Abstract
Vascular calcification contributes to the high risk of cardiovascular mortality in chronic kidney disease (CKD) patients. Dysregulation of calcium (Ca) and phosphate (P) metabolism is common in CKD patients, and drives vascular calcification. In this article, we review the physiological regulatory mechanisms for Ca and P homeostasis and the basis for their dysregulation in CKD. In addition, we highlight recent findings indicating that elevated Ca and P have direct effects on vascular smooth muscle cells (VSMCs) that promote vascular calcification, including stimulation of osteo/chondrogenic differentiation, vesicle release, apoptosis, loss of inhibitors, and ECM matrix degradation. These studies suggest a major role for elevated P in promoting osteo/chondrogenic differentiation of VSMC, whereas elevated Ca has a predominant role in promoting VSMC apoptosis and vesicle release. Furthermore, the effects of elevated Ca and P are synergistic providing a major stimulus for vascular calcification in CKD. Unravelling the complex regulatory pathways that mediate the effects of both Ca and P on VSMCs will ultimately provide novel targets and therapies to limit the destructive effects of vascular calcification in CKD patients.
Keywords: calcium, phosphate, vascular calcification, chronic kidney disease
INTRODUCTION
Vascular calcification is a prevalent pathology in ageing, atherosclerosis, hypertension and diabetes and correlates with increased vessel stiffening and increased risk of myocardial infarction. Its most devastating manifestation occurs in patients with chronic kidney disease (CKD) who exhibit a hugely elevated risk of cardiovascular mortality compared with age-matched controls. CKD patients develop accelerated medial as well as intimal calcification and this calcification rapidly progresses in patients on dialysis.1 The development of calcification in CKD patients is strongly linked to dysregulated mineral metabolism characterized by long term elevation of serum phosphate (P) levels as well as transient bouts of hypercalcemia. Importantly elevated extracellular levels of these minerals have been shown to affect the survival and phenotype of vascular smooth muscle cells (VSMCs) leading to a pattern of cellular adaptations and damage that ultimately promote calcification.
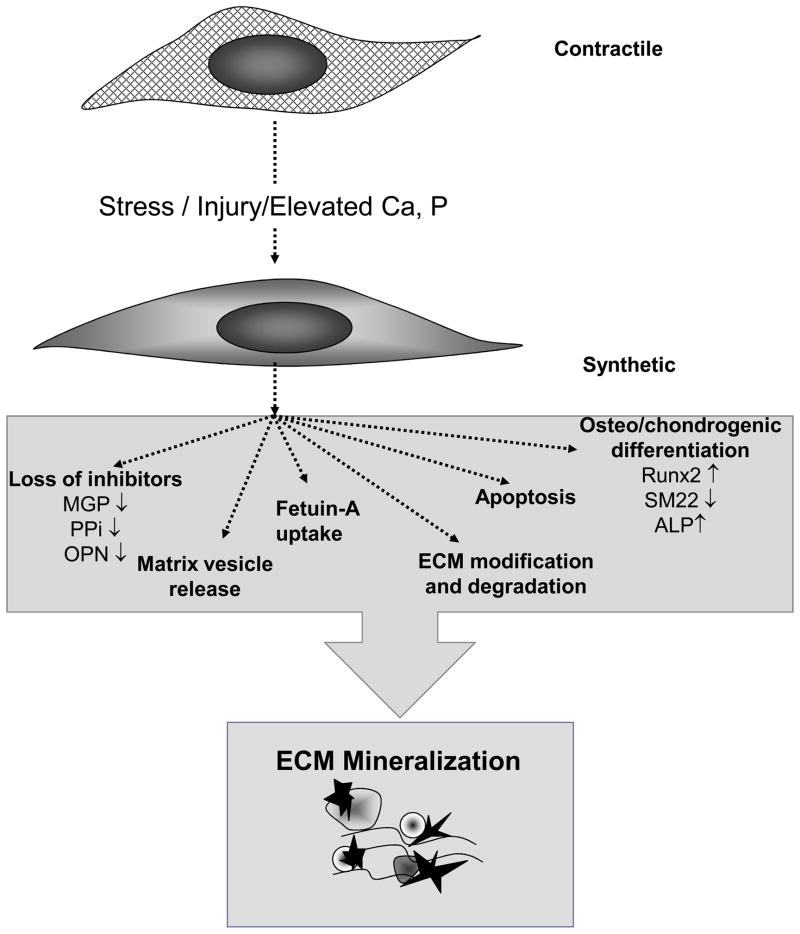
Like developmental skeletal formation, VSMC calcification is a cell-mediated process and over the last 15 years a large number of studies have revealed the key events required for its initiation and promotion (Summarized in Figure 1). These include loss of inhibitor function, development of a calcifiable extracellular matrix, and induction of apoptosis and vesicle release that are accompanied by osteo/chondrogenic differentiation of VSMCs. The key inhibitors of VSMC calcification include the endogenous inhibitors matrix Gla protein (MGP) and pyrophosphate (PPi) as well as the inducible inhibitor, osteopontin (OPN). There are also circulating inhibitors such as fetuin-A that are taken up by damaged VSMCs and utilized to inhibit vesicle calcification. Factors that induce VSMC death by apoptosis accelerate calcification by removing cells capable of inhibition. In addition, the resulting cellular vesicular debris can nucleate mineral to promote calcification. Concomitant with inhibitor loss and cell death VSMCs also undergo osteo/chondrogenic differentiation and produce a number of mineralization-regulating proteins that act to orchestrate the calcification process. Like chondrocytes and osteoblasts these osteo/chondrogenic VSMCs also release calcium (Ca)-enriched membrane bound bodies called matrix vesicles that can nucleate hydroxyapatite in the absence of inhibitors and form the first nidus for calcification.
Figure 1. Vascular calcification is mediated by VSMCs.
Alterations in Ca and P levels and/or vascular insult lead to osteo/chondrogenic conversion of VSMCs in the vascular wall. This is associated with dramatic loss of mineralization inhibitors, the production of calcifying matrix vesicles and ECM degradation. In addition, Ca and P induce VSMC apoptosis and release of apoptotic bodies, which in turn, form the initial nidus for vascular calcification.
Much work has shown that elevated levels of P and Ca have direct effects on VSMC function and promote calcification. These mineral ions can be taken up or sensed by VSMCs in a number of different ways and this affects the resultant response of the VSMC to the stimulus. In this review we will discuss studies that have revealed the mechanisms whereby P and Ca alone or together act to promote VSMC dysfunction and calcification in CKD.
ELEVATED SERUM P, CARDIOVASCULAR DISEASE, AND VASCULAR CALCIFICATION IN CKD
Elevated serum P is now recognized as a major risk factor for cardiovascular events in CKD2–4, as well as the general population.5–7 Mortality in end stage renal disease patients is strongly correlated with serum P levels greater than 5.5 mg/dL.8–10 Also, relatively small elevations in serum P in the high normal range (3.5–4.5 mg/dL) have been correlated with increased risk of cardiovascular and all-cause mortality in both CKD patients11 and the general population with normal renal function.12 Increased susceptibility of CKD patients to vascular calcification likely underlies this high risk of CVD related deaths in CKD patients.
DYSREGULATION OF P HOMEOSTASIS IN CKD
Phosphorus (mostly in the form of inorganic P (Pi)) is an important component of cellular and systemic homeostasis. It is needed for ATP generation, intracellular signaling and pH buffering, as well as being a prime component of bone, phospholipids and nucleic acids. In humans, the majority of P (85%) is found in bone while 14% is intracellular and 1% is in extracellular fluid.13 The western diet is rich in P sources, including meat, fish, dairy products and additives. Most of the ingested P is absorbed by the gastrointestinal tract via passive enterocyte paracellular pathways or the sodium-dependent P cotransporter (NaPi)-IIb.14 Under normal conditions, 80–90% of a filtered P load is reabsorbed in the renal proximal tubule predominantly via the active NaPi- IIa.15 While the kidney is the major regulator of serum P homeostasis under normal conditions, bone also serves as a P reservoir that can contribute to serum P regulation via bone formation and resorption.16
Hormonal regulators of P balance have been identified, including parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), klotho, and 1, 25-dihydroxyvitamin D. These hormones act mainly by modulating renal reabsorption or intestinal absorption of P.16 Both PTH and FGF-23 promote renal P wasting by stimulating the internalization and inactivation of the NaPi-IIa transporter, thereby decreasing renal P reabsorption. PTH also promotes bone remodeling, thereby moving P in and out of bone.16 Klotho is a required cofactor for the actions of FGF23, but it also promotes phosphaturia independently by inactivating NaPi-IIa.17 1, 25-dihydroxyvitamin D promotes intestinal absorption of P by increasing NaPi-IIb transporters. Normally, these systems act to maintain serum P levels in a normal range of 2.8–4.5 mg/dL.18
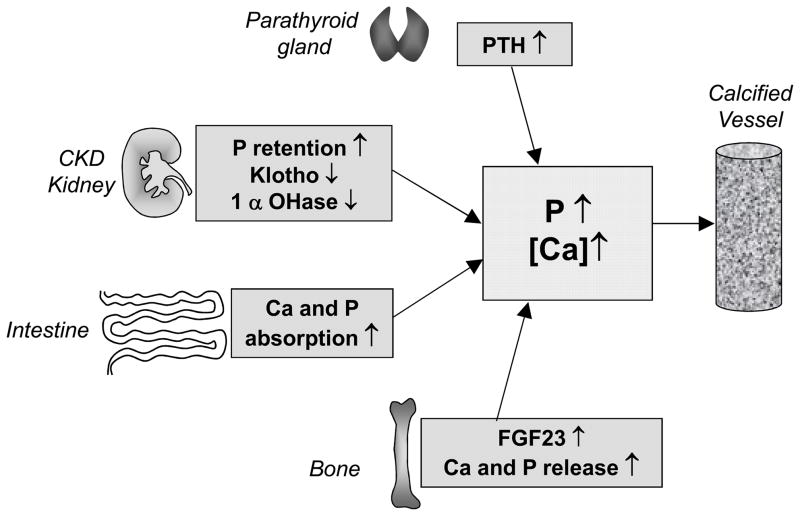
In early CKD, renal insufficiency leads to impaired P excretion and a decline in klotho levels, but serum P levels are maintained in the normal range by upregulation of FGF23 and PTH.19–21 Unfortunately, these defense mechanisms are overwhelmed as renal function continues to fall. As glomerular filtration rate declines in advanced CKD, inefficient urinary P excretion combined with disordered bone remodeling and continued ingestion of P results in hyperphosphatemia. A summary of factors controlling P homeostasis in CKD is shown in Figure 2.
Figure 2. Overview of the factors involved in dysregulated Ca and P homeostasis in CKD.
In early CKD renal insufficiency leads to a decline in klotho levels and impaired P excretion, but serum P levels are maintained in the normal range by upregulation of FGF23 from bone and PTH from the parathyroid gland. Declining renal function in CKD leads to 1, 25-dihydroxyvitamin D deficiency due to diminished activity of 1-alpha hydroxylase in the kidney as well as increased serum FGF23 levels (a direct inhibitor of 1 alpha hydroxylase activity). Low 1, 25-dihydroxyvitamin D levels lead to initial hypocalcemia, which together with hyperphosphatemia, provide a powerful stimulus for further PTH secretion and lead to the secondary hyperparathyrodism of CKD, and increased bone remodelling. Normal defence mechanisms (PTH, FGF23, and klotho) are overwhelmed as renal function continues to fall. Vitamin D deficiency and secondary hyperparathyroidism are treated with VDRAs that stimulate Ca and P uptake in the gut, often leading to transient hypercalcemic episodes (denoted by parentheses). Furthermore, Ca-containing P binders are commonly used to treat hyperphosphatemia, further increasing Ca burden in these individuals. Together with disordered bone remodelling, these factors contribute to dysregulated P and Ca metabolism, and promote vascular calcification in CKD.
HOW IS P SENSED BY VSMC?
The primary mechanism by which P enters cells is by NaPi cotransporters. There are three major families of NaPi cotransporters that are distinct based on structure, tissue expression, and biochemical characteristics.22, 23 Type I NaPi cotransporters (the SLC17 family) are found in the liver, kidney and brain. Type II (the SLC34 family) NaPi cotransporters are mainly present in the kidney, intestine and lung. In contrast, the type III NaPi cotransporters (the SLC20 family), known as PiT-1 and PiT-2 are ubiquitously expressed throughout the body, and are the major Pi transport proteins found in VSMCs.24, 25 The level of mRNA expression of PiT-1 and PiT-2 in VSMCs varies between species, as measured by RealTime polymerase chain reaction. In human VSMCs, PiT-1 was shown to be the predominant P transporter, with 8-fold higher RNA expression then PiT-2, while no expression of type I or type II NaPi cotransporters were detected.26 However, similar expression levels were found for PiT-1 and PiT-2 in rat VSMCs 27, and mouse VSMCs.
Pi uptake through PiT-1 and PiT-2 occurs in a time and concentration dependent manner. The uptake is also dependent on an inwardly facing sodium gradient, and follows a 2:1 Na+ to H2PO4− ratio.28, 29 Kinetic studies of the PiT transporters have shown an apparent affinity for H2PO4− following Michaelis-Menten kinetics with a Km= 25–100 μM, and saturation below physiological P levels (< 1.4mM) in rat VSMCs.27, 29 While the physiological roles of PiT-1 or PiT-2 have not been fully elucidated, PiT-1 appears critical for embryonic development. PiT-1 knockout embryos arrest between E11.5 and 13.5, and display severe anemia with abnormal yolk sac vasculature.30, 31 PiT-1 also appears to be critical for P-induced osteo/chondrogenic phenotype change and matrix mineralization in cultured VSMC as described in further detail below. Transgenic rats that overexpress PiT-1 have been created.32, 33 The effects of this overexpression included increased serum P level, decreased bone mass, and progressive proteinuria, while cultured osteoblasts had increased ALP activity and P uptake.32, 33 These transgenic rats died of cachexia around 32 weeks of age, and proteinuria appeared to be due to P-dependent podocyte injury induced by overexpression of PiT-1.32, 33 No knockout of PiT-2 has yet been reported.
Various cell and tissue types in the body are well known to have compensatory methods to respond to changes in extracellular P concentration (reviewed by Khoshniat et al34). Renal 35 and intestinal 36, 37 cells can increase uptake in low P media or decrease uptake in high P media. However, it is currently unclear how VSMCs sense changes in serum P. Some evidence suggests that PiT-1 and/or PiT-2 might function as P sensors in addition to transporters. Similar to VSMCs, PiT-1 was identified as the major NaPi cotransporter expressed in the parathyroid.38, 39 PTH secretion by the parathyroid gland is regulated by extracellular P 40, 41 and PiT-1 is hypothesized to function as the mediator between serum P concentrations and PTH secretion.38, 39 PiT-2 has also been implicated in P sensing after Salaun et al found that transport-deficient PiT-2 mutants still had the ability to bind P ions and undergo structural reorganization/ oligomerization.42 Whether other P sensors or receptors exist is currently unknown.
It is also unclear whether uptake of P and a subsequent rise in intracellular P levels is required to initiate VSMC changes in response to elevated extracellular P. In particular, a role for P transport as a key mediator of PiT-1 and PiT-2 function in vascular calcification was called into question by the studies of Villa-Bellosta et al. in rat VSMCs showing that PiT-1 and PiT-2, high affinity P transporters, are likely to be saturated under physiological P concentrations.27 Furthermore, most historical studies have used phosphonoformic acid (PFA), a weak inhibitor of type III NaPi cotransporters, to determine the requirement of P transport for VSMC calcification in response to elevated P. The Ki of PFA for PiT-1 and PiT-2 is in the range of 2.5–5 mM.29, 43 However, we now know that inhibition of VSMC calcification by PFA at these concentrations can also cause physicochemical inhibition of Ca-P crystal formation, confounding interpretation of results.44 Finally, as mentioned for PiT-2 above, evidence that PiT-1 can signal independently of P uptake was provided by Beck et al who showed that a transport deficient mutant of PiT-1 stimulated cell proliferation in HeLa and HepG2 cells.45 Whether the requirement for PiT-1 in P-induced VSMC calcification is P uptake-dependent or –independent remains a critical, unanswered question.
DIRECT EFFECTS OF HIGH P ON PATHWAYS MEDIATING VSMC CALCIFICATION
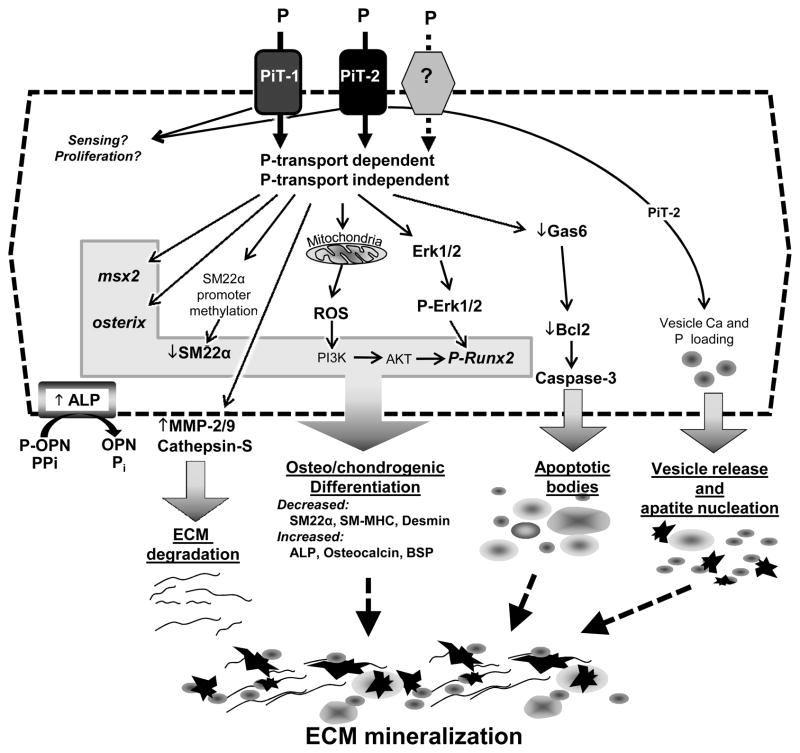
Numerous studies have shown that culturing VSMCs in elevated P conditions leads to matrix calcification. While other cell types such as pericytes 46 and vessel-derived stem cells 47 may also play a role in vascular calcification, this review will focus on VSMC since most of our understanding of effects of elevated P have come from this cell type. Potential mechanisms that mediate P-induced VSMC matrix calcification include osteo/chondrogenic conversion, apoptosis, and matrix remodeling, as shown in Figure 3, and discussed in detail below.
Figure 3. Role of P in VSMC Calcification.
Elevated extracellular P affects multiple signalling pathways that increase the susceptibility of VSMC to calcification including decreased calcification inhibitors, increased ECM degradation, osteo/chondrogenic differentiation, apoptosis and vesicle release. Some of the effects of P are mediated through sodium dependent phosphate co-transporters, Pit-1 and Pit-2, potentially via P transport-dependent and -independent activities. Whether other receptors exist that mediate specific downstream signalling pathways in response to P is not yet known, but cannot be excluded.
VSMC Osteo/chondrogenic conversion
P-induced vascular calcification has been hypothesized to be an adaptive VSMC transition from a contractile to an osteo/chondrogenic phenotype characterized by calcifying vesicle formation, downregulation of mineralization inhibitory molecules, and elaboration of a calcification prone matrix. In culture, elevated P exposure results in the upregulation of osteo/chondrogenic gene expression (Runx2, osterix, alkaline phosphatase (ALP), OPN), and simultaneous downregulation of smooth muscle lineage gene expression (SM alpha actin, SM22α).28, 48–52 Runx2 is a transcription factor important in osteoblastic and chondrocytic differentiation that induces the expression of major bone matrix components including type I collagen, osteocalcin, and OPN. Knockdown of Runx2 in VSMC inhibits osteogenic conversion 49, 53 and matrix mineralization.49 ALP is absolutely required for normal bone formation, and is thought to regulate vascular matrix mineralization by inactivating the mineralization inhibitors, PPi and P-OPN, and simultaneously liberating free P.54–56
Further, osteo/chondrogenic phenotype change induced by elevated P is characterized ultrastructurally by the appearance of matrix vesicles containing apatite and calcifying collagen fibrils on the surface of VSMCs.57 As in bone, these vesicles most likely act as early nucleation sites for calcification. In addition, hydroxyapatite (HA) nanocrystals shed from vesicles may further promote mineralization via direct effects on SMC phenotype. In support of this concept, synthetic HA nanocrystals and isolated high-P-induced nanocrystals induced osteo/chondrogenic gene expression in VSMC.58, 59
The receptors and signaling pathways important for P-mediated VSMC osteo/chondrogenic phenotype change and calcification are beginning to be unravelled. Our group has demonstrated a role for PiT-1 in human VSMC osteogenic conversion and matrix mineralization. Small hairpin RNA (shRNA) knockdown of PiT-1 suppressed P-induced calcification and blocked induction of osteogenic markers, including Runx2, but did not mediate P loading of matrix vesicles or apoptosis.26 Matrix vesicle loading of P may instead be mediated by PiT-2, as this transporter, and not PiT-1, was localized to vesicle membranes.26 Of interest, it was subsequently shown that shRNA knockdown of Pit-1 abrogated matrix mineralization in osteoblast cultures 60, 61 confirming the idea that Pit-1 is a key mediator of elevated P-induced calcification in mineralizing cell types.
The induction of Runx2 via PiT-1 signaling is believed to be though Erk1/2 activation. Treatment of mouse VSMCs with high P, and subsequent calcification and osteo/chondrogenic differentiation, occurred in conjunction with increased phosphorylation of Erk1/2.62 Prevention of Runx2 upregulation by high P was achieved through inhibition of Erk phosphorylation by the mitogen-activated protein kinase (MAPK) inhibitor U0126. This also stimulated expression of VSMC lineage markers.62
Bone morphogenetic protein 2 (BMP-2) and transglutaminase 2 (TG2) have also been implicated as downstream mediators of P-induced VSMC calcification. In a study with human VSMCs, addition of noggin, a BMP2 inhibitor, blocked mineralization due to high P media, and blocked expression of the osteogenic transcription factor osterix.63 Interestingly, BMP-2 levels are increased in the serum of uremic patients, and culturing bovine VSMCs in uremic serum resulted in increases of Runx2 that was inhibited by noggin.64 Furthermore, mouse VSMCs deficient in TG2 showed a blunted response to treatment with 2.5 mmol/L P, showing a lack of upregulation of ALP, PiT-1, Runx2, and the osteoblastic transcription factor, Msx2.65 TG2 also has a direct role in stabilization of extracellular matrices by producing protease-resistant isopeptide bonds in substrate proteins such as collagen I, fibronectin, and OPN.66
Montes de Oca et al found that human aortic smooth muscle cells and rat aortic rings incubated with high P media (3.3 mM) had increased methylation of the SM22α promoter leading to osteogenic conversion. The increased methylation resulted in reduced expression of SM22α, increased Runx2 expression, and increased ALP activity, which together resulted in an increased level of calcification.67 Inhibition of DNA methylation by procaine was able to prevent these transcriptional changes when VSMCs were grown in high P media, and this resulted in reduced calcification.
Reactive oxygen species (ROS) produced in response to high P loads have also been implicated as downstream mediators of osteogenic conversion of VSMCs during vascular calcification. In isolated mitochondria, P can regulate the mitochondrial membrane potential which is important in the production of ROS.68 Byon et al. demonstrated that oxidative stress in the form of H2O2 could increase the expression and activity of the Runx2 transcription factor in mouse VSMCs, leading to conversion to an osteogenic phenotype and increased calcification.69 Downregulation of Runx2 by shRNA eliminated the ability of H2O2 to cause the phenotypic change. They also determined that the Runx2 response was dependent on PI3K/AKT/Runx2 signaling.69 More recently, Zhao et al. used bovine aortic smooth muscle cells to show that ROS production is important in the osteogenic conversion process using β-glycerophosphate (BGP) exposure as their model.70 BGP is an organic P donor that leads to elevated P through the action of ALP, and in this study promoted downregulation of SMC lineage markers, an increase in osteogenic markers, and a 3-fold increase in ROS production. Inhibition of mitochondrial ROS formation by the superoxide dismutase (SOD) mimic MnTMPyP or the respiratory chain inhibitors rotenone or CCCP greatly reduced the level of mitochondrial ROS and significantly decreased Ca deposition compared to treatment with BGP alone. Further, it was determined that mitochondrial ROS uses NF-κB signaling during BGP mediated calcification. The receptor mediating elevated P-induced ROS was not determined.
In vivo evidence supporting a role for osteo/chondrogenic differentiation in elevated P induced vascular calcification has been obtained in both animal and clinical studies. In uremic mice, osteo/chondrogenic conversion was observed in calcified vessels associated with high P feeding.71, 72 In rats made uremic and hyperphosphatemic with adenine treatment, chondrocytic conversion was observed in the calcified arterial media73, and lowering P with lanthanum carbonate treatment significantly reduced vascular calcification as well as Runx2 and collagen type II expression.74 In people, increased OPN and decreased SM alpha actin was observed in biopsies from dialysis patients with calcific uremic arteriolopathy 75, and increased OPN, bone sialoprotein and Runx2 levels were characteristic of calcified inferior epigastric arteries of renal transplant patients.76, 77 Finally, increased Runx2, osterix, and ALP expression was observed in arteries from pediatric predialysis and dialysis patients.78
Apoptosis-dependent matrix mineralization
Another mechanism whereby elevated P might promote vascular matrix calcification is by stimulating VSMC apoptosis. Downregulation of growth arrest-specific gene 6 (Gas6) may be an important underlying mechanism. During P induced human aortic VSMC calcification, both Gas6 and its receptor Axl expression are reduced.79 The Gas6-Axl survival pathway was previously implicated in osteogenic differentiation of vascular pericytes.80 Its antiapoptotic effect is achieved through the Bcl2-mediated phosphatidylinositol 3-kinase/protein kinase B (PI3K-AKT) pathway; phosphorylation inactivates Bcl2 and activates the proapoptotic protein Bcl-2-associated death promoter, resulting in caspase-3 activation and apoptosis.81 Again, the receptor mediating this effect of elevated P was not identified.
In vivo evidence supporting a role for cell death in elevated P induced vascular calcification include the findings of smooth muscle cell drop out in the arterial media of uremic, high P fed mice concomitant with mineral deposition.71, 72 Furthermore, calcified vessels from pediatric dialysis patients exhibited extensive smooth muscle cell apoptosis.78
P Effects on Vascular Matrix Degradation
In vivo, VSMCs are surrounded by a complex, highly structured extracellular matrix (ECM) composed of collagen, elastin, fibronectin, heparan sulfate, proteoglycans, and chondroitin sulfate proteoglycans. A consistent feature of the predominant type of arterial medial calcification induced by CKD and hyperphosphatemia in people and animal models is the accumulation of linear mineral deposits along the arterial elastic lamina (elastocalcinosis).71, 82, 83 It is known that elastin degradation increases the ECMs affinity for Ca84, facilitating epitactic growth of hydroxyapatite along the elastic lamellae. Elastin fragments are also able to bind to elastin laminin receptors (ELRs) found on the surface of VSMCs, and through transforming growth factor (TGF)-β signaling 85, can increase proliferation and upregulate Runx2, resulting in osteogenic differentiation.86, 87 This process was seen in rat aortic rings treated with high P and warfarin which led to an early expression of matrix metalloproteinase (MMP)-9, an elastin degrading enzyme, closely followed by TGF-β signaling and VSMC osteogenic lineage reprogramming.88 Additionally, both MMP-2 and MMP-9 knock-out mice were resistant to elastin degradation and calcification.89 The mineralization process can also be markedly accelerated in human VSMCs when the soluble elastin-derived peptide α-elastin is added to the high (2.5mM) P media.90 Interestingly however, under normal P load (1.4mM) addition of α-elastin did not result in calcification. Because the α-elastin peptide did not induce VSMC mineralization under normal P conditions, it has been suggested that P-induced VSMC osteogenic differentiation needs to be present before α-elastin can exert procalcification effects.90 Indeed, in vivo, elastin degradation alone in the absence of a P load was insufficient to induce vascular calcification in uremic mice.83
In vivo evidence supporting a role for matrix remodeling in elevated P induced vascular calcification include studies in uremic, high P-fed mice that observed elastin remodelling and elevated elastase levels including MMP2, MMP9 and cathepsin S in calcified arterial medias.83 Furthermore, cathepsin S was required for arterial calcification in ApoE−/− mice with chronic renal insufficiency.91 Finally, a strong correlation between MMP-2 upregulation and elastic fiber disorganization, stiffness, calcification and vasomotor dysfunction was observed in the arterial vasculature in dialysis patients92, and upregulation of arterial MMP-2 and MMP-9 were correlated with arterial stiffening in diabetic CKD patients.92
ELEVATED CA, CARDIOVASCULAR DISEASE, AND VASCULAR CALCIFICATION IN CKD
Although much of the epidemiological and experimental evidence to date has focussed on the role of elevated serum P as the main trigger of calcification in CKD, evidence also implicates a pivotal role for elevated serum Ca and an elevated Ca × P product, in driving calcification.93 Historically elevated serum Ca has been associated with increased risk of myocardial infarction, coronary calcification and plaque thickness in the non-CKD population. More recently a number of studies have shown an association with elevated serum Ca and calcification in the CKD population.94–96 A strong correlation between calcification and an elevated Ca × P product has also been demonstrated and in many of these studies the association between cardiovascular dysfunction or mortality was found with Ca levels in the high normal range.10 It should be noted that serum Ca is tightly regulated and this may be one reason why Ca has been overlooked as a risk factor driving calcification when compared to P. However sporadic hypercalcemia is a relatively common occurrence in dialysis patients in response to a number of factors including dialysate composition, vitamin D therapy and potentially Ca containing P binders and these sporadic events are often not taken into account or may be missed in routine blood analysis.97 Overall these studies suggest that even sporadic elevations of Ca in a high P environment is highly detrimental 2, 10 and this idea is supported by clinical trials showing that use of non-Ca based P binders attenuated vascular calcification 98–101 and more controversially, mortality 99, 102, in dialysis patients.
DYSREGULATION OF CA HOMEOSTASIS IN CKD
Ca (in the diffusible, ionized form) is critical for a number of physiologic processes including neuronal signaling, muscle contraction, and blood clotting, in addition to being a major component of bone. In humans, 99% of Ca is found in bone with the remainder in blood and cells. Serum Ca is normally bound to protein such that the free, ionized Ca makes up roughly half of the total Ca. The American diet provides about 600–1000 mg Ca per day, primarily from dairy sources. Ca is absorbed in the small intestine by both diffusion driven paracellular processes and active transport mechanisms. Active transport occurs principally in the duodenum via the coordinated action of transient receptor potential vallinoid receptor type 6 (TRPV6) channels, calbindin-D and the Ca-adenosine triphosphatase, PMCA1b. 98% of the filtered Ca is reabsorbed by the kidney via paracellular processes and active transport through TRPV5 channels, calbindin-D and the Na-Ca exchanger, NCX1 and PMCA1b.103 In addition, about 500 mmole Ca per day normally moves in and out of bone through bone formation and resorptive processes. 104
The principal mechanisms controlling serum Ca levels are active vitamin D metabolites, PTH, klotho and calcitonin.103 1, 25-dihydroxyvitamin D increases serum Ca by increasing intestinal Ca absorption, decreasing Ca excretion, and increasing Ca resorption from bone. PTH serves to increase serum Ca levels by promoting 1 alpha hydroxylase activity in the kidney, thereby increasing the production of active 1,25-dihydroxyvitamin D. Furthermore, PTH decreases renal excretion of Ca and can have variable effects on bone, with Ca resorption increased at high doses, and bone formation increased at low doses. Finally, klotho also maintains serum Ca levels by stimulating TRPV5-mediated Ca reabsorption activity in renal cells.105 In contrast, calcitonin lowers serum Ca by decreasing osteoclast mediated bone resorption. These mechanisms serve to keep Ca levels tightly controlled in a normal range of 9–10.5 mg/dL.106
Declining renal function in CKD leads to 1, 25-dihydroxyvitamin D deficiency due to diminished activity of 1-alpha hydroxylase in the kidney 107 as well as increased serum FGF23 levels (a direct inhibitor of 1 alpha hydroxylase activity). Low 1, 25-dihydroxyvitamin D levels lead to hypocalcemia, that together with hyperphosphatemia, provide a powerful stimulus for PTH secretion and cause the secondary hyperparathyroidism commonly observed in CKD. Vitamin D deficiency and the secondary hyperparathyroidism of CKD are traditionally treated with activated Vitamin D receptor agonists (VDRAs), and have been associated with hypercalemic episodes. Furthermore, Ca-containing P binders are commonly used to treat hyperphosphatemia, further increasing Ca burden in these susceptible individuals.108 Together with disordered bone remodeling, these factors contribute to dysregulation of Ca homeostasis in CKD. A summary of factors controlling Ca homeostasis and their dysregulation in CKD is shown in Figure 2
It should be noted however that elevated serum Ca is not the only mechanism that can lead to the exposure of VSMCs to high extracellular Ca. Ca is released in the vessel wall at sites of apoptotic or necrotic cell death and this can lead to huge local elevations in extracellular Ca of up to 30 mM.109 Nanocrystalline Ca crystals can be taken up by VSMCs and this can lead to intracellular Ca overload 110 as can chronic dysregulated signalling events 111 and all of these have the capacity to impact on local VSMC Ca homeostasis.
HOW IS CA SENSED BY VSMCs?
Ca signaling in VSMCs is complex and involves Ca channels, exchangers and pumps that regulate extracellular Ca entry into the cell and maintain Ca concentrations in the cytosol and in the intracellular Ca stores (mainly sarcoplasmic reticulum). Ca uptake and release is also linked to mechanisms of Ca extrusion from the cell and all are tightly bound by fine spatial and temporal coordination and cross-talk.112 Ca regulation is also dependent on the VSMC phenotypic state.113, 114 Differentiated contractile VSMCs express high levels of the voltage-activated L-type Ca channels, that mediate extracellular Ca uptake and the sarcoplasmic reticulum intracellular Ca release channel the ryanodine receptor (RyR). When VSMCs undergo phenotypic modulation, which coincides with the initiation of osteo/chondrogenic differentiation, VSMCs downregulate L-type channel expression and increase expression of the low voltage-activated T-type channels. The RyR is also downregulated and this is compensated for by increased expression of other sarcoplasmic reticulum ion pumps.113 Thus changes in both intracellular and extracellular Ca pools are likely to impact dramatically on VSMC function with the impact of the change dependent on the phenotype of the VSMC. In addition to plasma membrane uptake mechanisms Ca can also be ‘sensed’ by VSMCs via the Ca sensing receptor (CaR).115 While some progress has been made on examining the role of Ca signalling in VSMC calcification this area is highly complex and still requires much exploration. Therefore, we have limited our discussion in this review to the relatively few aspects of Ca homeostasis that have been implicated or investigated as having a role in VSMC calcification.
Channel mediated Ca-uptake
Plasma membrane voltage dependent L-type Ca channels have long been recognised as important for Ca homeostasis in VSMCs. These channels as well as T-type Ca channels can be targeted by drugs known as Ca channel blockers (CCBs) widely used in the treatment of hypertension.116, 117 In a prospective clinical trial CCBs were shown to slow down the progression of calcification in hypertensive patients with and without renal failure118–121 while association studies have shown they can reduce mortality in renal patients.122
Pioneering studies by Fleckenstein119 showed that in response to Vitamin D overload in rats vascular calcification could be prevented by treatment with the CCBs verapamil or nifedipine. Although it was assumed that the mechanism of action was via prevention of Ca uptake in the hypercalcemic environment induced in the rats in response to vitamin D, other direct VSMC effects have not been excluded. For example, CCBs are effective in blocking calcification induced by either gluteraldehyde or warfarin treatments and these are not associated with hypercalcemia.123, 124 CCBs can also partially block atherogenesis in animal models and this may be due to their known effects on VSMC differentiation, proliferation and matrix synthesis.125, 126 Despite the importance of Ca uptake in VSMCs very few studies have investigated CCBs in the context of calcification in vitro. In a recent study Chen and colleagues using bovine VSMCs showed that verapamil and not nifedipine could block VSMC calcification, potentially via downregulation of ALP activity. Interestingly, verapamil also blocked mineralization of VSMC-derived matrix vesicles which did not express L-type Ca channels suggesting that its mode of action was not via L-type Ca channel blockade. The authors speculated that these effects on matrix vesicles may have been via effects on membrane phospholipid composition 127 therefore it remains unclear whether blockade of Ca uptake by VSMCs can directly effect calcification.
The Ca Sensing Receptor (CaR)
Elevated extracellular Ca also has a direct signalling role in VSMC calcification without any need for uptake.128 The CaR is a G-protein coupled receptor capable of sensing changes in extracellular Ca concentrations in the mmol/L range. The CaR was first identified in the parathyroid where it regulates Ca homeostasis by suppressing PTH secretion and renal Ca reabsorption. However it is also widely expressed on tissues that are not involved in the regulation of Ca homeostasis including VSMC and endothelial cells. In contractile VSMCs within the vessel wall the CaR has been shown to play a physiological role in regulating vascular myogenic tone.129 In cultured VSMCs it regulates proliferation129, 130 and survival with stimulation of the CaR leading to ERK1/2 activation suggesting the CaR may be important in both contractile and synthetic VSMC phenotypic contexts.131
In calcified arteries from CKD patients expression of the CaR is downregulated131 and studies in vitro have shown that when VSMCs are induced to calcify in response to elevated extracellular Ca, expression of the CaR is also downregulated.132 Moreover, ablation of CaR function increased VSMC calcification in response to both Ca and P, while calcimimetics, drugs that increase the sensitivity of the CaR to Ca, can ameliorate calcification in the same model.132 Although these studies suggest that the CaR is deregulated in CKD they were unable to identify a mechanism to account for reduced calcification in the presence of a functional CaR. However a role for the CaR in regulating expression of the key calcification inhibitor MGP has previously been shown.133 Increased extracellular Ca can increase MGP transcription and this transcriptional activation can be mimicked by treatment with CaR agonists. Thus Ca sensing maybe critical for the feedback response of VSMCs to raised extracellular Ca leading to the production of inhibitory proteins. This notion is supported by in vivo studies showing treatment of animals with or without renal failure with calcimimetics increases aortic MGP production.134 If calcimimetics have direct effects on VSMC calcification these drugs, which are in clinical use for hyperparathyroidism, may also be useful in blocking progression of calcification.
Regulation of Intracellular Ca in VSMCs
Because of their contractile properties VSMCs have large intracellular stores of Ca that must be regulated and buffered to prevent intracellular Ca overload. Intracellular Ca homeostasis can be disrupted by many stimuli including excess Ca uptake or release from these intracellular stores including the SR/ER, lysosomes and mitochondria. The SR is the main organelle which mediates spark alterations in intracellular Ca levels required for smooth muscle contraction while the mitochondria and lysosomes are involved in longer-term events that may also be crucial for VSMC calcification. Two recent studies have demonstrated that the release of intracellular Ca from lysosomes may act to promote calcification. In the first study human VSMCs were exposed to Ca-P nanocrystals of varying size. Phagocytotic uptake of the smallest crystals by VSMCs was shown to induce an intracellular Ca burst that resulted in necrotic/apoptotic cell death. This process could be inhibited by treatment with the lysosomal proton pump inhibitor bafilomycin A1 suggesting lysosomal processing of the mineral caused toxic intracellular Ca release.110 Importantly, the vascular wall in CKD patients has a very high Ca load and this Ca is deposited in the extracellular matrix surrounding VSMCs as nanocrystals.78, 135 Thus at the very early stages of mineral nidus formation in the vessel wall uptake of previously deposited mineral by VSMCs would be predicted to promote cell death. This notion is supported by evidence from an ex vivo model using human vessels treated with Ca/P where it was shown that VSMCs undergo a wave of rapid apoptosis concomitantly with the formation of the first crystalline nidus of calcification observable by electron microscopy.136
In a second study Ca/P nanocrystals were found to promote VSMC osteogenic differentiation. This phenotypic change was restricted to upregulation of BMP2 expression within 24 hours and was not associated with elevated Runx2.59 The mechanism that induced osteogenic gene expression remains unknown but may also involve Ca release from intracellular stores. More speculatively Ca overload has been associated with ageing of the vasculature and BMP2 was recently shown to be increased in senescent VSMCs.137 It is plausible that uptake of nanocrystals promotes Ca and/or P induced VSMC senescence which drives further osteogenic differentiation via the BMP2 pathway.138
ER stress can be induced by deregulated Ca homeostasis and emerging evidence indicates there maybe a role for ER stress in vascular calcification. Firstly, ER stress has recently been implicated as essential for bone development and mineralization as it regulates osteoblast differentiation via Runx2 and collagen I secretion.139–141 The ER stress markers Grp78, Grp94 and CHOP were found in homogenates from rat aortas where medial vascular calcification was induced by vitamin D treatment which is known to induce intracellular Ca overload in VSMCs. These ER stress markers were associated with increased VSMC apoptosis and signalling via JNK as well as activation of caspases 3 and 12.142 So far expression of these markers has not been explored in vessels from CKD patients.
Mitochondrial Ca overload is also a prominent feature of many calcified tissues and interestingly this was observed in normal arteries after long-term exposure to Ca and P ex vivo.136, 143, 144 This contrasted to the predominant calcification of the extracellular matrix with no sign of mitochondrial damage and Ca overload, observed in CKD arteries exposed to the same conditions. One explanation for this is possibly the different mechanisms of adaptation to elevated Ca by contractile and phenotypically modified VSMCs. Massive release of Ca loaded vesicles by phenotypically modified VSMCs may mitigate intracellular Ca overload and prevent mitochondrial damage. However further studies are required to fully explore the effects of extracellular Ca on intracellular Ca handling in VSMCs to further our understanding of homeostatic and pathological changes.
DIRECT EFFECTS OF HIGH CA THAT MEDIATE VSMC CALCIFICATION
Ca induces VSMC calcification in vitro and Ca and P are synergistic
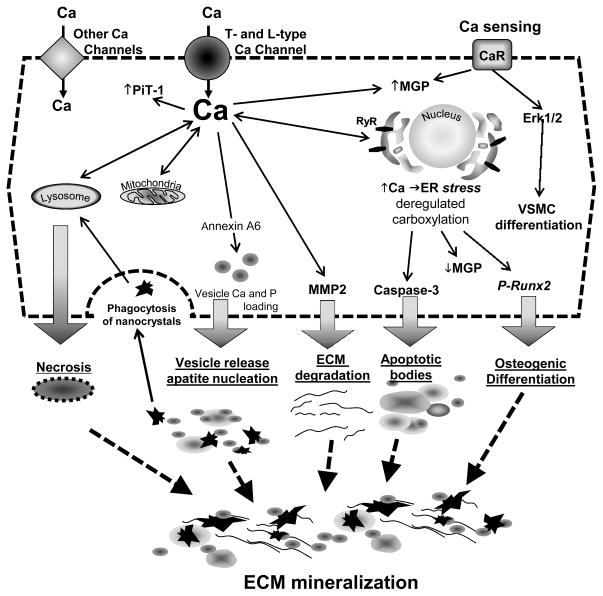
In addition to the changes in Ca handling that have been discussed above in vitro evidence also strongly supports a role for Ca in promoting VSMC calcification. Two key studies first demonstrated that Ca alone, when added to human VSMCs, promotes mineralization. Moreover when Ca and P were added together to the culture medium they were synergistic in inducing mineralization.145, 146 Further studies using vessel rings ex vivo demonstrated that for a given Ca × P product, elevated Ca was more potent at inducing VSMC calcification than elevated P.54, 55, 136 Importantly, these simple studies corroborated what had been found in human epidemiological studies and highlighted the role elevated levels of Ca have on the calcification process. Above we have already discussed how deregulated Ca uptake, release and signaling can impinge on VSMC calcification. In the next section we focus on the precise mechanisms whereby Ca itself can promote mineralization. These mechanisms differ from those of P and primarily implicate Ca as a major nucleator of crystalline hydroxyapatite (Figure 4).
Figure 4. Role of Ca in VSMC Calcification.
Ca elevation induces vesicle production and can lead to cell apoptosis and necrosis. High local levels of Ca triggers mineral nucleation in the matrix vesicles and apoptotic bodies. Accumulation of mineralized matrix vesicles and apoptotic bodies and their uptake accelerate cell death and facilitate further crystal growth. Degradation of ECM and nucleation of Ca apatite induces ECM protein mineralization.
Ca and VSMC osteogenic differentiation
Unlike P, which seems to have dramatic effects on promoting the osteogenic differentiation of VSMCs, elevated Ca alone does not seem to mediate this phenotypic transition. Synergism between Ca and P in driving Runx2 expression has been observed in dialysis vessels treated with Ca/P ex vivo however the mechanism may not be direct but involve increased PiT-1 expression or ROS production.136 Indeed, of note, in studies where calcification was induced in response to Ca alone or Ca/P, calcification proceeds in the absence of elevated ALP activity suggesting Ca may promote calcification by different mechanisms than P and this is consistent with their synergy.136
Ca and VSMC apoptosis, vesicle release and calcification
One of the major mechanisms whereby elevated extracellular Ca drives VSMC calcification is via its capacity to form mineral nucleation sites and participate in the very earliest events in the calcification cascade. Treatment of VSMCs in vitro with elevated Ca was found to promote apoptosis with marked synergism of apoptosis induced by combined Ca and P treatment. Apoptotic bodies have been shown to form a nidus for calcification and the propensity of these to calcify was markedly increased after Ca and P treatment.146, 147
Ca also acted to promote VSMC matrix vesicle release and calcification with Ca and P also synergistic in promoting this process.146 Using EM and EDX analysis Ca was shown to induce matrix vesicles to become what is known as ‘mineralization competent’ characterized by the presence of preformed crystalline hydroxyapatite mineral. P alone, even at very high concentrations, was unable to do this. The mechanisms whereby elevated Ca promotes vesicle calcification are still unclear however Ca can increase expression of the P-transporter PiT-1.145 This is present on the plasma membrane of VSMCs and also matrix vesicles and increased expression is likely to drive increased mineral accumulation.
However the major mechanism is more likely related to the ability of Ca to change the intrinsic properties of matrix vesicles.148 In bone a rise in intracellular Ca promotes matrix vesicle calcification via activating changes in annexin content, phospholipid composition and MMP activation.149, 150 Annexins together with phospholipids can bind Ca and form nucleation complexes for crystalline hydroxyapatite.151 VSMC-derived matrix vesicles are also enriched in annexins152, 153 and a recent study has shown that a similar rise in intracellular Ca in VSMCs, induced by uptake of excess Ca from the extracellular milieu, drives VSMC matrix vesicle calcification via annexin A6 and phosphatidyl serine complex formation. 148 Importantly, under normal conditions these Ca dependent changes in matrix vesicle properties are ameliorated by loading of the vesicles with calcification inhibitors. These include the endogenous inhibitor MGP and the circulating inhibitor fetuin-A which is taken up from the serum and loaded into matrix vesicles. The loading of fetuin-A into matrix vesicles is increased in the presence of Ca and this protein is a major buffer of excess Ca in the cell and in the matrix.146, 153, 154 Fetuin-A levels are reduced in patients on dialysis and this is likely to promote Ca dependent calcification mediated by matrix vesicles.155 Similarly, CKD patients have reduced levels of the non-carboxylated form of MGP that is not functional as a calcification inhibitor.156 Importantly, Ca has been shown to upregulate MGP production in a number of studies, potentially as an adaptive response aimed at inhibiting calcification.133, 157–159 However constant stimulation may lead to ER stress and act to deregulate carboxylation pathways localized in the ER, leading to the production of unprocessed MGP. In addition to this mechanism of MGP inactivation, a recent study has demonstrated that exposure of VSMCs to elevated Ca eventually depletes MGP from matrix vesicles further enhancing their calcification capacity. This study also showed that matrix vesicles are loaded with MMP2 and Ca acts to increase activation of this metalloproteinase which would further accelerate elastin degradation and calcification.148
Studies in vivo and ex vivo have led further support to these in vitro findings. Using vessels obtained from children with CKD or on dialysis it was shown that Ca load in the vessel wall correlated with time averaged Ca × P.78 Moreover in dialysis vessels calcification correlated with increased apoptosis and increased vesicle release shown by increased annexin A6 deposition in the vessel wall.78 Extending these studies by using vessels from these same children in organ culture experiments it was shown that control vessels were entirely resistant to the induction of calcification by Ca and/or P. In contrast vessels obtained from children on dialysis, extensively calcified in response to Ca/P. Again, the predominant mechanism appeared to be increased apoptosis and increased vesicle deposition leading to extracellular matrix calcification.136 In addition, increased deposition of both fetuin-A and uncarboxylated MGP was observed in vessels from dialysis patients and this deposition was dramatically increased in response to Ca/P treatment ex vivo. EM analysis of the vessels showed large numbers of calcified and non-calcified matrix vesicles deposited in the extracellular matrix consistent with the induction of vesicular calcification once inhibitors had been exhausted.
Further studies are now required to determine the cell biological mechanisms driving vesicle calcification and inhibitor dysfunction in VSMCs in response to Ca.
CONCLUSIONS
It is clear that dysregulated Ca and P homeostasis plays a major role in driving VSMC calcification in CKD. In addition to raising the Ca × P, elevated Ca and P can act directly on VSMC to drive distinct, as well as overlapping, pathways that predispose to calcification (Figure 5). The body of data presented in this review suggests that the maintenance of Ca and P levels in the normal range is the most important aim in CKD patients in order to minimize the vascular damage induced by dysregulated mineral metabolism. However there are many other factors associated with the CKD milieu that may also act to drive VSMC calcification. Some of these include advanced glycation end products 160, ROS 70, 161 and potentially vitamin D and PTH 162, although the calcific properties of these factors may be both dose and context dependent. In addition to these factors there are emerging areas such as the FGF23/Klotho signalling axis that may prove to be significant players in driving calcification. While these factors are key to regulating Ca and P homeostasis, long term exposure of VSMCs to elevated levels of FGF23 or reduced Klotho levels may potentially effect calcification. Indeed, recent data suggests that Klotho may be a direct inhibitor of VSMC calcification acting via effects on P-transport.163 However Klotho also regulates cellular ageing and vascular calcification is an age-associated pathology with the vasculature of CKD patients often regarded as ‘prematurely aged’. It will be interesting to determine whether there is an association between dysregulated mineral metabolism and cellular ageing given recent data suggesting that aged or senescent VSMCs exhibit a pro-osteogenic phenotype. 164, 165
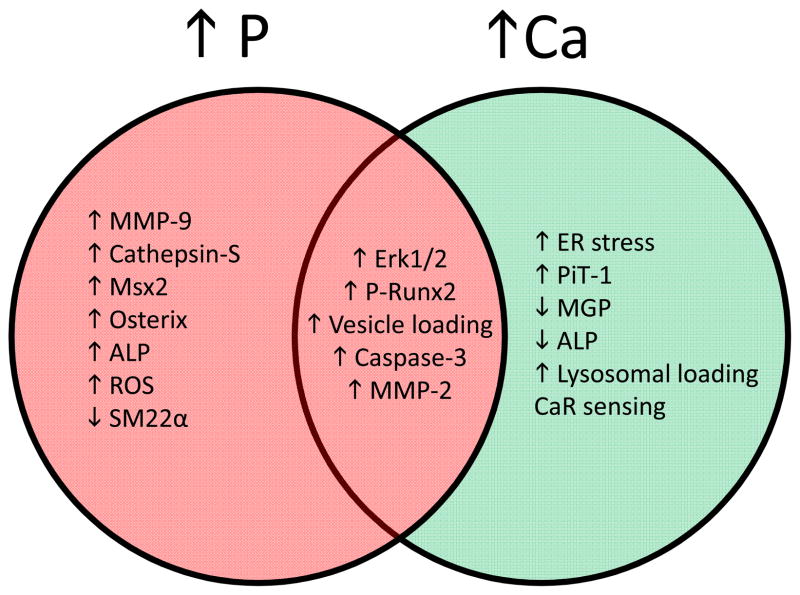
Figure 5. Overview of distinct and overlapping pathways initiated by elevated Ca and P in VSMC.
Pink shading indicates pathways specific to elevated serum Pi, green shading indicates pathways specific to elevated serum Ca, and brown area shows common pathways.
In summary, while we have made huge advances in understanding some of the mechanisms that drive VSMC calcification we still have many more questions than answers and hopefully these will be addressed in future studies.
Acknowledgments
This work was supported by grants from the British Heart Foundation to CMS, NIH grants HL081785 and HL62329 to CMG, and MHC is funded by NIH training grant T32 HL07828.
Non-standard Abbreviations and Acronyms
- ALP
alkaline phosphatase
- BGP
β-glycerophosphate
- BMP-2
bone morphogenetic protein 2
- CCBs
calcium channel blockers
- CaR
calcium sensing receptor
- Ca
calcium
- CKD
chronic kidney disease
- ELRs
elastin laminin receptors
- ECM
extracellular matrix
- FGF-23
fibroblast growth factor 23
- Gas6
growth arrest-specific gene 6
- HA
hydroxyapatite
- Pi
inorganic phosphate
- MGP
matrix Gla protein
- MMP
matrix metalloproteinase
- MAPK
mitogen-activated protein kinase
- OPN
osteopontin
- PTH
parathyroid hormone
- P
phosphate
- PI3K
phosphatidylinositol 3-kinase
- AKT
protein kinase B
- PPi
pyrophosphate
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- shRNA
small hairpin RNA
- NaPi
sodium-dependent phosphate cotransporter
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- TG2
transglutaminase 2
- TRPV6
transient receptor potential vallinoid receptor type 6
- VSMCs
vascular smooth muscle cells
- VDRAs
vitamin D receptor agonists
Footnotes
Disclosures
None
References
- 1.Bellasi A, Kooienga L, Block GA, Veledar E, Spiegel DM, Raggi P. How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol. 2009;22:255–262. [PubMed] [Google Scholar]
- 2.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 3.Young E, Albert J, Satayathum S, Goodkin D, Pisoni R, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port F. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhingra R, Sullivan L, Fox C, Wang T, D’Agostino RS, Gaziano J, Vasan R. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 6.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N, Muntner P. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–1792. doi: 10.1161/CIRCULATIONAHA.109.851873. [DOI] [PubMed] [Google Scholar]
- 8.Noordzij M, Korevaar JC, Bos WJ, Boeschoten EW, Dekker FW, Bossuyt PM, Krediet RT. Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant. 2006;21:2513–2520. doi: 10.1093/ndt/gfl257. [DOI] [PubMed] [Google Scholar]
- 9.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 11.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 13.Goldman L, Ausiello D. Cecil Medicine. 23. Saunders Elsevier; 2007. [Google Scholar]
- 14.Cross HS, Debiec H, Peterlik M. Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab. 1990;16:115–124. [PubMed] [Google Scholar]
- 15.Murer H, Hernando N, Forster I, Biber J. Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol. 2003;65:531–542. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 16.Hruska K, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. Faseb J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kestenbaum B. Phosphate metabolism in the setting of chronic kidney disease: significance and recommendations for treatment. Semin Dial. 2007;20:286–294. doi: 10.1111/j.1525-139X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 19.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 20.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 21.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner A, Dehmelt L, Nalbant P. Na+-dependent phosphate cotransporters: the NaPi protein families. J Exp Biol. 1998;201:3135–3142. doi: 10.1242/jeb.201.23.3135. [DOI] [PubMed] [Google Scholar]
- 23.Takeda E, Taketani Y, Morita K, Miyamoto K. Sodium-dependent phosphate co-transporters. Int J Biochem Cell Biol. 1999;31:377–381. doi: 10.1016/s1357-2725(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 24.Boyer CJ, Baines AD, Beaulieu E, Beliveau R. Immunodetection of a type III sodium-dependent phosphate cotransporter in tissues and OK cells. Biochim Biophys Acta. 1998;1368:73–83. doi: 10.1016/s0005-2736(97)00159-4. [DOI] [PubMed] [Google Scholar]
- 25.Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, Miller AD. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 27.Villa-Bellosta R, Bogaert YE, Levi M, Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. 2007;27:1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 28.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 29.Ravera S, Virkki LV, Murer H, Forster IC. Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol. 2007;293:C606–620. doi: 10.1152/ajpcell.00064.2007. [DOI] [PubMed] [Google Scholar]
- 30.Festing MH, Speer MY, Yang HY, Giachelli CM. Generation of mouse conditional and null alleles of the type III sodium-dependent phosphate cotransporter PiT-1. Genesis. 2009;47:858–863. doi: 10.1002/dvg.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck L, Leroy C, Beck-Cormier S, Forand A, Salaun C, Paris N, Bernier A, Urena-Torres P, Prie D, Ollero M, Coulombel L, Friedlander G. The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLoS One. 2010;5:e9148. doi: 10.1371/journal.pone.0009148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki A, Ammann P, Nishiwaki-Yasuda K, Sekiguchi S, Asano S, Nagao S, Kaneko R, Hirabayashi M, Oiso Y, Itoh M, Caverzasio J. Effects of transgenic Pit-1 overexpression on calcium phosphate and bone metabolism. J Bone Miner Metab. 2010;28:139–148. doi: 10.1007/s00774-009-0121-3. [DOI] [PubMed] [Google Scholar]
- 33.Sekiguchi S, Suzuki A, Asano S, Nishiwaki-Yasuda K, Shibata M, Nagao S, Yamamoto N, Matsuyama M, Sato Y, Yan K, Yaoita E, Itoh M. Phosphate overload induces podocyte injury via type III Na-dependent phosphate transporter. Am J Physiol Renal Physiol. 2011;300:F848–856. doi: 10.1152/ajprenal.00334.2010. [DOI] [PubMed] [Google Scholar]
- 34.Khoshniat S, Bourgine A, Julien M, Weiss P, Guicheux J, Beck L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci. 2011;68:205–218. doi: 10.1007/s00018-010-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markovich D, Verri T, Sorribas V, Forgo J, Biber J, Murer H. Regulation of opossum kidney (OK) cell Na/Pi cotransport by Pi deprivation involves mRNA stability. Pflugers Arch. 1995;430:459–463. doi: 10.1007/BF00373881. [DOI] [PubMed] [Google Scholar]
- 36.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 2004;287:F39–47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- 37.Rizzoli R, Fleisch H, Bonjour JP. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest. 1977;60:639–647. doi: 10.1172/JCI108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsumi S, Segawa H, Morita K, Haga H, Kouda T, Yamamoto H, Inoue Y, Nii T, Katai K, Taketani Y, Miyamoto KI, Takeda E. Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology. 1998;139:1692–1699. doi: 10.1210/endo.139.4.5925. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto K, Tatsumi S, Segawa H, Morita K, Nii T, Fujioka A, Kitano M, Inoue Y, Takeda E. Regulation of PiT-1, a sodium-dependent phosphate co-transporter in rat parathyroid glands. Nephrol Dial Transplant. 1999;14 (Suppl 1):73–75. doi: 10.1093/ndt/14.suppl_1.73. [DOI] [PubMed] [Google Scholar]
- 40.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ. Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest. 1996;97:2534–2540. doi: 10.1172/JCI118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res. 1996;11:970–976. doi: 10.1002/jbmr.5650110714. [DOI] [PubMed] [Google Scholar]
- 42.Salaun C, Marechal V, Heard JM. Transport-deficient Pit2 phosphate transporters still modify cell surface oligomers structure in response to inorganic phosphate. J Mol Biol. 2004;340:39–47. doi: 10.1016/j.jmb.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 43.Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol. 2007;293:F643–654. doi: 10.1152/ajprenal.00228.2007. [DOI] [PubMed] [Google Scholar]
- 44.Villa-Bellosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol. 2009;29:761–766. doi: 10.1161/ATVBAHA.108.183384. [DOI] [PubMed] [Google Scholar]
- 45.Beck L, Leroy C, Salaun C, Margall-Ducos G, Desdouets C, Friedlander G. Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J Biol Chem. 2009;284:31363–31374. doi: 10.1074/jbc.M109.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 47.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 49.Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem. 2010;110:935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shioi ANY, Jono S, Koyama H, Hosoi M, Morii H. B-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Throm Vasc Biol. 1995;17:1135–1142. doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 51.Chen NX, O’Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 52.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka T, Sato H, Doi H, Yoshida CA, Shimizu T, Matsui H, Yamazaki M, Akiyama H, Kawai-Kowase K, Iso T, Komori T, Arai M, Kurabayashi M. Runx2 represses myocardin-mediated differentiation and facilitates osteogenic conversion of vascular smooth muscle cells. Mol Cell Biol. 2008;28:1147–1160. doi: 10.1128/MCB.01771-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O’Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 55.Lomashvili K, Garg P, O’Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006;69:1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- 56.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 57.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84(2):166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 58.Villa-Bellosta R, Millan A, Sorribas V. Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am J Physiol Cell Physiol. 2011;300:C210–220. doi: 10.1152/ajpcell.00229.2010. [DOI] [PubMed] [Google Scholar]
- 59.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, Ono Y, Miura Y, Oiso Y, Itoh M, Caverzasio J. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21:674–683. doi: 10.1359/jbmr.020603. [DOI] [PubMed] [Google Scholar]
- 61.Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol. 2007;27:4465–4474. doi: 10.1128/MCB.00104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathew S, Lund RJ, Strebeck F, Tustison KS, Geurs T, Hruska KA. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 64.Chen NX, Duan D, O’Neill KD, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 65.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 67.Montes de Oca A, Madueño JA, Martinez-Moreno JM, Guerrero F, Muñoz-Castañeda J, Rodriguez-Ortiz ME, Mendoza FJ, Almaden Y, Lopez I, Rodriguez M, Aguilera-Tejero E. High-phosphate-induced calcification is related to SM22α promoter methylation in vascular smooth muscle cells. J Bone Miner Res. 2010;25:1996–2005. doi: 10.1002/jbmr.93. [DOI] [PubMed] [Google Scholar]
- 68.Selivanov VA, Zeak JA, Roca J, Cascante M, Trucco M, Votyakova TV. The role of external and matrix pH in mitochondrial reactive oxygen species generation. J Biol Chem. 2008;283:29292–29300. doi: 10.1074/jbc.M801019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao MM, Xu MJ, Cai Y, Zhao G, Guan Y, Kong W, Tang C, Wang X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011;79:1071–1079. doi: 10.1038/ki.2011.18. [DOI] [PubMed] [Google Scholar]
- 71.El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75:1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 73.Neven E, Persy V, Dauwe S, De Schutter T, De Broe ME, D’Haese PC. Chondrocyte rather than osteoblast conversion of vascular cells underlies medial calcification in uremic rats. Arterioscler Thromb Vasc Biol. 2010;30:1741–1750. doi: 10.1161/ATVBAHA.110.204834. [DOI] [PubMed] [Google Scholar]
- 74.Neven E, Dams G, Postnov A, Chen B, De Clerck N, De Broe ME, D’Haese PC, Persy V. Adequate phosphate binding with lanthanum carbonate attenuates arterial calcification in chronic renal failure rats. Nephrol Dial Transplant. 2009;24:1790–1799. doi: 10.1093/ndt/gfn737. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 76.Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 77.Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 78.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 79.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, Ouchi Y. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 80.Collett G, Wood A, Alexander MY, Varnum BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW, Canfield AE. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- 81.Son B, Kozaki K, Iijima K, Eto M, Nakano T, Akishita M, Ouchi Y. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol. 2007;556:1–8. doi: 10.1016/j.ejphar.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 82.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 83.Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rucker R. Calcium binding to elastin. Adv Exp Med Biol. 1974;48(0):185–209. doi: 10.1007/978-1-4684-0943-7_10. [DOI] [PubMed] [Google Scholar]
- 85.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 86.Heldin C, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 87.Lee K, Kim H, Li Q, Chi X, Ueta C, Komori T, Wozney J, Kim E, Choi J, Ryoo H, Bae S. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28:856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 89.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hosaka N, Mizobuchi M, Ogata H, Kumata C, Kondo F, Koiwa F, Kinugasa E, Akizawa T. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif Tissue Int. 2009;85:523–529. doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 91.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 92.Chung AW, Yang HH, Sigrist MK, Brin G, Chum E, Gourlay WA, Levin A. Matrix metalloproteinase-2 and -9 exacerbate arterial stiffening and angiogenesis in diabetes and chronic kidney disease. Cardiovasc Res. 2009;84:494–504. doi: 10.1093/cvr/cvp242. [DOI] [PubMed] [Google Scholar]
- 93.Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K. Outcomes associated with serum calcium level in men with non-dialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:468–476. doi: 10.2215/CJN.06040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, Sundstrom J. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–339. doi: 10.1161/ATVBAHA.109.196675. [DOI] [PubMed] [Google Scholar]
- 95.Yamada K, Fujimoto S, Nishiura R, Komatsu H, Tatsumoto M, Sato Y, Hara S, Hisanaga S, Ochiai H, Nakao H, Eto T. Risk factors of the progression of abdominal aortic calcification in patients on chronic haemodialysis. Nephrol Dial Transplant. 2007;22:2032–2037. doi: 10.1093/ndt/gfm031. [DOI] [PubMed] [Google Scholar]
- 96.West SL, Swan VJ, Jamal SA. Effects of calcium on cardiovascular events in patients with kidney disease and in a healthy population. Clin J Am Soc Nephrol. 5(Suppl 1):S41–47. doi: 10.2215/CJN.05860809. [DOI] [PubMed] [Google Scholar]
- 97.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5 (Suppl 1):S23–30. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 98.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 99.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 100.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 101.Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: A pilot randomized controlled trial. Nephrology (Carlton) 2011;16:290–298. doi: 10.1111/j.1440-1797.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 102.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 103.Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med. 2008;40:82–91. doi: 10.1080/07853890701689645. [DOI] [PubMed] [Google Scholar]
- 104.Ganong WF. Review of Medical Physiology. 19. Norwalk, Conn: Appleton & Lange; 1999. [Google Scholar]
- 105.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 106.Katzung BG. Basic and Clinical Pharmacology. 7. Apple and Lange; 1998. [Google Scholar]
- 107.Koenig KG, Lindberg JS, Zerwekh JE, Padalino PK, Cushner HM, Copley JB. Free and total 1,25-dihydroxyvitamin D levels in subjects with renal disease. Kidney Int. 1992;41:161–165. doi: 10.1038/ki.1992.22. [DOI] [PubMed] [Google Scholar]
- 108.Kestenbaum B, Belozeroff V. Mineral metabolism disturbances in patients with chronic kidney disease. Eur J Clin Invest. 2007;37:607–622. doi: 10.1111/j.1365-2362.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 109.Olszak IT, Poznansky MC, Evans RH, Olson D, Kos C, Pollak MR, Brown EM, Scadden DT. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest. 2000;105:1299–1305. doi: 10.1172/JCI9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 111.Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med. 2011;13:e11. doi: 10.1017/S1462399411001815. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sammels E, Parys JB, Missiaen L, De Smedt H, Bultynck G. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium. 2010;47:297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 116.Cribbs LL. T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium. 2006;40:221–230. doi: 10.1016/j.ceca.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 117.Richard S. Vascular effects of calcium channel antagonists: new evidence. Drugs. 2005;65 (Suppl 2):1–10. doi: 10.2165/00003495-200565002-00002. [DOI] [PubMed] [Google Scholar]
- 118.Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37:1410–1413. doi: 10.1161/01.hyp.37.6.1410. [DOI] [PubMed] [Google Scholar]
- 119.Fleckenstein-Grun G, Thimm F, Czirfuzs A, Matyas S, Frey M. Experimental vasoprotection by calcium antagonists against calcium-mediated arteriosclerotic alterations. J Cardiovasc Pharmacol. 1994;24 (Suppl 2):S75–84. [PubMed] [Google Scholar]
- 120.Motro M, Shemesh J, Grossman E. Coronary benefits of calcium antagonist therapy for patients with hypertension. Curr Opin Cardiol. 2001;16:349–355. doi: 10.1097/00001573-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Bursztyn M, Motro M, Grossman E, Shemesh J. Accelerated coronary artery calcification in mildly reduced renal function of high-risk hypertensives: a 3-year prospective observation. J Hypertens. 2003;21:1953–1959. doi: 10.1097/00004872-200310000-00024. [DOI] [PubMed] [Google Scholar]
- 122.Tepel M, Giet MV, Park A, Zidek W. Association of calcium channel blockers and mortality in haemodialysis patients. Clin Sci (Lond) 2002;103:511–515. doi: 10.1042/cs1030511. [DOI] [PubMed] [Google Scholar]
- 123.Kim KM. Cellular mechanism of calcification and its prevention in glutaraldehyde treated vascular tissue. Z Kardiol. 2001;90 (Suppl 3):99–105. doi: 10.1007/s003920170050. [DOI] [PubMed] [Google Scholar]
- 124.Essalihi R, Zandvliet ML, Moreau S, Gilbert LA, Bouvet C, Lenoel C, Nekka F, McKee MD, Moreau P. Distinct effects of amlodipine treatment on vascular elastocalcinosis and stiffness in a rat model of isolated systolic hypertension. J Hypertens. 2007;25:1879–1886. doi: 10.1097/HJH.0b013e328255e906. [DOI] [PubMed] [Google Scholar]
- 125.Gamble W. Atherosclerosis: the carbonic anhydrase, carbon dioxide, calcium concerted theory. J Theor Biol. 2006;239:16–21. doi: 10.1016/j.jtbi.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 126.Parmley WW. Calcium channel blockers and atherogenesis. Am J Med. 1987;82:3–8. doi: 10.1016/0002-9343(87)90204-x. [DOI] [PubMed] [Google Scholar]
- 127.Chen NX, Kircelli F, O’Neill KD, Chen X, Moe SM. Verapamil inhibits calcification and matrix vesicle activity of bovine vascular smooth muscle cells. Kidney Int. 2010;77(5):436–442. doi: 10.1038/ki.2009.481. [DOI] [PubMed] [Google Scholar]
- 128.Smajilovic S, Tfelt-Hansen J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc Res. 2007;75(3):457–467. doi: 10.1016/j.cardiores.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 129.Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1756–1762. doi: 10.1152/ajpheart.00739.2004. [DOI] [PubMed] [Google Scholar]
- 130.Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, Li HX, Zhang WH, Yang BF, Wu LY, Wang R, Yang GD, Xu CQ. The Calcium-Sensing Receptor Mediates Hypoxia-Induced Proliferation of Rat Pulmonary Artery Smooth Muscle Cells Through MEK1/ERK1,2 and PI3K Pathways. Basic Clin Pharmacol Toxicol. 2010;108:185–193. doi: 10.1111/j.1742-7843.2010.00639.x. [DOI] [PubMed] [Google Scholar]
- 131.Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, Zehnder D. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293:F946–955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- 132.Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, Vizard TN, Sage AP, Martin D, Ward DT, Alexander MY, Riccardi D, Canfield AE. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 133.Farzaneh-Far A, Proudfoot D, Weissberg PL, Shanahan CM. Matrix gla protein is regulated by a mechanism functionally related to the calcium-sensing receptor. Biochem Biophys Res Commun. 2000;277:736–740. doi: 10.1006/bbrc.2000.3747. [DOI] [PubMed] [Google Scholar]
- 134.Mendoza FJ, Martinez-Moreno J, Almaden Y, Rodriguez-Ortiz ME, Lopez I, Estepa JC, Henley C, Rodriguez M, Aguilera-Tejero E. Effect of Calcium and the Calcimimetic AMG 641 on Matrix-Gla Protein in Vascular Smooth Muscle Cells. Calcif Tissue Int. 2010;88:169–78. doi: 10.1007/s00223-010-9442-4. [DOI] [PubMed] [Google Scholar]
- 135.Schlieper G, Aretz A, Verberckmoes SC, Kruger T, Behets GJ, Ghadimi R, Weirich TE, Rohrmann D, Langer S, Tordoir JH, Amann K, Westenfeld R, Brandenburg VM, D’Haese PC, Mayer J, Ketteler M, McKee MD, Floege J. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burton DG, Giles PJ, Sheerin AN, Smith SK, Lawton JJ, Ostler EL, Rhys-Williams W, Kipling D, Faragher RG. Microarray analysis of senescent vascular smooth muscle cells: A link to atherosclerosis and vascular calcification. Exp Gerontol. 2009;44:659–665. doi: 10.1016/j.exger.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 138.Proudfoot D, Shanahan CM. Nanocrystals seed calcification in more ways than one. Kidney Int. 2011;79(4):379–382. doi: 10.1038/ki.2010.455. [DOI] [PubMed] [Google Scholar]
- 139.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 140.Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Imaizumi K. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11:1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- 141.Tohmonda T, Miyauchi Y, Ghosh R, Yoda M, Uchikawa S, Takito J, Morioka H, Nakamura M, Iwawaki T, Chiba K, Toyama Y, Urano F, Horiuchi K. The IRE1alpha-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep. 2011;12:451–457. doi: 10.1038/embor.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duan X, Zhou Y, Teng X, Tang C, Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem Biophys Res Commun. 2009;387:694–699. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 143.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976;35:156–162. [PubMed] [Google Scholar]
- 144.Kim KM. Cell injury and calcification of rat aorta in vitro. Scan Electron Microsc. 1984:1809–1818. [PubMed] [Google Scholar]
- 145.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 147.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 148.Kapustin A, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium Regulates Key Components of Vascular Smooth Muscle Cell-Derived Matrix Vesicles to Enhance Mineralization. Circ Res. 2011;109:e1–e12. doi: 10.1161/CIRCRESAHA.110.238808. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 149.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 150.Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taylor MG, Simkiss K, Simmons J, Wu LN, Wuthier RE. Structural studies of a phosphatidyl serine-amorphous calcium phosphate complex. Cell Mol Life Sci. 1998;54:196–202. doi: 10.1007/s000180050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen NX, O’Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 153.Chen NX, O’Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 155.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 156.Cranenburg EC, Brandenburg VM, Vermeer C, Stenger M, Muhlenbruch G, Mahnken AH, Gladziwa U, Ketteler M, Schurgers LJ. Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb Haemost. 2009;101:359–366. [PubMed] [Google Scholar]
- 157.Farzaneh-Far A, Weissberg PL, Proudfoot D, Shanahan CM. Transcriptional regulation of matrix gla protein. Z Kardiol. 2001;90 (Suppl 3):38–42. doi: 10.1007/s003920170040. [DOI] [PubMed] [Google Scholar]
- 158.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farzaneh-Far A, Davies JD, Braam LA, Spronk HM, Proudfoot D, Chan SW, O’Shaughnessy KM, Weissberg PL, Vermeer C, Shanahan CM. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem. 2001;276:32466–32473. doi: 10.1074/jbc.M104909200. [DOI] [PubMed] [Google Scholar]
- 160.Tanikawa T, Okada Y, Tanikawa R, Tanaka Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46:572–580. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- 161.Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24:2051–2058. doi: 10.1093/ndt/gfn757. [DOI] [PubMed] [Google Scholar]
- 162.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 163.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y. Sirtuin 1 Retards Hyperphosphatemia-Induced Calcification of Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.216739. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 165.Burton DG, Matsubara H, Ikeda K. Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Exp Gerontol. 2010;45:819–824. doi: 10.1016/j.exger.2010.07.005. [DOI] [PubMed] [Google Scholar]