Abstract
Estrogens and other endogenous steroids are known risk markers for cancer. Gas chromatography (GC) with mass spectrometry (MS) has traditionally predominated the analysis of estrogens and other endogenous steroids but liquid chromatography (LC) MS is increasingly favored. Direct comparisons of the two technologies have hitherto not been performed. Steroids were analyzed in urine from 232 premenopausal women in a blinded fashion by benchtop orbitrap LCMS and single quadrupole GCMS. 16 steroidal estrogens including oxidized metabolites could be analyzed by LCMS. LCMS-GCMS Spearman rank correlations of the major estrogens E1, E2, E3, 16α-OHE1, and 2-OHE1 were very high (r=0.72–0.91), and absolute concentrations also agreed (<5% difference for E1, E2, E3, 16α-OHE1). LCMS allowed reinterrogation of acquired data due to orbitrap technology, which permitted post-analysis quantitation of progesterone, cortisol, and cortisone (LCMS-GCMS Spearman rank correlations=0.80–0.84; absolute difference <7%; n=137). GCMS allows the measurement of a wide range of steroids including non-polar analytes that escape the presented LCMS assay. In contrast, orbitrap based LCMS can detect more estrogens, is faster, less costly, allows post data acquisition reinterrogation of certain analytes that had not been targeted a priori, and requires much less urine.
Keywords: estrogen metabolites, corticoids, progesterone, urine, breast cancer biomarkers
INTRODUCTION
Gas chromatography (GC) coupled with mass spectrometry (MS) traditionally served as the predominant method for steroid analyses due to high specificity, wide analyte coverage, and good sensitivity [1, 2]. However, its need for large sample amount, complex and time consuming sample preparation steps, and derivatization while having low throughput but high cost has advanced liquid chromatography (LC) MS to being the preferred technology because the latter overcomes these downsides [reviewed in 3, 4]. Immunoassays are also widely applied for steroid determinations, particularly in the clinical environment due to their affordability, ease of performance, and fast turn-around time. However, caution is needed due to their low specificity and susceptibility to unknown interferences, especially for low analyte concentrations, which LCMS can overcome [reviewed in 3, 4–6].
Endogenous sex steroids including progesterone, testosterone, DHEA and other precursors, but particularly estrogens are considered risk factors for cancers of the reproductive system, in particular breast cancer [7–9]. The metabolism of E1 and E2 yields potentially estrogenic and genotoxic products through the competing C2-, C4- and C16α-hydroxylation pathways, the probably less carcinogenic 2-hydroxy (OH) metabolites, and the more genotoxic 4-OH and 16α-OH metabolites [2, 10–12]. These hydroxylated products are methylated into the less toxic methoxy derivatives [13]. Since all metabolites have markedly different biological properties, the relative proportion of metabolites may influence breast cancer risk [2, 14, 15] as a larger proportion of 16α-OHE1 and a lower 2-OH/16α-OHE1 ratio is believed to represent a greater risk for breast cancer [2, 10].
In order to further evaluate the role of estrogens in premenopausal women on cancer risk, particularly the role of the 2-OHE1/16α-OHE1 (2:16) ratio, we improved previous LCMS methods by developing faster LC runs and applying high-resolution orbitrap based MS using a recently commercialized benchtop model to quantitate 16 steroidal estrogen metabolites with focus on the nine predominant urinary E1 and E2 metabolites [16]. We also investigated analysis of other urinary steroids using this technique such as cortisol (F), 6-hydroxycortisol (6-OHF), cortisone (E), and progesterone (P) from urine and compared results to classic single-quadrupole GCMS analyses. The advantages and limitations of orbitrap LCMS and single-quadrupole GCMS for steroid measurements from urine are discussed.
METHODS
Study Design and Sample Collection
The urine samples for this study were obtained from 27 women who were part of a randomized, crossover soy intervention study consisting of two 6-month periods (high-soy and low-soy) separated by a 1-month washout period (BEAN study). All women were premenopausal with a mean age of 37.6±4.9 years (range: 29.8–44.8). Overnight urine samples were collected from each woman at the study visits, which were scheduled during the mid-luteal phase based on self-reports of menstrual cycles. Participants voided their bladders before going to bed and then collected all urine during the night and the first morning urine. A mixture of boric and ascorbic acid was added to the urine in the air-tight plastic containers to reduce the urine pH. The urine samples were divided into aliquots and stored at −80°C until analyzed. The BEAN study protocol was approved by the Committee on Human Subjects at the University of Hawaii and by the participating clinics. All subjects signed an informed consent form at screening. Additional details of the study were reported recently [17].
Urinary Analysis
For comparison of performance of LCMS and GCMS, results were available from 232 urine samples for E1, E2, 16α-OHE1, estriol (E3), and 2-OH E1, and on 137 urine samples for F, and E (Table 1).
Table 1.
Analytes and internal standards of LCMS assay
| Analytes | Abbreviations | Internal standardsa | Internal standard abbreviations |
|---|---|---|---|
| Estrogens | |||
| Estroneb | E1 | E1-[2,4,16,16]-d4 | E1-d4 |
| Estradiolb | E2 | E2-[16,16,17]-d3 | E2-d3 |
| 16-pathway | |||
| Estriolb | E3 | E2-[16,16,17]-d3 | E2-d3 |
| 16α-Hydroxyestroneb | 16α-OHE1 | 16α-OHE1-[1,2,4,6,6]-d5 | 16α-OHE1-d5 |
| 16-Epiestriol | 16epi-E3 | E2-[16,16,17]-d3 | E2-d3 |
| 17-Epiestriol | 17epi-E3 | E2-[16,16,17]-d3 | E2-d3 |
| 16-Ketoestradiol | 16-ketoE2 | 16α-OHE1-[1,2,4,6,6]-d5 | 16α-OHE1-d5 |
| 2-pathway | |||
| 2-Hydroxyestroneb | 2-OHE1 | 4-OHE2-[1,2,16,16,17]-d5 | 4-OHE2-d5 |
| 2-Hydroxyestradiol | 2-OHE2 | 4-OHE2-[1,2,16,16,17]-d5 | 4-OHE2-d5 |
| 2-Methoxyestrone | 2-MeOE1 | 2-MeOE2-[1,4,16,16,17]-d5 | 2-MeOE2-d5 |
| 2-Methoxyestradiol | 2-MeOE2 | 2-MeOE2-[1,4,16,16,17]-d5 | 2-MeOE2-d5 |
| 2-Hydroxy-3-O-methylestrone | 2-OH-3-MeE1 | 2-MeOE2-[1,4,16,16,17]-d5 | 2-MeOE2-d5 |
| 4-pathway | |||
| 4-Hydroxyestrone | 4-OHE1 | 4-OHE2-[1,2,16,16,17]-d5 | 4-OHE2-d5 |
| 4-Hydroxyestradiol | 4-OHE2 | 4-OHE2-[1,2,16,16,17]-d5 | 4-OHE2-d5 |
| 4-Methoxyestrone | 4-MeOE1 | 2-MeOE2-[1,4,16,16,17]-d5 | 2-MeOE2-d5 |
| 4-Methoxyestradiol | 4-MeOE2 | 2-MeOE2-[1,4,16,16,17]-d5 | 2-MeOE2-d5 |
| Gestagen | |||
| Progesterone | P | ||
| Corticoids | |||
| Cortisolb | F | ||
| Cortisoneb | E | ||
Used for LCMS as listed; for GCMS 5-alpha-Androstan-3-alpha, 17-alpha diol was used for all analytes except E1–d4 as the internal standard for E1, E2–d4 for E2 and E3, and stigmasterol for F and E.
analytes included in the GCMS assay (in addition to 17β-dihydroandrosterone, 5-androstenediol, dihydroepiandrosterone, androstenetriol, androsterone, etiocholanolone, pregnanediol, 5-pregnenetriol, tetrahydrocortisol and allotetrahydrocortisol)
Extraction and liquid chromatography mass spectrometry (LCMS)
High-accuracy (± 5ppm) LCMS analyses were performed with a model Accela ultra-high performance liquid chromatography system coupled to a model Exactive benchtop orbitrap mass spectrometer (all from Thermo Electron, Waltham, MA). Injections were carried out with an HTC Pal autosampler (LEAP technologies, Carrboro, NC). E1, E2, and E3 were purchased from Sigma-Aldrich (St Louis, MO). 2-OHE1, 2-OHE2, 2-methoxyestrone (2-MeO E1), 4-OHE1, 16α-OHE1, and 16-keto E2 were obtained from Steraloids (Newport, RI). All solvents were LCMS grade and all chemicals were from Fisher (Los Angeles, CA). Deuterated internal standards (see Table 1) were purchased from Sigma-Aldrich, St Louis, MO (E2-d3,), from Steraloids, Newport, RI (E1-d4), from Medical Isotopes, Inc., Pelham, NH (16α-OHE1-d5), or from C/D/N Isotopes Inc., Point-Claire, Quebec, Canada (4-OHE2-d5, 2-MeOE2-d5). Stock solutions for LCMS calibration were prepared in methanol and concentrations were determined by absorbance readings using molar extinction coefficients as shown in Table 2. Urine was enzymatically hydrolyzed and dansylated [18] by mixing 0.3 mL clear urine with 15 μL of a mixture of internal standards composed of E1-d4, E2-d3,, 2-OHE1-d5, 2-MeOE2-d5, 4-OHE2-d5, 16α-OHE1-d5, all at 400 ng/mL in methanol. Aqueous ascorbic acid (15 μL 1% (w/V)) was added in order to preserve analytes during incubation, and 15 μL of β-glucuronidase (isolated from Escherichia coli, 80 U/mL at 25°C; Roche Applied Sciences Indianapolis, IN), and 15 μL of sulfatase (24,190 U/g, #9626 from Sigma, St. Louis, MO; 2 mg/ml in 1M pH6 sodium acetate buffer) followed by incubation for 2 hours at 37°C for hydrolysis. The deconjugated analytes were extracted with dichloromethane. The isolated dichloromethane phase was dried under nitrogen, then redissolved with 15 μL 1% aqueous ascorbic acid to preserve analytes during heating, 75 μL dansylchloride (3 mg/mL in acetone) and 75 μL sodium bicarbonate (100 mM; pH9) followed by keeping to 64°C for 15 minutes. After vortexing, 20 μL of this solution was injected into the LCMS system comprised of a ThermoFisher filter cartridge (2.1 mm ID; 0.2 μm) connected to a Supelco Ascentis Express C18 column (150 × 3.0 mm; 2.7 μm). Optimal selectivity was achieved by elution with a linear gradient of acetonitrile (A) and 0.1% (v/v) aq. formic acid (B) at 0.8 mL/min: A/B= 40/60 to 80/20 in 28 min, hold for 4 minutes and back to 40/60 in 1 minute followed by equilibration for 7 minutes before subsequent injection. The general MS conditions were as follows: source, ESI; ion polarity, positive; spray voltage, 4500 V; sheath and auxiliary and ion sweep gas, nitrogen; sheath gas pressure, 30 arbitrary units; auxiliary gas pressure, 5 arbitrary units; ion sweep gas pressure, 0 arbitrary units; ion transfer capillary temperature, 350°C; capillary voltage 65V; tube lens voltage 118V; skimmer voltage 29V; Scan range 250–1000m/z. Resolution 50,000 at 2Hz; automatic gain control 1,000,000 and maximum injection time 250 ms. Mass spectrometric monitoring was started immediately after sample injection. Quantitation was performed by extracting the respective exact masses, including internal standards as listed in tables 1–2: E1-d4 for E1,16α-OHE1-d5 for 16α-OHE1, E2-d3 for E2,16-ketoE2 and E3; 2-MeOE2-d5 for the methoxy compounds,and 4-OHE1-d5 for the hydroxy compounds. For quality control, we included several blinded samples into each batch. Tandem LCMS analyses were performed with a model TSQ-Ultra instrument using a UHPLC system model Accela (all from Thermo Electron).
Table 2.
Urinary steroids measured by LCMSa
| Analyte | εb | Protonated mass (m/z)c | Linear range (ng/mL) | Sloped | r2e |
|---|---|---|---|---|---|
| E1 | 2138 | 504.22031 | 0.025–100 | 2.26 × 10−4 | 0.999 |
| E2 | 2344 | 506.23596 | 0.025–100 | 1.14 × 10−4 | 0.998 |
| E3, 16epi-E3, 17epi-E3 | 2188 | 522.23087 | 0.05–100 | 7.70 × 10−5 | 0.999 |
| 16α-OHE1 | 2138 | 520.21522 | 0.05–100 | 9.84 × 10−5 | 0.999 |
| 16-ketoE2 | 2344 | 520.21522 | 0.05–100 | 7.29 × 10−5 | 0.999 |
| 2-OHE1 | 2138 | 753.26628 | 0.05–50 | 3.52 × 10−4 | 0.999 |
| 2-OHE2 | 2344 | 755.28193 | 0.05–50 | 3.40 × 10−4 | 0.999 |
| 2-MeOE1 | 2138 | 534.23087 | 0.05–50 | 5.94 × 10−5 | 0.999 |
| 2-MeOE2 | 2344 | 536.24652 | 0.05–50 | 2.71 × 10−5 | 0.998 |
| 2-OH-3-MeE1 | 2138 | 534. 23087 | 0.05–50 | 5.90 × 10−5 | 0.998 |
| 4-OHE1 | 2138 | 753.26628 | 0.05–50 | 3.64 × 10−4 | 0.999 |
| 4-OHE2 | 2344 | 755.28193 | 0.05–50 | 7.18 × 10−4 | 0.998 |
| 4-MeOE1 | 2138 | 534. 23087 | 0.05–50 | 6.40 × 10−5 | 0.998 |
| 4-MeOE2 | 2344 | 536.24652 | 0.05–50 | 3.37 × 10−5 | 0.998 |
| P | 16982 | 315.23186 | 0.05–100 | 9.28 × 10−5 | 0.998 |
| F | 15500 | 363.21660 | 1–1,000 | 2.93 × 10−3 | 0.998 |
| E | 15136 | 361.20095 | 1–1,000 | 1.97 × 10−3 | 0.996 |
| Internal Standards | |||||
| E1-d4 | 2138 | 508.24542 | |||
| E2-d3 | 2344 | 509.25479 | |||
| 2-MeOE2-d5 | 2344 | 541.27791 | |||
| 4-OHE2-d5 | 2344 | 760.31331 | |||
| 16α-OHE1-d5 | 2138 | 525.24661 | |||
using orbitrap MS (model Exactive, Thermo Scientific) that allowed exact mass measurements
molar extinction coefficient in methanol at 280 nm except for F (241 nm), E (238 nm), P (249 nm) used from CRC Handbook of Chemistry and Physics, 52nd ed. 1971; ε-values of E1 metabolites, E2 metabolites, and E3 metabolites were deduced from E1, E2, and E3, respectively
theoretical (monoisotopic) exact mass of each protonated analyte used to monitor in positive mode in a +/− 5 ppm range to account for mass spectrometric inaccuracies; all analytes dansylated except for P, F, E
slope of linear regression after forcing curve through zero (no intercept); relative standard error<6%
r2 = coefficient of determination for linear regression
Extraction and gas chromatography mass spectrometry (GCMS)
GCMS analyses, extensively validated previously [19], used a model HP-6890 gas chromatograph and a model 5973 single quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA). For quantitation of total steroids, which represented the sum of free, sulfated and glucuronidated steroids, internal standards were added to 10 ml of urine followed by extraction using C-18 (octadecylsilyl) solid phase extraction (SPE) columns (500 mg extraction bed, Varian, Walnut Creek, CA) and subsequent elution with methanol. The eluate, after drying, was subjected to hydrolysis using a mixture of β-glucuronidase/arylsulphatase (Helix pomatia, Roche Molecular Biochemicals, Indianapolis, IN) and crude β-glucuronidase (Helix pomatia, Sigma, St. Louis, MO) in sodium acetate buffer, pH 4.8. This digest was again extracted with the C-18 SPE cartridge and the adsorbed steroids eluted with methanol, dried down and derivatized in a two- step process to the methyloxime-trimethylsilyl (MO-TMS) ethers. The dried extract was first incubated at 60 °C for 1 hour in the presence of 2 % (w/V) methoxyamine hydrochloride (Sigma) in pyridine. The pyridine was then blown off and trimethylsilylimidazole (Regis Technologies, Morton Grove, IL) was added followed by incubation overnight at 100°C. Derivatization products were purified by taking them up in a small volume of cyclohexane and passing them through a Lipidex column (Hydroxyalkoxy-propyldextran, Type IX, Sigma). Finally, an aliquot (equal or less than 2 μL) of the concentrated eluate in cyclohexane was injected onto a temperature controlled non-polar capillary GC column (e.g. HP-1MS, 30 m, Agilent). Steroids were eluted essentially as described earlier [19] and identified and quantified by single ion monitoring (SIM) with HP-Chemstation software (Agilent) for integration of peak areas. A mixture of E1-d4, E2-d4, 5α-androstan-3α, 17α-diol, stigmasterol and cholesteryl butyrate (Steraloids, Newport, RI) served as internal standards. E1-d4 served as the internal standard for the quantitation of E1, E2-d4 for E2 and E3. For 16α-OHE1 and 2-OHE1,the 5α-androstan-3α, 17α-diol served as the internal standard, whereas stigmasterol served as internal standard for F and E.
In addition, levels of several other androgens and corticosteroids as well as some of their metabolites were also assessed but not included in the current comparison such as 17β-dihydroandrosterone, dihydroepiandrosterone, androstenetriol, androsterone, etiocholanolone, pregnanediol, 5-pregnenetriol, tetrahydrocortisone, tetrahydrocortisol, and allotetrahydrocortisol for which 5α-androstan-3α, 17α-diol served as the internal standard.
Blinded urine samples served as quality control samples and indicated both acceptable reproducibility and accuracy of the assay. Cholesteryl butyrate did not get derivatized and was used to monitor efficacy of the column.
Limits of quantitation (LOQ) were defined as the concentration that led to a signal that was at least 3 times higher than the noise and could be quantified with a coefficient of variation (CV) lower than 20% [20].
Statistical Analysis
The statistical analysis was performed using Microsoft Excel and SAS statistical software package version 9.2. (SAS Institute, Inc., Cary, NC). Non-normally distributed data were log-transformed prior to statistical analysis. We calculated unadjusted means and standard deviations for each metabolite by method and time of assessment. Spearman correlation coefficients were computed to compare the relation between the results derived through the two methods.
RESULTS
LCMS results
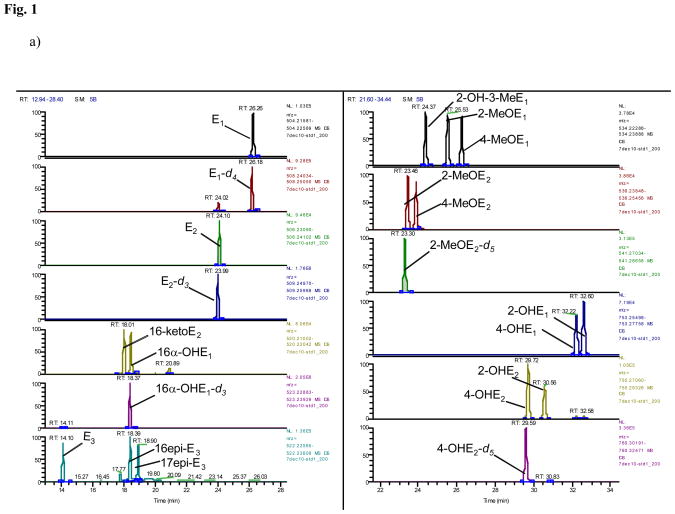
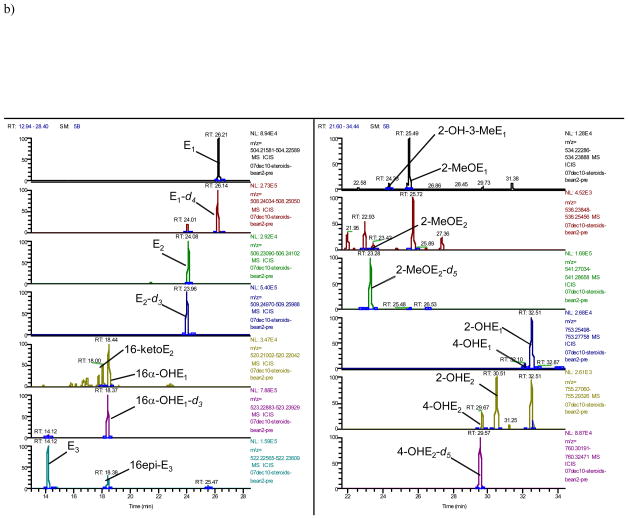
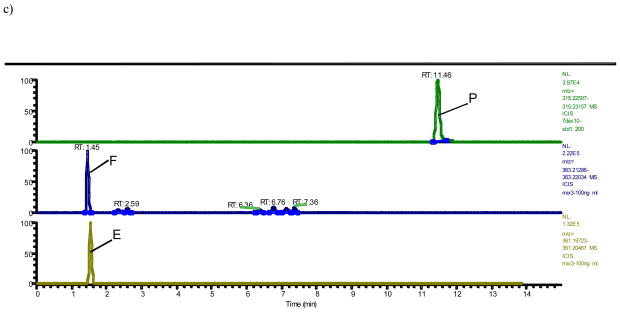
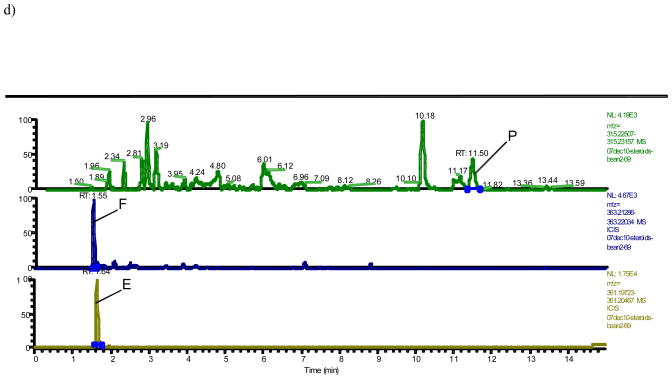
A typical trace of the newly developed benchtop orbitrap based LCMS analysis shows 16 steroidal estrogens (E1, E2, 16-ketoE2, 16α-OHE1, E3, 16epi-E3, 17epi-E3, 2-OH-3-MeE1, 2-MeOE1, 4-MeOE1, 2-MeOE2, 4-MeOE2, 4-OHE1, 2-OHE1, 4-OHE2 and 2-OHE2), in addition to P, F, and E separated either by mass or, in case of isobaric analytes (16α-OHE1+16-ketoE2; 2-OHE1+4-OHE1; 2-OHE2+4-OHE2; 2-OMeE1+4-OMeE1+2-OH-3-MeE1; 2-OMeE2+4-OMeE2; E3+16-epiE3+17-epiE3), by time without overlap of peaks (Fig. 1a–d). In order to separate the isobaric molecules of interest chromatographically, the LC run had to be extended to 35 minutes (Fig. 1). The array of steroids measured by LCMS with their respective internal standards are summarized in Table 1 and their respective exact masses used for monitoring (Fig. 1) are shown in Table 2.
Fig. 1. Orbitrap based LCMS trace of steroids after hydrolysis and dansylation.
a) Estrogens from standards at 5000 pg/mL
b) Estrogens from a urine extract obtained from a premenopausal woman at (pg/mL): 4340, 1543, 1076, 2153, 5846, 1461, 169, 1693, 59, 219, 1864, 17, and 207 for E1, E2, 16-ketoE2, 16α-OHE1, E3, 16epi-E3, 2-OH-3-MeE1, 2-MeOE1, 2-MeOE2, 4-OHE1, 2-OHE1, 4-OHE2 and 2-OHE2, respectively; other estrogens were below LOQ
c) Progesterone (P), cortisol (F), and cortisone (E) from standards at 5000 pg/mL, 100 ng/mL and 100 ng/mL, respectively
d) Progesterone (P), cortisol (F), and cortisone (E) in a urine extract obtained from a premenopausal at 372 pg/mL, 4670 ng/mL and 17500 ng/mL, respectively
Testing the repeatability of the LCMS assay revealed intra-assay CVs of 11% or less for most analytes (13% for 4-OHE1) and inter-assay CVs of 9% or less for most analytes (12% for 4-OHE1, 17% for 4-OHE1). GCMS intra-assay CVs were 5% or less and the inter-assay CVs were 9% or less (Table 3).
Table 3.
Quality control parameters of the most predominant estrogens using urine samples as external standards
| LCMS | GCMS | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall mean levels (ng/mL) | Intra-assay CVa (n=2) | Intra-assay CVa (n=3) | Inter-assay CVa (n=5) | Mean levels (ng/mL) | Intra-assay CVa (n=6) | Mean levels (ng/mL) | Inter-assay CVa (n=2) | |
| E1 | 8.2 | 6% | 4% | 5% | 7.9 | 2% | 7.2 | 6% |
| E2 | 3.3 | 10% | 2% | 5% | 3.1 | 4% | 3.8 | 9% |
| E3 | 5.9 | 12% | 10% | 9% | 7.8 | 3% | 6.3 | 5% |
| 16α-OHE1 | 2.9 | 7% | 4% | 9% | 4.3 | 5% | 2.1 | 7% |
| 16-ketoE2 | 1.0 | 10% | 5% | 17% | nd | - | nd | - |
| 2-OHE1 | 6.7 | 4% | 11% | 8% | 20.0 | 3% | 16.2 | 4% |
| 2-OHE2 | 0.7 | 10% | 7% | 7% | nd | - | nd | - |
| 2-MeOE1 | 2.3 | 7% | 7% | 6% | nd | - | nd | - |
| 4-OHE1 | 1.0 | 11% | 15% | 12% | nd | - | nd | - |
CV = coefficient of variation for repeat analysis starting with urine; intra-assay=repeat analysis on the same day; inter-assay=repeat analysis on different days; one quality control urine sample was used for LCMS and two for GCMS; cv values for other estrogens shown in Fig. 1 were very similar in the same concentration range.
Intra-assay cv <0.1% for all if repeat analysis on the same extract; to determine these parameters aliquoted urine sample from one individual were used for LCMS and aliquoted urine samples from separate two individuals for GCMS.
nd=not determined. Limit of quantitation for all analytes are shown in table 4; recoveries for LCMS and GCMS have been reported by Xu et al. 2005 and Shackleton et al. 1986, respectively.
LOQ for estrogens were 25–50 pg/mL using LCMS depending on the interferences in the urine sample, and 1 pg/mL using GCMS at a signal to noise ratio of 3 (Table 4). Further concentration of the urinary extract could improve the LCMS limits (data not shown).
Table 4.
Comparison between all LCMS and GCMS measured urinary steroids
| Steroid | Na | LCMS (ng/mL)
|
GCMS (ng/mL)
|
rd | pd | ||
|---|---|---|---|---|---|---|---|
| LOQbc | Median (Mean ±SD) | LOQb | Median (Mean ±SD) | ||||
| E1 | 232 | 0.05 | 7.0 (10.2±9.5) | 0.001 | 7.1 (9.9±9.9) | 0.91 | <0.0001 |
| E2 | 232 | 0.05 | 2.5 (3.7±3.7) | 0.001 | 2.3 (3.3±3.2) | 0.90 | <0.0001 |
| E3 | 232 | 0.05 | 7.8 (12.5±15.8) | 0.001 | 7.6 (11.8±14.7) | 0.89 | <0.0001 |
| 16α-OHE1 | 232 | 0.05 | 1.9 (3.4±5.2) | 0.001 | 1.5 (2.4±3.3) | 0.72 | <0.0001 |
| 2-OHE1 | 232 | 0.05 | 8.1 (13.0±16.7) | 0.001 | 4.5 (7.2±8.2) | 0.85 | <0.0001 |
| 2:16 ratio | 232 | --- | 4.4 (7.1±9.1) | --- | 3.3 (13.2±38.0) | 0.69 | <0.0001 |
| F | 137 | 0.1 | 67.2 (79.3±58.4) | 0.001 | 65.8 (81.7±64.3) | 0.84 | <0.0001 |
| E | 137 | 0.1 | 95.1 (106.6±63.1) | 0.001 | 102.6 (116.4±82.6) | 0.80 | <0.0001 |
urine specimens with undetectable levels were included in the analysis using as value one-tenth of the minimum detection limit; number of observations (N) below the minimum detectable levels were 1 for 2-OHE1 by GCMS; 32 for 16α-OHE1 by GCMS
LOQ=limit of quantitation (in μg) at a signal to noise ratio of 3; limit of quantitation was ≤2-fold higher
variable limits depending on noise levels
Spearman’s rank order correlation coefficient (r) and its p-value (p) comparing LCMS to GCMS
Comaprison of LCMS to GCMS results
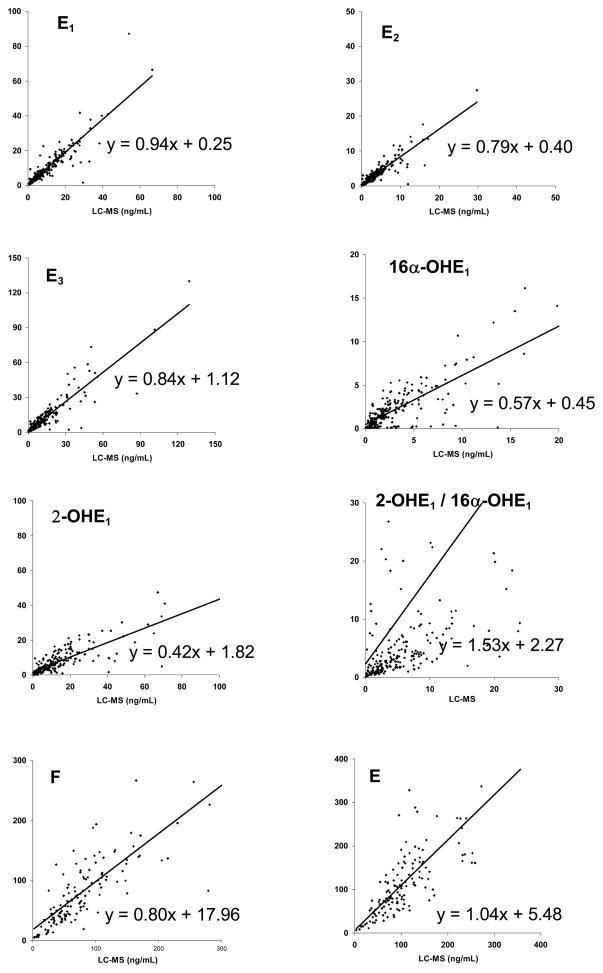
Comparing the LCMS results to those using traditional GCMS showed excellent agreement between these 2 methodologies for the steroidal estrogens (r=0.72–0.91; n=232) and also for the corticoids (r=0.80–0.84; n=137) (Table 4). Importantly, absolute concentrations also agreed; the median levels were almost identical (<5% difference) according to the two techniques for E1, E2, E3, 16α-OHE1, and F (Table 4). For E, the difference was only slightly higher (7%), but for 2-OHE1, LCMS found markedly higher median levels compared to GCMS (8.1 vs. 4.5 ng/mL). Despite this difference for 2-OHE1, the correlation of the 2 methodologies was very strong for this analyte (r=0.85) (Table 4).
DISCUSSION
The presented benchtop orbitrap based LCMS method measured after hydrolysis E1 and E2, five estrogen metabolites in the 16-OH pathway (16α-OHE1, E3, 16epi-E3, 17epi-E3, 16-ketoE2), five in the 2-OH pathway (2-OHE1, 2-OHE2, 2-MeOE1, 2-MeOE2, 2-OH-3-MeOE1), and four in the 4-catechol pathway (4-OHE1, 4-OHE2, 4-MeOE1, 4-MeOE2). The instability of catechol estrogens was taken into account by employing a catchol estrogen isotope as internal standard that corrects for potential losses of catecholic analytes. If separation of the isobaric analytes is not required, a much shorter LC run than the 35 minutes needed for the separation of all estrogens can be applied without impairing any results. The correlations between LCMS and GCMS results were very good; the Spearman correlations for steroidal estrogens were between 0.72 and 0.91 (n=232) and for the corticoids between 0.80 and 0.84 (n=137; table 4). The 2:16 ratio correlation was weaker (r=0.69) but agreed with a recent study that compared tandem LCMS with immunoassays (r=0.68) using urine collected from 264 premenopausal women in the luteal phase just as in the present study [5]. In fact, that study investigated the same estrogens as in our LCMS-GCMS comparison and found very similar LCMS-immunoassays correlations for all analytes (Table 4). Due to the suboptimal agreement in the 2:16 ratio found between the investigated methods, we recommend to express differences observed within a study on a relative basis. In this way, comparisons between studies using different methods are still valid due to the good correlations reported between these methods.
The absolute levels of the analytes also agreed very well in our comparison; the median levels were almost identical (<5% difference) between the two techniques for E1, E2, E3, 16α-OHE1, and F. For E, the difference was 7%. For 2-OHE1, however, LCMS found higher median levels compared to GCMS (8.1 vs. 4.5 ng/mL) probably because the catecholic internal standard used during LCMS analysis corrected for losses. Despite this difference, correlation between our 2 methodologies regarding 2-OHE1 was very good (r=0.85). LCMS-GCMS differences in absolute levels could be due to the differential internal standards used. While LCMS used the structurally closely related compound 4-OHE2-d5, GCMS used 5α-androstan-3α, a molecule that was less related to the analyte and would not adjust for losses due to catecholic oxidation. Another cause for discrepancy could be the instrument calibration. Determination of accurate stock solution concentrations needed for instrument calibration is best assured by absorbance readings instead of by weight measurements as large errors can be introduced, particularly when small amounts need to be weighed, when agents are hygroscopic, or only slightly soluble [21]. Therefore, we compiled the available information on extinction coefficients of estrogens (Table 2) and used those consistently for measuring the concentrations of all standards used for calibrating the LCMS instrument. ε-values of E1, E2, and E3 metabolites that were not available in the literature were deduced from those of E1, E2, and E3, respectively, since the additional hydroxy, methoxy, or keto groups in the metabolites will not act as or contribute to existing chromophores (Table 2).
The median levels we detected for E1, E2, 2-OHE1 (7.0, 2.5, 7.8 ng/mL)are in excellent agreement with the geometric means reported recently using tandem LCMS (6.3, 3.0, 7.0 ng/mL) [5] when units were converted to ng/mL [22].
A major improvement of our versus other LCMS methods is the short run time and better selectivity [4]. Also, our LCMS system allows the analysis of additional steroids such as P, F, E, and 6-hydroxycortisol (data not shown) even if they were not targeted a priori, by quantitation via reinterrogation of acquired data many months after the original project was completed. If time and resources had allowed, we could have extended the analysis to even more analytes. The scope of projects employing the presented technique in the future can chose the analytes of interest accordingly. This reinterrogation was possible due to the nature of orbitrap MS, which records all molecules with exact masses (± 5 ppm) that are detectable with the chosen MS parameters. However, we were unable to detect pregnanediol, the major gestagen in urine, or DHEA due to the MS conditions optimized for dansylated steroidal estrogens that required electrospray ionization (ESI) and monitoring in positive MS mode. Under these conditions, non-polar molecules such as pregnanediol or DHEA, although present at high levels in urine of premenopausal women, are not detectable [3].
The analytes that can be included in the presented LCMS assay during reinterrogation are limited to those that are not destroyed by the dansylation procedure, that elute within the chosen LC run, and that form positive ions by ESI. On the other hand, a very sensitive detection occurs for molecules containing an abstractable proton and, therefore, undergoing dansylation. Such molecules include phenols (other than estrogens), carboxylic acids, and amines [23]. In that fashion, steroidal estrogens and phytoestrogens can be analyzed in the same assay. The latter are known to be dansylated and to be measured by LCMS in the same way as steroidal estrogens [24]. Consequently, the post acquisition quantitation of the phytoestrogens daidzein and equol in the present urine analyses was easily possible (data not shown). Information on these phytoestrogens may be relevant as these agents have been shown to protect against breast cancer and to improve survival either by themselves or, in the case of isoflavonoids, by acting as markers of soy exposure [25–27]. This protective effect is particularly strong when exposure happens early in life [28–30] and changes of the estrogen metabolism may be responsible for these effects [14]. Including all these analytes in one assay may, therefore, be advisable and will also be very cost efficient.
If the array of analytes needs to be extended, the presented LCMS assay can be set up without compromising data quality to include all negatively charged molecules. The problem of ion suppression inherently connected to ESI can be overcome by adjusting for internal standards, i.e. stable isotopes (2H, 13C, 15N) of the analytes [3]. However, if additional analytes need to be included but had not been targeted initially, the quantitation during the reinterrogation is suboptimal since ideal internal standards are not available for adjustment. As an alternative, chemically related internal standards can be used, a procedure commonly performed for multiple analytes during MS analysis [31] including in the steroid field [19]. Our reinterrogated analytes were not adjusted for by any internal standards and overall correlations did not change after we used any of the available estrogen isotopes as internal standards. This lack of adjustment during LCMS analysis of the corticoids may have led to the lower LCMS-GCMS correlations.
In our experience, even when the same LC conditions were used, the benchtop orbitrap based method presented here was superior to our tandem MS (model TSQ, Thermo) based method [31] commonly used in steroid analyses [3, 32] due to less noise and the lack of need to divide the MS measurements into time dependent segments that could lead to major problems including total loss of data in case retention times change. The option to reinterrogate data reliably after the analyses are completed make orbitrap MS based methods much more attractive than tandem MS based methods.
We had not undertaken special efforts to lower the LOQs in our LCMS method since the levels occurring in urine of premenopausal women did not warrant this. The much lower LOQs found for GCMS (Table 4) are probably due to the much larger amount of starting material used (33 fold more). After adjustment for this difference, the LOQs were very comparable. In addition, we could fine tune our LCMS method to improve those limits by using a larger amount of starting material, by concentrating the extract several-fold higher, and/or by using smaller columns and eluent flows as reported recently [33].
Based on a recent analysis of urine samples from premenopausal women during the luteal phase within the Nurses Health Study, nine of the steroidal estrogens (E1, E2, 16α-OHE1, E3, 16-ketoE2, 2-OHE1, 2-OHE2, 2-MeOE1, 4-OHE1) represent more than 90% of 15 measured urinary estrogen metabolites [16]. Similarly, in a report that included 10 premenopausal women during luteal phase, these nine compounds represented 89% of all measured estrogen metabolites [18]. Therefore, we focused on these analytes regarding method validation (Table 3). Using our benchtop orbitrap based LCMS, intra-assay CVs were 11% or less for most analytes (13% for 4-OHE1) and inter-assay CVs were 9% or less for most analytes (12% for 4-OHE1, 17% for 4-OHE1). For GCMS, intra-assay CVs were less than 5% for E1, E2, E3, 2-OHE1 and 16α-OHE1. Inter-assay CVs for the same analytes were 9% or less for all analytes. (Table 3) Otherwise, LCMS [4, 18] and GCMS [19] methods have been extensively validated previously and did not require additional evaluations.
One advantage of GCMS is the ability to profile steroids broadly using one derivatization protocol. In addition to the analytes shown in table 4, 17β-dihydroandrosterone, 5-androstenediol, dihydroepiandrosterone, androstenetriol, androsterone, etiocholanolone, pregnanediol, 5-pregnenetriol, tetrahydrocortisol and allotetrahydrocortisol, all nonpolar molecules that escaped LCMS analysis, could be quantitated with the GCMS method described here. The latter will remain valuable for the inclusion of those analytes into one assay. However, reinterrogation of recorded GCMS data regarding non-targeted analytes is not possible as selected ion monitoring is chosen in order to maximize sensitivity. Also, the urine volume required for GCMS is 10 mL, which is greater than 33 fold more than the amount required for LCMS. While our chromatographic run times were approximately 35 minutes for both presented methods, in line with other reported GCMS assays of steroids [34], the overall turnaround time for the entire assay was approximately 3 times faster of our LC vs. GC based assays.
In conclusion, our orbitrap based LCMS is superior to traditional quadrupole based GCMS if productivity and sample material is an issue. Therefore, our LCMS may be more suitable than GCMS for projects with large number of samples, such as epidemiologic studies. However, the decision to use LCMS versus GCMS will ultimately depend on the array of analytes desired and the amount of urine available.
Fig. 2. LCMS-GCMS correlations.
Linear regression data for the urinary concentrations measured by LCMS and GCMS of estrone (E1), estradiol (E2), estriol(E3), 16α-hydroxyestrone (16α-OHE1), 2-hydroxyestrone (2-OHE1), the ratio of 2-hydroxyestrone to 16α-hydroxyestrone (2-OHE1/16α-OHE1), cortisol(F), and cortisone (E). All quantitations applied adjustments for internal standards but the latter were not identical for all analytes (see Methods for details)
Acknowledgments
Funding: This project was funded by grants from the National Cancer Institute R01 CA 80843 and P30 CA71789 and from the National Center for Research Resources S10 RR020890.
We thank Jennifer F. Lai (University of Hawaii Cancer Center) for the skillful assistance with manuscript preparations. Support for this study was obtained by grants from the National Cancer Institute R01 CA 80843 and P30 CA71789 and from the National Center for Research Resources S10 RR020890.
References
- 1.Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:1101–1108. [PubMed] [Google Scholar]
- 2.Sepkovic DW, Bradlow HL. Estrogen hydroxylation--the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 3.Rauh M. Steroid measurement with LC-MS/MS in pediatric endocrinology. Mol Cell Endocrinol. 2009;301:272–281. doi: 10.1016/j.mce.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 5.Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 7.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169:1251–1259. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, Barbieri RL, Hankinson SE. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:967–971. doi: 10.1158/1055-9965.EPI-05-0976. [DOI] [PubMed] [Google Scholar]
- 9.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 10.Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect. 1995;103(Suppl 7):147–150. doi: 10.1289/ehp.95103s7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebert DW. Elevated estrogen 16 alpha-hydroxylase activity: is this a genotoxic or nongenotoxic biomarker in human breast cancer risk? J Natl Cancer Inst. 1993;85:1888–1891. doi: 10.1093/jnci/85.23.1888. [DOI] [PubMed] [Google Scholar]
- 12.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 13.IARC. Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. Albany, NY, USA: World Health Organization; 2007. p. 543. [PMC free article] [PubMed] [Google Scholar]
- 14.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 15.Mense SM, Chhabra J, Bhat HK. Preferential induction of cytochrome P450 1A1 over cytochrome P450 1B1 in human breast epithelial cells following exposure to quercetin. J Steroid Biochem Mol Biol. 2008;110:157–162. doi: 10.1016/j.jsbmb.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maskarinec G, Morimoto Y, Conroy S, Pagano I, Franke A. No changes in nipple aspirate fluid volume during a randomized soy trial. J Nutr. 2011;141:626–630. doi: 10.3945/jn.110.133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shackleton CH. Jun 20) Profiling steroid hormones and urinary steroids. J Chromatogr. 1986;379:91–156. doi: 10.1016/s0378-4347(00)80683-0. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Departement of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validation. 2001. [Google Scholar]
- 21.Franke AA, Halm BM, Kakazu K, Li X. Metabolism, Bioavailability, and Analysis of Dietary Isoflavones. In: Fraga C, editor. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology The Wiley-IUBMB Series on Biochemistry and Molecular Biology. Wiley & Sons; 2009. [Google Scholar]
- 22.Franke A, Halm B, Ashburn L. Isoflavones In Children and Adults Consuming Soy. Arch Biochem Biophys. 2008;476:161–170. doi: 10.1016/j.abb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Bartzatt R. Dansylation of hydroxyl and carboxylic acid functional groups. J Biochem Biophys Methods. 2001;47:189–195. doi: 10.1016/s0165-022x(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–1037. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Q, Franke AA, Jin F, Shu X-O, Hebert JR, Custer LJ, Cheng J, Gao Y-T, Zheng W. Urinary Excretion of Phytoestrogens and Risk of Breast Cancer among Chinese Women in Shanghai. Cancer Epidemiol Biomarkers Prev. 2002;11:815–821. [PubMed] [Google Scholar]
- 27.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila Pa) 2009;2:887–894. doi: 10.1158/1940-6207.CAPR-09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korde LA, Wu AH, Fears T, Nomura AM, West DW, Kolonel LN, Pike MC, Hoover RN, Ziegler RG. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–1059. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- 29.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian- Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 30.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- 31.Franke AA, Halm BM, Kakazu K, Li X, Custer L. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Testing and Analysis. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clin Chem. 2009;55:1061–1066. doi: 10.1373/clinchem.2007.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blonder J, Johann DJ, Veenstra TD, Xiao Z, Emmert-Buck MR, Ziegler RG, Rodriguez-Canales J, Hanson JA, Xu X. Quantitation of steroid hormones in thin fresh frozen tissue sections. Anal Chem. 2008;80:8845–8852. doi: 10.1021/ac801402a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courant F, Aksglaede L, Antignac JP, Monteau F, Sorensen K, Andersson AM, Skakkebaek NE, Juul A, Bizec BL. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95:82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]