Background: Little is known about GlcCer catabolism in fungi because glucocerebrosidase has yet to be characterized.
Results: EGCrP1 specifically hydrolyzes GlcCer, and immature GlcCer accumulates in EGCrP1-deficient Cryptococcus neoformans.
Conclusion: EGCrP1 eliminates immature GlcCer to control the quality of GlcCer.
Significance: The finding of EGCrP1, the first glucocerebrosidase identified in fungi, provides insight into the quality control of fungus-specific GlcCer.
Keywords: Fungi, Glycolipids, Glycosidases, Lipid Metabolism, Sphingolipid, Cryptococcus neoformans, Endoglycoceramidase, Glucocerebrosidase, Glucosylceramide, Polysaccharide Capsule
Abstract
A fungus-specific glucosylceramide (GlcCer), which contains a unique sphingoid base possessing two double bonds and a methyl substitution, is essential for pathogenicity in fungi. Although the biosynthetic pathway of the GlcCer has been well elucidated, little is known about GlcCer catabolism because a GlcCer-degrading enzyme (glucocerebrosidase) has yet to be identified in fungi. We found a homologue of endoglycoceramidase tentatively designated endoglycoceramidase-related protein 1 (EGCrP1) in several fungal genomic databases. The recombinant EGCrP1 hydrolyzed GlcCer but not other glycosphingolipids, whereas endoglycoceramidase hydrolyzed oligosaccharide-linked glycosphingolipids but not GlcCer. Disruption of egcrp1 in Cryptococcus neoformans, a typical pathogenic fungus causing cryptococcosis, resulted in the accumulation of fungus-specific GlcCer and immature GlcCer that possess sphingoid bases without a methyl substitution concomitant with a dysfunction of polysaccharide capsule formation. These results indicated that EGCrP1 participates in the catabolism of GlcCer and especially functions to eliminate immature GlcCer in vivo that are generated as by-products due to the broad specificity of GlcCer synthase. We conclude that EGCrP1, a glucocerebrosidase identified for the first time in fungi, controls the quality of GlcCer by eliminating immature GlcCer incorrectly generated in C. neoformans, leading to accurate processing of fungus-specific GlcCer.
Introduction
Opportunistic fungi such as Cryptococcus neoformans, Aspergillus fumigatus, Rhizopus oryzae, and Candida albicans have attracted attention because of the increased numbers of patients with immunodeficiencies such as AIDS and in some cases cancer and diabetes (1). Very recently, highly virulent strains of Cryptococcus gattii that threaten immunocompetent people and animals have been found in the United States and Canada (2). These fungi synthesize a fungus-specific glucosylceramide (GlcCer)3 composed of a β-linked glucose and a ceramide possessing a characteristic sphingoid base that has two double bonds at C4/C8 in the trans conformation and a methyl substitution at C9 (3). Over the past decade, extensive studies have identified the enzymes involved in the biosynthesis of fungus-specific GlcCer. Dihydrosphingosine is acylated by ceramide synthase to form dihydroceramide (4), which is then modified by various enzymes, including fatty acid 2-hydroxylase (5), sphingolipid Δ4-desaturase (6), sphingolipid Δ8-desaturase (7), and sphingolipid 9-methyltransferase (8), generating a mature form of ceramide composed of a methyl d18:2 sphingoid base and an amide-linked α-hydroxy 16:0/18:0 fatty acid (see Fig. 7). The genes encoding Δ8-desaturase and sphingolipid 9-methyltransferase were identified in fungi but not mammals. Disruption of these enzymes resulted in the lack of fungal mature GlcCer and the significantly decreased virulence of C. albicans (9), indicating that the maturation process of ceramide in GlcCer is essential for virulence. In addition to its pathogenicity, mature GlcCer is related to various biological events such as budding and cell division (10–12), alkali tolerance (13, 14), hyphal elongation (15), and plant defensin sensitivity (16). Immature GlcCer possessing a non-methylated d18:1 or d18:2 sphingoid base could not compensate for the functions of mature GlcCer (7, 9, 15).
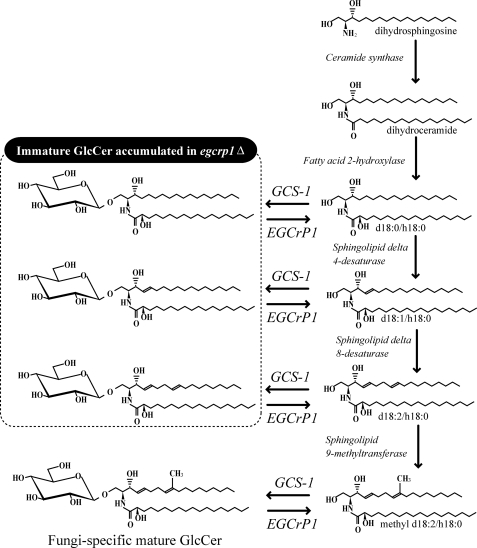
FIGURE 7.
Putative metabolic map of GlcCer synthesis in fungi. EGCrP1 controls the quality of GlcCer during production by converting the immature GlcCer to an immature ceramide, which follows the maturation pathway of ceramide. GlcCer surrounded by a dotted line shows the immature GlcCer found in egcrp1Δ.
As in mammals and plants, GlcCer is synthesized by the transfer of glucose from UDP-glucose to ceramide by glucosylceramide synthase-1 (GCS-1; also known as ceramide glucosyltransferase, EC 2.4.1.80) in fungi. GCS-1 adopts not only a mature ceramide but also an immature ceramide as a substrate (17); therefore, immature GlcCer could also be generated in vivo. Importantly, once immature ceramide is converted to GlcCer by GCS-1, the maturation step is stopped, and immature GlcCer accumulates because modification enzymes such as sphingolipid Δ4-desaturase and 9-methyltransferase utilize a ceramide, but not GlcCer, as an acceptor substrate (8, 18); however, GlcCer in pathogenic fungi exclusively consists of a mature form under normal conditions (9, 11–13, 19). The molecular mechanism underlying the elimination of immature GlcCer in vivo remains to be uncovered.
GlcCer is degraded by the detachment of glucose from GlcCer by glucocerebrosidase (GCase; also known as glucosylceramidase (EC 3.2.1.45)). Although knowledge about the synthesis of fungus-specific GlcCer has been accumulating, little is known about the catabolism of GlcCer because the gene encoding GCase has yet to be identified in fungi.
Endoglycoceramidase (EGCase) is an enzyme capable of cleaving the ceramide-glucosidic linkage of various glycosphingolipids (GSLs) to release an intact oligosaccharide and ceramide (20, 21). In the present study, a homologue of EGCase was found in C. neoformans, A. fumigatus, R. oryzae, and many other fungal genomic databases. Although GlcCer is resistant to hydrolysis by EGCase (20), we found that recombinant proteins of a homologue (tentatively designated EGCase-related protein 1 (EGCrP1)) from C. neoformans, A. fumigatus, and R. oryzae hydrolyzed GlcCer but not other GSLs; i.e. EGCrP1 was identified to be a novel GCase of fungi. Furthermore, an egcrp1-deficient mutant (egcrp1Δ) of C. neoformans exhibited not only the accumulation of fungus-specific GlcCer but also immature forms that are incorrectly synthesized in C. neoformans concomitantly with a decrease of GCase activity at neutral pH in the membranous fraction. Interestingly, disruption of egcrp1 in C. neoformans resulted in aberrant formation of the polysaccharide capsule, which is known to be required for virulence in C. neoformans (22). These results indicate that EGCrP1 is involved in the catabolism of GlcCer in fungi and especially eliminates the immature GlcCer generated as by-products due to the broad specificity of GCS-1 toward ceramide species.
This study provides mechanical insights into the quality control of GlcCer by EGCrP1 by which immature and less functional GlcCer is eliminated from the pathogenic fungi. This study uncovers a missing link in GlcCer metabolism in fungi through the discovery of a novel enzyme, EGCrP1 and should facilitate the development of drugs for pathogenic fungi based on the catabolism of fungus-specific GlcCer.
EXPERIMENTAL PROCEDURES
Materials
C6-7-nitro-2,1,3-benzoxadiazole (NBD)-ceramide, C6-NBD-GlcCer, and C12-NBD-Gb3Cer were purchased from Matreya, and C6-NBD-LacCer, C6-NBD-GalCer, and para-nitrophenol glycosides were from Sigma-Aldrich. C12-NBD-GM1, C12-NBD-sphingomyelin, C6:0-GlcCer, C16:0-GlcCer, C18:0-GlcCer, C22:0-GlcCer, C24:0-GlcCer, and α-hydroxy 24:0-GlcCer were prepared using sphingolipid ceramide N-deacylase by the method described (23). C6-NBD-trigalactosylceramide was prepared as described (24). The fungal GlcCer was extracted from R. oryzae cells with chloroform/methanol (2:1, v/v). The extract was partitioned using the Folch method, and the organic phase was applied to a Sep-Pak silica cartridge (Waters) previously equilibrated with chloroform/methanol (95:5, v/v). GlcCer was eluted from the cartridge with chloroform/methanol (85:15, v/v). GlcCer was identified by TLC plate (Merck) with orcinol-H2SO4 staining, and MALDI-TOF MS using 2,5-dihydroxybenzoic acid as a matrix. The sugar composition of GlcCer was determined by gas-liquid chromatography with a Shimadzu HiCap-CBP1 column (0.22 mm × 25 m) as a trimethylsilyl derivative.
Sequence Analysis
The phylogenic tree and alignment of EGCrP1 and EGCase were conducted with ClustalX (25). The phylogenic tree of full-length amino acid sequences of EGCrP1 was constructed by the neighbor-joining method (26) and visualized by NJplot. The alignment was shaded in ESPript 2.2 (27).
Fungi
C. neoformans var. grubii serotype A strain H99 (ATCC208821) was purchased from American Type Culture Collection, and R. oryzae (NBRC9364) and A. fumigatus (NBRC33022) were from the National Institute of Technology and Evaluation Biological Resource Center, Chiba, Japan. C. neoformans, A. fumigatus, and R. oryzae were cultured at 30 °C in YPD medium (2% Glc, 2% peptone, 1% yeast extract), PD medium (0.4% potato extract, 2% dextrose), and YPG medium (0.5% yeast extract, 0.5% peptone, 0.5% NaCl, 1% Glc), respectively.
Construction of Expression Vectors
Total RNA and mRNA were obtained from 1 g of fungus cells using Sepasol-RNA I (Nacalai Tesque) and a QuickPrep Micro mRNA purification kit (GE healthcare) according to the instructions of the manufacturers. First strand cDNA was synthesized from 1 μg of mRNA using an avian myeloblastosis virus reverse transcriptase first-strand cDNA synthesis kit (Roche Diagnostic). To introduce the restriction site, PCR was carried out using the first strand cDNA as a template and expression primers listed in supplemental Table S1. The amplification was conducted for 25 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 10 s, and extension at 72 °C for 150 s using PrimeSTAR HS DNA polymerase (Takara Bio Inc.). The amplified product was digested with appropriate restriction enzymes and inserted into the corresponding sites of pGEX4T3 (GE Healthcare) and pCold-TF DNA (Takara Bio Inc.) to express the GST-fused EGCrP1 and the trigger factor-tagged EGCrP1, respectively. The mutants of EGCrP1 (E254Q and E483Q) were generated with the PrimeSTAR mutagenesis basal kit (Takara Bio Inc.) using mutation primers listed in supplemental Table S1 (E254Q-S, E254Q-A, E483Q-S, and E483Q-A) according to the instructions provided by the manufacturer.
Expression of Recombinant EGCrP1
EGCrP1 of R. oryzae (RO3G_04172), C. neoformans (CNAG_00623), and A. fumigatus (AFUA_3G08820) were expressed in Escherichia coli BL21(DE3) transformed with a pCold-TF vector containing egcrp1 (DDBJ accession numbers AB669191 and AB669901). After incubating at 37 °C with shaking in medium A (Luria-Bertani medium supplemented with 100 μg/ml ampicillin) until the A600 reached ∼0.5, the culture medium was kept at 15 °C for 30 min. Then isopropyl β-d-thiogalactopyranoside was added to the culture at a final concentration of 1 mm to cause transcription. After being kept at 15 °C for 24 h, the cells were harvested by centrifugation (8,000 × g for 10 min), washed with PBS, suspended in 20 mm sodium phosphate buffer, pH 7.3, and kept in a sonic bath for 150 s. Cell debris was removed by centrifugation (25,000 × g for 30 min at 4 °C), and the supernatant was subjected to the enzyme assay.
Purification of Recombinant EGCrP1
E. coli BL21(DE3) cells transformed with the expression vector pGEX4T3 containing an intact or mutated egcrp1 were grown at 37 °C for 24 h in 200 ml of medium A with shaking. The cells were harvested by centrifugation (7,500 × g for 10 min) and suspended in PBS. After sonication for 150 s, cell debris was removed by centrifugation (25,000 × g for 30 min). The supernatant was applied to a GSTrap HP column (1 ml; GE Healthcare) that was equilibrated with PBS. Then the column was washed with PBS, and the recombinant enzyme was eluted with Tris-HCl buffer, pH 8.0 containing 10 mm reduced glutathione. The purified enzyme was dialyzed against 25 mm sodium phosphate buffer, pH 7.3.
Protein Assay
Protein content was determined by the bicinchoninic acid protein assay (Pierce) with bovine serum albumin as a standard. SDS-PAGE was carried out according to the method of Laemmli (28). The proteins were stained with Coomassie Brilliant Blue.
Enzyme Assay
An aliquot of 100 pmol of NBD-labeled GSL or 2.5 nmol of fungal GlcCer was incubated at 30 °C for an appropriate period with a suitable amount of enzyme in 20 μl of 50 mm sodium phosphate buffer, pH 7.3 containing 10% DMSO. The reaction mixture was then dried using a SpeedVac concentrator, dissolved in 10 μl of MeOH, and centrifuged (10,000 × g for 5 min), and the supernatant was applied to a TLC plate developed with chloroform/methanol/water (65:25:4, v/v/v). The plate was scanned in a Shimadzu CS-9300 chromatoscanner with the fluorescence mode set at 475-nm excitation for NBD-labeled GSLs. Unlabeled GlcCer and glucose were visualized by spraying the TLC plate with orcinol-H2SO4 reagent and scanned in the Shimadzu CS-9300 chromatoscanner with the reflection mode set at 540 nm. The extent of hydrolysis of NBD-labeled GSLs was calculated as follows: hydrolysis (%) = (peak area for NBD-ceramide) × 100/(peak area for NBD-ceramide + peak area for remaining NBD-GSLs). Alternatively, the extent of hydrolysis of unlabeled GlcCer was calculated as follows: hydrolysis (%) = (peak area for Glc) × 100/(peak area for Glc + peak area for remaining GlcCer).
Characterization of Recombinant EGCrP1
The pH dependence of EGCrP1 was determined in a pH range of 4–9 using the following buffers (at a final concentration of 50 mm): sodium acetate buffer (pH 4–5.5), MES (pH 6–7.5), sodium phosphate buffer (pH 7–8), HEPES (pH 8–9), Tris-HCl buffer (pH 8.0–8.5), and boric acid buffer (pH 8–9). The optimal temperature was determined in the range from 16 to 40 °C. The effect of organic solvents was examined using DMSO at a concentration of 0–50%. The effects of detergents were examined using Triton X-100 and sodium cholate at a concentration of 0–1.0%. The effects of metal ions were examined by adding Ca2+, Co2+, Cu2+, Fe2+, Fe3+, Hg2+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Zn2+, and EDTA to the reaction mixture at 1 mm. Kinetic constants of EGCrP1 were measured by using C6-NBD-GlcCer as a substrate at concentrations ranging from 1.5 to 50 μm.
Disruption of egcrp1 of C. neoformans with Nourseothricin Acetyltransferase (NAT) Split Marker
The EGCrP1 gene of C. neoformans (locus number CNAG_00623 in C. neoformans var. grubii serotype A genome database) was deleted with a NAT split marker (29). A gene-specific disruption cassette, which contains 350 bp of the 5′- and 3′-flanking regions of egcrp1, an 860-bp fragment of the promoter sequence and the ATG start codon of the C. neoformans actin gene (30), a 310-bp fragment of the terminator sequence and the stop codon of C. neoformans TRP1 (31), and a gene for the selectable marker NAT (32), was designed as shown in supplemental Fig. S1A. The DNA fragments were amplified in the first round of PCR using primers CN00623N-U and CN00623N-AP-D for the 5′-flanking region, CN00623C-U and CN00623C-D for the 3′-flanking region, ActinP-U and Act-Nat-Down for the actin promoter, Ttrp-Up and Ttrp-CN00623C-D for the TRP1 terminator with genomic DNA as a template, and Nat-Up and Nat-Ttrp-Down for NAT with pYL16 (WERNER BioAgents) as a template. The C. neoformans genomic DNA was prepared using ISOPLANT II (Nippon Gene). The sequences of these primers are listed in supplemental Table S1. The length of the flanking region of egcrp1 was determined according to a previous study (33). PCR products were analyzed in a 1% agarose gel, then extracted from the gel, and used as a template in overlap PCR to combine DNA fragments. All PCR amplifications were performed using PrimeSTAR GXL DNA polymerase (Takara Bio Inc.). Finally, the combined overlap PCR product was inserted into T-vector pMD20 to construct pΔegcrp1 after adding adenine overhang using the Mighty TA cloning kit for PrimeSTAR (Takara Bio Inc.). A NAT split marker containing the 200-bp overlapping sequence was PCR-amplified with primers CN00623N-U and NSL-2 (29) for the 5′-region of NAT and primers NSR-2 (29) and CN00623-D for the 3′-region of NAT (supplemental Fig. S1A) using pΔegcrp1 as a template. The two PCR fragments were extracted from the agarose gel, then precipitated onto 600 μg of gold microcarrier beads (0.6 μm; Bio-Rad), and introduced into C. neoformans H99 by biolistic transformation as described previously (34) using a Model PDS-1000/He Biolistic particle delivery system (Bio-Rad). Stable transformants were selected on YPD medium containing 100 μg/ml nourseothricin (WERNER BioAgents). Putative mutant strains were screened by PCR using expression primers neoEGCrP1KpnI-S and neoEGCrP1XbaI-A, and the results were confirmed by Southern blot analysis using genomic DNA digested with HindIII.
Fractionation of C. neoformans Cells
Wild type and egcrp1 deletion mutants (egcrp1Δ) of C. neoformans were incubated for 2 days at 30 °C in YPD medium with shaking, and the cells were collected by centrifugation (2,300 × g for 5 min). The fungal cells were washed with buffer A (50 mm sodium phosphate buffer, pH 7.3 containing protease inhibitor mixture (Roche Diagnostics)), resuspended with buffer A, and homogenized by using glass beads to prepare cell lysate (35). After centrifugation at 1,000 × g for 5 min, the precipitate was washed three times with buffer A and used as the cell wall fraction (36). The supernatant was centrifuged at 100,000 × g for 60 min, and the precipitate was resuspended with buffer A and used as the membranous fraction. The supernatant after ultracentrifugation was used as the cytosolic fraction. The culture supernatant was saturated with 70% ammonium sulfate and left overnight at 4 °C. The precipitate was collected with centrifugation at 17,000 × g for 30 min, dissolved in 50 mm sodium phosphate buffer, pH 7.3, and used as the supernatant fraction. Ten micrograms of protein of each fraction was used to measure the enzymatic activity at neutral pH, and 2 μg of the membranous fraction was used at low pH.
HPLC-based Quantitative Determination of GlcCer
Lipids were extracted from C. neoformans by the method of Bligh and Dyer (37). The GlcCer-containing lower phase was collected and dried using a SpeedVac concentrator. Five micrograms of each lipid sample was subjected to the HPLC-based quantitative determination of GlcCer after derivatization with o-phthalaldehyde (OPA) (38). The OPA derivatives were analyzed by HPLC using a normal phase column (Inertsil SIL 150A-5, 4.6 × 250 mm; GL Science) with an isocratic mobile phase consisting of n-hexane/isopropyl alchol/H2O (76:24:0.5, v/v/v) at a flow rate of 2.0 ml/min and detected by a fluorescence detector set to excitation and emission wavelengths of 340 and 455 nm, respectively. The amount of GlcCer in the samples was determined with a standard curve of OPA-labeled lyso-GlcCer. The hydrolysis of GlcCer by EGCrP1 measured with HPLC was calculated as follows: hydrolysis (%) = (peak area for control − peak area for EGCrP1 treatment) × 100/(peak area for control).
Structural Analysis of GlcCer
GlcCer was isolated from C. neoformans as described (13). Briefly, total lipid was dissolved in chloroform/methanol (95:5, v/v) and loaded onto a Sep-Pak silica cartridge (Waters) equilibrated with chloroform/methanol (95:5, v/v). The GlcCer-containing fraction was eluted with chloroform/methanol (90:10, v/v) and dried. Pellets were suspended in chloroform/methanol (50:50, v/v) containing 0.2 n KOH, incubated for 1 h at room temperature, and neutralized with an equal amount of acetic acid, and H2O was added. The lower phase was withdrawn and dried, then resuspended in chloroform/acetic acid (99:1, v/v), and applied to a Sep-Pak silica cartridge equilibrated with chloroform. The column was eluted with chloroform/acetic acid (99:1, v/v), chloroform/methanol (95:5, v/v), chloroform/methanol (90:10, v/v), chloroform/methanol (80:20, v/v), and finally methanol. The fractions were examined by TLC. The structure of GlcCer in the chloroform/methanol (90:10, v/v) fraction was analyzed by chip-based nanoelectrospray ionization MS/MS using a 4000Q TRAP (AB SCIEX) with a TriVersa NanoMate ionization source (Advion BioSystems) (39). The ion spray voltage, gas pressure, and flow rate were set at 1.25 kV, 0.3 p.s.i., and 200 nl/min, respectively. The mobile phase was chloroform/methanol (1:2, v/v) containing 5 mm ammonium formate. Each fraction of GlcCer was directly subjected to MS/MS with individual GlcCer molecular species ([M + Na]+) operated in the positive ion mode with a scan speed of 1,000 atomic mass units/s, scan range of m/z 200–1000, trap fill time of 5 ms, declustering potential of 100 V, collision energy of 70–75 eV, and resolutions of Q1/Q3.
Phenotypic Analysis of Wild Type and egcrp1Δ Mutant
Capsule production was measured by the method described (40). Briefly, C. neoformans cells were grown in Sabouraud medium overnight at 30 °C, and then the cultures were diluted to 1:100 in either Sabouraud medium (non-inducing conditions) or 10% Sabouraud medium buffered to pH 7.3 with 50 mm MOPS (capsule-inducing conditions) and grown at 30 °C for 2 days. India ink was added, and the capsules were visualized using light microscopy. To quantify capsule size, the diameters of the cell and capsule were measured with ImageJ 1.39u software (National Institutes of Health). The scale bar was estimated by the hematocytometer. To address the stress resistance, each strain was grown overnight, serially diluted in distilled water, and spotted onto YP (1% yeast extract, 2% peptone) or YPD medium containing 5 mm H2O2, 1 m NaCl, or 0.01% SDS. The pH of the medium was adjusted to 7.3 or 4.0.
RESULTS
Characterization of Recombinant EGCrP1
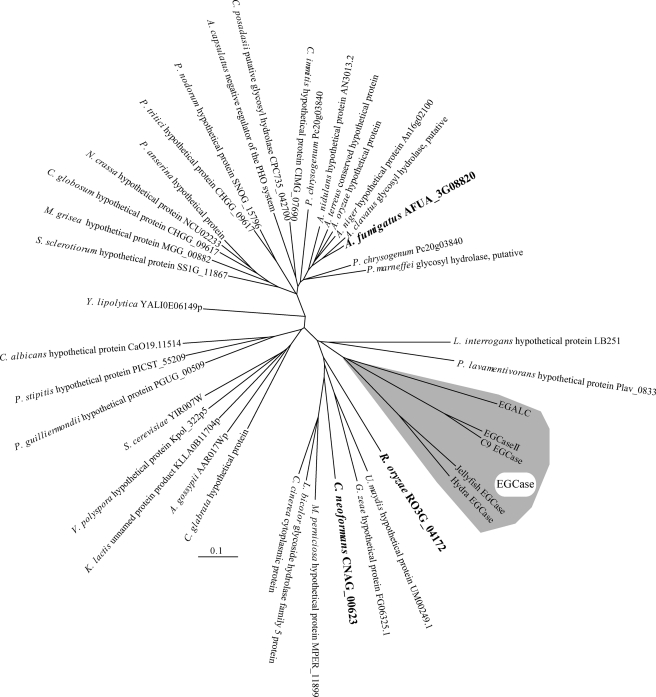
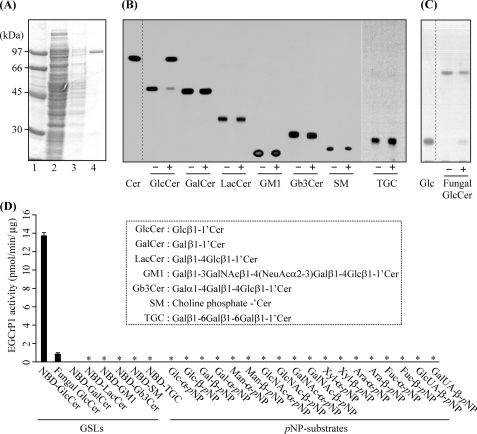
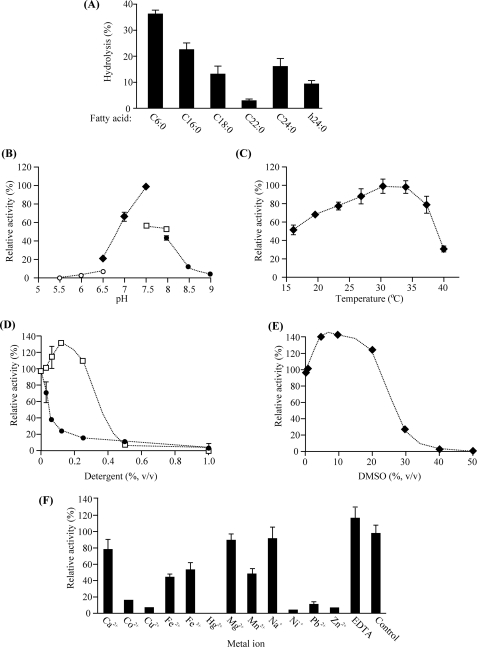
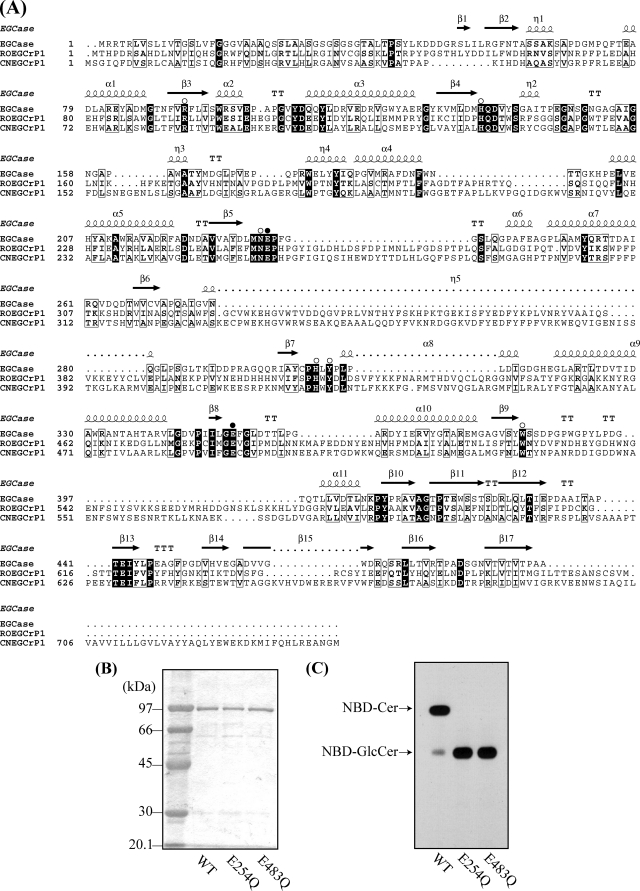
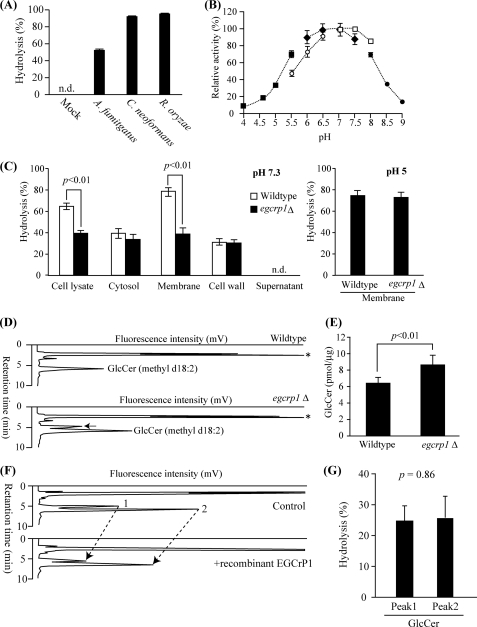
EGCase, an enzyme capable of cleaving the glucosidic linkage between an oligosaccharide and the ceramide of various GSLs, is distributed in bacteria and some invertebrates such as jellyfish and hydra (Fig. 1). We found that an EGCase orthologue (EGCrP1) is distributed across the phyla/genera of fungi, including in pathogens such as R. oryzae, C. neoformans, C. albicans, and A. fumigatus (Fig. 1). To characterize the EGCrP1, egcrp1 of R. oryzae was expressed in E. coli BL21(DE3) as a GST-fused protein. The recombinant EGCrP1 was purified as a single band on SDS-PAGE after elution from a GST affinity column (Fig. 2A). The specificity of the purified EGCrP1 was examined using various NBD-labeled fluorescent GSLs. Unexpectedly, EGCrP1 was not able to hydrolyze LacCer, GM1, and Gb3Cer, which are substrates for EGCase (Fig. 2B). Trigalactosylceramide and GalCer were also resistant to hydrolysis by EGCrP1 (Fig. 2B), although these substrates are susceptible to hydrolysis by endogalactosylceramidase, which is a paralogue of EGCase (41). Alternatively, EGCrP1 specifically hydrolyzed GlcCer (Fig. 2B) in contrast to EGCase and endogalactosylceramidase (20, 41). It was confirmed that not only NBD-labeled GlcCer but also native GlcCer isolated from R. oryzae was hydrolyzed by EGCrP1 (Fig. 2C). The ceramide moiety of R. oryzae GlcCer is composed of a methyl d18:2 sphingoid base and an amide-linked α-hydroxy 16:0 fatty acid. No para-nitrophenol glycosides tested, including para-nitrophenol glucose, were hydrolyzed by EGCrP1 (Fig. 2D). The hydrolysis of GlcCer by EGCrP1 was somewhat affected by chain length and the hydroxylation of fatty acid moieties of GlcCer under the conditions used (Fig. 3A). Maximal activity was observed at around pH 7.5, indicating that EGCrP1 is a neutral GCase (Fig. 3B). Compared with the mammalian neutral GCase Klotho-related protein (KLrP) (42), EGCrP1 exhibited lower kcat and similar Km values toward C6-NBD-GlcCer as a substrate (Table 1). The optimal temperature was around 30 °C, which is suitable for growth of R. oryzae (Fig. 3C). The activity was enhanced when 0.1% sodium cholate or 10% (v/v) DMSO was added to the reaction mixture (Fig. 3, D and E). However, Triton X-100, which stimulates the activity of EGCase and endogalactosylceramidase, strongly inhibited the activity of EGCrP1 even at a low concentration (Fig. 3D). No metal ions were required for the activity of EGCrP1 (Fig. 3F).
FIGURE 1.
Phylogenic tree of EGCrP1 and EGCase. Amino acid sequences of EGCrP1 of several fungi and EGCase were reconstructed by the neighbor-joining method. The shaded area indicates EGCase (41, 54–57). The genes investigated experimentally in this study are indicated in bold type. The scale bar represents 0.1 amino acid substitution per site. EGALC, endogalactosylceramidase.
FIGURE 2.
Substrate specificity of purified recombinant EGCrP1. A, purity of recombinant EGCrP1. The purified protein was subjected to SDS-PAGE and visualized with Coomassie Brilliant Blue. Lane 1, protein marker; lane 2, pass-through fractions; lane 3, wash fractions; lane 4, eluted fractions from a GSTrap HP column. B, TLC showing the fluorescent ceramide (Cer) released from NBD-GlcCer by EGCrP1. Each fluorescent GSL (100 pmol) was incubated with (+) or without (−) recombinant EGCrP1 at 30 °C for 16 h except for fluorescent GlcCer, which was incubated for 60 min. Samples were loaded onto a TLC plate that was developed with chloroform/methanol/water (65:35:8, v/v/v). Fluorescent GSLs and Cer were detected by AE-6935B Visirays. C, TLC showing the glucose (Glc) released from fungal GlcCer (methyl d18:2/α-hydroxy 16:0). GlcCer and glucose were visualized with orcinol-H2SO4 reagent. D, the hydrolysis of various substrates by EGCrP1. The structures of GSLs are shown in a dotted box. Error bars represent the mean ± S.D. of three experiments. An asterisk indicates no hydrolysis of substrates. SM, sphingomyelin; TGC, trigalactosylceramide; pNP, para-nitrophenol.
FIGURE 3.
General properties of recombinant EGCrP1. A, effects of fatty acid moieties of GlcCer on EGCrP1 activity. C6:0-GlcCer, C16:0-GlcCer, C18:0-GlcCer, C22:0-GlcCer, C24:0-GlcCer, and α-hydroxy 24:0 (h24:0)-GlcCer were prepared using sphingolipid ceramide N-deacylase as described under “Experimental Procedures.” Incubation was carried out at 30 °C for 16 h. B, pH dependence of the recombinant EGCrP1 of R. oryzae. MES (pH 5.5–6.5, open circles), sodium phosphate buffer (pH 6.5–7.5, closed diamonds), HEPES (pH 7.5–8, open squares), boric acid buffer (pH 8–9, closed squares). C, effects of temperature on EGCrP1 activity. D, effects of detergents on EGCrP1 activity. Open squares, sodium cholate; closed circles, Triton X-100. E, effects of DMSO on EGCrP1 activity. F, metal ion dependence of EGCrP1. Error bars represent the mean ± S.D. of three experiments.
TABLE 1.
Kinetic parameters of recombinant EGCrP1 and KLrP toward C6-NBD-GlcCer
The values for KLrP are from Ref. 42.
| Substrate | Enzyme | Km | kcat | kcat/Km |
|---|---|---|---|---|
| μm | s−1 | m−1s−1 | ||
| C6-NBD-GlcCer | EGCrP1 | 5.8 ± 0.3 | (38.3 ± 0.2) × 10−3 | (6.6 ± 0.4) × 103 |
| C6-NBD-GlcCer | KLrP | 4.6 ± 0.2 | (121.0 ± 5.5) × 10−3 | (26.2 ± 0.5) × 103 |
Determination of Catalytic Amino Acid Residues of EGCrP1
Alignment of the deduced amino acid sequences of R. oryzae EGCrP1, C. neoformans EGCrP1, and Rhodococcus EGCase (EGCase II) revealed that eight residues, which are essential for the catalytic activity of glycoside hydrolase (GH) family 5 glycosidases (43), were well conserved in the EGCrP1 (Fig. 4A). Notably, two glutamates, Glu254 and Glu483 at the end of β-strands 5 and 8 in R. oryzae EGCrP1, were thought to be an acid/base catalyst and nucleophile, respectively (Fig. 4A, black circles). To address whether they function as catalytic residues, Glu254 and Glu483 were replaced with Gln (E254Q and E483Q) by site-directed mutagenesis. The two mutant enzymes and wild type enzyme were separately expressed in E. coli BL21(DE3), purified (Fig. 4B), and subjected to the assay using C6-NBD-GlcCer as a substrate. In contrast to the wild type enzyme, no activity was detected in the E254Q and E483Q mutant enzymes (Fig. 4C), suggesting that Glu254 and Glu483 do function as an acid/base catalyst and nucleophile, respectively.
FIGURE 4.
Alignment of EGCrP1 with EGCase (A) and determination of catalytic residues of EGCrP1 (B and C). A, the amino acid sequences of Rhodococcus EGCase II (EGCase), R. oryzae EGCrP1 (ROEGCrP1), and C. neoformans EGCrP1 (CNEGCrP1) were aligned using ClustalX. Identical and similar residues are shown by white letters on a black background and black letters in an open box, respectively. Amino acid residues conserved in GH family 5 glycosidases are indicated by open circles. Two glutamates, Glu254 and Glu483 of R. oryzae EGCrP1 and Glu258 and Glu492 of C. neoformans EGCrP1 possibly functioning as an acid/base catalyst and nucleophile, respectively, are indicated by closed circles. The secondary structural elements are shown above the amino acid sequence of EGCase. B, SDS-PAGE of the purified wild type, E254Q, and E483Q R. oryzae EGCrP1. C, TLC showing the hydrolysis of NBD-GlcCer by the wild type and mutant R. oryzae EGCrP1.
GCase Activity and GlcCer Content in egcrp1-deficient Mutants of C. neoformans
The egcrp1 has been found in the genome of C. neoformans and A. fumigatus as well as R. oryzae as shown in Fig. 1. Thus, the egcrp1 genes of A. fumigatus and C. neoformans were cloned and expressed in E. coli, and the recombinant enzymes were characterized. The two enzymes specifically hydrolyzed GlcCer (Fig. 5A), the same as the enzyme from R. oryzae. C. neoformans EGCrP1 also exhibited maximal GCase activity at a neutral pH (Fig. 5B).
FIGURE 5.
Characterization of egcrp1 deletion mutants of C. neoformans. A, hydrolysis of NBD-GlcCer by cell lysate of E. coli transformed with pCold-TF (mock) harboring egcrp1 from A. fumigatus, C. neoformans, or R. oryzae. The enzyme assay was carried out as described in the legend of Fig. 2. n.d., products were not detectable. B, pH dependence of the recombinant EGCrP1 of C. neoformans. Fifty micromolar sodium acetate buffer (pH 4–5.5, closed squares), MES (pH 5.5–6.5, open circles), sodium phosphate buffer (pH 6–7.5, closed diamonds), HEPES (pH 7–8, open squares), and boric acid buffer (pH 8–9, closed circles) were used. C, hydrolysis of NBD-GlcCer at pH 7.3 by the cell lysate and cytosolic, membranous, cell wall, and supernatant fractions (left panel) and at pH 5.0 by the membranous fraction (right panel). D, HPLC-based quantification of GlcCer. GlcCer fractions were labeled with OPA and analyzed by normal phase HPLC (38). A black arrow shows the peak that emerged in only egcrp1Δ. An asterisk shows the OPA left after the reaction. E, amounts of fungus-specific GlcCer (methyl d18:2). F, HPLC showing the hydrolysis of fungus-specific mature (peak 2) and immature (peak 1) GlcCer by recombinant EGCrP1. G, extent of hydrolysis estimated from F. Error bars represent the mean ± S.D. of at least three experiments.
To verify whether EGCrP1 is involved in the catabolism of GlcCer in vivo, an egcrp1-deficient mutant (egcrp1Δ) of C. neoformans var. grubii serotype A strain H99 was generated by gene-targeting homologous recombination using the NAT split marker (supplemental Fig. S1A). The egcrp1Δ strains were selected by PCR (supplemental Fig. S1B) and GCase activity (supplemental Fig. S1C) and subjected to a Southern blot analysis using HindIII-digested genomic DNA (supplemental Fig. S1D). The GCase activity of the wild type and egcrp1Δ strains of C. neoformans was examined using C6-NBD-GlcCer as a substrate at pH 7.3 and 5.0. A significant decrease in GCase activity was observed in the lysate of egcrp1Δ cells at pH 7.3 (Fig. 5C). To verify the intracellular localization of EGCrP1, GCase activity was measured using the cytosolic, membranous, cell wall, and supernatant fractions of the wild type and egcrp1Δ strains. The GCase activity of egcrp1Δ significantly decreased in the membranes at a neutral pH but not at low pH (Fig. 5C), whereas the activity in the cytosol, cell wall, and supernatant was unchanged. These results suggest that EGCrP1 occurs in the membranes where it functions as a neutral GCase.
GlcCer levels in the wild type and egcrp1Δ strains were determined by HPLC-based methods. As expected, the amount of fungus-specific GlcCer containing the methyl d18:2 sphingoid base was increased in egcrp1Δ compared with the wild type (Fig. 5, D and E). In addition, a new peak emerged just before the peak of the fungus-specific GlcCer in egcrp1Δ but not in the wild type (Fig. 5D). Similar to the fungus-specific GlcCer (peak 2), the new peak (peak 1) was susceptible to hydrolysis by EGCrP1 (Fig. 5, F and G).
Structural Analysis of GlcCer Accumulated in egcrp1Δ
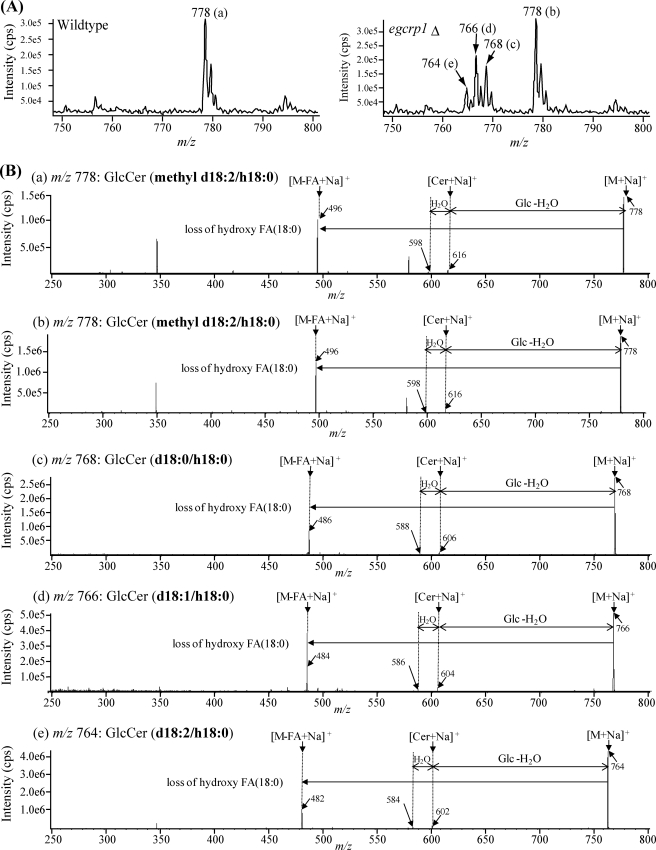
To clarify the structure of the GlcCer accumulated in egcrp1Δ, purified GlcCer fractions of wild type and mutant cells were analyzed by nanoelectrospray ionization MS/MS. Peaks a and b at m/z 778 were observed in both strains (Fig. 6A). They were identified by MS/MS to be a fungus-specific mature GlcCer composed of a methyl d18:2 sphingoid base and α-hydroxy 18:0 fatty acid (Fig. 6B). Meanwhile, three new peaks (peaks c, d, and e) were observed only in the egcrp1Δ strain (Fig. 6A, right panel). Similar but not identical to the mature GlcCer, peaks c, d, and e were assigned by MS/MS to GlcCer composed of d18:0 (m/z 768), d18:1 (m/z 766), and d18:2 (m/z 764) sphingoid bases, respectively (Fig. 6B). Three GlcCer species generated in the mutant cells contained α-hydroxy 18:0 fatty acid similar to the mature GlcCer. These results indicate that the three molecular species of GlcCer accumulated in the egcrp1Δ strain contain immature forms of ceramide that are intermediates generated from the pathway of GlcCer synthesis in C. neoformans (Fig. 7). No significant difference in susceptibility to EGCrP1 was observed between mature and immature GlcCer (Fig. 5, F and G).
FIGURE 6.
Structural analysis of GlcCer accumulated in egcrp1Δ strains. A, MS spectra of GlcCer isolated from the wild type (left panel) and egcrp1Δ (right panel). Each peak (a, b, c, d, and e) was further subjected to MS/MS. B, MS/MS spectra of GlcCer containing different sphingoid bases (methyl d18:2, d18:0, d18:1, and d18:2) and α-hydroxy 18:0 fatty acid (h18:0). FA, fatty acid.
Phenotype of egcrp1Δ
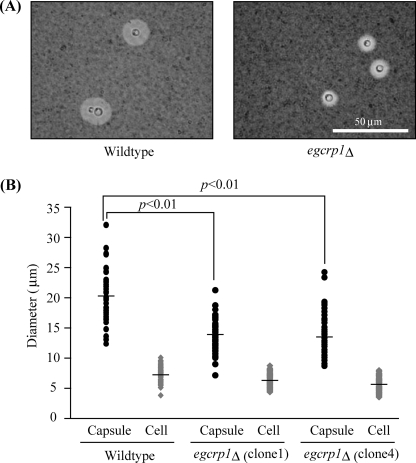
To estimate the biological functions of EGCrP1 in vivo, the growth of egcrp1Δ cells was compared with that of wild type cells under oxidative stress (hydrogen peroxide), high salt stress (NaCl), detergent stress (SDS), and different pH (pH 4.0 and 7.3). No significant difference was observed between the wild type and egcrp1Δ strains under these conditions (supplemental Fig. S2). On the other hand, the polysaccharide capsule, an important virulence factor in C. neoformans, was smaller in egcrp1Δ under capsule-inducing conditions (Fig. 8, A and B) but not under non-inducing conditions.
FIGURE 8.
Formation of polysaccharide capsule in egcrp1Δ. A, formation of a polysaccharide capsule in the wild type (left panel) and egcrp1Δ (right panel) strains. The capsule was visualized by India ink staining. B, size of the capsule (black circles) and cells (gray diamonds). Capsules from at least 50 cells per strain were measured. The bars represent the mean.
DISCUSSION
In this study, we showed that the recombinant EGCrP1 of R. oryzae, C. neoformans, and A. fumigatus specifically hydrolyzed GlcCer at neutral pH and that the disruption of egcrp1 in C. neoformans caused an accumulation of GlcCer concomitantly with a decrease in membrane-associated neutral GCase activity (Fig. 5). The egcrp1 gene is distributed across the phyla/genera of fungi, including Ascomycota (A. fumigatus), Basidiomycota (C. neoformans), and Zygomycota (R. oryzae), but is not found in mammals, plants, and bacteria. These results clearly indicate that EGCrP1 is a fungus-specific neutral GCase involved in the metabolism of GlcCer. However, in egcrp1Δ, the GCase activity of the cytosolic and cell wall fractions at pH 7.3 and membranous fraction at pH 5.0 was not decreased compared with that in the wild type, suggesting that some other GCases in addition to EGCrP1 are present in C. neoformans.
The gene encoding GCase had yet to be identified in fungi; however, in some protein databases, fungal β-1,6-glucanases are annotated as GCases because they show sequence similarity to the acid β-glucosidase 1 (GBA1), a mammalian lysosomal GCase. The β-1,6-glucanase of A. fumigatus is a β-1,6-glucan-specific glycosidase possibly involved in the degradation of cell walls (44). This enzyme is not likely to participate in the hydrolysis of GlcCer because Phytophthora infestans β-glucosidase BGX1, which shows significant sequence similarity to both GBA1 and β-1,6-glucanase, was not able to hydrolyze GlcCer (45).
In mammals, GlcCer is mainly catabolized by GBA1, which is assigned to GH family 30 (46). GBA2, assigned to GH family 116 and known as a bile acid β-glucosidase, was identified as a neutral GCase located in the endoplasmic reticulum (47). We also found that KLrP (GBA3; β-glucosidase (EC 3.2.1.21)) belonging to GH family 1 is a cytosolic neutral GCase that could be involved in the non-lysosomal catabolism of GlcCer (42). Additionally, lactase phlorizin hydrolase (glycosylceramidase (EC 3.2.1.62)/lactase (EC 3.2.1.108); GH family 1) was shown to participate in the digestion of GlcCer in mammalian intestines (48). A non-mammalian GCase belonging to GH family 3 has also been identified in Paenibacillus sp. (49, 50). An alignment of deduced amino acid sequences revealed that EGCrP1 and EGCase shared eight residues important for the catalytic activities of GH family 5 glycosidases (43). Among these residues, Glu254 and Glu483 of EGCrP1 were estimated to be the acid/base catalyst and nucleophile, respectively, by site-directed mutagenesis in the present study. These results show that EGCrP1 is the first GCase belonging to GH family 5.
Although EGCrP1 showed sequence similarity to EGCase at the protein level, the substrate specificity of the two enzymes is very different. EGCrP1 hydrolyzes the β-glucosidic linkage of GlcCer but not other GSLs. In contrast, EGCase hydrolyzes the β-glucosidic linkage between an oligosaccharide and ceramide; i.e. the minimum structure of the sugar moiety required for hydrolysis by EGCase is lactose (Galβ1,4Glcβ1-). Comparison of the primary structure of EGCrP1 with that of EGCase revealed several inserts between β-strand 5 and α-helix 6, between β-strands 6 and 7, and between β-strand 7 and α-helix 9 (Fig. 4A). Further study is required to elucidate the structure-function relationships of EGCrP1 and EGCase. Because the crystal structure of EGCase has been solved (51), an x-ray crystal analysis of EGCrP1 would provide valuable information about mutual relationships.
In this study, we found that GlcCer containing a mature ceramide accumulated in the egcrp1Δ concomitant with a decrease of GCase activity in C. neoformans. Furthermore, immature GlcCer containing d18:0, d18:1, or d18:2 emerged in egcrp1Δ but not in the wild type (Fig. 7). The mature ceramide is generated from dihydroceramide (d18:0/C18:0) through four steps catalyzed by a fatty acid 2-hydroxylase generating a ceramide composed of d18:0 and α-hydroxy 18:0 (h18:0), a sphingolipid Δ4-desaturase generating a ceramide composed of d18:1 and h18:0, a sphingolipid Δ8-desaturase generating a ceramide composed of d18:2 and h18:0, and finally a sphingolipid 9-methyltransfearase generating the mature ceramide composed of methyl d18:2 and h18:0 (Fig. 7). Immature GlcCer was probably generated because of the broad specificity of GCS-1; i.e. GCS-1 is likely to adopt not only a mature ceramide but also an immature ceramide as a substrate in vivo. This notion is supported by previous findings. Immature forms of GlcCer (d18:0, d18:1, and d18:2) were generated when GCS-1 was overexpressed in yeasts (17), an immature GlcCer possessing d18:2 was generated in sphingolipid 9-methyltransferase-disrupted Pichia pastoris, C. albicans, and Fusarium graminearum (4, 8, 15, 52), and GlcCer possessing d18:1 was generated in sphingolipid Δ8-desaturase-disrupted P. pastoris and C. albicans (4, 7).
Several reports have indicated the biological relevance of fungus-specific GlcCer possessing a methyl d18:2 sphingoid base e.g. to cell growth (52), hyphal elongation (15), and virulence (9, 13). On the other hand, immature GlcCer possessing d18:1 or d18:2 could not compensate for the functions of fungus-specific GlcCer (7, 9, 15). Sphingolipid Δ4-desaturase and 9-methyltransferase utilize ceramide, but not GlcCer, as an acceptor substrate (8, 18). Thus, once immature ceramide is converted to immature GlcCer by GCS-1, the process of maturation would be terminated, resulting in the accumulation of immature GlcCer. EGCrP1 seems to convert the immature GlcCer to immature ceramide by which the maturation of GlcCer would again proceed. Taken together, EGCrP1 is an enzyme responsible for controlling the quality of GlcCer during the course of its synthesis. EGCrP1 acts to eliminate less functional and wasteful forms of GlcCer that are generated as by-products due to the broad specificity of GCS-1. Kinetic analysis showed that the kcat of EGCrP1 is lower than that of another neutral GCase, KLrP (42), a property suitable for the quality control of GlcCer. Because the susceptibility of immature GlcCer to EGCrP1 is almost the same as that of mature GlcCer (Fig. 5, F and G), catalytic reactions that are too efficient may cause not only the elimination of immature forms but also a decrease in mature GlcCer during the course of production.
It is widely accepted that the polysaccharide capsule of C. neoformans is closely related to its pathogenicity (22). In this study, we found that the polysaccharide capsule was smaller in egcrp1Δ than in the wild type in the culture with capsule-inducing medium. The reason for this is unclear. However, it may be related to the transport of polysaccharide-containing vesicles to the outside of the cell, which triggers capsule formation, because the vesicles are mainly made of GlcCer and sterols (53). In contrast, the polysaccharide capsule in a gcs1-deficient mutant of C. neoformans was almost the same size as that of the wild type (13). Therefore, the accumulation of immature GlcCer in egcrp1Δ may affect the formation and/or transport of vesicles.
Opportunistic fungi such as C. neoformans have attracted attention over the past decade because of the increase in the numbers of patients with immunodeficiencies such as AIDS (1). Very recently, highly virulent strains of C. gattii have been found in the United States and Canada (2), and importantly, the gene encoding EGCrP1 was also found in the genome database of C. gattii. This study should facilitate the development of antifungal drugs based on the EGCrP1 involved in the metabolism of fungus-specific GlcCer across fungal phyla/genera.
Supplementary Material
Acknowledgments
We thank Dr. Kazuyuki Saito at Kyushu University for technical assistance and Dr. Yoshio Hirabayashi at RIKEN for critical reading of the manuscript.
This work was supported in part by the New Energy and Industrial Technology Development Organization (to M. I.), Basic Research B from the Japanese Ministry of Education, Culture, Sports, Science and Technology (to M. I.), the Naito Foundation (to Y. I.), and the Japan Society for the Promotion of Science for Young Scientists (to Y. I.).

This article contains supplemental Figs. S1 and S2 and Table S1.
- GlcCer
- glucosylceramide
- Cer
- ceramide
- EGCase
- endoglycoceramidase
- EGCrP1
- EGCase-related protein 1
- egcrp1Δ
- egcrp1-deficient mutant
- GBA
- acid β-glucosidase
- GCase
- glucocerebrosidase
- GCS-1
- glucosylceramide synthase-1
- GH
- glycoside hydrolase
- GSL
- glycosphingolipid
- KLrP
- Klotho-related protein
- NAT
- nourseothricin acetyltransferase
- NBD
- 7-nitro-2,1,3-benzoxadiazole
- OPA
- o-phthalaldehyde
- GM1
- Galβ1–3GalNAcβ1–4(NeuAcα2–3)Galβ1–4Glcβ1–1′Cer
- LacCer
- lactosylceramide
- GalCer
- galactosylceramide
- Gb3Cer
- globotriaosylceramide.
REFERENCES
- 1. Kauffman C. A. (2006) Proc. Am. Thorac. Soc. 3, 35–40 [DOI] [PubMed] [Google Scholar]
- 2. Byrnes E. J., 3rd, Bildfell R. J., Frank S. A., Mitchell T. G., Marr K. A., Heitman J. (2009) J. Infect. Dis. 199, 1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhome R., McQuiston T., Kechichian T., Bielawska A., Hennig M., Drago M., Morace G., Luberto C., Del Poeta M. (2007) Eukaryot. Cell 6, 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ternes P., Wobbe T., Schwarz M., Albrecht S., Feussner K., Riezman I., Cregg J. M., Heinz E., Riezman H., Feussner I., Warnecke D. (2011) J. Biol. Chem. 286, 11401–11414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haak D., Gable K., Beeler T., Dunn T. (1997) J. Biol. Chem. 272, 29704–29710 [DOI] [PubMed] [Google Scholar]
- 6. Michaelson L. V., Zäuner S., Markham J. E., Haslam R. P., Desikan R., Mugford S., Albrecht S., Warnecke D., Sperling P., Heinz E., Napier J. A. (2009) Plant Physiol. 149, 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oura T., Kajiwara S. (2008) Microbiology 154, 3795–3803 [DOI] [PubMed] [Google Scholar]
- 8. Ternes P., Sperling P., Albrecht S., Franke S., Cregg J. M., Warnecke D., Heinz E. (2006) J. Biol. Chem. 281, 5582–5592 [DOI] [PubMed] [Google Scholar]
- 9. Noble S. M., French S., Kohn L. A., Chen V., Johnson A. D. (2010) Nat. Genet. 42, 590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodrigues M. L., Travassos L. R., Miranda K. R., Franzen A. J., Rozental S., de Souza W., Alviano C. S., Barreto-Bergter E. (2000) Infect. Immun. 68, 7049–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levery S. B., Momany M., Lindsey R., Toledo M. S., Shayman J. A., Fuller M., Brooks K., Doong R. L., Straus A. H., Takahashi H. K. (2002) FEBS Lett. 525, 59–64 [DOI] [PubMed] [Google Scholar]
- 12. Pinto M. R., Rodrigues M. L., Travassos L. R., Haido R. M., Wait R., Barreto-Bergter E. (2002) Glycobiology 12, 251–260 [DOI] [PubMed] [Google Scholar]
- 13. Rittershaus P. C., Kechichian T. B., Allegood J. C., Merrill A. H., Jr., Hennig M., Luberto C., Del Poeta M. (2006) J. Clin. Investig. 116, 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saito K., Takakuwa N., Ohnishi M., Oda Y. (2006) Appl. Microbiol. Biotechnol. 71, 515–521 [DOI] [PubMed] [Google Scholar]
- 15. Oura T., Kajiwara S. (2010) Microbiology 156, 1234–1243 [DOI] [PubMed] [Google Scholar]
- 16. Ramamoorthy V., Cahoon E. B., Li J., Thokala M., Minto R. E., Shah D. M. (2007) Mol. Microbiol. 66, 771–786 [DOI] [PubMed] [Google Scholar]
- 17. Leipelt M., Warnecke D., Zähringer U., Ott C., Müller F., Hube B., Heinz E. (2001) J. Biol. Chem. 276, 33621–33629 [DOI] [PubMed] [Google Scholar]
- 18. Michel C., van Echten-Deckert G., Rother J., Sandhoff K., Wang E., Merrill A. H., Jr. (1997) J. Biol. Chem. 272, 22432–22437 [DOI] [PubMed] [Google Scholar]
- 19. Tsuge J., Hiratsuka H., Kamimiya H., Nozaki H., Kushi Y. (2008) Biosci. Biotechnol. Biochem. 72, 2667–2674 [DOI] [PubMed] [Google Scholar]
- 20. Ito M., Yamagata T. (1989) J. Biol. Chem. 264, 9510–9519 [PubMed] [Google Scholar]
- 21. Ito M., Yamagata T. (1986) J. Biol. Chem. 261, 14278–14282 [PubMed] [Google Scholar]
- 22. Doering T. L. (2009) Annu. Rev. Microbiol. 63, 223–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakagawa T., Tani M., Kita K., Ito M. (1999) J Biochem. 126, 604–611 [DOI] [PubMed] [Google Scholar]
- 24. Ishibashi Y., Kiyohara M., Okino N., Ito M. (2007) J. Biochem. 142, 239–246 [DOI] [PubMed] [Google Scholar]
- 25. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. (1997) Nucleic Acids Res. 25, 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saitou N., Nei M. (1987) Mol. Biol. Evol. 4, 406–425 [DOI] [PubMed] [Google Scholar]
- 27. Gouet P., Robert X., Courcelle E. (2003) Nucleic Acids Res. 31, 3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 29. Kim M. S., Kim S. Y., Yoon J. K., Lee Y. W., Bahn Y. S. (2009) Biochem. Biophys. Res. Commun. 390, 983–988 [DOI] [PubMed] [Google Scholar]
- 30. Cox G. M., Rude T. H., Dykstra C. C., Perfect J. R. (1995) J. Med. Vet. Mycol. 33, 261–266 [DOI] [PubMed] [Google Scholar]
- 31. Perfect J. R., Rude T. H., Penning L. M., Johnson S. A. (1992) Gene 122, 213–217 [DOI] [PubMed] [Google Scholar]
- 32. McDade H. C., Cox G. M. (2001) Med. Mycol. 39, 151–154 [DOI] [PubMed] [Google Scholar]
- 33. Nelson R. T., Pryor B. A., Lodge J. K. (2003) Fungal Genet. Biol. 38, 1–9 [DOI] [PubMed] [Google Scholar]
- 34. Davidson R. C., Cruz M. C., Sia R. A., Allen B., Alspaugh J. A., Heitman J. (2000) Fungal Genet. Biol. 29, 38–48 [DOI] [PubMed] [Google Scholar]
- 35. Erb-Downward J. R., Noggle R. M., Williamson P. R., Huffnagle G. B. (2008) Mol. Microbiol. 68, 1428–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreuder M. P., Brekelmans S., van den Ende H., Klis F. M. (1993) Yeast 9, 399–409 [DOI] [PubMed] [Google Scholar]
- 37. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 38. Zama K., Hayashi Y., Ito S., Hirabayashi Y., Inoue T., Ohno K., Okino N., Ito M. (2009) Glycobiology 19, 767–775 [DOI] [PubMed] [Google Scholar]
- 39. Ikeda K., Kubo A., Akahoshi N., Yamada H., Miura N., Hishiki T., Nagahata Y., Matsuura T., Suematsu M., Taguchi R., Ishii I. (2011) Anal. Bioanal. Chem. 400, 1853–1863 [DOI] [PubMed] [Google Scholar]
- 40. Liu O. W., Chun C. D., Chow E. D., Chen C., Madhani H. D., Noble S. M. (2008) Cell 135, 174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishibashi Y., Nakasone T., Kiyohara M., Horibata Y., Sakaguchi K., Hijikata A., Ichinose S., Omori A., Yasui Y., Imamura A., Ishida H., Kiso M., Okino N., Ito M. (2007) J. Biol. Chem. 282, 11386–11396 [DOI] [PubMed] [Google Scholar]
- 42. Hayashi Y., Okino N., Kakuta Y., Shikanai T., Tani M., Narimatsu H., Ito M. (2007) J. Biol. Chem. 282, 30889–30900 [DOI] [PubMed] [Google Scholar]
- 43. Sakon J., Adney W. S., Himmel M. E., Thomas S. R., Karplus P. A. (1996) Biochemistry 35, 10648–10660 [DOI] [PubMed] [Google Scholar]
- 44. Boisramé A., Gaillardin C. (2009) Appl. Microbiol. Biotechnol. 82, 663–669 [DOI] [PubMed] [Google Scholar]
- 45. Brunner F., Wirtz W., Rose J. K., Darvill A. G., Govers F., Scheel D., Nürnberger T. (2002) Phytochemistry 59, 689–696 [DOI] [PubMed] [Google Scholar]
- 46. Kolter T., Sandhoff K. (2005) Annu. Rev. Cell Dev. Biol. 21, 81–103 [DOI] [PubMed] [Google Scholar]
- 47. Matern H., Boermans H., Lottspeich F., Matern S. (2001) J. Biol. Chem. 276, 37929–37933 [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi T., Suzuki K. (1981) J. Biol. Chem. 256, 7768–7773 [PubMed] [Google Scholar]
- 49. Sumida T., Sueyoshi N., Ito M. (2002) J Biochem. 132, 237–243 [DOI] [PubMed] [Google Scholar]
- 50. Paal K., Ito M., Withers S. G. (2004) Biochem. J. 378, 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caines M. E., Vaughan M. D., Tarling C. A., Hancock S. M., Warren R. A., Withers S. G., Strynadka N. C. (2007) J. Biol. Chem. 282, 14300–14308 [DOI] [PubMed] [Google Scholar]
- 52. Ramamoorthy V., Cahoon E. B., Thokala M., Kaur J., Li J., Shah D. M. (2009) Eukaryot. Cell 8, 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodrigues M. L., Nimrichter L., Oliveira D. L., Frases S., Miranda K., Zaragoza O., Alvarez M., Nakouzi A., Feldmesser M., Casadevall A. (2007) Eukaryot. Cell 6, 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Izu H., Izumi Y., Kurome Y., Sano M., Kondo A., Kato I., Ito M. (1997) J. Biol. Chem. 272, 19846–19850 [DOI] [PubMed] [Google Scholar]
- 55. Horibata Y., Okino N., Ichinose S., Omori A., Ito M. (2000) J. Biol. Chem. 275, 31297–31304 [DOI] [PubMed] [Google Scholar]
- 56. Sakaguchi K., Okino N., Sueyoshi N., Izu H., Ito M. (2000) J. Biochem. 128, 145–152 [DOI] [PubMed] [Google Scholar]
- 57. Horibata Y., Sakaguchi K., Okino N., Iida H., Inagaki M., Fujisawa T., Hama Y., Ito M. (2004) J. Biol. Chem. 279, 33379–33389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.