Abstract
This review focuses on the recent advances in functions of spectrins in non-erythroid cells. We discuss new data concerning the commonly known role of the spectrin-based skeleton in control of membrane organization, stability and shape, and tethering protein mosaics to the cellular motors and to all major filament systems. Particular effort has been undertaken to highlight recent advances linking spectrin to cell signaling phenomena and its participation in signal transduction pathways in many cell types.
Keywords: Spectrin, Cell signaling, Spectrin-based skeleton, Membrane skeleton
Spectrins: several genes and numerous isoforms widely distributed in all metazoan cells
Spectrins are large flexible molecules that exist mainly as heterotetramers made of various α and β subunit isoforms. The α and β subunits are assembled side to side in an antiparallel fashion to form rod-like αβ dimers that in turn self-associate head to head to form tetramers. Tetramer formation involves the N-terminus of each α subunit with the C-terminus of each β subunit. The β-spectrin chains can also exist as homopolymeric complexes in skeletal muscle [1] and possibly in the Golgi apparatus [2, 3]. Each extremity of tetramers binds actin microfilaments via β-spectrin, allowing spectrin to form cross-links between actin filaments, thus generating an extended network.
Spectrins are expressed in all metazoan cells arising from numerous genes. Therefore, in mammals, the different spectrin isoforms originate by extensive mRNA splicing from seven genes. Two genes, SPTA1 and SPTAN1, encode αI- and αII-spectrin subunits, respectively. In contrast to SPTA1, the SPTAN1 gene codes for several αII-spectrin isoforms present in all non-erythroid cells, resulting from three alternative splicing processes [4–6]. Five genes code for β-spectrins: four “conventional” β genes, SPTB, SPTBN1, SPTBN2, SPTBN4, encoding the βI–βIV spectrins, respectively, and one gene, SPTBN5, encoding one large βV-spectrin (β-Heavy) [7, 8]. The expression of the diverse isoforms is regulated in a complex tissue- and developmental stage time-specific manner (Tables 1, 2).
Table 1.
Spectrin genes and their expression in mammalian tissue
| Subunit | Gene | Chromosome | Tissue expression | Ref. |
|---|---|---|---|---|
| Homo sapiens | ||||
| αI | SPTA1 | 1q21–q23 | Isoform αΙΣ1 RBC and isoform (αΙΣ*) in brain | [7, 8] |
| αII | SPTAN1 | 9q33–q34 | Several isoforms present in all non-erythroid cells | [4, 5, 132] |
| βI | SPTB | 14q22–q23.2 | βIΣ1 erythrocytes, βIΣ2 isoforms in brain and muscle, βI-spectrin was also detected in lymphocytes | [8, 18, 50, 100] |
| βII | SPTBN1 | 2q21 | All nucleated cells | [8, 50, 100] |
| βIII | SPTBN2 | 11q13 | Golgi and vesicular membrane skeletons, plasma membrane in neurons and epithelial cells | [49, 113] |
| βIV | SPTBN4 | 19q13.13 | Neurons (axon, initial segment, nodes of Ranvier) and pancreatic islets, nucleus | [6, 130] |
| βV | SPTBN5 | 15q21 | Low level in many tissues, outer segments of photoreceptor rods and cones, basolateral membrane of gastric epithelial cells and outer hair cell (OHC) | [59, 60] |
Table 2.
Examples of spectrin functions in cellular processes and signaling
| Spectrin isoforms | Function in cellular processes/signaling | Ref. |
|---|---|---|
| αI | Supports RBC shape and maintains cell membrane integrity and its mechanical properties | [14, 51, 52, 131] |
| αII | Engaged in maintaining cell architecture, morphology, and plasma membrane stability | [53–55] |
| Engaged in regulation of neurite outgrowth stimulated by NCAM | [64] | |
| Participates in the organization of specialized membranes—TRPC4 channels | [65, 66] | |
| Engaged in cell adhesion and spreading, regulation of actin dynamics | [84, 91] | |
| Modifies cell cycle by altering cell adhesion | [84] | |
| Engaged in DNA interstrand cross-links repair, connected to maintaining chromosomal stability | [85–87, 90] | |
| βI | Supports RBC shape and maintains cell membrane integrity and its mechanical properties | [51, 52] |
| Contributes to the formation of TCR complexes in lymphocytes | [99, 100, 109] | |
| Involved in early cellular apoptotic events | [110, 111] | |
| βII | Engaged in cell morphology and mechanical properties, compaction and accumulation of E-cadherin in the epithelial cell-cell contact | [56, 57] |
| Delivery of proteins and phospholipids to the membrane | [3, 30, 31, 47] | |
| Cell cycle regulation by involvement in TGFβ signaling | [75, 76, 80–82] | |
| βIII | Participates in the organization of the glutamate transporter EAAT4 in Purkinje cells | [70, 71] |
| Facilitates membrane protein transport via the secretory and endocytic pathways | [112–114] | |
| βIV | Regulates localisation of voltage-gated channels at the axon initial segment and node of Ranvier, synchronizes action potentials, provides multifunctional regulatory platform for sodium channels, plays an important role in the structure and stability of excitable membranes in heart and brain | [72, 73, 133] |
| Involved in targeting of critical structural and regulatory proteins | [134] | |
| βV | Engaged in cell flexibility | [59] |
| Engaged in OHCs’ electromotility | [60] |
Invertebrates have a smaller repertoire of spectrin genes. The Caenorhabditis elegans and Drosophila melanogaster genomes include a single gene coding for an α-spectrin closed to the mammalian αII-spectrin [spc-1 and I(3)dre3, respectively] [9, 10] and two genes coding for β-spectrin; one codes for a βG protein resembling the mammalian βII-spectrin referred to as “conventional β-spectrin” (Unc-70/bgs-1 and β-Spc) [11], and the other (sma 1 in C. elegans and karst in D. melanogaster) encodes βH-spectrin (β-Heavy similar to mammalian βV) [12, 13]. Greater sequence conservation is observed between spectrins from Drosophila and non-erythroid spectrin than between the erythroid and non-erythroid forms within the mammalian organism. Sequence analyses suggest that the erythroid spectrin genes arose during vertebrate evolution, and some of the sequence changes may correspond to neo-functionalization of the erythroid spectrin genes [14–16].
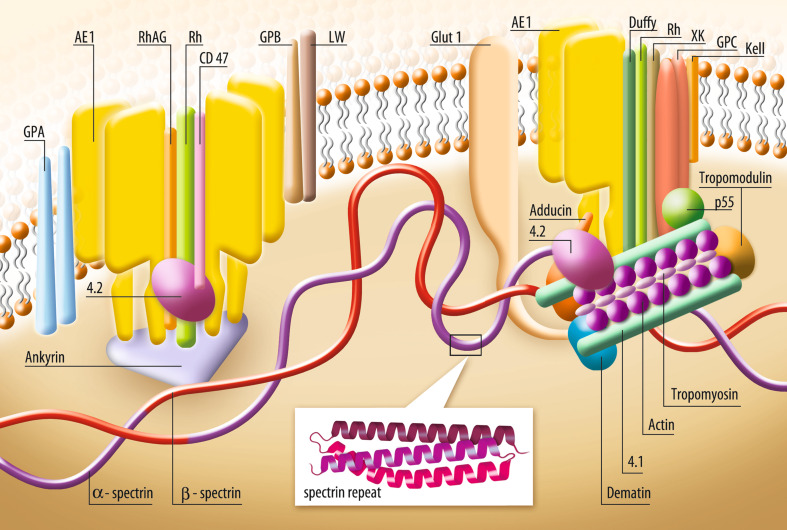
Despite the diversity of the genes, each spectrin subunit is made up of a succession of triple helical motifs called spectrin repeats (roughly 106 amino acid residues long), flanked by non-homologous N- and C-terminal sequences [7, 17, 18]. α-Spectrins contain 20 spectrin repeats (α1-α20), β-spectrins are made of 17 spectrin repeats (β1-β17), while the heavy βV-spectrins contain 30 repeats. The structure of the repeat unit (folded in a triple α-helical coiled-coil structure) and their interconnection are thought to be closely associated with spectrin flexibility [19]. When stretched, spectrin tetramer filaments can reach ~200 nm in length. Besides roles in the structure and the flexibility of spectrins, spectrin repeats can be considered as an interaction platform. Some spectrin repeats are involved in the formation of spectrin dimers and tetramers, such as the α17–α20 repeats with β1–β4 repeats, and the first helix of α-spectrin with the last incomplete β17 repeat of β-spectrin, respectively [20–22]. Moreover, they are also essential to the binding of the spectrin-based membrane skeleton to the membrane bilayer (Fig. 1). In erythrocytes, the β14–15 repeat region is bound to the anion exchanger (AE1) via ankyrin [23]. Another major membrane bilayer binding site is the protein 4.1 complex, which includes actin, dematin, adducin, tropomyosin and tropomodulin. This complex binds the spectrin-based network to glycophorin C and the anion AE1 by adapter proteins p55 and 4.2 [24–27]. The 4.1 complex also stabilizes the actin–spectrin interaction and maintains actin filament length. Spectrins also interact directly with phospholipids such as phosphatidylserine and phosphatidylethanolamine, a component actively confined to the inner leaflet of the lipid bilayer [28–34]. All these interactions are crucial for maintaining membrane mechanical properties. Recent data revealed that α-spectrin repeats can directly interact with membrane proteins such as the adhesion molecules Lu/BCAM (involving the α4 repeat) [35, 36]. Furthermore, there are still unexplained relationships between proteins exported by the malaria parasite Plasmodium and α-spectrin repeats (for a review, see [37].
Fig. 1.
A model of the human red cell membrane. The spectrin-actin interaction is modulated by accessory proteins such as protein 4.1, together with dematin, adducin, tropomyosin and tropomodulin. Their functions are to stabilize the actin-spectrin complex, to maintain actin filament length (adducin acts as a capping protein), and to bind the spectrin-based network to the transmembrane proteins (the glycophorin C, the anion exchanger AE1) via adapter proteins (protein p55 and protein 4.2). Another major binding site to membrane is mediated via ankyrin, which binds to β-spectrin and the anion exchanger AE1. The Rh/RhAG-ankyrin complex can be also a link between the red cell membrane and the spectrin-based skeleton. Spectrins also interact directly with phospholipids such as phosphatidylserine and phosphatidylethanolamine, membrane components actively confined to the inner leaflet of the lipid bilayer. The aminophospholipid-binding sites in β-spectrin are localized in close proximity to the attachment sites for both ankyrin and 4.1, the proteins engaged in spectrin links to the membrane. AE1 anion exchanger, GPA glycophorin A, GPB glycophorin B, GPC glycophorin C, GLUT 1 glucose transporter 1, Rh rhesus factor, RhAG Rh-associated glycoprotein
Besides the spectrin repeats, spectrins contain additional sequences that can facilitate some protein-protein or protein-lipid interactions. α-Spectrins contain an SH3 domain within the α9-spectrin repeat, which is well known to be engaged in cell signaling mainly by interacting with proline-rich stretches [38]. At the C-terminal end they also contain two EF-hand motifs related to calmodulin and involved in calcium binding [39–41]. αII-Spectrin differs from αI-spectrin by a 35-residue insert in the α10 repeat, which bears a Ca2+-dependent binding site for calmodulin [42] and cleavage sites for both caspases (2 and 3) [43] and for m and μ calpains [44]. All β-spectrins contain an actin-binding domain in their N-terminal region composed of a tandem of two CH (calponin homology) domains, which is present in many spectrin-related and unrelated skeletal proteins [45, 46]. The C-terminal region of the “long” isoforms of β-spectrins contains a PH (pleckstrin homology) domain responsible for phosphoinositide binding [47–49].
While mammalian erythrocytes contain only one type of spectrin tetramer made of αI and βI subunits, located at the inner surface of the membrane, nucleated cells can contain several spectrin species. Numerous isoforms of α- and β-spectrins derived from different genes are located in diverse cellular compartments (membrane, Golgi apparatus, endoplasmic reticulum, vesicles and nucleus). Some isoforms have a specific expression according to cell type or to cell organelle with very specific functions. The inactivation of the genes encoding canonical spectrin in D. melanogaster or in C. elegans indicated that these proteins are essential for the survival and normal development of these organisms (for review see [50]).
Spectrins are multifunctional proteins involved in regulation of cell morphology and mechanical properties
In erythrocytes the spectrin-based network supports cell shape, and maintains cell membrane integrity and its mechanical properties (for reviews, see [51, 52]). The role of spectrin in determining the physical properties of red blood cell membrane was clearly documented in hereditary hemolytic anemia associated with mutations in both αI- and βI-spectrins. Indeed molecular defects in erythroid spectrins are associated with abnormal shape, increased membrane fragility and reduced erythrocyte deformability.
Similarly, in nucleated cells the spectrin-based skeleton is involved in cell architecture, morphology, and plasma membrane stability [53–55]. In epithelial cells, knockdown of either βII-spectrin or ankyrin G results in loss of the lateral membrane, expansion of the apical and basal membrane area, and conversion of cells from columnar to squamous morphology [56, 57]. Both proteins are required for compaction and accumulation of E-cadherin in the epithelial cell-cell contact, and the delivery of proteins and phospholipids to the lateral membrane [56]. Recent data suggest that in Drosophila, βH-spectrin (homolog to mammal βV-spectrin) at the apical membrane coordinates the interaction between cadherin-based zonula adherens, with the immunoglobulin cell adhesion molecule Roughest during eye morphogenesis [4, 58].
Spectrins also participate in cell flexibility outside the red blood cells. This property is conferred by mammalian βV-spectrin and its homologs (βH spectrin in D. melanogaster and Sma-1 in C. elegans). These βV spectrin homologs have independently maintained an unusual 30-repeat length throughout evolution, which helps to cross-link membrane actin and confers extensive flexibility in cells [19, 59]. In the outer hair cells (OHC) αII-, βII- and βV-spectrins together with F-actin form the cortical network involved in the sound-induced electromotility. The main function of this spectrin-actin network is to provide flexible properties required for lateral wall contraction-elongation cycles. While βII-spectrin is restricted to the cuticular plate, a dense apical network of actin filaments, βV-spectrin is concentrated at the cortical lattice and is directly involved in the OHCs’ electromotility [60].
Both α- and β-spectrins are required during nervous system development. β-Spectrin interacts directly with the neural cell adhesion molecule NCAM, a synaptic adhesion molecule involved in mechanical stabilization of neuronal contacts [61, 62]. Genetic variations of NCAM are considered a risk factor in bipolar affective disease and schizophrenia [63]. In another way, αII-spectrin (α12 repeat) phosphorylation-dependent interaction with 14-3-3, a protein involved in neuronal migration and synaptic plasticity, acts as a switch between positive and negative regulation of neurite outgrowth stimulated by NCAM [64].
This short survey of published data presented in this paragraph suggests that various spectrins are strongly involved in supporting cell architecture and morphology in non-erythroid cells.
Spectrins are a structural platform for stabilization and activation of membrane microdomains
The spectrin-based skeleton participates in the organization of specialized membranes. When spectrin or its binding partner ankyrin is lost from or defective in cells, their interacting membrane partners do not accumulate at the appropriate site within the membrane (for review, see [65]). The surface expression and activation of the hTRPC4 channel (human Transient Receptor Potential Channel 4) is partially regulated by way of a direct interaction with spectrin. In αII-spectrin-depleted cells, the TRPC4 channels failed to undergo membrane insertion [66]. Mutations in βIII-spectrin are the cause of spinocerebellar ataxia type 5 (SCA5) and neurodegenerative disease [49, 67–69]. βIII-Spectrin defects are associated with mislocation of the glutamate transporter EAAT4 at the surface of the plasma membrane in Purkinje cells [70, 71]. βIV-Spectrin knock-out mice exhibit tremors and contraction of the hindlimbs. Loss of βIV-spectrin observed in quivering mice with hearing loss is associated with mislocation of voltage-gated channels at the axon initial segment and node of Ranvier. Alterations in the location of sodium and potassium channels at myelinated nerves slow propagation and desynchronize action potentials [72, 73]. So, βIV-spectrin acts as a multifunctional regulatory platform for sodium channels, and has important roles in the structure and stability of excitable membranes in heart and brain, targeting critical structural and regulatory proteins. In Drosophila, loss of β-spectrin led to the loss of Na+K+-ATPase from the basolateral domain of epithelial cells [74]. In an extreme case, loss of a variant of βII-spectrin in mice led to death in utero [75].
As described above, the spectrin-based membrane skeleton controls the disposition of selected membrane channels, receptors, transporters and adhesion molecules. Defects in spectrins result in destabilization of the membrane structure, lead to serious neurodegenerative diseases and are involved in pathological processes.
Spectrins, cell cycle and DNA repair
Other studies suggest the participation of spectrin in cell cycle regulation. Spectrin might be involved in TGFβ signaling. Proteomic studies revealed the presence of spectrin in a complex including TGFβ-R1 (transforming growth factor β receptor-1) [76]. The loss of β-spectrin results in defective TGFβ signaling as manifested by mislocation of proteins that modulate the activity of TGFβ—smads 3 and 4 [75]. Spectrins are also components of the G-protein-coupled receptor (GPCR) complex [77] and the synaptic multiprotein complex [29, 78, 79]. These data indicate that spectrins are involved in the cell cycle by regulating the expression of membrane receptors. It is also noteworthy that in a mouse model, downregulation of expression of ELF, an isoform of βII-spectrin, confers susceptibility to tumorigenesis: βII-Sp+/− mutant mice develop frequent tumors associated with deregulation of cell cycle control at the G1/S transition and defective TGFβ signaling [80–82]. Moreover, these βII-Sp+/− mice are born with many phenotypic characteristics observed in Beckwith-Wiedemann syndrome (BWS), a hereditary stem cell cancer syndrome. These include dramatic visceromegaly, followed in later months by the development of multiple cancers, including carcinomas of the gastrointestinal tract, as well as renal and adrenal adenocarcinomas. Epigenetic silencing of βII-spectrin expression in human BWS could be a potential causal factor in this stem cell disorder [83].
Furthermore, in αII-spectrin-depleted melanoma cells, increased expression of p21 (an inhibitor of cyclin-dependent kinase) was observed, which was associated with cell cycle arrest in the G1 phase. Spectrin depletion could secondarily modify the cell cycle by altering cell adhesion [84]. Although the detailed roles of spectrins in cell cycle regulation remain to be elucidated, spectrins should be considered as important elements in transduction pathways of extracellular signals controlling the cell cycle.
αII-Spectrin is present in nuclei of human cells and could play an important role in the repair of DNA interstrand cross-links. αII-Spectrin is deficient in cells from patients with Fanconi anemia (FA) [85]. It colocalizes with the cross-link repair protein XPF and FANCA, one of the Fanconi anemia proteins, in cross-link-induced nuclear foci [86, 87]. Another FA protein, FANCG, contains a motif that interacts directly with the SH3 domain of αII-spectrin. It plays a role in maintaining αII-spectrin stability in the cell [88]. αII-Spectrin could be particularly important in some of the initial steps of the cross-link repair process, which involves incision and unhooking of the cross-link via XPF/ERCC1 [89]. After cell damage, αII-spectrin binds to DNA at the sites of damage and acts as a scaffold, contributing to the recruitment of repair proteins. Moreover, αII-spectrin is involved in maintaining chromosomal stability. Depletion of αII-spectrin in normal human cells results in chromosomal instability, as evidenced by an increased number of interchromatid exchanges, fusions/radials and breaks. It leads to decreased cell growth and survival [90]. These studies demonstrate the importance of αII-spectrin in the repair of DNA interstrand cross-links.
Spectrin contributes with actin to cell adhesion and spreading
A newly proven role of α-spectrin is its participation in cell adhesion and spreading via its SH3 domain. αII-Spectrin is present in a specialized type of calpain-induced β3 integrin signaling complexes. The SH3 domain appears to transmit signals required for Rac activation and lamellipodia extension [91]. Cells overexpressing the SH3 domain adhered to the substratum, and their calpain-induced integrin signaling complexes were formed, but Rac activation, lamellipodia extension and cell spreading were inhibited. Spreading was restored by overexpressing constitutively active Rac. Other data supported the involvement of αII-spectrin in actin reorganization [84]. Spectrin loss by siRNA impaired cell adhesion and spreading. Spectrin-depleted cells exhibited modifications of the actin cytoskeleton, such as loss of stress fibers, alterations of focal contacts and modified expression of some integrins. Spectrin via its SH3 domain interacts with two members of the Ena/VASP (enabled/vasodilator-stimulated phosphoprotein) family: VASP [92] and EVL (Ena/VASP-like) [93, 94]. Ena/VASP proteins are found in focal contacts, cell-cell contacts and highly dynamic membrane regions such as lamellipodia. These proteins appear to regulate adhesion and to control actin dynamics. Proteins of the Ena/VASP family are essential for actin remodeling upon T cell activation, formation and extensions of lamellipodia. Ena/VASP proteins bind the adapter protein ADAP (expressed in T cells and myeloid cells), which participates in LFA-1 integrin clustering. Spectrin also interacts with other proteins involved in actin dynamics, such as Abi1 [95, 96] and proteins of the WASP (Wiskott-Aldrich syndrome protein) family. The T cells from patients with Wiskott-Aldrich syndrome show characteristic cytoskeletal defects [97] and impaired function [98].
Thus, these recent data pointed out an unexpected role of αII-spectrin in transmission of signals leading to Rac activation, adhesion, lamellipodia extension, and cell spreading through several ligands and partners regulating actin dynamics.
Control of activation of transmembrane proteins
The other example of spectrin participation in cell signaling is its contribution to the formation of TCR (T cell receptor) complexes in lymphocytes. It has been clearly demonstrated that the spectrin-based skeleton via its two major proteins, spectrin and ankyrin, directly binds CD45 in lymphocytes [99, 100]. CD45 plays a pivotal role in antigen-stimulated proliferation of T lymphocytes and in thymic development. The catalytic activity of CD45 is required for TCR signaling and regulation. Human mutations in the CD45-encoding gene are the cause of severe combined immunodeficiencies (SCID) [101–103]. CD45-deficient mice are severely immune-deficient, with very few peripheral T lymphocytes, defective thymocyte development and failed receptor-mediated activation [104]. The direct binding of spectrin to CD45 stimulates the PTPase activity of CD45 and also facilitates the movement of CD45 and CD3 to the lymphocyte surface [100].
In lymphoid-derived cell lines, spectrin is distributed in the cytoplasm, but appears very often as large aggregates [105]. These spectrin-rich large aggregates in lymphocytes contain several proteins, such as hsp70, receptor for activated C kinase-1 (RAC-1) and PKCθ (Ca2+-independent subfamily of serine/threonine specific protein kinase C) [106, 107]. Activation of lymphocytes by phorbol 12-myristate 13-acetate (PMA), T-receptor cross-linking and mild hyperthermia resulted in the formation of cytoplasmic spectrin aggregates [108]. Recruitment of intracellular proteins to the plasma membrane is a well-known event required for the initiation of signal transduction; the participation of spectrin in this event may indicate its signaling function in lymphocytes. These facts imply that occurrence of aggregation of spectrin and PKCθ in chemically and physically stimulated lymphocytes and formation of a large signaling complex at the site of TCR clustering in immunological synapses may be related phenomena [109].
Spectrin aggregation may also be associated with early cellular apoptotic events preceding a loss of membrane aminophospholipid asymmetry [110]. Concomitant PKCθ rearrangement in lymphocytes implies its relationship to spectrin aggregation and its participation in regulating early steps of apoptosis [111]. The redistribution of spectrin and PKCθ into a polar aggregate has also been observed in Jurkat T and HL60 cell lines during early apoptosis-induced by cytostatics. These changes seem to be restricted to spectrin and not to concern other cytoskeletal proteins such as actin or vimentin. Although spectrins are potential caspase -3, -7 and -8 substrates, these proteases exhibited minor involvement in the early apoptotic rearrangement of spectrin/PKCθ. Moreover, spectrin aggregation was shown to be at least partially dependent on PKCθ activity.
Taken together, we may state that spectrin also plays an important role in various pathways of regulation of cellular processes and signaling in lymphocytes, such as TCR formation, activation and early steps of apoptosis.
Spectrins interact with proteins involved in intracellular traffic
The multifunctional spectrin-based skeleton participates in the complexes linking various structures or organelles to the motors involved in microtubule-directed transport, and in the facilitation of membrane protein transport via the secretory and endocytic pathways [112]. βIII-Spectrin is present in the Golgi and vesicle membranes [49], and binds to the dynactin subunit ARP1, suggesting a possible role in transport [113]. In patients exhibiting spinocerebellar ataxia type 5 (SCA5), a mutation found in the calponin homology domain (CH) alters the interaction of βIII-spectrin with ARP1 and consequently affects the stabilization of membrane protein, or may cause alterations in EAAT4 transport by disrupting the binding to ARP1 and dynein motor complex. Cell culture studies reveal that the L253P mutant of βIII-spectrin, instead of being found at the cell membrane, appears trapped in the cytoplasm associated with the Golgi apparatus. Moreover, L253P βIII-spectrin prevents correct localization of wt βIII-spectrin and prevents EAAT4 from reaching the plasma membrane. These data provide evidence for a dominant-negative effect of an SCA5 mutation and show that trafficking of both βIII-spectrin and EAAT4 from the Golgi is disrupted through failure of the L253P mutation to interact with ARP1 [114].
Spectrin functions can be regulated by posttranslational modifications
Several pathways of spectrin posttranslational regulation have been correlated to apoptosis/necrosis [115] as well as to secretion/endocytosis, vertebrate lens development [116] and pathologies in the central nervous system [117, 118].
The regulatory pathways affecting spectrin include the action of calcium ions, calmodulin and Ca2+-activated proteolysis. Proteolysis of spectrin leads to destabilization of the membrane scaffold and membrane remodeling. This process is under the control of several proteases—m- and μ-calpains (Ca2+-activated proteases) and caspases 2, 3 and 7 (activated during apoptosis)—and is highly regulated by Ca2+/calmodulin and tyrosine phosphorylation. αII-Spectrin cleavage is highly influenced by Ca2+ homeostasis and calmodulin, which therefore represent a potential regulatory pathway for the stability and plasticity of the spectrin-based skeleton [43, 44]. In fusion of placental trophoblast cells, caspases rather than calpains mediate remodeling of the spectrin skeleton [119]. As was found recently, during early apoptosis, caspase 8 releases an N-terminal fragment containing ABD as well as a C-terminal fragment of βII-spectrin. The proteolysis in the N-terminal region depends on 4.1 protein (Kołodziejczyk, Dubielecka 2011 in preparation).
The other regulatory pathway important during membrane skeleton remodeling is spectrin phosphorylation. β-Spectrin phosphorylation was reported to be essential in destabilization of the erythrocyte membrane skeleton [120–122], disassembly of the skeleton during mitosis [123] and the control of Golgi stability [124]. Likewise, αII-spectrin is an important subject of tyrosine phosphorylation. Tyrosine phosphorylation/dephosphorylation in the calpain cleavage site of αII-spectrin by kinases and phosphatases is a mechanism that regulates this spectrin subunit’s sensitivity to cleavage [44, 125]. Spectrin is a key point of signal convergence between tyrosine/phosphatase and Ca2+-mediated signal cascades. This kind of control may be particularly important in vesicle trafficking, endocytosis, neurite outgrowth and NMDA receptor activation [126]. However, a study on homozygous mice expressing a mutant αII-spectrin designed to resist calpain and caspase cleavage questions the functional importance of this process in vivo [127].
Moreover, βIV-spectrin might be involved in a regulatory mechanism for Na+ channels (Nav1.5), via direct phosphorylation by βIV-spectrin targeted calcium/calmodulin-dependent kinase II [128]. These findings provide evidence for an unexpected yet commanding molecular platform involving spectrin that determines vertebrate membrane excitability.
Concluding remarks
The role of different spectrin subunits and domains has been studied and explained progressively. The first discovered role of αIβI-spectrin was to define the cell shape and to maintain cell membrane integrity and stability in erythrocytes. Defects in these skeletal proteins in red cells lead to hereditary hemolytic anemia.
In nucleated cells the functions of spectrins still remain to be elucidated. The occurrence of a variety of spectrin isoforms in different cells indicates that its functions may vary among different cells as a result of their specializations. In most cells spectrins are known to be engaged in determination of the cell shape, in maintaining cell flexibility, cell-cell contact, cell polarity and proliferation.
Moreover, spectrins are engaged in the organization and function of membrane integral proteins, such as ion channels, receptors and adhesion molecules in specialized membrane domains. The β-spectrin mutations induce destabilization of the membrane structure and mislocation of membrane receptors and channels, often leading to serious diseases, such as spinocerebellar ataxia and neurodegenerative diseases. Recent data have revealed that αII-spectrin mutations are associated with West syndrome, an epileptic encephalopathy [129]. Defects in this ortholog in Drosophila melanogaster and Caenorhabditis elegans larvae are lethal. These facts corroborate the crucial role of this protein. In the last few years, more and more reports providing new data concerning the previously unrecognized role of α-spectrins in signaling pathways have appeared. The SH3 domain of spectrin plays an essential role in Rac activation, initiation of actin network formation, adhesion, lamellipodia extension, cell spreading and DNA repair. The spectrins are also engaged in different pathways of cell transduction and signaling in lymphocytes, such as TCR formation, activation and early steps of apoptosis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bloch RJ, Morrow JS. An unusual beta-spectrin associated with clustered acetylcholine receptors. J Cell Biol. 1989;108:481–493. doi: 10.1083/jcb.108.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: identification of an erythroid beta-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, et al. Identification of a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that binds beta I sigma spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133:819–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianci CD, Zhang Z, Pradhan D, Morrow JS. Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry. 1999;38:15721–15730. doi: 10.1021/bi991458k. [DOI] [PubMed] [Google Scholar]
- 5.Moon RT, McMahon AP. Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990;265:4427–4433. [PubMed] [Google Scholar]
- 6.Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, et al. The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem. 1990;265:4434–4443. [PubMed] [Google Scholar]
- 8.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81:3173–3185. [PubMed] [Google Scholar]
- 9.Byers TJ, Dubreuil R, Branton D, Kiehart DP, Goldstein LS. Drosophila spectrin. II. Conserved features of the alpha-subunit are revealed by analysis of cDNA clones and fusion proteins. J Cell Biol. 1987;105:2103–2110. doi: 10.1083/jcb.105.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubreuil RR, Byers TJ, Sillman AL, Bar-Zvi D, Goldstein LS, et al. The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989;109:2197–2205. doi: 10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byers TJ, Brandin E, Lue RA, Winograd E, Branton D. The complete sequence of Drosophila beta-spectrin reveals supra-motifs comprising eight 106-residue segments. Proc Natl Acad Sci U S A. 1992;89:6187–6191. doi: 10.1073/pnas.89.13.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeown C, Praitis V, Austin J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 1998;125:2087–2098. doi: 10.1242/dev.125.11.2087. [DOI] [PubMed] [Google Scholar]
- 13.Dubreuil RR, Grushko T. Genetic studies of spectrin: new life for a ghost protein. Bioessays. 1998;20:875–878. doi: 10.1002/(SICI)1521-1878(199811)20:11<875::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Salomao M, An X, Guo X, Gratzer WB, Mohandas N, et al. Mammalian alpha I-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes. Proc Natl Acad Sci U S A. 2006;103:643–648. doi: 10.1073/pnas.0507661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37:796–803. doi: 10.1042/BST0370796. [DOI] [PubMed] [Google Scholar]
- 16.Baines AJ. Comprehensive analysis of all triple helical repeats in beta-spectrins reveals patterns of selective evolutionary conservation. Cell Mol Biol Lett. 2003;8:195–214. [PubMed] [Google Scholar]
- 17.Speicher DW, Ursitti JA. Spectrin motif. Conformation of a mammoth protein. Curr Biol. 1994;4:154–157. doi: 10.1016/s0960-9822(94)00037-0. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmann JC, Chang JG, Tse WT, Scarpa AL, Marchesi VT, et al. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990;265:11827–11832. [PubMed] [Google Scholar]
- 19.Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999;98:523–535. doi: 10.1016/s0092-8674(00)81980-7. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Tang HY, Speicher DW. A structural model of the erythrocyte spectrin heterodimer initiation site determined using homology modeling and chemical cross-linking. J Biol Chem. 2008;283:1553–1562. doi: 10.1074/jbc.M706981200. [DOI] [PubMed] [Google Scholar]
- 21.Speicher DW, Weglarz L, DeSilva TM. Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. J Biol Chem. 1992;267:14775–14782. [PubMed] [Google Scholar]
- 22.Ursitti JA, Kotula L, DeSilva TM, Curtis PJ, Speicher DW. Mapping the human erythrocyte beta-spectrin dimer initiation site using recombinant peptides and correlation of its phasing with the alpha-actinin dimer site. J Biol Chem. 1996;271:6636–6644. doi: 10.1074/jbc.271.12.6636. [DOI] [PubMed] [Google Scholar]
- 23.Ipsaro JJ, Mondragon A. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. doi: 10.1182/blood-2009-11-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korsgren C, Peters LL, Lux SE. Protein 4.2 binds to the carboxyl-terminal EF-hands of erythroid alpha-spectrin in a calcium- and calmodulin-dependent manner. J Biol Chem. 2010;285:4757–4770. doi: 10.1074/jbc.M109.056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ipsaro JJ, Huang L, Mondragon A. Structures of the spectrin-ankyrin interaction binding domains. Blood. 2009;113:5385–5393. doi: 10.1182/blood-2008-10-184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low PS. Where spectrin snuggles with ankyrin. Blood. 2009;113:5372–5373. doi: 10.1182/blood-2009-03-207712. [DOI] [PubMed] [Google Scholar]
- 27.Van Kim CL, Colin Y, Cartron JP. Rh proteins: key structural and functional components of the red cell membrane. Blood Rev. 2006;20:93–110. doi: 10.1016/j.blre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Diakowski W, Ozimek L, Bielska E, Bem S, Langner M, et al. Cholesterol affects spectrin-phospholipid interactions in a manner different from changes resulting from alterations in membrane fluidity due to fatty acyl chain composition. Biochim Biophys Acta. 2006;1758:4–12. doi: 10.1016/j.bbamem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Sikorski AF, Sangerman J, Goodman SR, Critz SD. Spectrin (betaSpIIsigma1) is an essential component of synaptic transmission. Brain Res. 2000;852:161–166. doi: 10.1016/s0006-8993(99)02253-2. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JM, Ellis RE, Green EM, Winlove CP, Petrov PG. Spectrin maintains the lateral order in phosphatidylserine monolayers. Chem Phys Lipids. 2008;151:66–68. doi: 10.1016/j.chemphyslip.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Grzybek M, Chorzalska A, Bok E, Hryniewicz-Jankowska A, Czogalla A, et al. Spectrin-phospholipid interactions. Existence of multiple kinds of binding sites? Chem Phys Lipids. 2006;141:133–141. doi: 10.1016/j.chemphyslip.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Chorzalska A, Lach A, Borowik T, Wolny M, Hryniewicz-Jankowska A, et al. The effect of the lipid-binding site of the ankyrin-binding domain of erythroid beta-spectrin on the properties of natural membranes and skeletal structures. Cell Mol Biol Lett. 2010;15:406–423. doi: 10.2478/s11658-010-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolny M, Grzybek M, Bok E, Chorzalska A, Lenoir M, et al. Key amino acid residues of ankyrin-sensitive phosphatidylethanolamine/phosphatidylcholine-lipid binding site of betal-spectrin. PLoS One. 2011;6:e21538. doi: 10.1371/journal.pone.0021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An X, Guo X, Gratzer W, Mohandas N. Phospholipid binding by proteins of the spectrin family: a comparative study. Biochem Biophys Res Commun. 2005;327:794–800. doi: 10.1016/j.bbrc.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 35.Collec E, Lecomte MC, El-Nemer W, Colin Y, Le Van Kim C (2011) Novel role for the Lu/BCAM-spectrin interaction in actin cytoskeleton reorganization. Biochem J [DOI] [PubMed]
- 36.Gauthier E, El Nemer W, Wautier MP, Renaud O, Tchernia G, et al. Role of the interaction between Lu/BCAM and the spectrin-based membrane skeleton in the increased adhesion of hereditary spherocytosis red cells to laminin. Br J Haematol. 2010;148:456–465. doi: 10.1111/j.1365-2141.2009.07973.x. [DOI] [PubMed] [Google Scholar]
- 37.Dhermy D, Schrevel J, Lecomte MC. Spectrin-based skeleton in red blood cells and malaria. Curr Opin Hematol. 2007;14:198–202. doi: 10.1097/MOH.0b013e3280d21afd. [DOI] [PubMed] [Google Scholar]
- 38.Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature. 1992;359:851–855. doi: 10.1038/359851a0. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg S, Buevich AV, Sethson I, Edlund U, Backman L. Calcium-binding mechanism of human nonerythroid alpha-spectrin EF-structures. Biochemistry. 1997;36:7199–7208. doi: 10.1021/bi9631531. [DOI] [PubMed] [Google Scholar]
- 40.Trave G, Lacombe PJ, Pfuhl M, Saraste M, Pastore A. Molecular mechanism of the calcium-induced conformational change in the spectrin EF-hands. Embo J. 1995;14:4922–4931. doi: 10.1002/j.1460-2075.1995.tb00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trave G, Pastore A, Hyvonen M, Saraste M. The C-terminal domain of alpha-spectrin is structurally related to calmodulin. Eur J Biochem. 1995;227:35–42. doi: 10.1111/j.1432-1033.1995.tb20357.x. [DOI] [PubMed] [Google Scholar]
- 42.Okabe T, Sobue K. Identification of a new 84/82 kDa calmodulin-binding protein, which also interacts with actin filaments, tubulin and spectrin, as synapsin I. FEBS Lett. 1987;213:184–188. doi: 10.1016/0014-5793(87)81488-6. [DOI] [PubMed] [Google Scholar]
- 43.Rotter B, Kroviarski Y, Nicolas G, Dhermy D, Lecomte MC. AlphaII-spectrin is an in vitro target for caspase-2, and its cleavage is regulated by calmodulin binding. Biochem J. 2004;378:161–168. doi: 10.1042/BJ20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, et al. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banuelos S, Saraste M, Djinovic Carugo K. Structural comparisons of calponin homology domains: implications for actin binding. Structure. 1998;6:1419–1431. doi: 10.1016/s0969-2126(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 46.Karinch AM, Zimmer WE, Goodman SR. The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem. 1990;265:11833–11840. [PubMed] [Google Scholar]
- 47.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 48.Saraste M, Hyvonen M. Pleckstrin homology domains: a fact file. Curr Opin Struct Biol. 1995;5:403–408. doi: 10.1016/0959-440x(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 49.Stankewich MC, Tse WT, Peters LL, Ch’ng Y, John KM, et al. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 51.Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1–20. doi: 10.1016/j.blre.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411–1426. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 53.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Goodman SR, Zagon IS, Kulikowski RR. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci USA. 1981;78:7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu S, Sangerman J, Li M, Brough GH, Goodman SR, et al. Essential control of an endothelial cell ISOC by the spectrin membrane skeleton. J Cell Biol. 2001;154:1225–1233. doi: 10.1083/jcb.200106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, et al. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 57.Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, et al. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 58.Lee HG, Zarnescu DC, MacIver B, Thomas GH. The cell adhesion molecule Roughest depends on beta(Heavy)-spectrin during eye morphogenesis in Drosophila . J Cell Sci. 2010;123:277–285. doi: 10.1242/jcs.056853. [DOI] [PubMed] [Google Scholar]
- 59.Stabach PR, Morrow JS. Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem. 2000;275:21385–21395. doi: 10.1074/jbc.C000159200. [DOI] [PubMed] [Google Scholar]
- 60.Legendre K, Safieddine S, Kussel-Andermann P, Petit C, El-Amraoui A. alphaII-betaV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. J Cell Sci. 2008;121:3347–3356. doi: 10.1242/jcs.028134. [DOI] [PubMed] [Google Scholar]
- 61.Leshchyns’ka I, Tanaka M, Schachner M, Sytnyk V (2011) Immobilized pool of NCAM180 in the postsynaptic membrane is homeostatically replenished by the flux of NCAM180 from extrasynaptic regions. J Biol Chem [DOI] [PMC free article] [PubMed]
- 62.Leshchyns’ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol. 2003;161:625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramser EM, Buck F, Schachner M, Tilling T. Binding of alphaII spectrin to 14–3-3beta is involved in NCAM-dependent neurite outgrowth. Mol Cell Neurosci. 2010;45:66–74. doi: 10.1016/j.mcn.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Djinovic-Carugo K, Gautel M, Ylanne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- 66.Odell AF, Van Helden DF, Scott JL. The spectrin cytoskeleton influences the surface expression and activation of human transient receptor potential channel 4 channels. J Biol Chem. 2008;283:4395–4407. doi: 10.1074/jbc.M709729200. [DOI] [PubMed] [Google Scholar]
- 67.Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 68.Lorenzo DN, Li MG, Mische SE, Armbrust KR, Ranum LP, et al. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila . J Cell Biol. 2010;189:143–158. doi: 10.1083/jcb.200905158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, et al. Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proc Natl Acad Sci USA. 2010;107:6022–6027. doi: 10.1073/pnas.1001522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- 71.Perkins EM, Clarkson YL, Sabatier N, Longhurst DM, Millward CP, et al. Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J Neurosci. 2010;30:4857–4867. doi: 10.1523/JNEUROSCI.6065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parkinson NJ, Olsson CL, Hallows JL, McKee-Johnson J, Keogh BP, et al. Mutant beta-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat Genet. 2001;29:61–65. doi: 10.1038/ng710. [DOI] [PubMed] [Google Scholar]
- 73.Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubreuil RR, Wang P, Dahl S, Lee J, Goldstein LS. Drosophila beta spectrin functions independently of alpha spectrin to polarize the Na, K ATPase in epithelial cells. J Cell Biol. 2000;149:647–656. doi: 10.1083/jcb.149.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, et al. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 76.Conrotto P, Yakymovych I, Yakymovych M, Souchelnytskyi S. Interactome of transforming growth factor-beta type I receptor (TbetaRI): inhibition of TGFbeta signaling by Epac1. J Proteome Res. 2007;6:287–297. doi: 10.1021/pr060427q. [DOI] [PubMed] [Google Scholar]
- 77.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, et al. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 78.Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, et al. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- 79.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 80.Baek HJ, Kim SS, da Silva FM, Volpe EA, Evans S, et al. Inactivation of TGF-beta signaling in lung cancer results in increased CDK4 activity that can be rescued by ELF. Biochem Biophys Res Commun. 2006;346:1150–1157. doi: 10.1016/j.bbrc.2006.05.195. [DOI] [PubMed] [Google Scholar]
- 81.Kim SS, Shetty K, Katuri V, Kitisin K, Baek HJ, et al. TGF-beta signaling pathway inactivation and cell cycle deregulation in the development of gastric cancer: role of the beta-spectrin, ELF. Biochem Biophys Res Commun. 2006;344:1216–1223. doi: 10.1016/j.bbrc.2006.03.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, et al (2007) Tgf-Beta signaling in development. Sci STKE 2007: cm1 [DOI] [PubMed]
- 83.Yao ZX, Jogunoori W, Choufani S, Rashid A, Blake T, et al. Epigenetic silencing of beta-spectrin, a TGF-beta signaling/scaffolding protein in a human cancer stem cell disorder: Beckwith–Wiedemann syndrome. J Biol Chem. 2010;285:36112–36120. doi: 10.1074/jbc.M110.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, et al. AlphaII-spectrin is critical for cell adhesion and cell cycle. J Biol Chem. 2009;284:2409–2418. doi: 10.1074/jbc.M801324200. [DOI] [PubMed] [Google Scholar]
- 85.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry. 2001;40:7025–7034. doi: 10.1021/bi002917g. [DOI] [PubMed] [Google Scholar]
- 86.Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW. Nonerythroid alphaII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Sci. 2003;116:823–835. doi: 10.1242/jcs.00294. [DOI] [PubMed] [Google Scholar]
- 87.Sridharan DM, McMahon LW, Lambert MW. alphaII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol Int. 2006;30:866–878. doi: 10.1016/j.cellbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Lefferts JA, Wang C, Sridharan D, Baralt M, Lambert MW. The SH3 domain of alphaII spectrin is a target for the Fanconi anemia protein, FANCG. Biochemistry. 2009;48:254–263. doi: 10.1021/bi801483u. [DOI] [PubMed] [Google Scholar]
- 89.Wang C, Lambert MW. The Fanconi anemia protein, FANCG, binds to the ERCC1-XPF endonuclease via its tetratricopeptide repeats and the central domain of ERCC1. Biochemistry. 2010;49:5560–5569. doi: 10.1021/bi100584c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McMahon LW, Zhang P, Sridharan DM, Lefferts JA, Lambert MW. Knockdown of alphaII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem Biophys Res Commun. 2009;381:288–293. doi: 10.1016/j.bbrc.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bialkowska K, Saido TC, Fox JE. SH3 domain of spectrin participates in the activation of Rac in specialized calpain-induced integrin signaling complexes. J Cell Sci. 2005;118:381–395. doi: 10.1242/jcs.01625. [DOI] [PubMed] [Google Scholar]
- 92.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, et al. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180:205–219. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rotter B, Bournier O, Nicolas G, Dhermy D, Lecomte MC. AlphaII-spectrin interacts with Tes and EVL, two actin-binding proteins located at cell contacts. Biochem J. 2005;388:631–638. doi: 10.1042/BJ20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bournier O, Kroviarski Y, Rotter B, Nicolas G, Lecomte MC, et al. Spectrin interacts with EVL (Enabled/vasodilator-stimulated phosphoprotein-like protein), a protein involved in actin polymerization. Biol Cell. 2006;98:279–293. doi: 10.1042/BC20050024. [DOI] [PubMed] [Google Scholar]
- 95.Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, et al. Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem. 1998;273:13681–13692. doi: 10.1074/jbc.273.22.13681. [DOI] [PubMed] [Google Scholar]
- 96.Dubielecka PM, Ladwein KI, Xiong X, Migeotte I, Chorzalska A, et al. Essential role for Abi1 in embryonic survival and WAVE2 complex integrity. Proc Natl Acad Sci USA. 2011;108:7022–7027. doi: 10.1073/pnas.1016811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell. 1994;79:922. [PubMed] [Google Scholar]
- 98.Nonoyama S, Ochs HD. Characterization of the Wiskott–Aldrich syndrome protein and its role in the disease. Curr Opin Immunol. 1998;10:407–412. doi: 10.1016/s0952-7915(98)80113-1. [DOI] [PubMed] [Google Scholar]
- 99.Iida N, Lokeshwar VB, Bourguignon LY. Mapping the fodrin binding domain in CD45, a leukocyte membrane-associated tyrosine phosphatase. J Biol Chem. 1994;269:28576–28583. [PubMed] [Google Scholar]
- 100.Pradhan D, Morrow J. The spectrin-ankyrin skeleton controls CD45 surface display and interleukin-2 production. Immunity. 2002;17:303–315. doi: 10.1016/s1074-7613(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 101.Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 102.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–153. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, et al. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001;166:1308–1313. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 104.Wallace VA, Penninger JM, Kishihara K, Timms E, Shahinian A, et al. Alterations in the level of CD45 surface expression affect the outcome of thymic selection. J Immunol. 1997;158:3205–3214. [PubMed] [Google Scholar]
- 105.Repasky EA, Pollina CM, Menold MM, Hudecki MS. Increased concentration of spectrin is observed in avian dystrophic muscle. Proc Natl Acad Sci USA. 1986;83:802–806. doi: 10.1073/pnas.83.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghaffari-Tabrizi N, Bauer B, Villunger A, Baier-Bitterlich G, Altman A, et al. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur J Immunol. 1999;29:132–142. doi: 10.1002/(SICI)1521-4141(199901)29:01<132::AID-IMMU132>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 107.Trushin SA, Pennington KN, Algeciras-Schimnich A, Paya CV. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J Biol Chem. 1999;274:22923–22931. doi: 10.1074/jbc.274.33.22923. [DOI] [PubMed] [Google Scholar]
- 108.Masso-Welch PA, Black JD, Erikson J, Repasky EA. Polarized expression of immunoglobulin, spectrin, and protein kinase C beta II occurs in B cells from normal BALB/c, autoimmune lpr, and anti-ssDNA transgenic, tolerant mice. J Leukoc Biol. 1999;66:617–624. doi: 10.1002/jlb.66.4.617. [DOI] [PubMed] [Google Scholar]
- 109.Kwiatkowska K, Sobota A. Engagement of spectrin and actin in capping of FcgammaRII revealed by studies on permeabilized U937 cells. Biochem Biophys Res Commun. 1999;259:287–293. doi: 10.1006/bbrc.1999.0769. [DOI] [PubMed] [Google Scholar]
- 110.Dubielecka PM, Grzybek M, Kolondra A, Jazwiec B, Draga A, et al. Aggregation of spectrin and PKCtheta is an early hallmark of fludarabine/mitoxantrone/dexamethasone-induced apoptosis in Jurkat T and HL60 cells. Mol Cell Biochem. 2010;339:63–77. doi: 10.1007/s11010-009-0370-4. [DOI] [PubMed] [Google Scholar]
- 111.Dubielecka PM, Jazwiec B, Potoczek S, Wrobel T, Miloszewska J, et al. Changes in spectrin organisation in leukaemic and lymphoid cells upon chemotherapy. Biochem Pharmacol. 2005;69:73–85. doi: 10.1016/j.bcp.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 112.Devarajan P, Stabach PR, De Matteis MA, Morrow JS. Na, K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, et al. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 114.Clarkson YL, Gillespie T, Perkins EM, Lyndon AR, Jackson M. Beta-III spectrin mutation L253P associated with spinocerebellar ataxia type 5 interferes with binding to Arp1 and protein trafficking from the Golgi. Hum Mol Genet. 2010;19:3634–3641. doi: 10.1093/hmg/ddq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Czogalla A, Sikorski AF. Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee A, Morrow JS, Fowler VM. Caspase remodeling of the spectrin membrane skeleton during lens development and aging. J Biol Chem. 2001;276:20735–20742. doi: 10.1074/jbc.M009723200. [DOI] [PubMed] [Google Scholar]
- 117.Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, et al. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- 118.Glantz SB, Cianci CD, Iyer R, Pradhan D, Wang KK, et al. Sequential degradation of alphaII and betaII spectrin by calpain in glutamate or maitotoxin-stimulated cells. Biochemistry. 2007;46:502–513. doi: 10.1021/bi061504y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gauster M, Siwetz M, Orendi K, Moser G, Desoye G, et al. Caspases rather than calpains mediate remodelling of the fodrin skeleton during human placental trophoblast fusion. Cell Death Differ. 2010;17:336–345. doi: 10.1038/cdd.2009.133. [DOI] [PubMed] [Google Scholar]
- 120.Manno S, Takakuwa Y, Nagao K, Mohandas N. Modulation of erythrocyte membrane mechanical function by beta-spectrin phosphorylation and dephosphorylation. J Biol Chem. 1995;270:5659–5665. doi: 10.1074/jbc.270.10.5659. [DOI] [PubMed] [Google Scholar]
- 121.Perrotta S, del Giudice EM, Iolascon A, De Vivo M, Di Pinto D, et al. Reversible erythrocyte skeleton destabilization is modulated by beta-spectrin phosphorylation in childhood leukemia. Leukemia. 2001;15:440–444. doi: 10.1038/sj.leu.2402047. [DOI] [PubMed] [Google Scholar]
- 122.Pinder JC, Bray D, Gratzer WB. Control of interaction of spectrin and actin by phosphorylation. Nature. 1977;270:752–754. doi: 10.1038/270752a0. [DOI] [PubMed] [Google Scholar]
- 123.Fowler VM, Adam EJ. Spectrin redistributes to the cytosol and is phosphorylated during mitosis in cultured cells. J Cell Biol. 1992;119:1559–1572. doi: 10.1083/jcb.119.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siddhanta A, Radulescu A, Stankewich MC, Morrow JS, Shields D. Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 2003;278:1957–1965. doi: 10.1074/jbc.M209137200. [DOI] [PubMed] [Google Scholar]
- 125.Nedrelow JH, Cianci CD, Morrow JS. c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem. 2003;278:7735–7741. doi: 10.1074/jbc.M210988200. [DOI] [PubMed] [Google Scholar]
- 126.Kamal A, Ying Y, Anderson RG. Annexin VI-mediated loss of spectrin during coated pit budding is coupled to delivery of LDL to lysosomes. J Cell Biol. 1998;142:937–947. doi: 10.1083/jcb.142.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meary F, Metral S, Ferreira C, Eladari D, Colin Y, et al. A mutant alphaII-spectrin designed to resist calpain and caspase cleavage questions the functional importance of this process in vivo. J Biol Chem. 2007;282:14226–14237. doi: 10.1074/jbc.M700028200. [DOI] [PubMed] [Google Scholar]
- 128.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saitsu H, Tohyama J, Kumada T, Egawa K, Hamada K, et al. Dominant-negative mutations in alpha-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am J Hum Genet. 2010;86:881–891. doi: 10.1016/j.ajhg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tse WT, Tang J, Jin O, Korsgren C, John KM, et al. A new spectrin, beta IV, has a major truncated isoform that associates with promyelocytic leukemia protein nuclear bodies and the nuclear matrix. J Biol Chem. 2001;276:23974–23985. doi: 10.1074/jbc.M009307200. [DOI] [PubMed] [Google Scholar]
- 131.Krieger CC, An X, Tang HY, Mohandas N, Speicher DW, et al. Cysteine shotgun-mass spectrometry (CS-MS) reveals dynamic sequence of protein structure changes within mutant and stressed cells. Proc Natl Acad Sci USA. 2011;108:8269–8274. doi: 10.1073/pnas.1018887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Resneck WG, Lee PC, Randall WR, Bloch RJ, et al. Characterization and expression of a heart-selective alternatively spliced variant of alpha II-spectrin, cardi+, during development in the rat. J Mol Cell Cardiol. 2010;48:1050–1059. doi: 10.1016/j.yjmcc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin PM, Cifuentes-Diaz C, Garcia M, Goutebroze L, Girault JA. [Axon and Schwann cells... so far away, so close] Rev Neurol (Paris) 2008;164:1057–1062. doi: 10.1016/j.neurol.2008.10.003. [DOI] [PubMed] [Google Scholar]