylase activity was determined by measuring 14CO2 production. As shown in Fig. 4b, high enzyme activity was detected in the cell-free extracts of the psd1Δpsd2Δdpl1Δ strain harboring a PkPSD cDNA, whereas no activity was found in the psd1Δpsd2Δdpl1Δ strain harboring the empty vector. The levels of PkPSD expression produced nearly three times the catalytic activity of the endogenous yeast PSD1 gene. Interestingly, significant levels (49%) of PkPSD enzyme activity were detected in the soluble fractions prepared from cell extracts (Fig. 4c). Thus far, all eukaryotic PSD enzymes have been localized to membrane compartments (30–32), and these findings suggest unusual properties of the malarial enzyme. The relatively large soluble population of the PkPSD protein could be an intrinsic feature of the enzyme that renders the molecule amphitropic.
The phospholipid compositions of the psd1Δpsd2Δdpl1Δ strains with the PkPSD cDNA were analyzed and compared with that of a psd2Δ strain that contains wild type PSD1 and DPL1 genes. Lipids extracted from the cells grown on medium in the absence of Etn were separated by two-dimensional TLC and visualized by iodine staining. The appearance of a PtdEtn spot indicates that the lipid was synthesized by the action of PkPSD enzyme (Fig. 5a). Fig. 5b shows the quantification of each lipid extracted from the TLC plates. There was a slight increase in PtdEtn level and a 42% reduction in PtdSer in the psd1Δpsd2Δdpl1Δ strains expressing the PkPSD cDNA compared with that in the psd2Δ strain. Taken together, the results from Figs. 4 and 5 demonstrate that the PkPSD enzyme expressed in the psd1Δpsd2Δdpl1Δ strains was fully functional and complemented the biochemical defect of the mutant strain.
FIGURE 5.
Yeast cells harboring PkPSD produce normal levels of PtdEtn. The parental strain (psd2Δ) and psd1Δpsd2Δdpl1Δ mutant strain harboring pBEVY-PkPSD were grown to log phase in SC-U medium, and lipids were separated by two-dimensional TLC. a, lipids were visualized by iodine straining. b, isolated lipids were quantified by phosphorus assay, and the results are expressed as the percentage of total lipid phosphorus. Data are expressed as means ± S.E. of four experiments.
N-terminal Deletion of PkPSD Yields Active Forms of Enzyme
The N-terminal 137 amino acids of yeast PSD1 contain mitochondrial targeting and inner membrane sorting sequences. The sequence is not only required for the mitochondrial targeting but also for the correct processing into β and α fragments (33). Previously, plant PSD sequences from tomato and Arabidopsis were identified and expressed in the yeast psd1psd2 double mutant strain (10). The N-terminal mitochondrial targeting sequence of plant PSD protein was inhibitory to its functional expression in yeast. A deletion construct that removed the plant-specific mitochondrial targeting sequence and a chimeric construct in which the yeast PSD1 targeting sequence replaced the plant sequence could readily suppress the Etn auxotrophy of the yeast double mutant strain.
The N-terminal 55-amino acid sequence of the PkPSD protein does not show any significant amino acid homology with any other PSDs. When the sequence was analyzed with TMpred software, a trans-membrane domain was predicted at positions 18–34. To investigate the role of the N-terminal sequence, two truncated PkPSD cDNAs were constructed to encode proteins with deletions of residues 2–34 (PkPSDΔ34) and 2–55 (PkPSDΔ55) (Fig. 6a). The constructs were placed under control of the GAL1 promoter in an episomal vector (pYES2.1, Invitrogen) and introduced into the psd1Δpsd2Δ double mutant strain. Enzyme assay showed that the PkPSD with a 34-amino acid deletion produced robust activity, whereas the PkPSD with a 55-amino acid deletion produced very weak activity (Fig. 6b). The catalytic activity of the PkPSD with the 34-amino acid deletion was also evenly distributed between membrane and soluble fractions (data not shown). This indicates that the PkPSD residues between 2 and 34 were not required for enzyme maturation, catalysis, or membrane association in the yeast expression system. In contrast, the amino acids between positions 35 and 55 appear crucial for the functional activity of PkPSD.
FIGURE 6.
N-terminal transmembrane domain of PkPSD is not required for enzyme activity. a, schematic depiction of full-length PkPSD and constructs deleted for amino acids 1–34 (PkPSDΔ34) and deleted for amino acids 1–55 (PkPSDΔ55). TM denotes the predicted trans-membrane domain, and the α and β chains indicate the processed forms of PkPSD. b, PkPSD constructs were placed into a pYES2.1 vector. PSD enzyme assays were performed with cell-free extracts from psd1Δpsd2Δmutant strains harboring pYES2.1, pYES2.1 containing PkPSDΔ55, PkPSDΔ34, or full-length PkPSD as indicated. Assay for PSD utilized Ptd[1′-14C]Ser as the substrate, and the reaction product was trapped as 14CO2 on 2 m KOH impregnated filter paper. Data are means ± S.D. for two experiments each performed in duplicate.
Expression of PkPSD Fusion Proteins in Bacteria and Yeast
To further characterize the activity and processing of PkPSD, we generated PkPSD-His6 and PkPSD-V5 fusion proteins expressed in E. coli and yeast, respectively. A PkPSD-His6 fusion protein was expressed in BL21 and Rosetta strains of E. coli. Protein extracts were separated on SDS-PAGE, and the fusion protein was detected with anti-His6 antibody. As seen in Fig. 7a, the fusion protein was detected only with the Rosetta host strain, which provides six tRNAs for codons that are rarely used in E. coli but common in eukaryotic organisms, thus enabling the efficient translation of DNA from P. knowlesi. Two immunoreactive bands were detected with the anti-His6 antibody. The upper 43-kDa band corresponds to the unprocessed proenzyme and the 7-kDa lower band corresponds to the α subunit containing the active site of the enzyme (28). The majority of the E. coli-expressed PkPSD was in the form of proenzyme, which indicates that the endoproteolytic processing of the PkPSD fusion protein was not efficient. The Rosetta strain expresses its endogenous PSD, but the total catalytic activity in cell extracts increased 2-fold with PkPSD cDNA expression (Fig. 7b).
FIGURE 7.
Expression of the PkPSD-His6 protein in E. coli. a, Western blot analysis of PkPSD-His6 fusion proteins from a BL21 strain harboring an empty vector (lane 1), pET45-PkPSDHis6 (lanes 2 and 3), a Rosetta strain harboring an empty vector (lane 4), or pET45-PkPSDHis6 (lanes 5 and 6). b, PSD enzyme assays were performed with cell-free extracts from Rosetta strain harboring pET45 and pET45-PkPSDHis6. Data are means ± S.E. for three experiments each performed in duplicate.
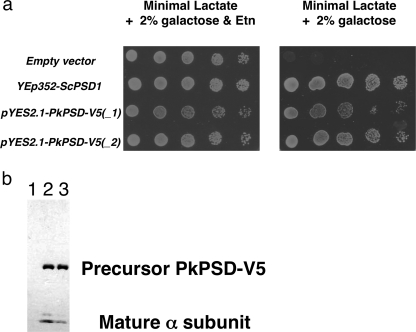
A multicopy yeast vector encoding a PkPSD-V5 fusion protein under control of the GAL1 promoter was constructed and transformed into a yeast psd1Δpsd2Δ strain. Fig. 8a shows the growth of the psd1Δpsd2Δ strains harboring either an empty vector, a multicopy vector with the yeast PSD1, or a multicopy vector containing the PkPSD-V5 cDNA fusion construct. As expected, the psd1Δpsd2Δ strains harboring empty vector failed to grow in the absence of Etn, because the strains had no PSD enzymes. Expression of the PkPSD fusion protein readily suppressed the growth defect of the mutant strain in the absence of Etn. Extracts from the yeast cells were analyzed by SDS-PAGE followed by Western blotting using an anti-V5 antibody (Fig. 8b). Two protein bands, corresponding to the proenzyme and the active form of the α subunit, were detected demonstrating the maturation of the enzyme in vivo. These findings with the fusion proteins demonstrated that epitope-tagged versions of PkPSD could undergo processing in vivo and maturation to active enzyme.
FIGURE 8.
Expression of the PkPSD-V5 fusion protein in the psd1Δpsd2Δdpl1Δ strain. a, psd1Δpsd2Δdpl1Δ mutant strains harboring an empty vector, YEp352-ScPSD1, pYES2.1-PkPSD-V5 (isolate 1), or pYES2.1-PkPSD-V5 (isolate 2) were cultured in SL-U medium plus 2% galactose, with or without 2 mm Etn, as indicated. 5-Fold serial dilutions of cells were plated. Galactose was used to induce expression of PkPSD from the pYES2.1 vector. Plates were incubated at 30 °C for 3 days. b, Western blot analysis was performed to detect the V5 epitope in proteins extracted from the psd1Δpsd2Δdpl1Δ mutant strains harboring an empty vector (lane 1), pYES2.1-PkPSD-V5 (isolate 1) (lane 2), or pYES2.1-PkPSD-V5 (isolate 2) (lane 3).
In Vitro Processing of PkPSD Demonstrates PtdSer Post-translationally Enhances Enzyme Maturation
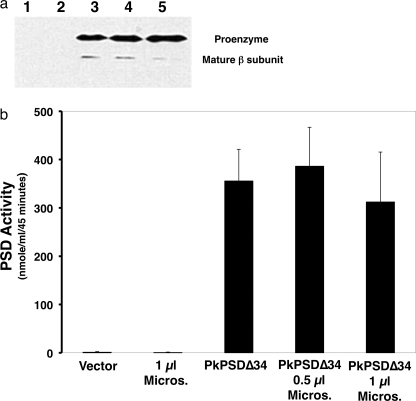
The retention of enzyme activity by PkPSDΔ34 (see Fig. 6b) indicated that the predicted membrane binding N terminus of the protein was not required for processing of the proenzyme to its mature form, and it raised the possibility that processing of a soluble form of the enzyme could be examined in vitro using epitope-tagged versions of the enzyme. We utilized a coupled in vitro transcription-translation (TnT) system to examine nascent enzyme formation and its subsequent processing to mature enzyme. We empirically determined that an N-terminal His6 tag appended to PkPSDΔ34 produced the most robust catalytic activity from the in vitro TnT reaction. In our initial experiments, we observed significant production of the PkPSDΔ34 proenzyme (39 kDa), with modest processing to the mature enzyme (seen as the 33-kDa β subunit), detected by both immunoblotting and measurement of catalytic activity (see Fig. 9, a and b). We tested whether the presence of a membrane compartment, canine pancreatic microsomes, could augment processing of the enzyme, and these experiments did not demonstrate any influence of added membranes upon the process (Fig. 9, a and b).
FIGURE 9.
In vitro expression of His6-PkPSDΔ34 fusion protein produces an active PSD enzyme. A TnT reaction containing pET45 vector only, 1 μl of canine pancreatic microsomal membranes (Promega), pET45-His6-PkPSDΔ34, pET45-His6-PkPSDΔ34 + 0.5 μl of canine pancreatic microsomal membranes, or pET45-His6-PkPSDΔ34 + 1 μl of canine pancreatic microsomal membranes was performed for 90 min at 30 °C. Upon completion of the TnT reaction, aliquots were used for PSD assays and Western blot analysis. a, Western blot analysis was conducted to detect the His6 epitope present in the nascent enzyme generated in the TnT reaction. The lane numbers correspond to plasmids and microsome additions to the TnT reaction. Lane 1, empty vector; lane 2, no vector + microsomes; lane 3, pET45-His6-PkPSDΔ34; lane 4, pET-His6-PkPSDΔ34 + 0.5 μl of microsomes; lane 5, pET45-His6-PkPSDΔ34 + 1 μl of microsomes. b, PSD enzyme assay with the samples from the reactions was performed with Ptd[1′-14C]Ser as the substrate. Data are means ± S.E. for three experiments each performed in duplicate. Micros, microsomal.
Because several pyruvoyl enzymes have been reported to be stabilized via a Schiff base conjugate with their substrates and reaction products (34), we first tested whether inclusion of the substrate (PtdSer) for the reaction might be required to promote proenzyme processing. As detailed in Fig. 10a, the inclusion of increasing concentrations of DOPS in the TnT reaction, greatly enhanced the processing of the PkPSD enzyme to its mature form as shown by the reduction in the proenzyme content and increased appearance of the β subunit, detected by immunoblotting. The enhanced processing of the proenzyme was also accompanied by significant quantitative increases (3–4-fold) in the amount of detectable catalytic activity as shown in Fig. 10b.
FIGURE 10.
Endoproteolytic maturation of His6-PkPSΔD34 is enhanced by PtdSer. TnT reactions were conducted for 90 min at 30 °C in either the absence or presence of lipids. Upon the completion of the TnT reactions, aliquots were analyzed by Western blotting or PSD assay. a, Western blot analysis was conducted to detect the His6 epitope present in the nascent and mature proteins produced in the TnT reaction. Lane numbers correspond to the plasmid and lipid additions to the TnT reactions. Lane 1, pET45 empty vector; lane 2, pET45-His6-PkPSDΔ34 + no lipid; lane 3, pET45-His6-PkPSDΔ34 + 50 μg/ml DOPS and 50 μg/ml DOPC; lane 4, pET45-His6-PkPSDΔ34 + 100 μg/ml DOPS; lane 5, pET45-His6-PkPSΔD34 + 200 μg/ml DOPS. b, PSD assays were performed on replicate samples corresponding to the conditions in lanes 1–5 of a. Lane 1, empty vector; lane 2, no lipid; lane 3, DOPS 0.5× + DOPC 0.5×; lane 4, DOPS 1×; lane 5, DOPS 2×. Values are means ± S.D. for three experiments each performed in duplicate. c, Western blot analysis was conducted to detect the His6 epitope present in proteins appearing as a function of time following translational arrest with 0.2 mm cycloheximide at the end of a 60-min TnT reaction. Following the addition of cycloheximide, the samples were further incubated for the indicated times up to 80 min, either in the absence (−DOPS), or presence (+DOPS) of lipid. d, Western blot analysis was performed to examine the effects of liposome size upon PkPSD maturation. The TnT reaction was conducted as described for c using pET45-His6-PkPSD as the plasmid template and a 60-min reaction that was terminated with 0.2 mm cycloheximide. Following translational arrest, the maturation of the PkPSD was allowed to proceed for 40 min in the presence of either no added lipid (lane 1) or 200 μg/ml DOPS in the form of 50 nm vesicles (lane 2), or 200 nm vesicles (lane 3), 1000 nm vesicles (lane 4), or large multilamellar vesicles (lane 5).
Next, we examined if enzyme processing is a co-translational event or occurs in a time-dependent manner after translation. First, in vitro synthesis of PkPSD was conducted for 60 min in the absence of DOPS. Subsequently, the translation reactions were halted by addition of cycloheximide, and the reaction aliquots were incubated further to allow processing of PkPSD in the absence or presence of DOPS. The reaction aliquots were removed at increasing times of incubation after translation arrest and analyzed as shown in Fig. 10c. The data reveal that during the initial 60-min translation reaction, significant precursor PkPSDs were made, but with very low levels of processed β subunits. With increasing time after translational arrest, some mature forms were produced even in the absence of DOPS, indicating that PkPSD processing did not need to be co-translational. The lipid supplementation after translational arrest revealed that DOPS significantly enhanced processing of the newly synthesized proenzyme, which resulted in a time-dependent decline in the proenzyme content and an increase in the mature β subunit content.
The DOPS used to stimulate PkPSD processing was prepared as a sonicated suspension of unilamellar vesicles. We also examined whether the diameter of the vesicles affected the processing reaction by preparing liposomes of various sizes, using extrusion through polycarbonate filters. Liposomes of 50, 200, and 1000 nm diameter all produced similar levels of enzyme processing (Fig. 10d). In addition, large multilamellar liposomes also produced robust enhancement of PkPSD proenzyme processing.
Processing of PkPSD Is Down-regulated by the Anionic Lipids, DOPA, DOPG, and PI
We next investigated if regulation of PkPSD processing is PtdSer-specific. PtdSer is an anionic phospholipid, and we compared its activity with other anionic phospholipids, including DOPA, DOPG, and bovine liver PI. Comparisons were also made with the zwitterionic phospholipids, DOPE, and DOPC. Fig. 11a shows that supplementation of the in vitro processing reactions with DOPC or a DOPC/DOPE mixture had only a modest stimulatory effect on the process. In contrast, the anionic lipids, DOPA and DOPG, exerted a strong inhibitory effect upon maturation of PkPSD, which resulted in concomitant reduction of β subunit formation and catalytic activity. Strong inhibition of the processing of the proenzyme by DOPG and DOPA resulted in reduced PSD activities (90 and 85% reduction by DOPG and DOPA, respectively) as seen in Fig. 11b. DOPC and a DOPC/DOPE mixture show only mild increases in the catalytic activities consistent with their mild stimulatory effects on the processing of the proenzyme. Interestingly, although other lipids did not affect overall synthesis of the PkPSD proenzyme, PI strongly inhibited it, indicating disruption of either transcription or translation by the in vitro reaction.
FIGURE 11.
Anionic phospholipids inhibit maturation of PkPSD. The influence of multiple phospholipids upon the maturation of PkPSD was examined by Western blotting, and enzyme assay was performed in conjunction with TnT reactions that used pET45-His6-PkPSDΔ34 as a template. a, TnT reactions were performed for 90 min at 30 °C in the presence of empty vector, or plasmid harboring His6-PkPSDΔ34 in the absence of lipids (No lipid), or the presence of 100 μg/ml DOPC, or 50 μg/ml DOPC + 50 μg/ml DOPE, or 100 μg/ml DOPG, or 100 μg/ml DOPA. Following these incubations, aliquots of the reactions were analyzed by Western blotting. a also contains incubations that included soybean PI. b, same reactions were performed as shown in a with the corresponding plasmid and lipid additions indicated in the figure and then added to PSD assays, and the enzyme activity was quantified. c, TnT reaction using a pET45-His6-PkPSDΔ34 template was performed for 60 min and then arrested by the addition of 0.2 mm cycloheximide. Subsequently, the maturation reaction was conducted at 30 °C for an additional 40 min in either the absence of lipid or the presence of DOPA, DOPG, PI, DOPC, DOPE, or DOPS. Aliquots from the maturation reaction were examined by Western blotting.
To bypass the inhibitory effect on expression of precursor PkPSDs by PI, the reaction was conducted for 40 min in the absence of lipid, followed by translational arrest with cycloheximide and further incubation with the various lipids. The Western blot analysis showed that DOPE and DOPC did not alter the basal level of processing, but PI along with DOPA and DOPG strongly inhibited processing (Fig. 11c). Taken together, the data demonstrate that anionic lipids, other than PtdSer, have a strong inhibitory effect on the post-translational processing of the PkPSD precursor. These findings suggest that membrane lipid composition plays an important regulatory role in PSD processing.
Regulating Lipids Do Not Bind to PkPSD Precursors with High Affinity
Because there was strong activation/inhibition by anionic phospholipids of the processing of the PkPSD precursor, we investigated whether there was any stable physical association between lipids and the proenzyme. Using multilamellar liposomes, we tested if the precursor PkPSDs generated in the TnT reactions could be sedimented with the lipid by centrifugation. The data in Fig. 12 shows that there is no strong physical association between any of the lipids and the PkPSD precursor. These results suggest that both DOPS stimulation of proenzyme processing and DOPG, DOPA, and PI-mediated inhibition of proenzyme processing occur by low affinity interactions between the protein and lipids.
FIGURE 12.
Regulatory lipids do not bind to the His6-PkPSDΔ34 precursor with high affinity. The TnT reaction for in vitro expression of His6-PkPSDΔ34 was carried out for 40 min at 30 °C. Translation was arrested by addition of 0.2 mm cycloheximide, and maturation reactions were continued for an additional 40 min in either the absence (None) or presence of 100 μg/ml lipids (DOPS, DOPC, PI, and DOPG) as indicated. The lipids were in the form of multilamellar liposomes that could be sedimented by centrifugation. Following the maturation reaction, aliquots were shifted to 0 °C and either unprocessed (R) or centrifuged at 13 × 104 × g for 20 min to recover liposomes (P). Volume normalized aliquots of the total reaction and the liposome pellet were analyzed by Western blotting.
DISCUSSION
The successful cloning of the PkPSD cDNA using a library-based genetic complementation approach in yeast demonstrates the utility of this system for assigning function to numerous genes within the Plasmodium genus. We screened ∼130 P. knowlesi genome equivalents and identified 127 isolates of PkPSD. These data indicate that the library will likely cover very rare cDNAs expressed at very low levels. The bootstrapping approach of functional genetic screening with PkPSD libraries and cross-correlating to the P. falciparum genome effectively bypasses the problem of the A + T-rich genome of P. falciparum. We foresee this work as an important stepping stone toward large scale functional annotation of the P. falciparum genome. In addition, our approach has immediately yielded important new insights into the biochemistry of the parasite PSD, which have not otherwise been tractable with other eukaryotic PSD enzymes. More detailed understanding of the mechanism of PkPSD processing has the potential to reveal new and unsuspected vulnerabilities of the parasite to disruption of membrane biogenesis.
Sequence alignment between PkPSD and E. coli PSD reveals that the PkPSD sequence starting at position 56 shares homology with E. coli sequence at position 16 (Fig. 2). The sequence homology between PkPSD and ScPSD1 begins at position 67 of the parasite protein and position 138 of the yeast protein. The N-terminal 137 amino acids of yeast PSD1 contain mitochondrial targeting and inner membrane sorting sequences. The truncated yeast PSD1 with a 137-amino acid deletion was reported as a nonfunctional enzyme because the encoded construct failed to be processed to functional α and β fragments (33). Unlike PSDs from other organisms, including E. coli, yeast, mammals, and plants, that contain a strong hydrophobic trans-membrane domain in the C terminus of their β fragments, there is no trans-membrane domain in the corresponding region of PkPSD. Instead, PkPSD contains a predicted transmembrane domain at the N terminus at positions 18–34. When the PkPSD cDNA was expressed in yeast, nearly half of the enzyme activity was recovered in the soluble fraction. Western blot analysis of epitope-tagged versions of the full-length proenzyme did not reveal any discernible size differences between the soluble and membrane-associated proteins, suggesting that the protein was amphitropic. The amphitropic properties of the enzyme are likely to allow access to its substrate in multiple membranes, including the outer mitochondrial membrane. Decarboxylation of PtdSer at the outer mitochondrial membrane is the likely reason that expression of PkPSD in yeast enables strain growth under respiratory conditions. The factors that dictate membrane association remain unidentified.
Truncated versions of the PkPSD lacking the N terminus and predicted transmembrane domain (amino acids 2–34) were expressed in yeast, and the PkPSDΔ34 version resulted in a highly active form of the enzyme. Epitope-tagged versions of PkPSD also resulted in catalytically active forms of the enzyme in both bacteria and yeast. Epitope-tagged forms of PkPSDΔ34 provided a convenient variant of the enzyme for examination of processing events in vitro, and the His6-PkPSDΔ34 cDNA was transcribed and translated with a TnT system that contains reticulocyte lysate components for protein synthesis. In the absence of lipid supplementation, a modest level of the nascent enzyme is processed to the mature form. Inclusion of DOPS in the transcription-translation reaction increases the processing of proenzyme 3–4-fold, as evidenced by the diminution in the amount of unprocessed form, and a concomitant increase in the amount of mature β subunit. The increased processing observed on Western blotting correlates with definitive quantitative increases in catalytic activity of the enzyme detected in PSD assays. The action of DOPS appears specific, insofar as DOPE and DOPC have little or no effect upon enzyme maturation, whereas DOPG, DOPA, and PI are inhibitory. The observed low level of maturation of the PkPSD in the absence of lipid addition may be a consequence of endogenous levels of phosphatidylserine present in the reticulocyte lysate.
The application of the TnT system also enabled us to determine that the P. knowlesi PSD undergoes processing after translation is complete. This timing may be of important regulatory value insofar as it provides the nascent proenzyme with a time window for sampling membrane content of PtdSer and other anionic phospholipids. Membrane environments rich in PtdSer will promote processing, whereas those with high content of other anionic phospholipids will inhibit processing. A schematic summary of lipid modulation of PkPSD proenzyme processing appears in Fig. 13.
FIGURE 13.
Anionic phospholipid regulation of the maturation of PkPSD. PkPSD is synthesized as a proenzyme that undergoes auto-endoproteolytic processing to form a mature enzyme containing a large β subunit and a small α subunit with an N-terminal pyruvoyl prosthetic group. An engineered form of PkPSD lacking the N-terminal transmembrane domain and containing a His6 epitope tag undergoes maturation in vitro. The maturation reaction is a post-translational event that is enhanced by the presence of the substrate for the enzyme (DOPS) and inhibited by other anionic phospholipids PI, DOPG, and DOPA. These findings suggest that intracellular substrate abundance and local membrane phospholipid composition play an important role in regulating the quantity of active PSD within the cell.
The maturation of PSD enzymes broadly appears to take place in three steps (35). In the first step, the hydroxyl group of a critical serine, which is destined to form the active site pyruvoyl group, attacks the proximal (N-terminally located) peptide bond, effecting serinolytic cleavage that results in the β subunit being attached to the α subunit by an ester bond. In the second step, the β subunit is liberated by an elimination reaction, which also yields an α subunit harboring an N-terminal dehydroalanine. In the third step, the reactive dehydroalanine undergoes water addition and ammonia elimination to form the pyruvoyl prosthetic group on the α subunit. The mechanism of action of DOPS in promoting proenzyme processing is not yet known, but two possibilities seem likely. One mechanism could involve DOPS forming a Schiff base with the newly formed carbonyl moiety of the α subunit immediately as it is formed, which would function to shift the equilibrium of the processing reaction in the direction of mature enzyme. A second mechanism might involve DOPS binding to the β subunit as an allosteric enhancer of the auto-endoproteolytic reaction. The availability of soluble versions of PkPSD should enable testing of these two mechanisms in the future.
Acknowledgment
We thank Dr. Manoj Duraisingh for providing P. knowlesi RNA for library construction.
This work was supported, in whole or in part, by National Institutes of Health Grant 5R37 GM32543 (to D. R. V.). This work was also supported by the Burroughs Welcome Fund (to C. B. M.).
- PtdEtn
- phosphatidylethanolamine
- Etn
- ethanolamine
- Pf
- P. falciparum
- Pk
- P. knowlesi
- Cho
- choline
- PtdSer
- phosphatidylserine
- PtdCho
- phosphatidylcholine
- ER
- endoplasmic reticulum
- PSD
- PtdSer decarboxylase
- CDP
- cytidine diphosphate
- DOPS
- dioleoyl phosphatidylserine
- DOPC
- dioleoyl phosphatidylcholine
- DOPG
- dioleoyl phosphatidylglycerol
- DOPA
- dioleoyl phosphatidic acid
- DOPE
- dioleoyl phosphatidylethanolamine
- PI
- phosphatidylinositol.
REFERENCES
- 1. Rosenthal P. J. (2003) J. Exp. Biol. 206, 3735–3744 [DOI] [PubMed] [Google Scholar]
- 2. Pessi G., Mamoun C. B. (2006) Future Lipidol. 1, 173–180 [Google Scholar]
- 3. Bobenchik A. M., Choi J. Y., Mishra A., Rujan I. N., Hao B., Voelker D. R., Hoch J. C., Mamoun C. B. (2010) BMC Biochem. 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vial H. J., Ancelin M. L. (1998) in Malaria: Parasite Biology, Pathogenesis, and Protection (Sherman I., ed) pp. 159–175, American Society for Microbiology, Washington, D. C [Google Scholar]
- 5. Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. (1985) Annu. Rev. Biophys. Biophys. Chem. 14, 211–238 [DOI] [PubMed] [Google Scholar]
- 6. Cullis P. R., de Kruijff B. (1978) Biochim. Biophys. Acta 513, 31–42 [DOI] [PubMed] [Google Scholar]
- 7. Elabbadi N., Ancelin M. L., Vial H. J. (1997) Biochem. J. 324, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elabbadi N., Ancelin M. L., Vial H. J. (1992) Antimicrob. Agents Chemother. 36, 50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rontein D., Nishida I., Tashiro G., Yoshioka K., Wu W. I., Voelker D. R., Basset G., Hanson A. D. (2001) J. Biol. Chem. 276, 35523–35529 [DOI] [PubMed] [Google Scholar]
- 10. Baunaure F., Eldin P., Cathiard A. M., Vial H. (2004) Mol. Microbiol. 51, 33–46 [DOI] [PubMed] [Google Scholar]
- 11. Achleitner G., Zweytick D., Trotter P. J., Voelker D. R., Daum G. (1995) J. Biol. Chem. 270, 29836–29842 [DOI] [PubMed] [Google Scholar]
- 12. Achleitner G., Gaigg B., Krasser A., Kainersdorfer E., Kohlwein S. D., Perktold A., Zellnig G., Daum G. (1999) Eur. J. Biochem. 264, 545–553 [DOI] [PubMed] [Google Scholar]
- 13. Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnóczky G. (2006) J. Cell Biol. 174, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trotter P. J., Pedretti J., Voelker D. R. (1993) J. Biol. Chem. 268, 21416–21424 [PubMed] [Google Scholar]
- 15. Wu W. I., Voelker D. R. (2001) J. Biol. Chem. 276, 7114–7121 [DOI] [PubMed] [Google Scholar]
- 16. Reynolds J. M., Takebe S., Choi J. Y., El Bissati K., Witola W. H., Bobenchik A. M., Hoch J. C., Voelker D. R., Mamoun C. B. (2008) J. Biol. Chem. 283, 7894–7900 [DOI] [PubMed] [Google Scholar]
- 17. Pessi G., Choi J. Y., Reynolds J. M., Voelker D. R., Mamoun C. B. (2005) J. Biol. Chem. 280, 12461–12466 [DOI] [PubMed] [Google Scholar]
- 18. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pain A., Böhme U., Berry A. E., Mungall K., Finn R. D., Jackson A. P., Mourier T., Mistry J., Pasini E. M., Aslett M. A., Balasubrammaniam S., Borgwardt K., Brooks K., Carret C., Carver T. J., Cherevach I., Chillingworth T., Clark T. G., Galinski M. R., Hall N., Harper D., Harris D., Hauser H., Ivens A., Janssen C. S., Keane T., Larke N., Lapp S., Marti M., Moule S., Meyer I. M., Ormond D., Peters N., Sanders M., Sanders S., Sargeant T. J., Simmonds M., Smith F., Squares R., Thurston S., Tivey A. R., Walker D., White B., Zuiderwijk E., Churcher C., Quail M. A., Cowman A. F., Turner C. M., Rajandream M. A., Kocken C. H., Thomas A. W., Newbold C. I., Barrell B. G., Berriman M. (2008) Nature 455, 799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller C. A., 3rd, Martinat M. A., Hyman L. E. (1998) Nucleic Acids Res. 26, 3577–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Storey M. K., Clay K. L., Kutateladze T., Murphy R. C., Overduin M., Voelker D. R. (2001) J. Biol. Chem. 276, 48539–48548 [DOI] [PubMed] [Google Scholar]
- 22. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (eds) (1993) Current Protocols in Molecular Biology, John Wiley & Sons, Inc., New York [Google Scholar]
- 23. Trotter P. J., Voelker D. R. (1995) J. Biol. Chem. 270, 6062–6070 [DOI] [PubMed] [Google Scholar]
- 24. Schumacher M. M., Choi J. Y., Voelker D. R. (2002) J. Biol. Chem. 277, 51033–51042 [DOI] [PubMed] [Google Scholar]
- 25. Trotter P. J., Wu W. I., Pedretti J., Yates R., Voelker D. R. (1998) J. Biol. Chem. 273, 13189–13196 [DOI] [PubMed] [Google Scholar]
- 26. Choi J. Y., Martin W. E., Murphy R. C., Voelker D. R. (2004) J. Biol. Chem. 279, 42321–42330 [DOI] [PubMed] [Google Scholar]
- 27. Rouser G., Siakotos A. N., Fleischer S. (1966) Lipids 1, 85–86 [DOI] [PubMed] [Google Scholar]
- 28. Dowhan W., Li Q. X. (1992) Methods Enzymol. 209, 348–359 [DOI] [PubMed] [Google Scholar]
- 29. Li Q. X., Dowhan W. (1988) J. Biol. Chem. 263, 11516–11522 [PubMed] [Google Scholar]
- 30. Voelker D. R., Golden E. B. (1992) Methods Enzymol. 209, 360–365 [DOI] [PubMed] [Google Scholar]
- 31. Rontein D., Wu W. I., Voelker D. R., Hanson A. D. (2003) Plant Physiol. 132, 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dowhan W., Wickner W. T., Kennedy E. P. (1974) J. Biol. Chem. 249, 3079–3084 [PubMed] [Google Scholar]
- 33. Gulshan K., Schmidt J. A., Shahi P., Moye-Rowley W. S. (2008) Mol. Cell. Biol. 28, 5851–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Poelje P. D., Snell E. E. (1990) Annu. Rev. Biochem. 59, 29–59 [DOI] [PubMed] [Google Scholar]
- 35. Voelker D. R. (1997) Biochim. Biophys. Acta 1348, 236–244 [DOI] [PubMed] [Google Scholar]