Abstract
The highly synchronized formations that characterize schooling in fish and the flight of certain bird groups have frequently been explained as reducing energy expenditure. I present an alternative, or complimentary, hypothesis that synchronization of group movements may improve hearing perception. Although incidental sounds produced as a by-product of locomotion (ISOL) will be an almost constant presence to most animals, the impact on perception and cognition has been little discussed. A consequence of ISOL may be masking of critical sound signals in the surroundings. Birds in flight may generate significant noise; some produce wing beats that are readily heard on the ground at some distance from the source. Synchronization of group movements might reduce auditory masking through periods of relative silence and facilitate auditory grouping processes. Respiratory locomotor coupling and intermittent flight may be other means of reducing masking and improving hearing perception. A distinct border between ISOL and communicative signals is difficult to delineate. ISOL seems to be used by schooling fish as an aid to staying in formation and avoiding collisions. Bird and bat flocks may use ISOL in an analogous way. ISOL and interaction with animal perception, cognition, and synchronized behavior provide an interesting area for future study.
Keywords: Hearing, Schooling fish, Organized flight, Intermittent flight, Synchronization, Respiratory locomotor coupling
Introduction
Animal locomotion results in vibrations and turbulence in the substratum (e.g., air, water, or the ground), which will emit sound waves. Incidental sounds produced as a by-product of locomotion (ISOL) will be an almost constant presence to most animals. It is important for animals to recognize and discriminate salient acoustic information, e.g., sound of predators or interspecific communication. Natural environments are usually filled with noise from several sources, such as wind, moving water, rustling leaves, and sounds from other animals. The energy from all these different sources is combined to reach the ears of the animal as a single pressure signal that varies in time (Lu and Vicario 2011). The animal’s auditory system is challenged to interpret this combined pressure signal, integrating information at multiple time scales and extracting specific patterns from variable backgrounds (Lu and Vicario 2011). Own-produced ISOL is likely to be a component of this auditory signal when an animal moves. The impact of ISOL on perception, cognition, and behavior has been little discussed.
The highly synchronized formations that characterize schooling in fish and the flight of certain bird groups have frequently been explained as reducing energy expenditure. I present an alternative, or complimentary, hypothesis that synchronization of group movements and some other little understood behavior may improve hearing perception. Intermittent flight in birds, acoustical consequences of respiratory locomotor coupling in vertebrates, and, to some extent, intervertebrates, as well as potential acoustical advantages of synchronization will be discussed. Other areas addressed are the role of ISOL as an aid to staying in formation in animal groups and the relationship between ISOL and intentionally modulated communicative sound.
Adaptations to avoid masking by self-produced ISOL
Sounds produced during locomotion in birds
Animal auditory systems may be stimulated by the signals produced by their own vocalizations, breathing, and movements. Detection of relevant sensory signals requires the filtering out of irrelevant noise, including noise created by the animal’s own movements (reafference) (von Holst and Mittelstaedt 1950; Zhang and Bodznick 2008). Noise resulting from an animal’s locomotion might interfere with the perception of signals emanating from the surrounding environment. This has been explored in individual animals, including fish and salamanders (Russell 1968; Roberts and Russell 1972; Montgomery and Bodznick 1994).1 In birds, sensory reafference associated with ISOL, its amplitude, and other characteristics have scarcely been investigated. During bird flight, the movement of air across and around wing feathers and vibrations generated by feather-to-feather friction produce sound (Coleman 2008). Hingee and Magrath (2009) recorded wing whistles2 produced by the crested pigeon, Ocyphaps lophotes. They found the alarm whistle, which produced escape response in con-specifics, to have a mean amplitude of 68 dB, while the non-alarm type had a mean of 62 dB. The latter sound was produced when the bird flew away for no obvious reason, causing no reaction in the surrounding birds, i.e., the non-alarm type whistle fulfills criteria as ISOL. The sound was recorded approximately 1 m from the bird (Hingee and Magrath 2009). The level of sound energy will be negatively correlated to the distance from the sound source. A distance from the sound source (the wing) to the pigeon’s ear of 0.1 m would indicate an amplitude of over 80 dB at the bird’s ear.3 The masking potential of ISOL depends heavily on the frequency of simultaneously produced signals. The ability to hear vocal signals will in most species have a high adaptive value, and therefore, natural selection is expected to favor sensitive hearing across the frequency range of vocal signals (Henry and Lucas 2010a, b). Hingee and Magrath (2009) demonstrated the frequency of the two tonal elements in the crested pigeon wing whistle and found that Tone 1 had a mean fundamental peak frequency (+SD) of 1.303 + 0.100 kHz, while Tone 2 had fundamental peak frequency of 2.937 + 0.209 kHz. In pigeons, 1–2 kHz is suggested to be the middle of the hearing range (Lewald 1990). In pigeons, vocal signals between 0.250 and 1.000 kHz are the most prevalent (Larsen, 2011, personal communication). Although this interval does not overlap with the mean fundamental frequency of wing whistles, vocal calls as well as wing whistles produce harmonics (Moore 2003). The fundamental frequency determines the spacing and number of harmonic components (Moore 2003). Overlap of the frequencies (band-width) of harmonics of maskers and the harmonics of signals may significantly influence perception of signals (i.e., increase masking) in birds and humans (Dooling et al. 2001). However, little is known about acoustic interaction between wing whistles and vocal calls in doves. Alarm calls of heterospecifics may provide birds with information about attacking raptors (Magrath et al. 2009), which could present another potential masking problem since wing whistles (1.3–2.9 kHz) overlap with the range at which the major portion of vocal communication in songbirds is produced (1–2 kHz) (Lewald 1990).
Substantial masking due to ISOL seems likely in waterfowl, which commonly produce wing beats that are readily heard on the ground, in many cases tens of meters from the source. The British Library sound archive provides an example of wing beats from the mute swan, Cygnus olor, recorded 25 m from the sound source (Wingbeats from the mute swan Co 2010). When many animals move randomly in close proximity to one another, the sound produced contains more energy than that of a single individual, and quiet intervals are few. The masking potential of group locomotion therefore should be significant, especially for environmental sounds or vocal calls of frequencies similar to ISOL. However, to the degree that members of a group move concurrently, resulting noise will be synchronized (Larsson 2009). Movement cessations will also coincide, resulting in regular relatively quiet intervals that may facilitate enhanced detection of sounds of the surroundings.
Intermittent flight
Three modes of intermittent flight have been recognized in birds (Tobalske and Dial 1994; Rayner 1985): “bounding flight” (also known as flap-bounding) (Tobalske and Dial 1994), in which periods of flapping are interspersed with periods with the wings folded against the body; “undulating flight” (flap-gliding) (Tobalske and Dial 1994), in which periods of flapping are interspersed with periods of gliding; and “chattering” or “alternate flapping”, in which wing-beat frequency varies between two values while in sustained flapping flight (Rayner et al. 2000). The most widely accepted hypothesis is that the bounding flight mode is driven primarily by performance optimization in flight. Other suggestions are to confuse potential predators, display or communication, and crypsis or camouflage (Rayner et al. 2000). Body size has profound effects upon intermittent flight. Species that use both flap-gliding and flap-bounding have been shown to be of a body mass less than 300 g or to have pointed wings of relatively high aspect ratio (Tobalske 1996). Species larger than 300 g, pigeons for example, use intermittent gliding but do not exhibit bounds. The percentage of time spent flapping increases with the body mass (Tobalske 2007). Flap-bounding is extremely common in the most diverse birds, the passerines. This behavior has been suggested to be puzzling in light of the estimated higher aerodynamic power required for flight for flap-bounding during slow flight compared with continuous flapping, i.e., flap-bounding during slow flight increases energy demand (Rayner 1985; Rayner et al. 2000; Tobalske 2007).
Acoustic effects of intermittent flight
Compared with flapping, noise is likely to be reduced during bounding, when the wings are folded against the body. This could temporarily enhance the perception of critical signals such as intra-specific sound communication or sounds of approaching predators. Periods of glide flight would also be likely to reduce masking compared with flapping. Flap-gliding may be synchronized, as in small groups of jackdaws, Corvus monedula (personal observation), which may provide intervals of substantially reduced masking due to ISOL of the group.
Air rushing past the ears at high speed is likely to cause significant noise. However, masking is largely relevant only for signals of similar sound frequencies, temporal patterns, and harmonics (Moore 2003). ISOL produced by wing beats will most likely result in sound patterns different from those produced by air rushing past the ears. The latter is likely to be monotonous. Wing beats will emit oscillating sounds of different frequencies, temporal patterns, and harmonics (Hingee and Magrath 2009). Thus, wing beats are likely to be heard.
Organized flight
Birds that fly in organized groups usually do so in line formation or, alternatively, in cluster formation (Gould and Heppner 1974; Lebar Bajec and Heppner 2009). Line formation is typical of large birds such as waterfowl, where birds fly arranged in single lines, often joined together, as in the V-formation (Lebar Bajec and Heppner 2009). Forbush (1912) and Bent (1925) suggested that linear formations enable birds to supervise other flock members and maintain a clear field of vision to the front. Other functions of formations could be to maintain flock unity and aid in navigation (Gould and Heppner 1974). Another predominant idea is that birds gain an aerodynamic advantage when in a linear formation (Weimerskirch et al. 2001). A related hypothesis is that this invokes kin selection and reciprocation (Andersson and Wallander 2004). These hypotheses (vision and aerodynamics) require a bird to track the lateral position of its predecessor (Seiler et al. 2003). Seiler et al. (2003) suggested that it is inherently difficult for birds to track the lateral position of the predecessor, i.e., staying in the most favorable position for visual communication and/or aerodynamics will be a challenge. An error made by a follower will be amplified through a chain of birds flying in formation. Williams et al. (1976) reported the angle of V formations to vary substantially among groups (38–124°). The angle was not correlated with cloud cover, temperature, wind speed, or direction. Gould and Heppner (1974) measured several parameters of formation flight by Canada geese, Branta canadensis, and found extensive variation in their formation flight, suggesting that factors other than aerodynamic advantage may lead to the V flight formation.
One of the most cited studies included a group of domesticated pelicans (Weimerskirch et al. 2001). Energy consumption during flight was indirectly estimated from heart rate (HR). The HRs of birds in formation were 11–15% lower than in solitary birds. However, Lebar Bajec and Heppner (2009) suggested an alternative interpretation might be that, since pelicans are highly social animals, flying solo might have been stressful, i.e., cause more anxiety compared with group flight. This is analogous to findings in laboratory mice, Mus musculus. Mice housed alone had a 4% higher HR than mice housed in pairs (Spani et al. 2003). Moreover, Wascher et al. (2008) showed that HR in the graylag goose, Anser anser, is significantly modulated by social contexts.
Acoustic consequences of formation flight
Periods of relative silence
A group of birds flapping their wings simultaneously will for a moment increase the locomotion noise. That will momentarily increase auditory masking of critical signals such as vocal calls of con-specifics or sound coming from predators (possibly also increase the risk of detection by a predator). On the other hand, an interval of reduced noise is an obvious result of birds synchronized in glide flight. During such periods, masking from ISOL is reduced and the capacity to detect critical signals is likely to be increased.
Auditory grouping of self-produced ISOL
In nature, the environment typically contains several active sound sources, and various strategies are used to organize them into distinct auditory events (Bregman 1990; Ciocca 2008). Common onset, the harmonic relations between frequency components, continuity of pitch, timbre, and overall sound level are important cues for grouping of sounds (Bregman 1990; Darwin 2008). The acoustic situation in a flying bird flock seems not to have been studied, but what may be inferred from other research? Wing beat synchrony has been proposed by Schweppenburg (1952) and Nachtigall (1970). Later studies by Gould and Heppner (1974) and Berger (1972) did not discover synchrony or phase relationships in geese flying in formation but they demonstrated that mean wing-beat frequencies differed little among individual birds. Birds that are similar in size and body shape, and fly with similar wing-beat frequencies will be likely to produce ISOL of similar shape (harmonics, pitch, timbre) and sound level, which may facilitate auditory grouping of flock-produced ISOL. Auditory grouping of such sounds could help the brain to create an auditory scene analysis in which ISOL of group members emanating from various directions are perceived as a single sound source. Auditory grouping is also influenced by distance. Gould and Heppner (1974) demonstrated a mean distance of 4.1 m (SD = 0.79) between adjacent birds along the legs of V formations. This covered a range of 2.5–12.8 m; thus, formations were far from perfect in symmetry. However, in a small majority (8/15) of birds that had both a predecessor and a follower, the difference in distance between the predecessor-reference bird and follower-reference bird was equal to or less than 0.5 m. How might the order of birds influence perception during flight? Suppose two birds a meter apart are flying at 16 m/s. Since the speed of sound is 330 m/s, sound traveling from the rear bird to the front bird is effectively traveling at 314 m/s relative to the birds and will take 1/314 = 0.0032 s to travel 1 m. Sound traveling in the opposite direction will take 1/346 = 0.0029 s. Since sound intensity obeys an inverse square law, the ratio of the intensities will be (0.0032/0.0029)2 = 1.22 so one sound will be 22% louder than the other, which is likely to be noticeable (personal communication Oliver Linton). According to Ciocca (2008), an equal distance to almost identical sound sources (in this case it might be wingbeats, breathing, or vocal calls) facilitates auditory grouping. On page 16 is hypothesized that the distance to a neighbor may be assessed from such stereotypic sounds.
Respiratory locomotor coupling
Respiratory locomotor coupling is evident in all classes of vertebrates (Bramble and Carrier 1983; Funk et al. 1992). When two oscillating systems with different periods assume rhythmic synchronization, it is referred to as entrainment. The two oscillators may fall into synchrony, but other phase relationships are also possible. Phase locking of limb and respiratory frequency has been recorded in dogs, horses, humans, and geese (Bramble and Carrier 1983; Funk et al. 1992). In flying birds, the coordination ratio of wing beats to breathing varies among and within species. The most commonly observed ratio is 3:1, but 2:1, 5:1, 5:2, and 1:1 have also been described (Boggs 2001). Quadrupedal species normally synchronize locomotor and respiratory cycles at a constant ratio of 1:1 (strides per breath). Flying bats also have a 1:1 pattern of coordination (Boggs 2001). The tendency of humans to entrain respiration and locomotion is stronger in runners than in walkers (Bechbache and Duffin 1977). Human runners employ several phase-locked patterns (4:1, 3:1 2:1 1:1, 5:2, and 3:2), although 2:1 appears to be favored (Bramble and Carrier 1983).
The adaptive value of respiratory locomotor coupling is poorly understood. Energy saving has been suggested, but evidence for that is weak (Boggs 2001). Bluegill, Lepomis macrochirus, tend to ventilate the gills every second or third pectoral fin beat, with a regular phase relationship between locomotion and ventilation (Tytell and Alexander 2007). During pectoral fin abduction, a jet is produced by the pumping operculum (the hard bony flap covering and protecting the gills) ending just after the fin is fully abducted and the adduction begins. “The opercular flow wraps around the base of the fin during peak abduction, when it is likely to have little hydrodynamic effect” (Tytell and Alexander 2007). They suggested that if the benefits were small, synchronization might be expected to disappear. Neither the locomotor nor the respiratory capabilities of the bluegill were challenged; however, synchronization has remained (Tytell and Alexander 2007). Wing-beat and respiration frequencies are coupled primarily at a 3:1 ratio during free flight in Canada geese. Usually, the inspiration begins at the top of the upstroke and it ends at the completion of the downstroke of the following cycle, making two downstrokes and one upstroke for each inspiratory event (Funk et al. 1993). With this breathing pattern, the energy saving (the difference in the external work required to ventilate birds mechanically during simulated flight) was modest (9%) compared with out-of-phase coordination (Funk et al. 1997). The energy required to ventilate lungs mechanically will be only a fraction of the total energy consumption required for wing movements, CNS metabolism, and other organ functions. Moreover, in flying geese, locomotor respiratory coupling has been shown to be clearly related to wing beats, not to thoracic compression per se (Funk et al. 1992). Studies have not shown energy gain due to coupling of locomotion and breathing in humans. Wilke et al. (1975) found that the mechanical effect of the step cycle was very small and suggested that the tendency of humans to entrain respiratory to locomotor cycles does not reflect mechanical effects on the respiratory system. Banzett et al. (1992) showed that the work of respiratory muscles in humans is not reduced by locomotion. In other words, stride does not appear to assist ventilation during ordinary walking and running. Bernasconi and Kohl (1993) found no change in oxygen uptake of a single subject during running when switching naturally from one phase-locked pattern to another.
Acoustical consequences
The synchronization (timing of the jet), which minimizes interaction between the flow from the operculum and the flow over the pectoral fins in bluegill, might have acoustical consequences. The reduced hydrodynamic effect would result in minimized turbulence. The pectoral fin abduction, as well as the operculum jet, produces pressure waves and other water movements close to the inner ear, which could have the potential to mask extrinsic sounds. Hence, reduced turbulence could also mean reduced auditory masking. No doubt evolution favored other mechanisms that reduce the masking potential of water movements caused by breathing. It has been shown that an adaptive filter in the medullary nuclei cancels self-induced breathing noise in the electrosensory and lateral line (LL) mechanosensory systems of fish (Montgomery and Bodznick 1994). Furthermore, second-order electrosensory neurons in elasmobranch fish and mechanosensory neurons in teleost fish have adapted to cancel the effects of stimuli that are coupled with the fish respiratory movements (Montgomery and Bodznick 1994). The amplitude of sounds produced by breathing seems not to have been investigated in birds; however, the potential to act as a masker seems likely, not least since breathing noise will also include sound transmitted by bone conduction (Moore 2003).
In humans, breathing sounds have been recorded. Inspiratory sound recorded outside of the mouth at a flow rate of 60 L/min has been shown to have a mean amplitude of 51 dB (Forgacs et al. 1971). Groger and Wiegrebe (2006) reported external human breathing sounds in non-exercise and calm nose breathing to range from 25 to 35 dB SPL. The amplitude of breathing sound is positively correlated to the flow rate (Forgacs et al. 1971). Therefore, inspiratory sounds are likely to increase during exercise (as well as the amplitude of ISOL). The tendency of humans to entrain respiration and locomotion is stronger in running than when walking (Bechbache and Duffin 1977). Since most vertebrates should produce higher amplitude sound and breathe more intensely during locomotion, they might experience analogous masking challenges. Wilke et al. (1975) suggested that the entrainment of breathing and locomotory cycles in humans is an expression of the ease with which breathing becomes entrained to various rhythmic events. Breathing in humans can be unconsciously entrained to many kinds of rhythmic events, such as finger tapping, that have no mechanical link to the respiratory pump (Haas et al. 1986). Banzett et al. (1992) concluded that the coordination of breathing and stride in humans is this kind of neural phenomenon and has no obvious mechanical advantage. A benefit of respiratory locomotor coupling may be enhanced hearing perception through concurrent noise production and silent intervals and auditory grouping of own-produced noise. In addition, respiratory locomotor coupling will produce rhythmic and more predictable noise. In humans, predictability may contribute to reducing auditory masking due to a learning effect, specifically for background masking (Moore 2003).
Intervertebrates
While there is a lack of studies investigating acoustic interaction between locomotion and breathing in vertebrates, in insects, ventilation and other motor activities have a strong impact on hearing. Because the tympanic membrane of grasshoppers is immediately adjacent to air sacs in the tracheal system, it is deflected inward and outward during the respiratory cycle (Meyer and Elsner 1995; Meyer and Hedwig 1995). These slow movements change its auditory response properties and modulate the afferent activity. Ventilation thus distorts the perception of conspecific communication signals. There is some evidence that singing males of Chorthippus biguttulus arrange their ventilatory and stridulatory activity in such a way as to leave “windows” open for listening (Meyer and Elsner 1995; Meyer and Hedwig 1995).
The stridulatory mechanisms involving the wings or legs in Orthoptera make use of some muscles also used in locomotion, and they probably evolved from incidental sound production during flight or walking (Huber 1962; Elsner 1994; Heinrich and Elsner 1997). It has been proposed that hearing organs in insects were generally derived from proprioreceptors monitoring body movements and that an early step was the development of vibration sensitivity. Thus, the animals could have made use of a pre-existing, information-processing system that had already evolved for the perception of body movements and vibrations (Elsner and Popov 1978).
ISOL in animal communication
ISOL in fish communication
The notion that ISOL may mask important signals does not contradict a role of ISOL in animal communication. Pitcher et al. (1976) showed that the LL has an important role in fish schooling. Fish with a temporarily disabled LL school differently, making less accurate distance adjustments (Partridge and Pitcher 1980). Firehead tetras, Hemigrammus bleheri, totally deprived of the lateral system were unable to maintain a shoal (Faucher et al. 2010). Thus, it seems likely that the LL may be used by fish to transfer information about position in space, such as direction, distance, and (relative) speed of neighbors in a school.
Gray and Denton (1991) suggested that the relative merits of communication by light rather than by sound signals diminish as the speed of movement increases and that sound-transmitted information is more important for fast movements than for slow movements. Gray and Denton (1991) also suggested that there are many means by which a fish might assess the distance to another fish from the sound that it makes, including changes in amplitude with distance and the phases of pressure and pressure gradients in the near-field. When shoals of fish meet, the major factors determining whether individuals will join are body length and species. The exact mechanisms behind such join, leave, or stay decisions are not known (Krause et al. 2000; Gomez-Laplaza and Gerlai 2011). However, as fish of similar shape and size will emit similar ISOL, and vice versa for fish differing in form, it has been suggested that ISOL from fish encountered could provide information about size and shape that is useful in making decisions whether fish should join (Larsson 2009).
ISOL and intentionally modulated communicative sound
Coleman (2008) showed that wing beats of certain characteristics, i.e., wing whistles might serve as a predator alarm in the mourning dove, Zenaida macroura, and this has also been shown in the crested pigeon (Hingee and Magrath 2009). Coleman (2008) suggested that wing whistles may contain important information, and it’s likely that individuals of many species give attention to acoustic characteristics of wing whistles. The alarm whistle cannot be considered incidental. Although the non-alarm whistle may fulfill this criterion, it does produce a signal, roughly saying “no danger, just leaving.” Thus, the line between ISOL and intentionally modulated communicative sound may not be clear.
“Sonations” is the suggested term for intentionally modulated communicative sounds produced by non-syringeal structures such as bills, feet, and feathers, or combinations thereof (Bostwick and Prum 2003). The flappet lark, Mirafra rufocinnamomea, intermittently doubles its wing beat rate during flight, producing series of bursts of rattling wing beats. This behavior has been suggested to play a role in mate selection, and local dialects of wing-clapping have been described (Payne 1973; Norberg 1991). Hunter (2008) suggested that male wing trill is an important component of hummingbird communication. Bostwick (2006) suggested that the diversity in feather generated sonations indicates that these are important mechanisms in bird communication and that advanced and frequent use of sonations can be observed in the neotropical manakins, Pipridae. The development of field video technology has resulted in increased knowledge of the underlying mechanisms and purpose of sonations (Fusani et al. 2004, 2007; Bostwick 2006 ). Fusani et al. (2007) found that numerous elements of the displays of male golden-collared manakins, Manacus vitellinus, differed significantly among individuals and suggested that individual features of the displays may form the basis for female choice. Although manakins probably process visual information much faster than do humans, the movements in manakin male display are rapid, and the authors question to what degree female manakins are able to distinguish the male activity.
Intentional body movements resulting in vibrations of the substratum are used by many animals in communication. Tremulation display has been reported to be an important signal in agonistic interactions of red-eyed treefrogs, Agalychnis callidryas, and suggested to transmit information through vibrations in the surrounding plant substrate (Caldwell et al. 2010). Various mammal species use vibrations caused by body movements in communication, e.g., foot-stamping in kangaroo rats, Dipodomys, or elephant shrews, Elephantulus rufescens (Randall 2001). Examples of locomotion-related sound used in sound communication can be found across phyla. Wing movements in the courtship behavior of drosophila have been explored (Bennet-Clark et al. 1980; Tauber and Eberl 2003). Other examples are mosquitoes (Gibson and Russell 2006) and moths (Bailey 1991) that may produce audible intersexual advertisements by wing movements during flight. Thus, many examples can be found where there is no clear delineation between ISOL and intentionally modulated communicative sound.
Rapid transmission of information
Little is known of how the rapid transmission of information is accomplished in a fish school or in a cluster formation of birds; for example, how animals avoid collisions or reach a consensus to move away from a predator. Cluster formations are typically made up of many small birds flying in irregular three-dimensional arrangements. Synchronized and apparently simultaneous rapid changes in direction are typical traits of such groups (Lebar Bajec and Heppner 2009). Ballerini et al. (2008) observed that, in a flock of starlings, neighbors were less likely to be found along the direction of motion. Instead, they concentrated laterally, and each individual interacted with up to six or seven neighbors, irrespective of the distance to them. Ballerini et al. (2008) also suggested that aerodynamic arguments be ruled out as explanation for this spatial anisotropy, since individual interactions depend on the order of neighbors rather than on the distance to them. Interacting with seven laterally flying neighbors using only visual information might be problematic, since some birds may obscure the sight of others. Sound cues may give supplementary information. Clark (2008) suggested that sounds produced continuously during flight potentially play important roles in bird communication. Hingee and Magrath (2009) suggested that the audible “whooshing” of flight could be a general mechanism by which individuals in flocks gather information and, moreover, that such sounds may have contributed to the evolution of grouping. The perception of ISOL may provide birds with potentially useful information during flight, such as the speed, location in 3-dimensional space (distance and direction), and the wing-beat frequency of neighbors (Fig. 1). Information embedded in ISOL will travel in all directions; hence, it might be used in mutual adaptation among neighbors. The distance to a neighbor may be assessed from a stereotypic sound with a stable sound level (ISOL, breathing, or vocal call) that the neighbor produces, since the relative amplitude will be influenced by distance. Moreover, when a complex sound travels through air, its timbre changes, since higher frequencies are damped more than lower frequencies.
Fig. 1.

The perception of ISOL may provide birds with valuable information during flight, such as the speed, location in 3-dimensional space, and the wing-beat frequency of neighbors. Such information will travel in all directions; hence, it might be used in mutual adaptation among neighbors. The distance may be assessed from a stereotypic sound, such as ISOL, breathing, or vocal call, since the amplitude will be influenced by distance. Moreover, when a sound travels through air, its timbre changes, since higher frequencies are damped more (Photo: Torbjörn Arvidson)
Coleman (1962) showed that humans can use change in timbre effectively to estimate distance when a familiar sound is heard. Bird wing-beat frequency is expected to decrease relative to its body size (mass) (Rayner 1979). Similarity in size and phenotype may produce more predictable ISOL in birds. Perhaps sound cues might be a complement to visual information to aid in staying in formation and to avoid collisions.
Animal locomotion often displays a rhythmic alternating character, e.g., coast and burst swimming in fish or flapping in birds. Thus, a school of fish or birds flying in cluster formation might be depicted as a group of oscillators. A large system of biological oscillators such as singing crickets may occasionally spontaneously lock to a common frequency despite differences in the natural frequencies of individual oscillators (Strogatz 2003). When coupling is sufficiently strong, a fully synchronized solution is possible. In that situation, all the oscillators share a common frequency, although their phases are different. In biological oscillators, the ability to send and receive signals is crucial (Strogatz 2003). Flying birds and swimming fish fulfill this criterion (the signals could be visual or auditory (ISOL) or combinations). Sounds or water movements produced by locomotion seem to play a communicative role in fish schooling (Larsson 2011), but it remains to be studied if ISOL serves a similar purpose in flight formations of birds.
Synchronized locomotion in diverse ecological niches
Larsson (2009, 2011) suggested that the evolutionary development of the octavolateralis system (OLS) led to an increased potential for cannibalism within the shoal and also gave small individuals a chance to escape or to never join with larger fish. This produced increased homogeneity of size within groups, which increased the capacity for moving in synchrony. Synchronized locomotion might confuse the OLS of predators due to overlapping hydrodynamic signals (Larsson 2009) (Fig. 2). This consequence has been little discussed but might be significant. In the muskelunge, Esox masquinongy, vision has been found to be used to localize prey, but the LL was of principal importance in the later stages of an attack (New et al. 2001). Two objects need to be at least five body widths apart in order to be clearly distinguished by the electrosensory system (Babineau et al. 2007); thus, overlapping electrical fields of individual fish in a school might blur the electrosensory systems (ESS) of predatory fish (Larsson 2009) (Fig. 3). Hence, bird ancestors (fish) might have received substantial benefit from a well-developed capacity to move in synchrony.
Fig. 2.
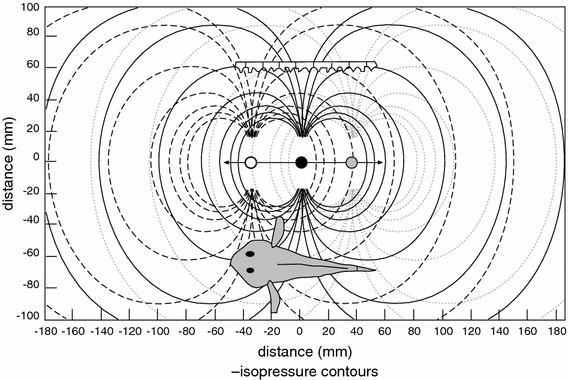
Schooling may confuse the lateral line of predators. Fin movements of a single fish emit a gradient that will approximate a point-shaped wave source. The unfilled, black, and gray dots represent point-shaped wave sources (prey-fish). A schematized lateral line (LL) organ of a predator is depicted in the upper part of the figure. The predatory fish LL may exploit the gradients produced by prey-fish movements. A lone fish (see pressure gradients of the black prey-fish in the center) would produce a symmetric gradient, while the combined gradients of three nearby fish will be more complicated. The complexity is likely to increase with the number of fish. The synchronized movements of many nearby fish may create a flat wave-front, possibly mimicking the pressure waves of a large animal. Developed from Braun and Coombs (2000). The figure is reproduced from Larsson (2009) with kind permission from Fish and Fisheries, Wiley-Blackwell
Fig. 3.
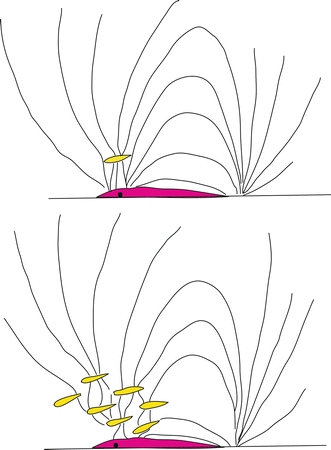
Schooling may confuse the electro-sensory system of predators. The electric field surrounding a fish with an electrosensory system is perceptually distorted by objects of differing electrical conductivity. Individual prey must be about five body widths apart to be perceived as separate images by a predator
Gregarious fish species have been found to show lateralization for turning biases at the population level, while most species that did not shoal have been found to be lateralized at the individual level (Bisazza et al. 2000; Vallortigara 2006). Vallortigara (2006) suggested that turning biases at the population level reduce the risk of a shoal splitting. Larsson (2011) hypothesized that confusion of predator’s OLS and ESS adds to the adaptive value of turning biases. Central positions in the schools are more protected (Pitcher 2001); moreover, aforementioned predator confusion might be less effective in the periphery. Larsson (2011) proposed that well-functioning sense organs, good health, skillful motor performance, and turning bias may be important to avoid occupying the periphery and reduce the probability of separation.
Schooling fish and bird groups display many similarities (Ballerini et al. 2008). However, the adaptive value of swimming in synchrony may differ from that of flying in formation. For example, the confusion of the electrosensory system of predatory fish suggested by Larsson (2009) will not be relevant to birds. In birds, effects such as reduced energy expenditure, group cohesion, optical advantages, and a collision risk for predators attacking a cluster formation (Figs. 4, 5) have been suggested (Lebar Bajec and Heppner 2009). Could birds flying in formation achieve an analogous confusion of a raptor’s auditory system as was suggested for schooling fish?
Fig. 4.
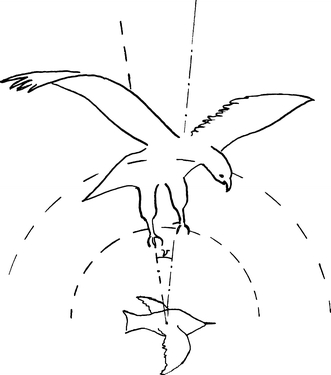
Visual and auditory cues may be integrated in diurnal raptors to locate prey. A bird flying alone will represent a single sound source, and thus, an easy target for a raptor taking advantage of sound cues
Fig. 5.
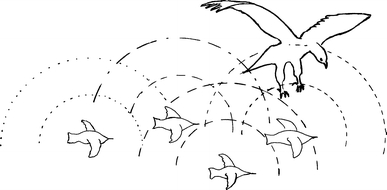
Possible advantages of flying in formation. a Mechanical protection: one or more birds closely following a bird that is attacked may collide with the predator, causing injury. b Birds flying in a formation will produce complex and overlapping sounds. This may confuse diurnal raptors that use sound cues, alone or together with vision, to localize the prey
Location of prey using acoustic cues is well documented in owls. One example is the barn owl, Tyto alba, which can accurately locate prey up to 7 m in complete darkness (Iwaniuk et al. (2006). Iwaniuk et al. (2006) reviewed and sampled data on auditory abilities of several bird species and suggested that the minimum absolute resolvable angle was lowest in raptors (2–14°) (low angle means the accuracy is high when a bird navigate toward a hidden sound source). The marsh hawk, Circus cyaneus, also demonstrate the capability to locate prey through sonic cues while in total darkness (Rice 1982). Rice (1982) suggested that empirical and theoretical research indicates that, in birds, the ability to locate prey by sound is restricted to a relatively short distance. Rice also suggested several mechanisms by which visual and auditory cues may be integrated in diurnal raptors to locate prey. It is unclear whether auditory cues may contribute to the efficiency of raptors in aerial predation. However, if that should be the case, complex and overlapping sound signals (ISOL) produced by flying in formation might contribute to predator confusion (Figs. 4, 5). Starlings, Sturnus vulgaris, form huge flocks shortly before dusk (Cavagna et al. 2008), a period during which the importance of auditory cues may increase in raptors. Evaluating the use and significance of multisensory cues in animals is complicated; however, it may be demonstrated in humans. For example, blocking the hearing in human tennis players was shown to result in reduced performance (Takeuchi 1993).
Modern comparative research has changed our view of vertebrate brain evolution. “The metaphor of the vertebrate brain climbing up the ladder of progress from fish to human has been replaced by the theme of a largely conservative bauplan of vertebrate brain organization.” (Wulliman and Vernier 2007). This could also mean that fish descendants, such as birds, possess anatomical structures, wiring, and processing abilities in the brain that may be reused if it is of adaptive value in their ecological niche to form coordinated animal groups. It is a huge step in the vertebrate tree from fish to birds. A crucial question for this hypothesis is whether ISOL or breathing sounds might influence flock behavior in an analogous manner in other vertebrate groups. Larsson (2009) suggested that concurrent surface diving of dolphin dyads will result in simultaneous splash down, providing longer periods of relative silence compared with non-synchronized diving. Contagious yawning is well documented in humans (Wilkinson et al. 2011), in non-human primates (chimpanzees Pan troglodytes), (Anderson et al. 2004); stump-tailed macaques Macaca arctoides, (Paukner and Anderson 2006); gelada baboons Theropithecus gelada, (Palagi et al. 2009); and dogs Canis familiaris, (Joly-Mascheroni et al. 2008). The function of contagious yawning is poorly understood (Wilkinson et al. 2011). Postulated hypothesis has included communicating drowsiness, social stress, or even boredom (Guggisberg et al. 2007). Daquin et al. (2001) suggest that yawning is a form of communication used to synchronize group behavior. Most studies of contagious yawning have focused on visual cues; however, hearing seems to be involved as well. A contagion effect has been found in audio presentations of yawning to blind subjects (Moore 1942). Arnott et al. (2009) showed that hearing a yawn increases a person’s urge to yawn. Hearing a yawn has also been shown to activate brain areas involved in hearing and executing mouth actions (Gazzola et al. 2006), which are also necessary for recognizing the actions of others (Pazzaglia et al. 2008). In the humpback whale, Megaptera novaeangliae, synchronized breathing is commonly observed. Whales will often breathe in synchrony when resting (Cynthia D’Vincent, personal communication). Social cohesion has been suggested to be the source of this behavior (Connor 2007). However, reduced masking could be an alternative (or complimentary) hypothesis, since one effect will be long periods of silence, which may facilitate detection of critical signals in the surrounding.
By comparison with birds, bats have a 50% higher wing-beat frequency for a given size range, and bat flight is less variable (Bullen and McKenzie 2002). If bat body mass is known, the wing-beat frequency (fw) for any bat can be estimated at low or high flight speed to within ±1.5 Hz. At and above cruising speed, fw appears to remain almost constant until the bats attain their extreme high speed (Bullen and McKenzie 2002). Low variability in fw is likely to produce similar SOL in a group of flying bats, which may favor auditory grouping processes. In bats, auditory scene analysis is intricate, it must be resolved extremely rapidly, at flight speeds up to 10 m per second, with ultra-sonar echoes from the ground, branches, insects, etc., and in some cases with thousands of animals in the air simultaneously, nearly brushing wings with each other (Ulanovsky and Moss 2008). How can bats avoid collisions in these situations? The vast majority of such studies have focused on the processing of echoes (Ulanovsky and Moss 2008). The question of whether bats use information about neighbors' positions embedded in ISOL seems not to have been raised.
Conclusions
Incidental sounds produced during locomotion (ISOL) are likely to be among the most common sounds heard during the life of many vertebrates. The impact of ISOL on animal cognition and behavior has scarcely been studied. ISOL may have a potential to mask important signals, such as sounds of predators or prey or of vocal communication. Theoretical models suggest that intermittent flight, respiratory locomotor coupling of individual animals, and synchronized locomotion in animal groups may be used to reduce masking problems as well as to achieve enhanced auditory grouping of ISOL. Several authors (Payne 1973; Norberg 1991; Coleman 2008; Hingee and Magrath 2009) have proposed that sound produced as a by-product of locomotion may play a significant role in animal communication. This review emphasizes that the border between ISOL and intentionally modulated communicative sound may be hard to define. ISOL seems to be used by schooling fish as an aid in staying in formation and avoid collisions. A more speculative hypothesis is that ISOL also may provide flying bird and bat groups with potentially useful information such as the speed, location, and fin/wing-beat frequency of neighbors. Theoretical models are persuasive; however, due to the lack of empirical studies these premises are highly speculative.
What might be of value for future study? Masking properties of ISOL will mainly be relevant for signals of similar frequency; therefore, comparative studies of ISOL and frequencies of intra-specific calls would be pertinent. It may have echological implications. Halfwerk et al. (2011) showed that masking due to traffic noise had a negative impact on reproductive success and argued that knowledge of the spatial, temporal, and spectral overlap between noise and species-specific acoustic behavior in birds is important for effective noise management. Play-back experiments as those conducted with mourning doves (Coleman 2008) and the crested pigeon (Hingee and Magrath 2009) would be of interest in other bird species. The possible role of ISOL in communicative signals in highly synchronized bird groups might be studied by assessing overall performance, collisions, and nearest neighbor distance in deaf birds or birds with temporarily impaired hearing. A reduction in individual vigilance with an increase in group size is frequently reported (Roberts 1996). Although this is generally considered to have a basis in visual cues (Fernandez-Juricic et al. 2004), it may also be of interest to study whether the amount of ISOL (produced by group members) contributes in the individual animal’s assessment of the group size. Investigations in vertebrate species might include whether hearing perception influences the tendency toward respiratory locomotor coupling. Schooling behavior, join, leave, or stay decisions in fish, and intermittent flight in birds might also be worthy of study in an acoustical context. Sound incidental to locomotion and its interaction with behavior has been little investigated and may provide an interesting area for future study.
Acknowledgments
I thank three anonymous reviewers, Associate Prof. Ole N. Larsen, Associate Prof. Rob Magrath for valuable suggestions about the content. I am grateful to Ms Kathleen Hills and Dr Alan Pike at The Lucidus Consultancy for engaged and skillful help with the English language and editorial comments. I thank Cynthia D’Vincent, Prof. Hal Whitehead, and Dr. Amir Perelberg, for help and comments about cetacean behavior, Oliver Linton, Old Mill Cottage, Crook, Kendal, UK, for comments about moving sound sources. Prof. Claes Möller for consultations about auditory perception, and Maria Bergman for help in the production of figures.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The lateral line (LL) and the inner ear in fish will have several overlapping functions (Braun and Coombs 2000; Popper and Fay 1993). Many principles concerning perception and masking will be analogous (Larsson 2009). For simplicity this article will not consequently differentiate between effects on the inner ear and the LL and the term ISOL will be used for different types of hydrodynamic effects caused by locomotion. Thus, ISOL may refer to pressure waves, at other times water-movements and sometimes a combination of both.
Wing-whistles: “a variety of pulsed and tonal sounds produced in flight…such sounds are universally attributed to vibrations caused when air is forced through flight feathers” Bostwick (2006).
The level of sound energy (p) will be negatively correlated to the distance (r) of the sound source p = k 1/r (an idealization because it assumes equal sound pressure in all directions). Doubling the distance drops the sound pressure p to a half (0.5), which corresponds to a sound pressure level of about 6 dB. A distance from the sound source (the wing) to the bird’s ear of 0.1 m and a distance to the microphone of 1 m would reduce recorded sound pressure to a tenth (0.1), a drop of 20 dB, indicating an amplitude of over 80 dB at the bird’s ear.
References
- Anderson JR, Myowa-Yamakoshi M, Matsuzawa T. Contagious yawning in chimpanzees. Proc R Soc Lond Ser B Biol Sci. 2004;271:S468–S470. doi: 10.1098/rsbl.2004.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Wallander J. Kin selection and reciprocity in flight formation? Behav Ecol. 2004;15(1):158–162. doi: 10.1093/beheco/arg109. [DOI] [Google Scholar]
- Arnott SR, Singhal A, Goodale MA. An investigation of auditory contagious yawning. Cogn Affect Behav Neurosci. 2009;9(3):335–342. doi: 10.3758/CABN.9.3.335. [DOI] [PubMed] [Google Scholar]
- Babineau D, Lewis JE, Longtin A. Spatial acuity and prey detection in weakly electric fish. PLoS Comput Biol. 2007;3(3):402–411. doi: 10.1371/journal.pcbi.0030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey WJ (1991) Acoustic behaviour of insects: an evolutionary perspective. Chapman & Hall, London
- Ballerini M, Cabibbo N, Candelier R, Cavagna A, Cisbani E, Giardina I, Orlandi A, Parisi G, Procaccini A, Viale M, Zdravkovic V. Empirical investigation of starling flocks: a benchmark study in collective animal behaviour. Anim Behav. 2008;76:201–215. doi: 10.1016/j.anbehav.2008.02.004. [DOI] [Google Scholar]
- Banzett RB, Mead J, Reid MB, Topulos GP. Locomotion in men has no appreciable mechanical effect on breathing. J Appl Physiol. 1992;72(5):1922–1926. doi: 10.1152/jappl.1992.72.5.1922. [DOI] [PubMed] [Google Scholar]
- Bechbache RR, Duffin J. Entrainment of breathing frequency by exercise rhythm. J Physiol. 1977;272(3):553–561. doi: 10.1113/jphysiol.1977.sp012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet-Clark HC, Leroy Y, Tsacas L (1980) Species and sex-specific songs and courtship behaviour in the genus Zaprionus (Diptera-Drosophilidae). Anim Behav 28:230–255
- Bent AC (1925) Life histories of North American wild fowl, part 2. The United States National Museum Bulletin 130
- Berger M. Formationsflug ohne Phasenbesiehung der Flügelshläge. J Ornithol. 1972;113:161–169. doi: 10.1007/BF01640499. [DOI] [Google Scholar]
- Bernasconi P, Kohl J. Analysis of co-ordination between breathing and exercise rhythms in man. J Physiol. 1993;471:693–706. doi: 10.1113/jphysiol.1993.sp019923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A, Cantalupo C, Capocchiano M, Vallortigara G. Population lateralisation and social behaviour: a study with 16 species of fish. Laterality. 2000;5(3):269–284. doi: 10.1080/135765000406111. [DOI] [PubMed] [Google Scholar]
- Boggs DF (2001) Interactions between locomotion and ventilation in tetrapods. In: Annual meeting of the society for experimental biology, Canterbury, England. Elsevier, pp 269–288
- Bostwick KS. Mechanisms of feather sonation in Aves: unanticipated levels of diversity. Acta Zool Sin. 2006;52S:68–71. [Google Scholar]
- Bostwick KS, Prum RO. High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves) J Exp Biol. 2003;206(Pt 20):3693–3706. doi: 10.1242/jeb.00598. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Carrier DR. Running and breathing in mammals. Science. 1983;219(4582):251–256. doi: 10.1126/science.6849136. [DOI] [PubMed] [Google Scholar]
- Braun CB, Coombs S. The overlapping roles of the inner ear and lateral line: the active space of dipole source detection. Philos Trans R Soc Lond B Biol Sci. 2000;355(1401):1115–1119. doi: 10.1098/rstb.2000.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS. Auditory scene analysis: the perceptual organization of sound. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Bullen RD, McKenzie NL. Scaling bat wingbeat frequency and amplitude. J Exp Biol. 2002;205(Pt 17):2615–2626. doi: 10.1242/jeb.205.17.2615. [DOI] [PubMed] [Google Scholar]
- Caldwell MS, Johnston GR, McDaniel JG, Warkentin KM. Vibrational signaling in the agonistic interactions of red-eyed treefrogs. Curr Biol. 2010;20(11):1012–1017. doi: 10.1016/j.cub.2010.03.069. [DOI] [PubMed] [Google Scholar]
- Cavagna A, Cimarelli A, Giardina I, Orlandi A, Parisi G, Procaccini A, Santagati R, Stefanini F. New statistical tools for analyzing the structure of animal groups. Math Biosci. 2008;214(1–2):32–37. doi: 10.1016/j.mbs.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Ciocca V. The auditory organization of complex sounds. Front Biosci. 2008;13:148–169. doi: 10.2741/2666. [DOI] [PubMed] [Google Scholar]
- Clark CJ. Fluttering wing feathers produce the flight sounds of male streamertail hummingbirds. Biol Lett. 2008;4(4):341–344. doi: 10.1098/rsbl.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD. Failure to localize the source distance of an unfamiliar sound. J Acoust Soc Am. 1962;34(3):345–346. doi: 10.1121/1.1928121. [DOI] [Google Scholar]
- Coleman SW. Mourning dove (Zenaida macroura) wing-whistles may contain threat-related information for con- and hetero-specifics. Naturwissenschaften. 2008;95(10):981–986. doi: 10.1007/s00114-008-0404-x. [DOI] [PubMed] [Google Scholar]
- Connor RC. Dolphin social intelligence: complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):587–602. doi: 10.1098/rstb.2006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquin G, Micallef J, Blin O. Yawning. Sleep Med Rev. 2001;5(4):299–312. doi: 10.1053/smrv.2001.0175. [DOI] [PubMed] [Google Scholar]
- Darwin CJ. Listening to speech in the presence of other sounds. Philos Trans R Soc Lond B Biol Sci. 2008;363(1493):1011–1021. doi: 10.1098/rstb.2007.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Dent ML, Leek MR, Gleich O. Masking by harmonic complexes in birds: behavioral thresholds and cochlear responses. Hear Res. 2001;152(1–2):159–172. doi: 10.1016/S0378-5955(00)00249-5. [DOI] [PubMed] [Google Scholar]
- Elsner N (1994) The search for the neural centers of cricket and grasshopper song. In: Schilberger K, Elsner N (eds) Neural basis of behavioral adaptations. Fortschritte der Zoologie, vol 39, pp 167–193
- Elsner N, Popov A (1978) Neuroethology of acoustic communication. In: Advances in insect physiology 13. Academic Press, London, pp 229–355
- Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P. Fish lateral system is required for accurate control of shoaling behaviour. Anim Behav. 2010;79(3):679–687. doi: 10.1016/j.anbehav.2009.12.020. [DOI] [Google Scholar]
- Fernandez-Juricic E, Kerr B, Bednekoff PA, Stephens DW. When are two heads better than one? Visual perception and information transfer affect vigilance coordination in foraging groups. Behav Ecol. 2004;15(6):898–906. doi: 10.1093/beheco/arh092. [DOI] [Google Scholar]
- Forbush EH. A history of the game birds, Wildfowl, and shore birds of Massachusetts and adjacent states. Boston: Massachusetts State Board of Agr. Wright and Potter; 1912. [Google Scholar]
- Forgacs P, Nathoo AR, Richards H. Breath sounds. Thorax. 1971;26(3):288–295. doi: 10.1136/thx.26.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Milsom WK, Steeves JD (1992) Coordination of wingbeat and respiration in the Canada goose. I. Passive wing flapping. J Appl Physiol 73(3):1014–1024 [DOI] [PubMed]
- Funk GD, Sholomenko GN, Valenzuela IJ, Steeves JD, Milsom WK. Coordination of wing beat and respiration in Canada Geese during free flight. J Exp Biol. 1993;175:317–323. [Google Scholar]
- Funk GD, Valenzuela IJ, Milsom WK. Energetic consequences of coordinating wingbeat and respiratory rhythms in birds. J Exp Biol. 1997;200(5):915–920. doi: 10.1242/jeb.200.5.915. [DOI] [PubMed] [Google Scholar]
- Fusani L, Goslow GE, Schlinger BA. The courtship display of the Golden-collared manakin: a slow-motion analysis. Horm Behav. 2004;46(1):88–89. [Google Scholar]
- Fusani L, Giordano M, Day LB, Schlinger BA. High-speed video analysis reveals individual variability in the courtship displays of male Golden-collared manakins. Ethology. 2007;113(10):964–972. doi: 10.1111/j.1439-0310.2007.01395.x. [DOI] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16(18):1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gibson G, Russell I. Flying in tune: sexual recognition in mosquitoes. Curr Biol. 2006;16(13):1311–1316. doi: 10.1016/j.cub.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Gomez-Laplaza LM, Gerlai R. Can angelfish (Pterophyllum scalare) count? Discrimination between different shoal sizes follows Weber’s law. Anim Cogn. 2011;14(1):1–9. doi: 10.1007/s10071-010-0337-6. [DOI] [PubMed] [Google Scholar]
- Gould LL, Heppner F. Vee formation of Canada Geese. Auk. 1974;91(3):494–506. [Google Scholar]
- Gray JAB, Denton EJ. Fast pressure pulses and communication between fish. J Mar Biol Assoc UK. 1991;71(1):83–106. doi: 10.1017/S0025315400037413. [DOI] [Google Scholar]
- Groger U, Wiegrebe L (2006) Classification of human breathing sounds by the common vampire bat, Desmodus rotundus. Bmc Biol 4. doi:10.1186/1741-7007-4-18 [DOI] [PMC free article] [PubMed]
- Guggisberg AG, Mathis J, Herrmann US, Hess CW. The functional relationship between yawning and vigilance. Behav Brain Res. 2007;179(1):159–166. doi: 10.1016/j.bbr.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Haas F, Distenfeld S, Axen K. Effects of perceived musical rhythm on respiratory pattern. J Appl Physiol. 1986;61(3):1185–1191. doi: 10.1152/jappl.1986.61.3.1185. [DOI] [PubMed] [Google Scholar]
- Halfwerk W, Holleman LJM, Lessells CM, Slabbekoorn H. Negative impact of traffic noise on avian reproductive success. J Appl Ecol. 2011;48(1):210–219. doi: 10.1111/j.1365-2664.2010.01914.x. [DOI] [Google Scholar]
- Heinrich R, Elsner N. Central nervous control of hindleg coordination in stridulating grasshoppers. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1997;180(3):257–269. doi: 10.1007/s003590050046. [DOI] [Google Scholar]
- Henry KS, Lucas JR. Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Anim Behav. 2010;80(3):497–507. doi: 10.1016/j.anbehav.2010.06.012. [DOI] [Google Scholar]
- Henry KS, Lucas JR. Habitat-related differences in the frequency selectivity of auditory filters in songbirds. Funct Ecol. 2010;24(3):614–624. doi: 10.1111/j.1365-2435.2009.01674.x. [DOI] [Google Scholar]
- Hingee M, Magrath RD. Flights of fear: a mechanical wing whistle sounds the alarm in a flocking bird. Proc R Soc Lond B Biol Sci. 2009;276(1676):4173–4179. doi: 10.1098/rspb.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber F. Central nervous control of sound production in crickets and some speculations on its evolution. Evolution. 1962;16(4):429–442. doi: 10.2307/2406177. [DOI] [Google Scholar]
- Hunter T. On the role of wing sounds in hummingbird communication. Auk. 2008;125(3):532–541. doi: 10.1525/auk.2008.06222. [DOI] [Google Scholar]
- Iwaniuk AN, Clayton DH, Wylie DRW. Echolocation, vocal learning, auditory localization and the relative size of the avian auditory midbrain nucleus (MLd) Behav Brain Res. 2006;167(2):305–317. doi: 10.1016/j.bbr.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Joly-Mascheroni RM, Senju A, Shepherd AJ. Dogs catch human yawns. Biol Lett. 2008;4(5):446–448. doi: 10.1098/rsbl.2008.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Butlin RK, Peuhkuri N, Pritchard VL. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol Rev Camb Philos Soc. 2000;75(4):477–501. doi: 10.1111/j.1469-185x.2000.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Larsson M. Possible functions of the octavolateralis system in fish schooling. Fish Fish. 2009;10:344–355. [Google Scholar]
- Larsson M (2011) Why do fish school? Curr Zool
- Lebar Bajec I, Heppner FH. Organized flight in birds. Anim Behav. 2009;78(4):777–789. doi: 10.1016/j.anbehav.2009.07.007. [DOI] [Google Scholar]
- Lewald J. Neural mechanisms of directional hearing in the pigeon. Exp Brain Res. 1990;82(2):423–436. doi: 10.1007/BF00231262. [DOI] [PubMed] [Google Scholar]
- Lu K, Vicario DS (2011) Toward a neurobiology of auditory object perception: what can we learn from the songbird forebrain? Curr Zool
- Magrath RD, Pitcher BJ, Gardner JL. An avian eavesdropping network: alarm signal reliability and heterospecific response. Behav Ecol. 2009;20(4):745–752. doi: 10.1093/beheco/arp055. [DOI] [Google Scholar]
- Meyer J, Elsner N (1995) How respiration affects auditory sensitivity in the grasshopper Chorthippus biguttulus (L.). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 176(4):563–573
- Meyer J, Hedwig B. The influence of tracheal pressure changes on the responses of the tympanal membrane and auditory receptors in the locust Locusta migratoria L. J Exp Biol. 1995;198(6):1327–1339. doi: 10.1242/jeb.198.6.1327. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Bodznick D. An adaptive filter that cancels self-induced noise in the electrosensory and lateral line mechanosensory systems of fish. Neurosci Lett. 1994;174(2):145–148. doi: 10.1016/0304-3940(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Moore JE (1942) Some psychological aspects of yawning. J Gen Psychol 27:289–294
- Moore BJC. An introduction to the psychology of hearing. 5. San Diego: Academic Press; 2003. [Google Scholar]
- Nachtigall W. Verbandflug der Gänse. Z vergleichende Physiol. 1970;67:414–422. doi: 10.1007/BF00297909. [DOI] [Google Scholar]
- New JG, Fewkes LA, Khan AN. Strike feeding behavior in the muskellunge, Esox masquinongy: contributions of the lateral line and visual sensory systems. J Exp Biol. 2001;204(6):1207–1221. doi: 10.1242/jeb.204.6.1207. [DOI] [PubMed] [Google Scholar]
- Norberg Å. The flappet lark Mirafra rufocinnamomea doubles its wingbeat rate to 24 Hz in wing-clap display flight: a sexually selected feat. J Exp Biol. 1991;159:515–523. [Google Scholar]
- Palagi E, Leone A, Mancini G, Ferrari PF. Contagious yawning in gelada baboons as a possible expression of empathy. Proc Natl Acad Sci USA. 2009;106(46):19262–19267. doi: 10.1073/pnas.0910891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge BL, Pitcher TJ. The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Physiol. 1980;135(4):315–325. doi: 10.1007/BF00657647. [DOI] [Google Scholar]
- Paukner A, Anderson JR. Video-induced yawning in stumptail macaques (Macaca arctoides) Biol Lett. 2006;2(1):36–38. doi: 10.1098/rsbl.2005.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RB. Wingflap dialect in flapplet lark Mirafra rufocinnamomea. Ibis. 1973;115(2):270–274. doi: 10.1111/j.1474-919X.1973.tb02644.x. [DOI] [Google Scholar]
- Pazzaglia M, Pizzamiglio L, Pes E, Aglioti SM. The sound of actions in apraxia. Curr Biol. 2008;18(22):1766–1772. doi: 10.1016/j.cub.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ. Fish schooling: implications for pattern in the oceans and impacts on human fisheries. UK: Encyclopedia of Ocean Sciences Academic Press; 2001. [Google Scholar]
- Pitcher TJ, Partridge BL, Wardle CS. Blind fish can school. Science. 1976;194(4268):963–965. doi: 10.1126/science.982056. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. Sound detection and processing by fish: critical review and major research questions. Brain Behav Evol. 1993;41(1):14–38. doi: 10.1159/000113821. [DOI] [PubMed] [Google Scholar]
- Randall JA. Evolution and function of drumming as communication in mammals. Am Zool. 2001;41(5):1143–1156. doi: 10.1668/0003-1569(2001)041[1143:EAFODA]2.0.CO;2. [DOI] [Google Scholar]
- Rayner JMV (1979) New approach to animal flight mechanics. J Exp Biol 80:17–54
- Rayner JMV. Bounding and undulating flight in birds. J Theor Biol. 1985;117(1):47–77. doi: 10.1016/S0022-5193(85)80164-8. [DOI] [Google Scholar]
- Rayner JMV, Viscardi PW, Ward S, Speakman JR (2000) Aerodynamics and energetics of intermittent flight in birds. In: Annual meeting of the society for integrative and comparative biology, Atlanta, Georgia, Jan 04–08, pp 188–204
- Rice WR. Sonic prey detection by the marsh hawk: adaptation to concealed prey. Auk. 1982;99(3):403–413. [Google Scholar]
- Roberts G. Why individual vigilance declines as group size increases. Anim Behav. 1996;51:1077–1086. doi: 10.1006/anbe.1996.0109. [DOI] [Google Scholar]
- Roberts BL, Russell IJ. Activity of lateral-line efferent neurones in stationary and swimming dogfish. J Exp Biol. 1972;57(2):435–448. doi: 10.1242/jeb.57.2.435. [DOI] [PubMed] [Google Scholar]
- Russell IJ. Influence of efferent fibres on a receptor. Nature. 1968;219(5150):177–178. doi: 10.1038/219177a0. [DOI] [PubMed] [Google Scholar]
- Schweppenburg GV. Vorteile der Zuggeselligkeit. Vogelwarte. 1952;16:116–119. [Google Scholar]
- Seiler P, Pant A, Hedrick JK. A systems interpretation for observations of bird V-formations. J Theor Biol. 2003;221(2):279–287. doi: 10.1006/jtbi.2003.3191. [DOI] [PubMed] [Google Scholar]
- Spani D, Arras M, Konig B, Rulicke T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab Anim. 2003;37(1):54–62. doi: 10.1258/002367703762226692. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Synch. The emerging science of spontaneous order. NY: Hyperion; 2003. [Google Scholar]
- Takeuchi T. Auditory information in playing tennis. Percept Mot Skills. 1993;76(3):1323–1328. doi: 10.2466/pms.1993.76.3c.1323. [DOI] [PubMed] [Google Scholar]
- Tauber E, Eberl DF. Acoustic communication in Drosophila. Behav Process. 2003;64(2):197–210. doi: 10.1016/S0376-6357(03)00135-9. [DOI] [Google Scholar]
- Tobalske BW. Scaling of muscle composition, wing morphology, and intermittent flight behavior in woodpeckers. Auk. 1996;113(1):151–177. [Google Scholar]
- Tobalske BW. Biomechanics of bird flight. J Exp Biol. 2007;210(18):3135–3146. doi: 10.1242/jeb.000273. [DOI] [PubMed] [Google Scholar]
- Tobalske BW, Dial KP. Neuromuscular control and kinematics of intermittent flight in budgerigars (Melopsittacus-Undulatus) J Exp Biol. 1994;187:1–18. doi: 10.1242/jeb.187.1.1. [DOI] [PubMed] [Google Scholar]
- Tytell ED, Alexander JK. Bluegill Lepomis macrochirus synchronize pectoral fin motion and opercular pumping. J Fish Biol. 2007;70(4):1268–1279. doi: 10.1111/j.1095-8649.2007.01416.x. [DOI] [Google Scholar]
- Ulanovsky N, Moss CF. What the bat’s voice tells the bat’s brain. Proc Natl Acad Sci USA. 2008;105(25):8491–8498. doi: 10.1073/pnas.0703550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara G. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev Psychobiol. 2006;48(6):418–427. doi: 10.1002/dev.20166. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip. (Wechselwirkungen zwischen Zentralnervensystem und Peripheri) Naturwissenschaften. 1950;37:464–476. doi: 10.1007/BF00622503. [DOI] [Google Scholar]
- Wascher CAF, Amold W, Kotrschal K. Heart rate modulation by social contexts in greylag geese (Anser anser) J Comp Psychol. 2008;122(1):100–107. doi: 10.1037/0735-7036.122.1.100. [DOI] [PubMed] [Google Scholar]
- Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. Energy saving in flight formation: pelicans flying in a ‘V’ can glide for extended periods using the other birds’ air streams. Nature. 2001;413(6857):697–698. doi: 10.1038/35099670. [DOI] [PubMed] [Google Scholar]
- Wilke JT, Lansing RW, Rogers CA. Entrainment of respiration to repetitive finger tapping. Physiol Psychol. 1975;3(4):345–349. [Google Scholar]
- Wilkinson A, Sebanz N, Mand I, Huber L. No evidence of contagious yawning in the red-footed tortoise Geochelone carbonaria. Curr Zool. 2011;57(4):477–484. [Google Scholar]
- Williams TC, Klonowski TJ, Berkeley P. Angle of Canada Goose V-flight formation measured by radar. Auk. 1976;93(3):554–559. [Google Scholar]
- Wingbeats from the mute swan (2010) The British Library sound archive. http://cadensa.bl.uk/uhtbin/cgisirsi/x/0/0/5?searchdata1=mute_swan01&library=ALL. Accessed 30 Jun 2010
- Wulliman MF, Vernier P. Evolution of the nervous system in fishes. In: Kaas JK, editor. Evolution of nervous system. Amsterdam: Elsevier; 2007. pp. 39–60. [Google Scholar]
- Zhang Z, Bodznick D. Plasticity in a cerebellar-like structure: suppressing reafference during episodic behaviors. J Exp Biol. 2008;211(23):3720–3728. doi: 10.1242/jeb.020099. [DOI] [PubMed] [Google Scholar]


