Abstract
The creatine transporter (CRTR) defect is a recently discovered cause of X-linked intellectual disability for which treatment options have been explored. Creatine monotherapy has not proved effective, and the effect of treatment with L-arginine is still controversial. Nine boys between 8 months and 10 years old with molecularly confirmed CRTR defect were followed with repeated 1H-MRS and neuropsychological assessments during 4–6 years of combination treatment with creatine monohydrate, L-arginine, and glycine. Treatment did not lead to a significant increase in cerebral creatine content as observed with H1-MRS. After an initial improvement in locomotor and personal-social IQ subscales, no lasting clinical improvement was recorded. Additionally, we noticed an age-related decline in IQ subscales in boys affected with the CRTR defect.
Introduction
Creatine is important for the energy homeostasis in the central nervous system (CNS) (Wyss and Kaddurah-Daouk 2000) and might act as a neuromodulator (Almeida et al. 2006). Creatine is both taken up from the diet and synthesized endogenously, mainly in the kidney, pancreas, and liver. In the first step of the biosynthesis, catalyzed by arginine:glycine amidinotransferase (AGAT), guanidinoacetic acid and ornithine are formed from arginine and glycine. In the second step, catalyzed by guanidinoacetate methyltransferase (GAMT), guanidinoacetic acid is methylated into creatine. Creatine is then transported through the blood to creatine-requiring organs, mainly the brain and muscles, where it is taken up via the creatine transporter (CRTR) (Wyss and Kaddurah-Daouk 2000).
Cerebral creatine deficiency, which can be diagnosed by in vivo proton magnetic resonance (1H-MRS) of the brain, causes neurological symptoms. The deficiency can be caused by autosomal recessive biosynthesis defects (AGAT or GAMT deficiency) or X-linked CRTR defect. The first male patient with CRTR defect was described in 2001 (Cecil et al. 2001; Salomons et al. 2001). Patients present with intellectual disability (ID), severe speech delay, behavior disturbances, and epilepsy (Stöckler and Salomons 2006).
In AGAT and GAMT deficiency, creatine supplementation has led to a partial restoration of the cerebral creatine content and attenuation of the symptoms (Mercimek-Mahmutoglu and Stockler-Ipsiroglu 2009). In CRTR defect, high plasma creatine levels might result in some cellular creatine uptake via alternative mechanisms or residual activity of CRTR. CRTR-deficient fibroblasts take up creatine when incubated in high creatine concentrations (DeGrauw et al. 2002). However creatine monotherapy has not proved to be successful in patients with CRTR defect (Anselm et al. 2006, 2008; Bizzi et al. 2002; DeGrauw et al. 2002; Dezortova et al. 2008; Poo-Arguelles et al. 2006).
Brain cells appear to be capable of endogenous creatine synthesis since AGAT and GAMT are expressed in all brain cells (Braissant et al. 2001) and synthesis has been observed in brain cell cultures (Dringen et al. 1998). Provision of arginine increased guanidinoacetic acid (GAA) and creatine synthesis in astroglial cells (Dringen et al. 1998), the rat kidney (Edison et al. 2007) and, in combination with glycine, in human CRTR-deficient lymphoblasts (Leuzzi et al. 2008). Supplementation with creatine precursors L-arginine and glycine might, therefore, increase endogenous cerebral creatine synthesis. Chilosi et al. observed improvement and an increase in cerebral creatine (although still well below normal) after 1-year supplementation with L-arginine in an 8.6-year-old male patient with CRTR defect (Chilosi et al. 2008). Treatment with creatine combined with arginine and glycine also resulted in resolution of severe seizures in a 9-year-old girl with CRTR defect (Mercimek-Mahmutoglu et al. 2010). However Fons et al. (2010) reported no effect of a 9 month L-arginine supplementation in four male patients. The effectiveness of L-arginine supplementation therefore remains controversial.
We describe the 4–6 year follow-up with repeated 1H-MRS and neuropsychological assessments during a pilot study in nine boys who were diagnosed in the Netherlands shortly after the discovery of the creatine transporter defect in 2001 and were treated with creatine monohydrate, L-arginine, and glycine supplementation.
Subjects and methods
Subjects
Between 2003 and 2005, nine boys (four sib pairs, including one twin, and one single case), between 8 months and 10 years old (mean 5.3 years; median 3.9 years) were diagnosed with CRTR defect based on elevated urinary creatine/creatinine ratio, cerebral creatine depletion on 1H-MRS, and mutation in the SLC6A8 gene and were started on treatment with creatine monohydrate, L-arginine, and glycine. Clinical details of the patients are summarized in Table 1. Two sibpairs patients have been published before (Mancini et al. 2005).
Table 1.
Clinical details at start of and during treatment
| Patient | Age at start | Creatine uptake (pmol/μgprotein) | Brain tCr at starta (% normal mean) | SLC6A8 mutation | Total IQ at start | Psychiatric assesment (DSM-IV Axis I) | Epilepsy and age of onset | Urine cr/crn ratio | Urine GAAb (mmol/mol creatinine) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At start | During | At start | During | At start | During | |||||||
| 1 | 10 years 3 months | 2.89 | 28 | c.1631 C > T; p.(Pro544Leu) | 35 | ADHD, combined type; some signs of PDD NOS; mixed receptive-expressive language disorder | No (epileptic activity on EEG at 6 years) | No | 1.8 | 6.2 | 56 | 80 |
| 2 | 8 years 4 months | 3.16 | 29 | c.1631 C > T; p.(Pro544Leu) | 38 | ADHD, combined type; some features PDD NOS and ODD; mixed receptive-expressive language disorder | No (epileptic activity on EEG at 4 years) | No | 1.5 | 5.6 | 58 | 98 |
| 3 | 8 years | 1.47 | 29 | c.1495 + 5 G > C | 50 | ADHD, combined type | No | 12 years 5 months | 2.7 | 11.3 | 141 | 68 |
| 4 | 5 years 11 months | 1.21 | 24 | c.1495 + 5 G > C | 50 | Diagnosis deferred; some signs of ADHD and PDD NOS | No | No | nd | 11.7 | 40 | 112 |
| 5 | 3 years 11 months | 0 | 23 | c.570_571del; p.(Ala191GlnfsX10) | 47 | PDD NOS; signs of ADHD | No | No | 4.0 | 4.7 | 114 | 179 |
| 6 | 3 years 11 months | nd | 25 | c.428_430del; p.(Tyr143del) | 37 | PDD NOS; signs of ADHD | 3 years 11 months | Unchanged | nd | 15.3 | nd | 139 |
| 7 | 3 years 11 months | nd | 22 | c.428_430del; p.(Tyr143del) | 35 | PDD NOS; signs of ADHD | No | 4 years 6 months | 3.5 | 10.4 | 67 | 112 |
| 8 | 3 years | nd | 21 | c.92delC; p.(Pro31ArgfsX66) | 51 | Autistic disorder | Possible insult at 1 year 7 months | 4 years 6 months | 3.5 | 17.0 | 96 | 244 |
| 9 | 8 months | nd | 22 | c.92delC; p.(Pro31ArgfsX66) | 65 | nd | No | 2 years 11 months | 8.3 | 24.6 | 354 | 239 |
| Normal controls | 27.8 ± 5.6 | 0.006–1.2 (<4 years)c | 4–220 (<15 years)c | |||||||||
| 0.017–0.72 (4–12 years)c | 3–78 (>15 years)c | |||||||||||
| 0.011–0.24 (>12 years)c | ||||||||||||
tCr Total creatine, cr/crn creatine/creatinine, GAA guanidinoacetate, ADHD attention-deficit hyperactivity disorder, PDD NOS pervasive developmental disorder not otherwise specified, ODD oppositional defiant disorder, nd not determined
aMean of means of four brain regions
bMean values from several measurements
cReference values from Almeida et al. (2004)
Treatment
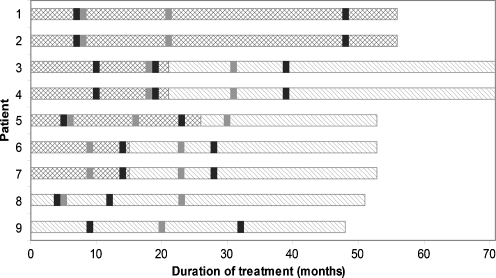
All patients were started between 2003 and 2005 on oral creatine monohydrate 400 mg/kg and oral L-arginine 400 mg/kg per day, divided into two to three doses. In the two youngest patients (patients 8 and 9), 150 mg/kg glycine per day was added from the start. In five other patients, glycine was added after 15–21 months. Two brothers (patients 1 and 2) were never started on glycine treatment due to family circumstances. The regimen was chosen on the basis of the creatine supplement used for GAMT deficiency and L-arginine for patients with urea cycle defects. The glycine dose was chosen in a 1:1 molar ratio with L-arginine. These dosages are known to lack side effects and to be palatable and well tolerated. The individual treatment protocols are depicted in Fig. 1. Treatment was discontinued in 2009–2010 because of lack of evident response. The study protocol was discussed and supported by the Metabolic Section of the Dutch Pediatric Society.
Fig. 1.
Treatment protocol and timing of neuropsychological assessments and 1H-MRS in the individual patients. Double-hatched bars depict treatment with creatine and L-arginine. Hatched bars depict treatment with creatine, L-arginine, and glycine. Black rectangles depict neuropsychological assessments and gray rectangles 1H-MRS assessments
Follow-up
Neuropsychological assessment and measurement of cerebral creatine by 1H-MRS were performed before the start of treatment and one to three times during treatment. The treatment duration at the last neuropsychological assessment was 12–48 months (mean 33) and at the last 1H-MRS 20–31 months (mean 25) (Fig. 1). Evaluation further consisted of check-ups twice yearly (history and physical examination) and biochemical analysis of urinary and plasma creatine, guanidinoacetate, and amino acids. A renal ultrasound was performed to screen for renal stone formation during the treatment. Eight of the nine boys were seen by a child psychiatrist to classify their behavioral problems (Table 1).
Methods
Neuropsychological function was assessed with the Griffiths Mental Developmental Scales (with locomotor, personal-social, hearing and speech, hand and eye coordination, and performance subscales). In patients 3 and 4, the Snijders-Oomen Non-Verbal Intelligence Test (SON-R 2½-7) and the Denver Developmental Screening Test (DDST) were used instead of the Griffiths scales at the neuropsychological assessment before treatment.
1H-MRS spectroscopy of the brain was performed at 1.5 T (Siemens Vision, Erlangen, Germany) using a standard CP head coil. Single-voxel STEAM spectroscopy [repetition time (TR)/echo time (TE)/mixing time (TM) = 6,000/20/10 ms; 64 acquisitions] was obtained from volumes-of-interest (VOIs) in parietal gray matter (10 ml), parietal white matter (5 ml), basal ganglia (5 ml), and cerebellar vermis (8 ml) (Pouwels et al. 1999). Spectra were quantified using LCModel as described previously (Pouwels et al. 1999; Provencher 1993). In the current study we focused on the concentration of total creatine (Cr) (sum of creatine and phosphocreatine), which was expressed in mmol/l VOI (mM).
GAA and creatine were measured in plasma and urine using stable isotope dilution gas chromatography-mass spectrometry according to Almeida et al. (2004).
The creatine uptake assay in cultured skin fibroblasts after incubation at 25 μM creatine and genomic sequence analysis of the SLC6A8 gene was performed as previously described (Rosenberg et al. 2007).
Statistical analysis
Statistical analysis was performed on the neuropsychological tests, 1H-MRS measurements, and growth parameters. To account for repeated measurements within subjects, data were analyzed using a linear mixed model in SPSS (v. 15.0). In longitudinal studies it is possible to separate cross-sectional and longitudinal age effects (e.g., Fitzmaurice et al. 2004). First a model with treatment, cross-sectional and longitudinal age effects was fitted. Since the cross-sectional and longitudinal effects were equal, a simpler model with treatment, region of measurement (only in analysis of 1H-MRS) and a common age effect was fitted, and results for the latter model are presented. Significance value was set at P < 0.05.
Results
Adverse effects and compliance
In general, no adverse effects were reported. The parents of patients 3 and 4 reported that their sons’ hyperactive behavior increased after the addition of glycine to the treatment and decreased after the discontinuation of the creatine/arginine/glycine treatment. Renal ultrasounds (performed in eight boys) did not show calcifications or renal stones.
The urinary creatine/creatinine ratio (Table 1) increased during treatment and could be used to follow compliance of the creatine treatment. However in three patients (patients 1, 2, and 4) collection of urinary samples was sometimes difficult for the families. Little increase was seen in patient 5 and his parents reported problems administering the supplements. Increases in urinary GAA were found during treatment but not consistently (Table 1). No consistent changes were found in ornithine, L-arginine, and glycine levels in urine or plasma, making assessment of compliance with these supplements difficult. Therefore the results of the neuropsychological assessment and 1H-MRS in all patients were used for statistical evaluation irrespective of compliance.
Clinical assessment
Epileptic seizures occurred, with low seizure frequency, in five patients (Table 1). In four patients the onset was after the start of the supplementation treatment, although one patient probably had seizures before. Seizures were easily controlled by valproate monotherapy, as reported for untreated CRTR patients. Initially improved concentration, speech, and locomotion were reported in several patients. Height and weight increased more than two standard deviation (SD) scores in two and three patients, respectively, and between one and two SD scores in an additional three and two patients, respectively, but decreased more than two SD scores in one other patient. Head circumference did not change more than one SD score in any of the patients. Statistical analysis of the whole group did not show any significant changes of the growth parameters with treatment.
Neuropsychological assessment
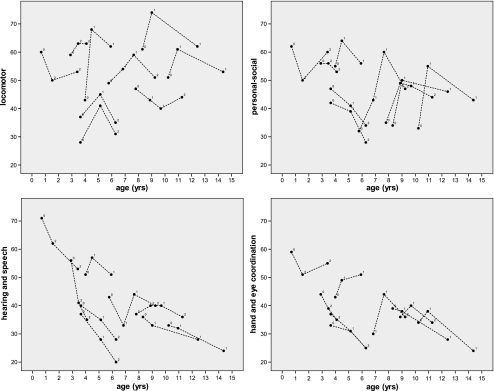
The developmental courses on the Griffiths subscales (Fig. 2) show in the whole group a decrease in scores for hearing and speech and for hand and eye coordination with time. Notably, some patients had an initial increase in scores on locomotor and personal-social subscales followed by a decrease. Statistical analysis (Table 2) confirmed a significant negative correlation between age and scores on the hearing and speech (P = 0.019) and the hand and eye coordination (P = 0.017) subscales, although there was also still a just significant negative effect (P = 0.043) of treatment with creatine plus L-arginine and glycine. Statistical analysis also confirmed a significant treatment effect on locomotor (P = 0.004) and personal-social (P = 0.008) subscales during treatment with creatine combined with L-arginine alone. However after addition of glycine this effect was not significant anymore.
Fig. 2.
Griffiths developmental scores (locomotor, personal-social, hearing and speech, and hand and eye coordination subscales) of the nine patients plotted against age. The dots depict the measurements, the lines connect the measurements of one patient, and the numbers stand for the treatment conditions (0 before start, 1 during treatment with creatine and arginine, 2 during treatment with creatine, arginine, and glycine)
Table 2.
Estimated effects of age and treatment on Griffiths developmental scores
| Griffiths scales | Age effect | Treatment effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment vs no treatment | cr + arg vs no treatment | cr + arg + gly vs no treatment | cr + arg + gly vs cr + arg | |||||||
| Estimated effect (95% CI) | Significance (P) | Estimated effect (95% CI) | Significance (P) | Estimated effect (95% CI) | Significance (P) | Estimated effect (95% CI) | Significance (P) | Estimated effect (95% CI) | Significance (P) | |
| Total | −0.88 (−2.1; 0.3) | 0.164 | −1.0 (−5.1; 3.2) | 0.623 | 1.3 (−3.0; 5.7) | 0.528 | −3.4 (−8.5; 1.8) | 0.190 | −4.7 (−9.3; −0.1) | 0.047* |
| Locomotor | −0.9 (−2.6; 0.8) | 0.301 | 5.6 (0.18; 11.1) | 0.043* | 8.9 (3.2; 14.6) | 0.004* | 2.4 (−4.4; 9.1) | 0.475 | −6.5 (−12.5; −0.5) | 0.035* |
| Hearing and speech | −1.8 (−3.2; −0.3) | 0.019* | −3.9 (−8.7; 1.0) | 0.113 | −1.6 (−6.7; 3.5) | 0.517 | −6.1 (−12.0; −0.1) | 0.045* | −4.5 (−9.9; 0.9) | 0.10 |
| Personal-social | −1.4 (−2.8; 0.1) | 0.066 | 6.4 (0.0; 12.9) | 0.050* | 10.2 (3.0; 17.5) | 0.008* | 2.6 (−5.0; 10.3) | 0.485 | −7.6 (−15.2; −0.03) | 0.049* |
| Hand and eye coordination | −1.6 (−2.8; −0.3) | 0.017* | −1.3 (−1.6; 3.6) | 0.575 | 0.2 (−5.1; 5.5) | 0.95 | −2.9 (−8.6; 2.9) | 0.317 | −3.0 (−8.1; 2.1) | 0.23 |
| Performance | 0.4 (−1.6; 2.4) | 0.684 | 3.2 (−4.3; 10.6) | 0.390 | 5.8 (−2.5; 14.0) | 0.160 | 0.5 (−8.5; 9.6) | 0.903 | −5.3 (−14.0; 3.5) | 0.22 |
CI Confidence interval, cr creatine, arg arginine, gly glycine
*P ≤ 0.05
1H-MRS spectroscopy of the brain
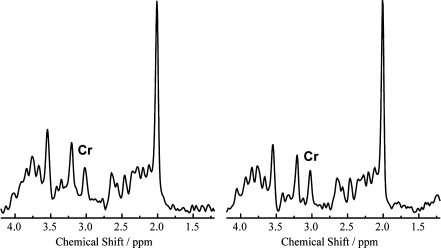
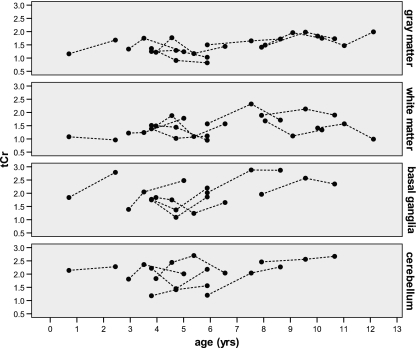
Figure 3 shows the 1H-MRS spectra of one patient before and during treatment. The course of the Cr concentration during treatment in the four brain regions of all nine patients is shown in Fig. 4. There was no significant increase in Cr during treatment in any of the regions. The slight variation in Cr concentration can be entirely due to the reproducibility of the measurement. Based on the error estimate of the LCModel analysis and the actual low concentration of Cr, the obtained values will have a variability of at least 0.5 mM. Overall, Cr concentrations in cerebellum (mean 2.0 ± 0.5 mM) and basal ganglia (2.0 ± 0.5 mM) of the whole group were significantly higher than in cortex (1.5 ± 0.3 mM) and white matter (1.5 ± 0.4 mM) (P < 0.05). There was no clearly detectable GAA signal in any of the patients’ spectra either before or during treatment. With LCModel a low concentration of GAA may be fitted, but this is a small value that is unreliable, comparable to the value in healthy controls.
Fig. 3.
Gray matter 1H-MRS spectra of patient 4 at start of treatment at age 5 years 11 months and during treatment at age 8 years 8 months. Cr concentrations in this brain region are 1.5 and 1.7 mM, respectively
Fig. 4.
Total creatine concentrations in gray matter, white matter, basal ganglia and cerebellum of the nine patients plotted against age. The dots depict the individual values, the lines connect the measurements of one patient. The first measurements are before the start of treatment
Discussion
During the 4–6 years of follow-up of nine boys with the CRTR defect, we did not find enough evidence to conclude that treatment with creatine monohydrate, L-arginine, and glycine effectively improves cerebral creatine and/or development, as assessed by neuropsychological tests and 1H-MRS.
Nonetheless, subjective improvements were reported by parents and caretakers during the first months of treatment. Also formal neuropsychological testing did indeed show significant improvements in motor and social skills in the whole group during the first assessments, while most patients were still on creatine monohydrate plus L-arginine treatment without glycine. However this effect did not last. It is unlikely that this is caused by the addition of glycine because scores also decreased in patients who were not (yet) started on glycine. Scores on the hearing and speech and the hand-eye coordination subscales even declined during treatment. Probably this is mainly age-related and not due to treatment because older patients already had lower scores at the start of treatment. Statistical analysis confirmed a significant negative correlation with age. An age-related decline in IQ scores is also known to occur in Down’s syndrome and fragile X syndrome (Fisch et al. 2007) and might be common to more mental retardation disorders. This does not necessarily mean that these conditions are progressive. When the rate of development slows down and reaches an early ceiling, the IQ score (ratio of developmental age and chronological age) declines while the patient remains unchanged. As the developmental ages did not decrease, we saw in our cohort no indication for regression. An age-related decline in IQ scores is also consistent with the fact that IQ scores described in adult patients with the creatine transporter defect are lower than described in affected children (Hahn et al. 2002). Speech and coordination appear to be specific weaknesses in CRTR-deficient patients and a ceiling appears to be reached earlier than for the other subscales. The age-related decline in IQ scores complicates the evaluation of treatment in children, and further studies of the natural course of the CRTR defect are therefore needed.
No significant increase in brain creatine content upon treatment was seen in 1H-MRS. It should be noted that creatine measurements varied inter- and intraindividually, which can be temporarily (mis)taken for improvements. Creatine measurements also differed over the various brain regions, being significantly higher in the basal ganglia and cerebellum than in cortex and white matter. These differences must be taken into account when comparing H1-MRS creatine levels in a single individual or in small numbers of observations. The regional differences are comparable to those in healthy subjects (Pouwels et al. 1999).
There are several possible explanations why treatment failed to increase cerebral creatine content. It is possible that the uptake of arginine and glycine through the blood-brain barrier was insufficient. In future trials, this could be monitored by measurements of cerebrospinal fluid concentrations of amino acids. Other explanations are related to the central question why the CRTR defect leads to cerebral creatine deficiency to begin with if the brain is capable of endogenous creatine synthesis. It is possible that the cerebral creatine synthesis is limited and that the expected upregulation of the AGAT reaction, the rate-limiting step in creatine synthesis, by decreasing creatine (Wyss and Kaddurah-Daouk 2000) or by supplementation of precursors L-arginine and glycine, does not occur in the brain. This is, however, in contrast with the hypothesis that the CNS mainly derives its creatine from endogenous synthesis (Braissant et al. 2001) because the permeability of the blood-brain barrier for creatine appears limited (Wyss and Schulze 2002), and astrocytes, contacting the capillary endothelial cells forming the blood-brain barrier, do not express CRTR (Braissant et al. 2001). In addition, CRTR might also be important in the cerebral creatine synthesis. Braissant et al. found that AGAT and GAMT, although expressed in all CNS cell types, are rarely co-expressed within the same cell and hypothesized that GAA must be transported by CRTR between brain cells for creatine synthesis to occur (Braissant and Henry 2008; Braissant et al. 2007, 2010). In this model GAA accumulation would be expected in cases of CRTR deficiency (Braissant and Henry 2008), which was indeed suggested in one patient (Sijens et al. 2005). Arginine and glycine supplementation would then not lead to the aimed for creatine increase but to further GAA accumulation, an adverse effect because GAA is considered to be epileptogenic (Tachikawa et al. 2008). However, we saw no GAA accumulation in our cohort of CRTR-deficient patients nor an increase during treatment.
It is possible that our treatment has been effective outside the brain, for instance on muscle function. The temporary improvements in motor skills may have been caused by improved muscle function. Unfortunately, formal muscle function tests were not performed. An increase in body weight has been noted upon creatine monohydrate treatment in patients with the CRTR defect (Anselm et al. 2006; Poo-Arguelles et al. 2006). Five patients in our study showed an increase of more than one to two SD scores in height and weight, although not statistically significant in the whole group.
Our follow-up has been too short to decide whether the treatment might prevent complications later in life, such as myopathy and intestinal dysfunction, which have been described in adult patients (Kleefstra et al. 2005; Hahn et al. 2002). Personal observations of creatine monohydrate plus L-arginine and glycine treatment in four male adult patients aged between 17 and 54 years registered positive effects with improved behavior in all, amelioration of severe constipation in one, and achievement of urinary continence in another (Mancini, unpublished).
Though this study represents the largest and longest treated cohort so far and includes repeated neuropsychological assessments and repeated brain 1H-MRS, there are still several limitations that should be addressed in future studies. The cohort was heterogeneous in age at the start of treatment. This complicates the evaluation of treatment because neuropsychological assessments may be less reliable in the first years and IQ scores may decline with age as we noticed for certain subscales. Furthermore it is possible that treatment starting at a younger age is more successful. Due to the sample size, this could not be evaluated in this study. The treatment conditions in the cohort differed in the addition of glycine, and neuropsychological assessments and brain 1H-MRS were performed at different treatment durations. This limited the power of the statistical analyses. The analyses were performed irrespective of compliance because the compliance could not be optimally monitored.
In future therapeutic trials, additional or other treatment options need to be considered. S-adenosylmethionine (SAMe) supplementation might be another way to strengthen the cerebral creatine synthesis. SAMe acts as methyl donor in the GAMT reaction, which in fact accounts for the main percentage of total utilization of methyl groups in the body (Wyss and Kaddurah-Daouk 2000). SAMe crosses the blood-brain barrier and increases cerebral phosphocreatine (Moxon-Lester et al. 2009). Folic acid and vitamins B6 and B12 could be added to increase methionine synthesis and maintain low concentrations of S-adenosylhomocysteine, which inhibits GAMT (Moxon-Lester et al. 2009). Other alternative treatment could be with lipophilic creatine analogs, which cross the blood-brain barrier independent of the CRTR. Uptake of lipophilic creatine analogs was found in CRTR-blocked mouse hippocampal slices (Lunardi et al. 2006) and in human CRTR-deficient and control fibroblasts, however no effect of treatment with creatine ethyl ester (CEE) was found in CRTR patients (Fons et al. 2010).
It is possible that creatine is released from central neurons and acts as a neuromodulator (Almeida et al. 2006). Therefore CRTR might also be essential for creatine reuptake and termination of synapsis (Peral et al. 2010) and/or for release of creatine from neurons (Mak et al. 2009). Treatment directed at increasing the levels of creatine might not solve disturbed neuromodulation in CRTR deficiency.
Acknowledgements
We thank Ofir T. Betsalel for his help with the figures.
Funding
The work of Gajja S. Salomons is supported by the Dutch Society for Scientific Research (ZonMW/NWO), VIDI grant number 917.56.349.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Competing interests: None declared
References
- Almeida LS, Verhoeven NM, Roos B, et al. Creatine and guanidinoacetate: diagnostic markers for inborn errors in creatine biosynthesis and transport. Mol Genet Metab. 2004;82(3):214–219. doi: 10.1016/j.ymgme.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer ANM. Exocytotic release of creatine in rat brain. Synapse. 2006;60(2):118–123. doi: 10.1002/syn.20280. [DOI] [PubMed] [Google Scholar]
- Anselm IA, Alkuraya FS, Salomons GS, et al. X-linked creatine transporter defect: a report on two unrelated boys with a severe clinical phenotype. J Inherit Metab Dis. 2006;29(1):214–219. doi: 10.1007/s10545-006-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselm IA, Coulter DL, Darras BT. Cardiac manifestations in a child with a novel mutation in creatine transporter gene SLC6A8. Neurology. 2008;70(18):1642–1644. doi: 10.1212/01.wnl.0000310987.04106.45. [DOI] [PubMed] [Google Scholar]
- Bizzi A, Bugiani M, Salomons GS, et al. X-linked creatine deficiency syndrome: a novel mutation in creatine transporter gene SLC6A8. Ann Neurol. 2002;52(2):227–231. doi: 10.1002/ana.10246. [DOI] [PubMed] [Google Scholar]
- Braissant O, Henry H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. J Inherit Metab Dis. 2008;31(2):230–239. doi: 10.1007/s10545-008-0826-9. [DOI] [PubMed] [Google Scholar]
- Braissant O, Henry H, Loup M, Eilers B, Bachmann C. Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 2001;86(1–2):193–201. doi: 10.1016/S0169-328X(00)00269-2. [DOI] [PubMed] [Google Scholar]
- Braissant O, Bachmann C, Henry H. Expression and function of AGAT, GAMT and CT1 in the mammalian brain. Subcell Biochem. 2007;46:67–81. doi: 10.1007/978-1-4020-6486-9_4. [DOI] [PubMed] [Google Scholar]
- Braissant O, Beard E, Torrent C, Henry H. Dissociation of AGAT, GAMT and SLC6A8 in CNS: relevance to creatine deficiency syndromes. Neurobiol Dis. 2010;37(2):423–433. doi: 10.1016/j.nbd.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Salomons GS, Ball WSJ, et al. Irreversible brain creatine deficiency with elevated serum and urine creatine: a creatine transporter defect? Ann Neurol. 2001;49(3):401–404. doi: 10.1002/ana.79. [DOI] [PubMed] [Google Scholar]
- Chilosi A, Leuzzi V, Battini R, et al. Treatment with L-arginine improves neuropsychological disorders in a child with creatine transporter defect. Neurocase. 2008;14(2):151–161. doi: 10.1080/13554790802060821. [DOI] [PubMed] [Google Scholar]
- DeGrauw TJ, Salomons GS, Cecil KM, et al. Congenital creatine transporter deficiency. Neuropediatrics. 2002;33(5):232–238. doi: 10.1055/s-2002-36743. [DOI] [PubMed] [Google Scholar]
- Dezortova M, Jiru F, Petrasek J, et al. 1H MR spectroscopy as a diagnostic tool for cerebral creatine deficiency. Magma. 2008;21(5):327–332. doi: 10.1007/s10334-008-0137-z. [DOI] [PubMed] [Google Scholar]
- Dringen R, Verleysdonk S, Hamprecht B, Willker W, Leibfritz D, Brand A. Metabolism of glycine in primary astroglial cells: synthesis of creatine, serine, and glutathione. J Neurochem. 1998;70(2):835–840. doi: 10.1046/j.1471-4159.1998.70020835.x. [DOI] [PubMed] [Google Scholar]
- Edison EE, Brosnan ME, Meyer C, Brosnan JT. Creatine synthesis: production of guanidinoacetate by the rat and human kidney in vivo. Am J Physiol Renal Physiol. 2007;293(6):F1799–1804. doi: 10.1152/ajprenal.00356.2007. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, et al. Studies of age-correlated features of cognitive-behavioral development in children and adolescents with genetic disorders. Am J Med Genet A. 2007;143A(20):2478–2489. doi: 10.1002/ajmg.a.31915. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH (2004) Longitudinal and cross-sectional information. In: Applied longitudinal analysis. Wiley, New York, pp 418–423
- Fons C, Arias A, Sempere A, et al. Response to creatine analogs in fibroblasts and patients with creatine transporter deficiency. Mol Genet Metab. 2010;99(3):296–299. doi: 10.1016/j.ymgme.2009.10.186. [DOI] [PubMed] [Google Scholar]
- Hahn KA, Salomons GS, Tackels-Horne D, et al. X-linked mental retardation with seizures and carrier manifestations is caused by a mutation in the creatine-transporter gene (SLC6A8) located in Xq28. Am J Hum Genet. 2002;70(5):1349–1356. doi: 10.1086/340092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, Rosenberg EH, Salomons GS, et al. Progressive intestinal, neurological and psychiatric problems in two adult males with cerebral creatine deficiency caused by an SLC6A8 mutation. Clin Genet. 2005;68(4):379–381. doi: 10.1111/j.1399-0004.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- Leuzzi V, Alessandri MG, Casarano M, Battini R, Cioni G. Arginine and glycine stimulate creatine synthesis in creatine transporter 1-deficient lymphoblasts. Anal Biochem. 2008;375(1):153–155. doi: 10.1016/j.ab.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Lunardi G, Parodi A, Perasso L, et al. The creatine transporter mediates the uptake of creatine by brain tissue, but not the uptake of two creatine-derived compounds. Neuroscience. 2006;142(4):991–997. doi: 10.1016/j.neuroscience.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Mak CSW, Waldvogel HJ, Dodd JR, et al. Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience. 2009;163(2):571–585. doi: 10.1016/j.neuroscience.2009.06.065. [DOI] [PubMed] [Google Scholar]
- Mancini GMS, Catsman-Berrevoets CE, de Coo IFM, et al. Two novel mutations in SLC6A8 cause creatine transporter defect and distinctive X-linked mental retardation in two unrelated Dutch families. Am J Med Genet A. 2005;132A(3):288–295. doi: 10.1002/ajmg.a.30473. [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Stockler-Ipsiroglu S (2009) Creatine deficiency syndromes. In: GeneReviews at GeneTests: medical genetics information resource (database online). University of Washington, Seattle. http://www.genetests.org.
- Mercimek-Mahmutoglu S, Connolly M, Poskitt K, et al. Treatment of intractable epilepsy in a female with SLC6A8 deficiency. Mol Genet Metab. 2010;101(4):409–412. doi: 10.1016/j.ymgme.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Moxon-Lester L, Takamoto K, Colditz PB, Barnett NL. S-adenosyl-L-methionine restores photoreceptor function following acute retinal ischemia. Vis Neurosci. 2009;26(5–6):429–41. doi: 10.1017/S0952523809990241. [DOI] [PubMed] [Google Scholar]
- Peral MJ, Vazquez-Carretero MD, Ilundain AA. Na(+)/Cl(−)/creatine transporter activity and expression in rat brain synaptosomes. Neuroscience. 2010;165(1):53–60. doi: 10.1016/j.neuroscience.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Poo-Arguelles P, Arias A, Vilaseca MA, et al. X-Linked creatine transporter deficiency in two patients with severe mental retardation and autism. J Inherit Metab Dis. 2006;29(1):220–223. doi: 10.1007/s10545-006-0212-4. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res. 1999;46(4):474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rosenberg EH, Martinez Munoz C, Betsalel OT, et al. Functional characterization of missense variants in the creatine transporter gene (SLC6A8): improved diagnostic application. Hum Mutat. 2007;28(9):890–896. doi: 10.1002/humu.20532. [DOI] [PubMed] [Google Scholar]
- Salomons GS, van Dooren SJ, Verhoeven NM, et al. X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet. 2001;68(6):1497–1500. doi: 10.1086/320595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijens PE, Verbruggen KT, Oudkerk M, van Spronsen FJ, Soorani-Lunsing RJ. 1H MR spectroscopy of the brain in Cr transporter defect. Mol Genet Metab. 2005;86(3):421–422. doi: 10.1016/j.ymgme.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Stöckler SG, Salomons G, et al. Creatine deficiency syndromes. In: Fernandez J, et al., editors. Inborn metabolic diseases, diagnosis and treatment. Heidelberg: Springer; 2006. pp. 211–216. [Google Scholar]
- Tachikawa M, Fujinawa J, Takahashi M, et al. Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. J Neurochem. 2008;107(3):768–778. doi: 10.1111/j.1471-4159.2008.05652.x. [DOI] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience. 2002;112(2):243–260. doi: 10.1016/S0306-4522(02)00088-X. [DOI] [PubMed] [Google Scholar]