Abstract
The AP2/EREBP genes play various roles in developmental processes and in stress-related responses in plants. Genome-wide microarrays based on the gene expression profiles of the AP2/EREBP family were analyzed under conditions of normal growth and drought stress. The preferential expression of fifteen genes was observed in specific tissues, suggesting that these genes may play important roles in vegetative and reproductive stages of growth. A large number of redundant genes were differentially expressed following phytohormone treatments (NAA, GA3, KT, SA, JA, and ABA). To investigate the gene expression responses in the root, leaf, and panicle of three rice genotypes, two drought stress conditions were applied using the fraction of transpirable soil water (FTSW) under severe (0.2 FTSW), mild (0.5 FTSW), and control (1.0 FTSW) conditions. Following treatment, transcriptomic analysis using a 44-K oligoarray from Agilent was performed on all the tissue samples. We identified common and specific genes in all tissues from two near-isogenic lines, IR77298-14-1-2-B-10 (drought tolerant) and IR77298-14-1-2-B-13 (drought susceptible), under drought stress conditions. The majority of the genes that were activated in the IR77298-14-1-2-B-10 line were members of the AP2/EREBP gene family. Non-redundant genes (sixteen) were found in the drought-tolerant line, and four genes were selected as candidate novel reference genes because of their higher expression levels in IR77298-14-1-2-B-10. Most of the genes in the AP2, B3, and B5 subgroups were involved in the panicle under severe stress conditions, but genes from the B1 and B2 subgroups were down-regulated in the root. Of the four subfamilies, RAV exhibited the highest number of up-regulated genes (80%) in the panicle under severe stress conditions in the drought-tolerant line compared to Minghui 63 under normal conditions, and the gene structures of the RAV subfamily may be involved in the response to drought stress in the flowering stage. These results provide a useful reference for the cloning of candidate genes from the specific subgroup for further functional analysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00438-011-0659-3) contains supplementary material, which is available to authorized users.
Keywords: AP2/EREBP, Microarray, Expression profile, Hormone, Stress, NILs
Introduction
Under different adverse environmental conditions, genes show specific expression patterns in accordance with their biological and physiological functions. Hence, an important goal in developmental biology is to detect the expression of unique genes or groups of genes and to determine their expression patterns to identify the exact functional network of that gene(s) and whether it is linked to specific or multiple tissues. Transcription factors (TFs) are important for maintaining the expression of genes that encode functional proteins in the genome. In plants, the AP2/EREBP gene family encodes a large number of TF genes. TFs that are encoded by AP2/EREBP genes contain the highly conserved AP2/ERF DNA-binding domain (Riechmann and Meyerowitz 1998). Jofuku et al. (1994) first described this conserved DNA-binding domain in the homeotic gene APETALA2 (AP2) and in ethylene-responsive element binding proteins (EREBPs) in tobacco (Ohme-Takagi and Shinshi 1995). Based on the number of AP2/ERF domains and the gene structure, the AP2/EREBP gene family has been divided into four subfamilies, AP2, RAV, dehydration-responsive element binding protein (DREB), and ERF (ethylene-responsive element binding factor) (Sakuma et al. 2002; Sharoni et al. 2011). The RAV, DREB, and ERF subfamilies are of particular interest due to their involvement in plant responses to stress. The C-terminal domain of RAV subfamily members is similar to that of the ABI3 and VP1 transcription factors, which have two core motifs CAACA and CACCTG (Kagaya et al. 1999). TFs encoded by genes in the DREB subfamily bind to both GCC and the DRE (dehydration-responsive element) cis-elements (Park et al. 2001; Sakuma et al. 2002). The ERF subfamily of genes encode a large number of ERFs (Fujimoto et al. 2000) that participate in plant responses to biotic stresses, such as pathogens, by recognizing the cis-acting element AGCCGCC, known as the GCC box (Hao et al. 1998). The ERF- and DREB-subfamily transcription factors have been discovered in several plant species including rice (Quan et al. 2010), Arabidopsis (Sakuma et al. 2006) and tobacco (Agarwal et al. 2010). Furthermore, the functions of the ERF and DREB transcription factors in the plant response to biotic and abiotic stress have been extensively documented (Agarwal et al. 2006, 2010). A combination of genetic engineering and molecular approaches have been used to characterize a series of regulatory genes of the AP2/EREBP gene family that are involved in various pathways, including genes related to drought and high salinity (Dubouzet et al. 2003), diseases (Gutterson and Reuber, 2004; Agarwal et al. 2006), cold (Qin et al. 2007a), and the control of flowering (Elliott et al. 1996). The overexpression of DREB1A (CBF3) or DREB1B (CBF1) in transgenic Arabidopsis induces the strong expression of target stress-inducible genes and results in increased tolerance to drought and high salt (Quan et al. 2010; Zhu et al. 2010). Similarly, the overexpression of a number of ERF genes enhances the resistance to biotic and abiotic stresses in tobacco (Park et al. 2001). In addition, TERF1 and TSRF1 exhibit drought and salinity tolerance in rice (Gao et al. 2008; Quan et al. 2010). Members of the RAV subfamily are involved in the ethylene response (Alonso et al. 2003), the brassinosteroid response (Hu et al. 2004), and biotic and abiotic stress responses in pepper (Sohn et al. 2006). A microarray analysis in Arabidopsis has shown that there are different pathways that independently respond to abiotic stress, and one such important pathway involves the DREB regulon (Fowler and Thomashow 2002).
Drought is a serious abiotic stress that imposes a constraint on rice production and severely reduces the grain yield potential of rice (Serraj et al. 2009). Among cereal crops, rice is very sensitive to soil water deficits and evaporative demands, and the greatest sensitivity is found in the lowland-adapted genotypes (Parent et al. 2010). Different mechanisms of the drought response can be measured at many different levels, from the whole plant down to the molecular level. In plants, knowledge of the molecular aspects of drought control or drought tolerance is limited. Therefore, a promising approach is to examine near-isogenic lines (NILs) that have a common genetic background but contrasting levels of tolerance to water deficits in a long-term drought stress situation that is similar to field conditions, using a dry-down method. Isogenic lines and NILs are invaluable tools for testing hypotheses in physiological and genetic studies because there is no interference from variations in other traits (Lafitte et al. 2006). Therefore, the use of NILs reduces the noise of gene expression data by comparing it with the IR64 recurrent parent. Through selection in the drought-breeding program of IRRI (International Rice Research Institute), a series of advanced backcrossed rice plant lines was developed by backcrossing Aday Selection (Aday Sel), a drought-tolerant traditional variety, to IR64 (Khush et al. 2004). IR64 is resistant to blast, bacterial leaf blight, and brown planthopper, but it is susceptible to tungro (Khush et al. 2004) and drought stress (Guan et al. 2010). IR64 is the most widely grown high-yielding rice variety in the tropics and has important agronomic traits. Using the IR64 as a recurrent parent, the following pair of NILs with contrasting drought tolerances (severe and mild) was selected (Venuprasad et al. 2007) from the IR77298-14-1-2-B family: IR77298-14-1-2-B-10 (highly drought tolerant) and IR77298-14-1-2-B-13 (susceptible to drought). These advanced backcrossed lines are considered pre-NILs because they are sister lines that were derived from a single family that was segregated for drought-tolerant phenotypes. Venuprasad et al. (2011) reported that the two NILs of the IR77298-14-1-2-B family are 96.5% genetically similar using 491 SSR markers. The genome-wide identification of genes that are regulated by drought treatments has manifold significance (Degenkolbe et al. 2009). The identification of these genes enables a more detailed understanding of the transcriptional response to stress and provides a clue for further elucidating the role of single genes in the stress response, which will have a significant impact on crop engineering. The rice genome is thought to contain 163 OsAP2/EREBP genes (Sharoni et al. 2011); however, only a few of these genes have been characterized in this species. The characterization of genes from the OsAP2/EREBP family in rice facilitate the understanding of the molecular mechanisms of resistance to stress and thus, will aid in the development of drought-resistant rice varieties using transgenic technology. In this study, we used Affymetrix microarray studies to systematically characterize the expression of the entire OsAP2/EREBP gene family in the rice Minghui 63 genotype to gain a better understanding of the role each family/subfamily member or subgroup plays in several developmental stages under normal conditions and following phytohormone treatments. Furthermore, we used the Agilent 44 K oligoarray system to profile the transcriptomes of root, leaf, and panicle tissues from three rice genotypes (IR77298-14-1-2-B-10, IR77298-14-1-2-B-13, and IR64) grown under different drought stress conditions to identify the putative drought responsive genes in tolerant NIL by examining their different gene expression patterns and comparing them with the control. Initially, global gene expression analyses in root tissue from these NILs grown under drought stress conditions were performed, and the gene expression data were analyzed using gene ontology profiling (Moumeni et al. 2011, personal communication). Although there were many more genes in this array, this study focuses only on OsAP2/EREBP gene family members to select the best candidate genes for further functional analysis. A number of specific subfamily genes or subgroup family genes were differentially expressed, and the data revealed novel information about their roles in the plant response to different stress conditions. The conserved cis-elements in the 2-kb upstream sequence of the promoter region of differentially expressed genes (DEGs) under different stress conditions were revealed. Introgressed regions were examined and Gene Ontology (GO) enrichment tests were performed. We used RT-PCR to confirm the expression of selected OsAP2/EREBP genes under stressed and non-stressed conditions. To our knowledge, this is the first report that focuses on OsAP2/EREBP genes to identify family-level expression patterns, and these results provide a solid basis for future functional genomic research of the OsAP2/EREBP gene family.
Materials and methods
Expression profile analysis (Minghui 63)
The expression profile data for OsAP2/EREBP genes in Minghui 63 and in different hormone-treated seedlings were extracted from the NCBI-GEO database (GSE 19024, GSE7567, GSE32634, and GSM26043; http://www.ncbi.nlm.nih.gov/). This database contains Affymetrix rice microarray data from 26 tissues that cover the entire life cycle of rice. As shown in Table 1, vegetative and reproductive tissues at different developmental stages covering the whole rice plant body were used for the OsAP2/EREBP gene expression profile analyses used in this study. After normalization, the average signal value of two biological replicates of each sample was used for analysis. To identify preferentially expressed genes, Student’s t test was performed. A gene in a given tissue was defined as being differentially expressed only if the expression value of the gene in this tissue was more than twofold (P ≤ 0.05) greater than that in the control (CK). In the phytohormone-treated samples, genes that were up- or down-regulated more than 1.5-fold (P ≤ 0.05) compared with the control or an untreated sample were considered differentially expressed. Trefoil-stage seedlings were exposed to 100 mM concentrations of gibberellic acid (GA3), kinetin (KT), and naphthalene acetic acid (NAA) for 15, 30, and 60 min, and the samples were then mixed (Nuruzzaman et al. 2008). The different hormone treatments were performed in seedlings growing under normal agricultural conditions of temperature, light, and humidity. Benzothiadiazole (BTH) is a functional analog of salicylic acid (SA) and protects plants from disease by activating the SA signaling pathway. The single-color method was used to compare four biological replicates of BTH-treated leaves with mock-treated leaves. Leaf disks (6 mm diameter) from seedlings were floated on jasmonic acid (JA, 500 μM) and incubated under light at 25°C. The samples were harvested at 6, 12, 24, and 48 h after the treatment. The japonica rice variety Nipponbare were grown for 3 weeks at 28°C in the dark and were treated with abscisic acid (ABA, 100 μM) for 5 h. Plants treated with H2O for 5 h were used as controls. Whole plants were used for RNA extractions (Yazaki et al. 2003). The log2 ratio values of the means were normalized, and up- or down-regulated genes with values >0.585 or <−0.585 were selected for the JA and ABA treatments.
Table 1.
Sample vegetative and reproductive developmental stage analyses in Minghui 63
| Sample | Developmental tissues |
|---|---|
| CK | Seed: germination (72 h after imbibition) |
| 1 | Calli 1: 15 days after subculture |
| 2 | Calli 2: screening stage |
| 3 | Calli 3: 5 days after regeneration |
| 4 | Seedling 1: 3 days after sowing |
| 5 | Seedling 2: root and leaf at the three-leaf stage |
| 6 | Shoot: seedling with 2 tillers |
| 7 | Root : seedling with 2 tillers |
| 8 | Stem 1: 5 days before heading |
| 9 | Stem 2: heading stage |
| 10 | Flag leaf 1: 5 days before heading |
| 11 | Flag leaf 2: 14 days after heading |
| 12 | Leaf 1: young panicle at stage 3 |
| 13 | Leaf 2: 4–5 cm young panicle |
| 14 | Sheath 1: young panicle at stage 3 |
| 15 | Sheath 2: 4–5 cm young panicle |
| 16 | Panicle 1: young panicle at stage 3 |
| 17 | Panicle 2: young panicle at stage 4 |
| 18 | Panicle 3: young panicle at stage 5 |
| 19 | Panicle 4: 4–5 cm young panicle |
| 20 | Panicle 5: heading stage |
| 21 | Stamen: just before heading |
| 22 | Spikelet: 3 days after pollination |
| 23 | Endosperm 1: 7 days after pollination |
| 24 | Endosperm 2: 14 days after pollination |
| 25 | Endosperm 3: 21 days after pollination |
Plant growth and drought-treatment conditions for rice
The two NILs and the IR64 plants used for this study were chosen because of their different rice yields under drought stress conditions (DSCs). The two NILs plants were derived from the IR77298-14-1-2 family and developed at IRRI. Under stress, IR77298-14-1-2-B-10 is a high-yielding (highly drought-tolerant) plant, whereas IR77298-14-1-2-B-13 is a low-yielding (drought-susceptible) plant. Both IR77298-14-1-2-B-10 and IR77298-14-1-2-B-13 NILs plants exhibit similar yield potentials under normal growth conditions (Venuprasad et al. 2011). Plants were grown in a PVC pipe filled with 10 kg of a soil and sand mixture (2:1) in which all the soil nutrients were controlled. The test was initially conducted in a green house, and the plants were moved outside to natural conditions before imposing drought stress. Before planting, the saturated soil in the pots was covered with white or transparent plastic covers with an opening in the center to facilitate planting. Feeder pipes were fixed to the pots to facilitate watering. In one pot, five germinated seeds were transplanted, and the plants were subsequently separated to one plant per pot when they reached the three-leaf stage. This test was carried out on eight replicate samples using a randomized complete block design. All of the pots were watered twice daily to keep the soil saturated. The day before beginning the progressive soil drying process, the soil in each pot was saturated with water. Stress was imposed by initiating a soil dry-down protocol starting 35 days after sowing. The dry-down process continued until the pot reached the target fraction of transpirable soil water (FTSW) (Sinclair and Ludlow 1986). The weight of each pot was determined every day during the dry-down process to estimate the transpiration of soil water. The DSCs were as follows: a control (1.0 FTSW) consisting of well-watered plants with soil that was kept saturated throughout the experiment, and two drought stresses, one severe (0.2 FTSW) and one mild (0.5 FTSW). No water was applied to the soil during the dry-down period. The target FTSW was maintained in all of the pots until the plants were harvested. The water-use efficiency in the different rice genotypes was calculated using the lysimeter system.
Rice oligo microarray (44 K) and data analysis
Cyanine-3 (Cy3) and cyanine-5 (Cy5)-labeled target complementary RNA (cRNA) samples were prepared from 850 ng total mRNA using a fluorescent linear amplification kit (Agilent Technologies) in accordance with the manufacturer’s instructions. Transcriptome profiles specific to stressed plants were examined by the direct comparison of transcription activities between stressed and non-stressed plants on the same oligoarray. We hybridized 1 μg of fluorescent linear-amplified, Cy3- and Cy5-labeled cRNA (825 ng of each cRNA) to a customized rice oligo microarray. The fragmented cRNAs were added to the hybridization buffer, applied to the microarray, and hybridized for 17 h at 60°C. The slides were washed with SSC (10 min in 6 × SSC and 0.005% Triton X-102 at room temperature, followed by 5 min in 0.1 × SSC and 0.005% Triton X-102 at 4°C). The slides were dried and scanned using an Agilent Technologies G2565BA microarray scanner system. The scanned microarray images were analyzed using Feature Extraction 6.1.1 software (Agilent Technologies), and the dye-normalized, background-subtracted intensity and ratio data were exported to a text file. This software flags corrupted spots and detects a lack of differences between sample spots and the background. The slide images were scanned using a DNA microarray scanner (G2505B, Agilent Technologies).
The image files of the slides were processed using Feature Extraction version 9.5 (Agilent Technologies). The Cy3 and Cy5 signal intensities were normalized using rank-consistency filtering and the LOWESS method. The signal intensities of all the samples were transformed into log2-based numbers and normalized according to the quantile method for standardization among the array data using Expander version 5.0 (Shamir et al. 2005). A significantly DEG was defined as an expressed gene with a log2-based ratio (stressed sample and control sample) >1 or <−1, and significance in changes in gene expression between two plants was defined as P ≤ 0.05 using a paired t test (permutations, all possible combinations, FDR correction, adjusted Bonferroni method). Data processing was performed using MeV version 4.5 (Saeed et al. 2003). The Michigan State University (Osa1, Ouyang et al. 2007) database contained at least 163 AP2/EREBP genes in the rice genome and contained corresponding locus IDs. We identified 140 OsAP2/EREBP genes from our 44 K microarray data collected under DSCs. The probe arrangement of the array data (platform number GPL7252) is available at NCBI-GEO (series number GSE30464, Barrett et al. 2007).
Transcript-level analysis
Total RNA was extracted from the root, leaf, and panicle of all of the rice genotypes using an RNeasy Maxi Kit (Qiagen) in accordance with the manufacturer’s instructions. The concentration and quality of the RNA samples were determined using a spectrophotometer (Nanodrop ND-1000, Nanodrop Technologies) and a model 2100 bioanalyzer (Agilent Technologies). The RNA samples were pretreated with RNase-free DNase I (Invitrogen) to eliminate any contaminating genomic DNA. The first-strand cDNA was synthesized from 1 μg of total RNA in a 20-μl reaction volume using Superscript II Reverse Transcriptase (Invitrogen), and 2 μl of the reaction mixture was subsequently used for RT-PCR in a 50-μl reaction volume. The RT-PCR was performed using SuperScriptII Reverse Transcriptase in accordance with the manufacturer’s instructions to synthesize first-strand cDNA from the DNase I-treated total RNA. The same RNA samples were used for hybridization and RT-PCR experiments. Approximately 1/20 of the first-strand cDNA that was generated from 1 μg total RNA was used as a template for PCR (reaction volume 50 μl) using rTaq DNA polymerase (Takara). The RT-PCR procedures consisted of 25–35 cycles, depending on the linear range of the PCR amplification for each gene. Each PCR was performed (in triplicate) in an ABI 9700 thermocycler (Applied Biosystems). The program entailed incubation at 94°C for 45 s, at 55°C for 50 s, and at 72°C for 1 min. The rice actin gene (LOC_Os05g36290) was used as an internal control (Nuruzzaman et al. 2010; Sharoni et al. 2011), and the primers are listed in Supplementary Table 1. For convenience, all the “LOC_” prefixes in the Osa1 (Ouyang et al. 2007) locus ID are omitted in the remainder of the manuscript.
Results
Expression patterns of OsAP2/EREBP genes under normal growth conditions using Affymetrix arrays
The rice tissues from the vegetative and reproductive stages that were selected for microarray analysis cover the complete life cycle of the rice plant. Detailed information on the selected tissues is presented in Table 1. The expression pattern of the microarray data was confirmed to reveal the expression profiles of the various genes (Nuruzzaman et al. 2008; Ye et al. 2009). Probes for 116 of the 163 OsAP2/EREBP genes that were identified in the microarray and demonstrated differential expression during various stages of development when compared with their expression in the seed are summarized in Supplementary Table 2, and the P values and the fold-change values are included in this table. The AP2/EREBP genes were classified into four subfamilies and two subfamilies of those (ERF and DREB), and these were further divided into different subgroups, e.g., DREB-A1 to subgroup DREB-A6 and ERF-B1 to subgroup ERF-B7 (Sharoni et al. 2011). A number of up-regulated genes were found in the calli, shoot, leaf-2, and spikelet stages. In contrast, a higher number of down-regulated genes were observed in Minghui 63 in most of the stages of development (Fig. 1a). During the vegetative stages, 26 OsAP2/EREBP genes were up-regulated and 25 genes were down-regulated by two-fold or more in Minghui 63. These data show that the differentially regulated OsAP2/EREBP genes did not exhibit high variations, implying that their expression may be somewhat conservative during vegetative development. In contrast, of the 52 genes differentially regulated in reproductive development, 22 OsAP2/EREBP genes were up-regulated more than twofold and 30 were down-regulated. This observation suggests that the expression pattern of OsAP2/EREBP genes in reproductive development is complex and implies the activation of different genes at different developmental stages. A biphasic expression pattern was also observed for OsAP2/EREBP genes during reproductive development, nine genes (e.g., Os06g11860, Os09g11480, and Os06g07030) were up-regulated during several stages but were down-regulated during the other stages. Specific genes were up-regulated or down-regulated during vegetative and reproductive development, probably because of the prevalence of similar processes in these stages. OsAP2/EREBP genes, nine in total (e.g., Os02g43940, Os03g09170, and Os08g31580, subfamily DREB), exhibited up-regulated expression during both vegetative and reproductive development, whereas five genes were down-regulated (e.g., Os02g34260, Os01g12440, and Os04g48350, subfamily ERF). Overall, the DREB subfamily exhibited the highest number of up-regulated genes, followed by ERF, AP2, and RAV, whereas the AP2 subfamily demonstrated a higher number of down-regulated genes, followed by RAV, ERF, and DREB, respectively (Supplementary Table 2). Moreover, we found 15 up-regulated genes in specific tissues of plants grown under normal growth conditions (Fig. 1b; Supplementary Table 2). For example, three genes (Os03g12950, Os05g25260, and Os08g36920) were specifically expressed in the panicle-2 stage under non-stressed conditions (Fig. 1b). The number of differentially expressed genes under normal conditions was compared with the number of differentially expressed genes under different drought stresses, as described later. Taken together, these results provide a global overview of the OsAP2/EREBP transcriptome in relation to rice tissues and the developmental stages of the plant life cycle.
Fig. 1.
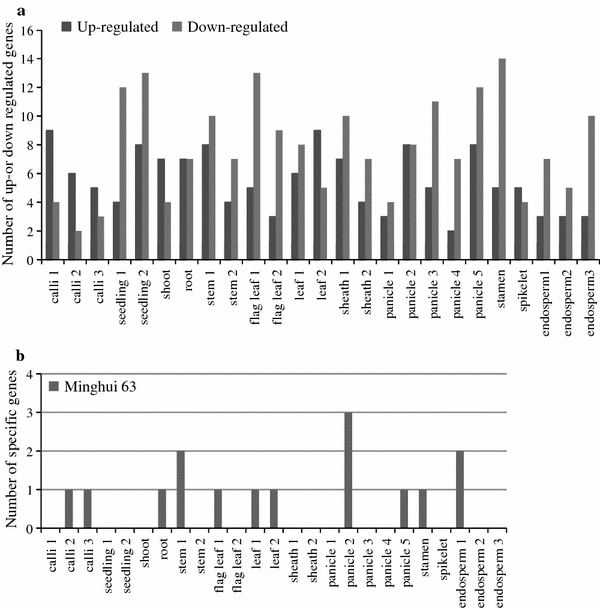
a Number of up- or down-regulated genes in different tissues under normal conditions in Minghui 63 are presented. b Tissue-specific up-regulated genes in Minghui 63: calli 2, Os02g32040; calli 3, Os06g44750; root, Os04g44670; stem 1, Os02g51300 and Os09g28440; flag leaf 1, Os08g45110; leaf 1, Os09g13940; leaf 2, Os08g42550; panicle 2, Os03g12950, Os05g25260, and Os08g36920; panicle 5, Os02g45450; stamen, Os09g11460, and endosperm 1, Os05g03040 and Os08g41030. The number of genes is shown on the Y-axis, and the different developmental stages are indicated on the X-axis. Tissue-specific up-regulated genes are shown in Supplemental Table 2
Responses of OsAP2/EREBP genes to GA3, KT, NAA, SA, JA, and ABA treatments
Phytohormones play a critical role in plant growth and development. To investigate the response of OsAP2/EREBP genes to phytohormone treatment, microarray analysis was performed. To understand the expression patterns of the OsAP2/EREBP gene family, data were extracted from the NCBI database as described previously. The Minghui 63 rice cultivar was used for microarray expression analysis following three different hormonal treatments of plants at the seedling stage. We identified a total of 21 redundant OsAP2/EREBP genes out of 116 from Gene Chip that were differentially expressed following treatments with one or more of the phytohormone GA3, KT, and NAA in the Minghui 63 genotypes compared with the control (Fig. 2). The fold-change values of differentially expressed genes in the seedling stage with respect to the control (CK, untreated sample) are shown in Supplementary Table 3. Of these 116 genes, 18 genes were up-regulated, whereas three genes were down-regulated following different hormone treatments. Interestingly, two genes (Os02g51300 and Os05g03040, subfamily AP2) were up-regulated following treatment with all three phytohormones. For instance, the Os03g60430 gene of the AP2 subfamily was up-regulated specifically by NAA treatment of Minghui 63, and the Os06g40150 gene of the B3 subgroup of the ERF subfamily was up-regulated by GA3 and NAA treatments in seedlings (Supplementary Table 3). Furthermore, 12 DEGs were found between the SA and ABA treatments, whereas 41, 54, 63, and 57 redundant DEGs were observed following JA treatment at four different time points (Supplementary Table 3). We observed 24 up- or down-regulated genes that were common to all the time points in JA-treated plants. The gene expression profiles of up- or down-regulated genes following treatment of Minghui 63 with different phytohormones were compared in the root, leaf, and panicle of the NILs under severe and mild drought stress conditions.
Fig. 2.
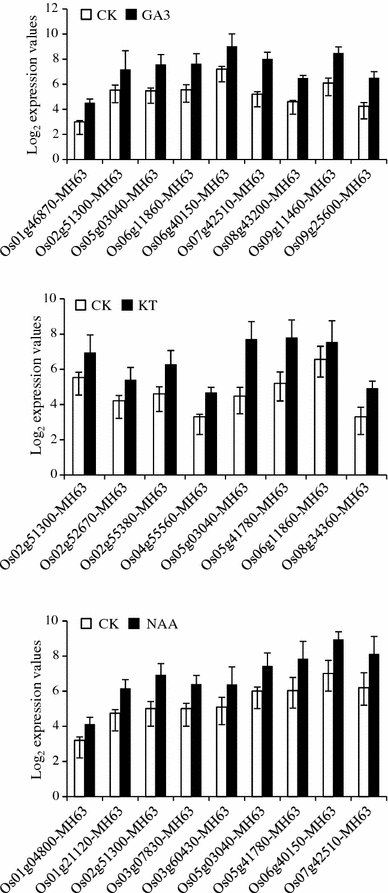
Expression profiles of differentially expressed OsAP2/EREBP genes in 7-day-old seedlings subjected to three phytohormones (GA3 gibberellic acid, KT kinetin, and NAA naphthalene acetic acid). X-axis represents differentially expressed genes. Y-axis represents log2 expression values. Each treatment is indicated at the top of each diagram. CK control
Gene expression patterns in different rice genotypes under different stress conditions
IR64 and two NILs, IR77298-14-1-2-B-10 and IR77298-14-1-2-B-13, were used in this study. In these NILs, gene identification and the determination of gene expression patterns are important to understand the molecular basis of plant responses and to improve drought stress tolerance in the progeny from a common genetic background using transgenic techniques. The three water regimes used were severe stress (0.2 FTSW), mild stress (0.5 FTSW), and control (1.0 FTSW), and gene expression in the root, leaf, and panicle tissues of the two NILs and IR64 at the reproductive stage were examined. Therefore, the goals of this study are to identify DEGs in IR77298-14-1-2-B-10 versus IR77298-14-1-2-B-13 and IR64, to determine the number of DEGs that are specifically induced in each NIL, to determine the number of DEGs that are common to both NILs under both DSCs in multiple tissues, and to identify putative drought responsive genes in the drought-tolerant line for further functional analysis. Altogether, the results of the expression patterns and analyses indicate the involvement of this large gene family in a number of signaling pathways that are triggered by the drought response.
The physical appearance of the plants, such as plant height, transpiration, flowering, the total biomass (root, shoot, and panicle) at harvest, the spikelet fertility score and the grain yield were recorded for the IR77298-14-1-2-B family (Moumeni et al. 2011; Venuprasad et al. 2011). The drought-tolerant NIL in the IR77298-14-1-2-B family exhibited a significantly higher grain yield compared with the drought-susceptible NIL under drought-stress but not under non-stress conditions (Venuprasad et al. 2011). We found 162 out of a possible 163 OsAP2/EREBP genes in our 44 K array data. Among these genes, 140 were differentially expressed under both DSCs in different experimental tissues. A gene was defined as preferentially responsive if the signal values of the gene in this tissue increased more than twofold and had a P value less than 0.05 compared with the control (Supplementary Table 4). We compared gene expression profiles of three rice genotypes under two DSCs. Supplementary Table 5 shows the number of DEGs obtained from microarray experiments using tissues from the different rice genotypes under both DSCs. The differentiation of the expression patterns in the three tissues of the rice genotypes indicated that a higher number of DEGs was observed under severe stress compared with mild stress conditions. We observed that the tolerant line IR77298-14-1-2-B-10 exhibited the highest number of up-regulated genes, followed by IR77298-14-1-2-B-13 and IR64, in all tissues under both stresses, with the exception of the root and the leaf under severe and mild stress conditions. In response to severe stress, this tolerant line demonstrated the highest number of up-regulated genes (61) in the panicle. Therefore, despite their common genetic background as backcrossed progeny from Aday Sel x IR64, the two NILs showed distinctive differences in their gene expression patterns in response to drought. We further analyzed the DEGs in two NILs of the IR77298-14-1-2-B family and compared their expression profiles under normal conditions and after the hormone treatment of seedlings, as outlined below.
AP2/EREBP gene expression in the root common to both NILs under severe and mild stress conditions
In this study, gene expression profiles were compared between the drought-tolerant and drought-susceptible lines. It is believed that genotypes responding differentially to drought stress exhibit variations in gene expression and that these differences are strongly related to drought avoidance or to drought response. Spollen et al. (1993) reported that mild osmotic stress rapidly inhibits the growth of the leaves and stems, whereas the roots continue to elongate, and the root architecture is a key trait for dissecting genotypic differences in rice responses to DSCs. Thus, the analysis of the genes observed entirely in two NILs i.e. up-regulated genes in both NILs and in individual is of interest to identify the most promising candidate genes among three tissues at procreative stage under both DSCs, which will provide a starting point for further elucidating role of single gene in stress response. In the root under severe stress, fourteen genes were shared common in IR77298-14-1-2-B-10 and in IR77298-14-1-2-B-13 (Fig. 3; Supplementary Table 6), while most of them were also up- or down-regulated in different tissues under both DSCs in two NILs and assigned to DREB subfamily (Supplementary Table 4). We considered a gene specifically expressed in one NIL if that gene was not differentially expressed and exhibited no change in expression levels or a low level of expression compared with susceptible NIL under severe or mild stress in a certain tissue. In IR77298-14-1-2-B-10, we identified two specific genes (Os06g42990 and Os11g06770, DREB subfamily) that were expressed in the root under severe stress but were down-regulated or highly sensitive in the root under severe stress in IR77298-14-1-2-B-13. Similarly, in IR77298-14-1-2-B-13, four genes (Os02g40070, Os04g46440, Os04g55970, and Os06g09390, ERF and AP2 subfamilies) were specifically induced in the root under severe stress, and all of the genes were down-regulated, their expression levels did not change or these genes were highly sensitive in IR77298-14-1-2-B-10 under conditions of severe stress (Supplementary Table 6). Os02g40070 was down-regulated in the root under non-stressed conditions. This study assumed that these genes are line and tissue specific under conditions of severe stress. In the root under mild stress conditions, 13 up-regulated genes were common to both NILs (Fig. 3; Supplementary Table 6). Out of these 13 genes, eight genes belong to the DREB subfamily. A total of six genes (Os02g52670, Os03g08500, Os09g39850, Os02g32140, Os03g19900, and Os06g05340) assigned to subfamilies DRED, ERF, and AP2 were specifically up-regulated in IR77298-14-1-2-B-10 under mild stress conditions, but they were down-regulated or exhibited no change in expression in IR77298-14-1-2-B-13 in the root. These genes were not up- or down-regulated following treatment with the different plant hormones, whereas Os03g19900 of the AP2 subfamily was expressed with a sixfold increase in the drought-tolerant line compared with the drought-susceptible line in the root, and its expression was down-regulated in root tissue under normal growth conditions. In IR77298-14-1-2-B-13, four genes (Os02g54160, Os05g32270, Os01g67410, and Os04g42570, DREB and AP2 subfamilies) were up-regulated under mild stress conditions. Os01g67410 and Os05g32270 expression was induced in leaf-1 and panicle-1, respectively, under normal conditions, whereas Os01g67410 was up-regulated and Os05g32270 was down-regulated at all time points of the JA treatment. Interestingly, two genes of the AP2 subfamily were severely down-regulated in IR77298-14-1-2-B-10 under mild stress conditions. In the root, under both DSCs, the two NILs shared eight common genes (Fig. 3; Supplementary Table 6). Of these eight genes, Os02g51300 is from the AP2 subfamily and was up-regulated following treatment of seedlings with GA3, KT, and NAA. Under both DSCs in the root, three genes were up-regulated specifically in IR77298-14-1-2-B-10. Among these three genes, Os11g06770 (subgroup B4, ERF subfamily) was down-regulated under severe stress conditions, and its expression was induced in the root under mild stress conditions in IR77298-14-1-2-B-13. Os02g52670 (A5 subgroup, DREB subfamily) and Os03g19900 (AP2 subfamily) exhibited a low level of expression in the root in IR77298-14-1-2-B-13 under mild stress conditions, whereas Os03g19900 was down-regulated under non-stress conditions in the same tissue. Similarly, five genes were commonly found in IR77298-14-1-2-B-13 under both stress conditions, although the majority of these genes exhibited high expression levels under either DSC in the drought-tolerant line compared with the drought-susceptible line. We speculate that expression responses of these genes are root tissue- and NILs-specific under both DSCs.
Fig. 3.
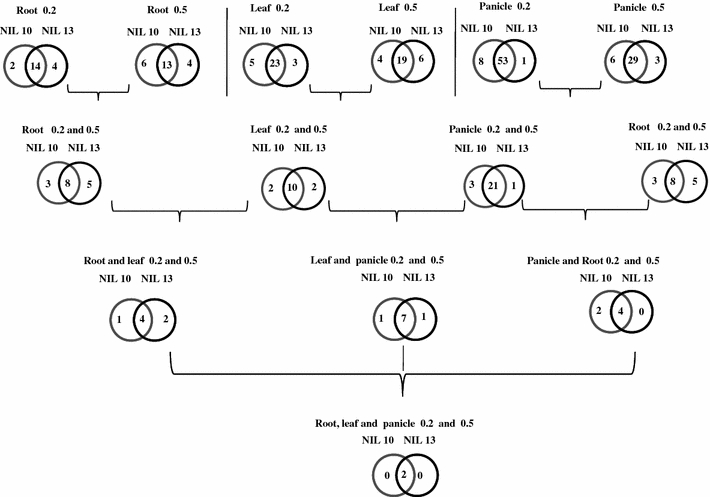
Up-regulated overlapped and specific genes were detected in NIL10 and NIL13 lines under severe and mild stress conditions in the root, leaf, and panicle. NIL10 = R77298-14-1-2-B-10 and NIL13 = IR77298-14-1-2-B-13, 0.2 = severe stress and 0.5 = mild stress
AP2/EREBP gene expression in the leaf common to both NILs under severe and mild stress conditions
We found that 23 up-regulated genes in the leaf were common to both lines under severe stress conditions (Fig. 3; Supplementary Table 6). Of these 23 genes, 14 were not differentially expressed in the root under both DSCs (Supplementary Table 4). Under severe stress conditions, five genes (Os08g31580, Os10g38000, Os03g08500, Os01g10370, and Os07g22770, subfamilies DREB and ERF) were up-regulated in the leaf, specifically in IR77298-14-1-2-B-10. With the exception of Os03g08500, these genes were up-regulated at least once in the JA treatment time point. All of these genes were expressed in the leaf at a low level or was highly sensitive in IR77298-14-1-2-B-13 under severe stress conditions (Supplementary Table 6). In IR77298-14-1-2-B-13, three genes (Os01g64790, Os04g57340, and Os11g03540; AP2, DREB, and ERF subfamilies) were highly activated in the leaf under severe stress conditions and down-regulated or expressed at a low level in that tissue in IR77298-14-1-2-B-10 under severe stress conditions. These genes are specific to NILs and to leaf tissue under severe stress. Of the genes expressed at high levels in the leaf under mild stress, 19 were common to both NILs (Fig. 3; Supplementary Table 6). In IR77298-14-1-2-B-10, four genes (Os02g43940, Os09g11460, Os04g44670, and Os02g51300, subfamilies AP2, DREB, and ERF) were highly responsive, specifically in IR77298-14-1-2-B-10, under mild conditions in the leaf and not differentially expressed or highly responsive in IR77298-14-1-2-B-13. Of these four genes, two (Os09g11460 and Os02g43940) were up-regulated by JA treatment at the seedling stage (Supplementary Table 3), whereas Os04g44670 was highly induced specifically in the root under normal conditions and was down- and up-regulated in seedlings following SA and JA treatments, respectively (Fig. 1b; Supplementary Tables 2 and 3). Similarly, six genes (Os08g45110, Os06g07030, Os04g34970, Os06g09390, Os06g10780, and Os02g54050, ERF and DREB subfamilies) were highly activated in the leaf, specifically in IR77298-14-1-2-B-13 under mild stress condition, but were not differentially expressed and did not exhibit lower intensities in IR77298-14-1-2-B-10. Os04g34970 was activated in root tissue under normal conditions. During both stress conditions, ten genes were up-regulated in the leaf in both NILs (Fig. 3; Supplementary Table 6). All of these genes were expressed in the different experimental tissues under both DSCs (Supplementary Table 4). Os08g31580 (DREB-A5) and Os09g11460 (ERF-B2) were up-regulated in the leaf under both stress conditions in IR77298-14-1-2-B-10, but one of them (Os09g11460) was more highly expressed in IR77298-14-1-2-B-13. Os09g11460 exhibited up-regulation specifically in the stamen under normal conditions and following GA3 and JA treatments (Fig. 1b; Supplementary Tables 2 and 3). On the other hand, Os06g07030 and Os04g34970 genes (subgroups A3 and A4 of the DREB subfamily) were expressed at high levels in IR77298-14-1-2-B-13 under both DSCs, whereas both genes were down-regulated in drought-tolerant NILs under mild stress conditions (Fig. 3; Supplementary Table 6). These Os06g07030 and Os04g34970 genes were up-regulated in the root and the leaf-1 tissue under normal conditions in Minghui 63, respectively.
AP2/EREBP panicle genes expression common in two NILs under severe and mild stress conditions
Plants are very susceptible to drought during the reproductive stage of development. Under severe stress conditions, a greater number up-regulated genes (53) common to both NILs were found in the panicle compared with the root and leaf (Fig. 3; Supplementary Table 6). Of these 53 genes, thirteen genes (e.g., Os02g34270 and Os04g46250) were up-regulated in the panicle in both NILs under severe stress conditions, but they were not up-regulated in any other tissue (Supplementary Table 4). Os02g34270 exhibited down-regulation in the root under non-stressed conditions. Interestingly, eight genes (Os05g27930, Os03g08500, Os06g08340, Os01g46870, Os11g06770, Os07g22770, Os12g39330, and Os06g05340) belonged to the subfamilies AP2, DREB, and ERF and were up-regulated specifically in IR77298-14-1-2-B-10 in the panicle; however, they were not differentially expressed or only expressed at a low level in IR77298-14-1-2-B-13 under severe stress. Out of these eight genes, five belong to the ERF subfamily, and one gene (Os01g46870, ERF-B3) was up-regulated in the 3-leaf stage following treatment with GA3 (Supplementary Table 3). Similarly, Os02g45420 (subfamily DREB-A1) was up-regulated specifically in the IR77298-14-1-2-B-13 panicle under severe stress (Fig. 3; Supplementary Table 6). This study detected 29 common genes in the panicles of IR77298-14-1-2-B-10 and IR77298-14-1-2-B-13 under mild stress conditions (Fig. 3; Supplementary Table 6). All of these genes were differentially expressed in at least one tissue in both NILs (Supplementary Table 4). In IR77298-14-1-2-B-10 under mild stress conditions, six genes (Os04g55520, Os03g08500, Os05g49010, Os01g21120, Os10g25170, and Os09g28440; subfamilies DREB and ERF) were up-regulated specifically in the panicle but were not differentially expressed or highly sensitive in IR77298-14-1-2-B-13. Of these six genes, four (Os04g55520, Os01g21120, Os10g25170, and Os09g28440) were up-regulated following JA treatment at all the time points, whereas Os01g21120 was up-regulated following NAA treatment. In leaf-1 tissue, Os04g55520 gene was down-regulated under normal conditions. Similarly, three genes (Os03g22170, Os07g22730, and Os01g58420 from ERF) were activated specifically in the IR77298-14-1-2-B-13 panicle under mild stress conditions but exhibited low expression levels in IR77298-14-1-2-B-10 (Supplementary Table 6). In the panicle under both DSCs, both NILs shared 21 common genes, and these genes were expressed in at least one of the stress conditions in the root or leaf, as indicated above. The Os03g08500 gene (subgroup A6, DREB subfamily) and the Os01g21120 and Os11g06770 (subgroups B1 and B4, ERF subfamily) genes were highly induced in the panicle under both DSCs in IR77298-14-1-2-B-10, whereas Os03g08500 in the panicle was highly sensitive in IR77298-14-1-2-B-13 under both DSCs. In the drought-tolerant line, the expression intensity of the Os01g21120 gene under severe stress conditions was higher than that under mild stress. Similarly, the Os07g22730 (subgroup B3, ERF subfamily) was activated in the panicle in IR77298-14-1-2-B-13 under both DSCs but up-regulated approximately ninefold in IR77298-14-1-2-B-10 under severe stress conditions. The rapid change in the expression level of this gene under the drought stress conditions in the drought-tolerant line suggests the recruitment of genes that are normally expressed at lower level for emergencies. In contrast, some genes exhibit ubiquitous expression under normal conditions and during phytohormone and drought stress treatments. A number of genes were up-regulated in the drought-tolerant line, and these genes may be required to maintain grain yield in response to severe drought. Functional analyses are needed to further explore these possibilities.
AP2/EREBP genes expression in both NILs under both DSCs in root vs. all tissues and in leaf vs. panicle and all tissues
The up-regulation of four genes was observed in both lines under the two DSCs in the root and the leaf (Fig. 3; Supplementary Table 6). Os02g52670 (subfamily DREB-A5) was expressed in the root and in the leaf in both NILs under both stress conditions but was highly sensitive in the root in IR77298-14-1-2-B-13 under mild stress. Similarly, Os02g54160 and Os04g34970 (DREB subfamily) were activated in the root and leaf under severe and mild stress conditions in both lines but were not differentially expressed in the root and leaf under mild stress in IR77298-14-1-2-B-10. We observed that seven overlapping genes were up-regulated in both the leaf and panicle in both NILs under both DSCs (Fig. 3; Supplementary Table 6). The Os08g31580 (DREB-A5) gene exhibited low expression levels in the leaf under severe stress in IR77298-14-1-2-B-13. Os06g07030 (DREB-A3) was down-regulated in the leaf under mild stress in IR77298-14-1-2-B-10. The expression responses of both genes were higher in IR77298-14-1-2-B-10 than in IR77298-14-1-2-B-13 in all tissues (Fig. 3; Supplementary Table 6). The induction of four genes in both the panicle and the root were observed in the tolerant and susceptible lines under both DSCs (Fig. 3; Supplementary Table 6). The Os05g49700 and Os01g07120 (BREB subfamily) genes were uniformly expressed in all tissues in both lines, suggesting that these are housekeeping genes. The Os05g49700 gene response was higher in the root and panicle under both DSCs in IR77298-14-1-2-B-10 when compared with IR77298-14-1-2-B-13 (Fig. 3; Supplementary Table 6). On a broad scale, our findings contribute to the understanding of gene responses to different abiotic stresses.
Identification of putative drought responsive genes, subgroups, and subfamilies
The specific AP2/EREBP genes that were activated during the drought stress response in the drought-tolerant line are of particular interest. In total, sixteen non-redundant genes were up-regulated in IR77298-14-1-2-B-10, and these genes were expressed at a low level or were not differentially expressed in the drought-susceptible IR77298-14-1-2-B-13 line under both DSCs in all tissues (Table 2). To identify putative genes that are responsible for drought avoidance in the tolerant NIL, this study focused on DEGs with high levels of expression in response to two DSCs in the tolerant line versus the susceptible line and IR64. In the tolerant line, two (Os05g49700 and Os06g42990) out of four genes demonstrated higher expression levels in the root than in the leaf and panicle under severe stress and two of these genes (Os01g66270 and Os03g12950) demonstrated higher expression levels in the panicle compared with the root and leaf under severe and mild stress (Fig. 4). Os03g12950 is induced in the panicle and Os05g49700 is down-regulated in the endosperm under normal conditions, whereas Os01g66270 and Os06g42990 are absent in the Affymetrix Gene Chip. Therefore, we suspected that these genes might play functional roles in the specific tissue under both stress conditions. However, further work is required to distinguish these possibilities. We found that most of the genes belonging to subgroups B3 and B5 and to the RAV subfamily were responsive in the panicle under severe stress, but the genes belonging to subgroups B and B2 of the ERF subfamily were down-regulated in the root. The genes belonging to subgroup A2 of the DREB subfamily were involved in the root and panicle, whereas other stress-responsive genes are randomly distributed in the other phylogenetic subgroups or subfamilies (Supplementary Table 7). Among the four subfamilies, we compared the DEGs of two rice genotypes, Minghui 63 and IR77298-14-1-2-B-10, in the root, leaf, and panicle tissues under normal and severe drought stress conditions. Table 3 shows that none of the RAV subfamily genes were differentially expressed in any tissue under normal conditions in Minghui 63, but this subfamily exhibited a large number of up-regulated genes (80%) in the panicle under severe drought stress condition in IR77298-14-1-2-B-10, followed by the ERF, DREB, and AP2 subfamilies. Our data suggest that the up-regulation of RAV subfamily genes may be tissue specific during severe drought stress conditions. Furthermore, the expression intensities of a number of up-regulated genes in IR77298-14-1-2-B-10 were higher than or equal to their expression intensities in IR77298-14-1-2-B-13 or IR64 in all examined tissues (Fig. 5). Taken together, these results enhance our knowledge of the involvement of OsAP2/EREBP TFs in plant resistance to drought and show that members of certain subgroups or subfamilies of the OsAP2/EREBP gene family are activated in response to drought stress.
Table 2.
Specific up-regulated genes in the tolerant line IR77298-14-1-2-B-10 compared with the sister line IR77298-14-1-2-B-13 and control IR64
| Root severe | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os06g42990 | 1.293 | 0 | 0 |
| Root mild | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os09g39850 | 1.528 | 0 | 0 |
| Os06g05340 | 1.12 | 0 | 0 |
| Leaf severe | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os10g38000 | 1.11 | 0 | 0 |
| Os03g08500 | 1.007 | 0 | 0 |
| Leaf mild | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os02g43940 | 1.619 | 0 | 0 |
| Os04g44670 | 1.271 | 0 | 0 |
| Os02g51300 | 1.106 | 0 | 0 |
| Panicle severe | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os03g08500 | 1.424 | 0 | 0 |
| Os06g08340 | 1.745 | 0 | 0 |
| Os01g46870 | 1.283 | 0 | 0 |
| Os07g22770 | 1.238 | 0 | 0 |
| Os06g05340 | 1.297 | 0 | 0 |
| Panicle mild | |||
| NIL10 specific | IR77298-14-1-2-B-10 | IR77298-14-1-2-B-13 | IR64 |
| Os04g55520 | 1.257 | 0 | 0 |
| Os03g08500 | 1.036 | 0 | 0 |
| Os05g49010 | 1.358 | 0 | 0 |
| Os01g21120 | 1.274 | 0 | 0 |
| Os10g25170 | 1.015 | 0 | 0 |
| Os09g28440 | 1.229 | 0 | 0 |
0 = genes not differentially expressed in IR77298-14-1-2-B-13 and IR64
Fig. 4.
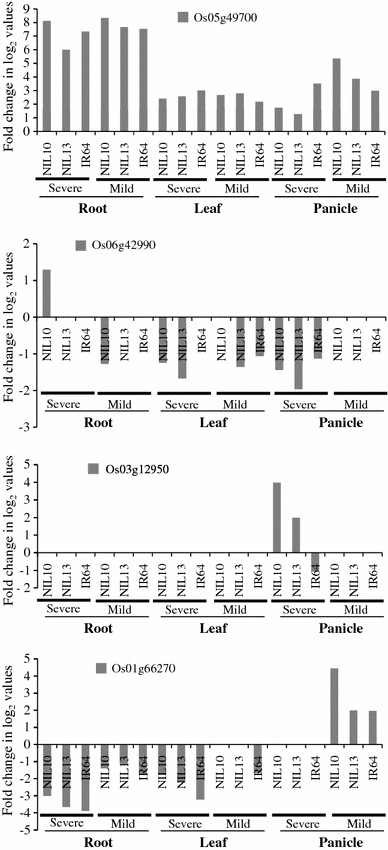
Identification of the reference genes showing the highest expression in the tolerant NIL compared with its counterparts under severe and mild stress conditions in the root, leaf, and panicle. Fold changes in log2 values are shown on the Y-axis, and the different genotypes are indicated on the X-axis. NIL10 = IR77298-14-1-2-B-10 and NIL13 = IR77298-14-1-2-B-13
Table 3.
The number of differentially expressed gene patterns in Minghui 63 under non-stress conditions and severe stress conditions in the drought-tolerant IR77298-14-1-2-B-10
| Subfamily | No. of gene | Root | Leaf | Panicle | |||
|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | ||
| Minghui 63 under normal conditions | |||||||
| AP2 | 20 (24) | 2 (10%) | 2 | 1 | 2 | 0 | 1 |
| RAV | 5 (5) | 0 | 0 | 0 | 0 | 0 | 0 |
| DREB | 35 (57) | 3 (8.5%) | 1 | 2 (5.7%) | 2 | 3 (8.5%) | 0 |
| ERF | 56 (77) | 2 (3.5%) | 4 | 2 (3.5%) | 8 | 0 | 3 |
| Total | 7 | 7 | 5 | 12 | 3 | 4 | |
| IR77298-14-1-2-B-10 under stress conditions | |||||||
| AP2 | 18 (24) | 3 (16.6%) | 5 | 1 | 3 | 6 (33%) | 4 |
| RAV | 5 (5) | 0 | 3 | 1 | 2 | 4 (80%) | 0 |
| DREB | 49 (57) | 9 (18.3%) | 15 (30.5%) | 13 (26.5%) | 7 (14.2%) | 19 (38.7%) | 13 (26.5%) |
| ERF | 68 (77) | 4 (5.8%) | 28 (41.1%) | 13 (19.1%) | 14 (20.5%) | 32 (47.1%) | 7 (10.2%) |
| Total | 16 | 31 | 28 | 26 | 61 | 24 | |
The number of genes in each subfamily is indicated in parentheses, classification by Sharoni et al. (2011). Selected tissues of Minghui 63, Root = seedling with 2 tillers, Leaf 1 = young panicle at stage 3, Panicle 1 = young panicle at stage 3, Up = up-regulated genes, Down = down-regulated genes. Bold values indicate greater difference between normal and stressed conditions
Fig. 5.
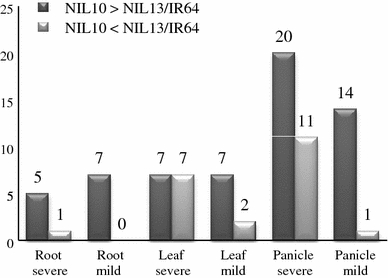
The expression intensity of up-regulated genes was higher in NIL10 than in NIL13 or IR64 in all tissues except the leaf under severe stress conditions. The X-axis represents the selected tissues, and the Y-axis represents the number of up-regulated genes. The number of genes is provided at the top of each diagram. NIL10 = IR77298-14-1-2-B-10 and NIL13 = IR77298-14-1-2-B-13
Consensus cis-regulatory elements in tolerant NIL
The interaction between TFs and cis-regulatory DNA sequences controls gene expression and constitute the essential functional linkage of gene regulatory networks. We examined the cis-elements in the promoter sequences (2-kb upstream region) of genes that were specifically up-regulated in tolerant NIL using a Rice Cis-Element Searching tool (RiCES, Doi et al. 2008). We determined that in the tolerant NIL under both DSCs, the most important cis-elements contained cis-motifs matching the published ARF, ASF-1/2, Dof1, GT-1, RAV1, WRKY, W-box, and MYB/C motifs. The different types of cis-elements that are involved in the responses to abiotic and biotic stresses are listed in Supplementary Table 8. The majority of the OsAP2/EREBP up-regulated genes with tissue-specific expression patterns contained at least two of these cis-elements (Supplementary Table 8), whereas uncommon cis-elements were found in genes in the susceptible line (data not shown). With the help of bioinformational analysis, we predict that further analysis of a number of these TFs will contribute to a better understanding of gene regulatory networks in rice under different kinds of drought stress conditions.
Introgressed regions of the OsAP2/EREBP gene in the IR64 background
Advanced backcross quantitative trait loci (QTLs) analysis facilitates simultaneous identification and introgression of identified QTLs into a recipient genotype. We observed introgressed lines in our present study that can be used as an important genetic material to decipher the physiological and molecular basis of tolerance in individual genomic regions. In different rice genotypes, this study identified four OsAP2/EREBP genes that were introgressed (McNally et al. 2009) on chromosomes 3, 5, 7, and 10 in the IR64 background (Supplementary Fig. 1). From this genome-wide comparison, four regions showed differential hybridization; these regions were further genotyped using SSR markers to confirm polymorphism between the parental lines. The four regions mapped for QTL identification were at 6.8–7.3, 6.7–7.2, 14.6–16.5 and 18.6-19.3 Mb on chromosomes 2, 4, 9 and 10, respectively (personal communication, Arvind Kumar). Of these four genes, one (Os07g23710) was not differentially expressed under both DSCs in the tissues that were examined. The Os03g08460 gene was down-regulated in the root under both DSCs conditions in all genotypes, whereas the Os09g30840 gene was down-regulated in the root and leaf under mild stress and in the panicle under severe stress in the drought-susceptible line. Only Os05g29810 was up-regulated in the leaf and panicle under severe stress in all genotypes (Supplementary Table 4). The data show that the effect of the introgressed QTL of the OsAP2/EREBP genes on grain yield under drought conditions is unclear. The Os09g25070, Os09g24490, and Os06g48950 genes are from WRKY, MYC, and ARF families, which are placed in the QTL region (personal communication, Arvind Kumar). We observed that the cis-element motif of OsAP2/EREBP genes is found in the different transcription factors mentioned above. AP2/EREBP may regulate target genes directly or indirectly thorough intermediary WRKY, MYB/C, and ARF transcription factors in tolerant NILs. Functional analysis is required to substantiate this suggestion. Bernier et al. (2007) have suggested that marker-assisted mapping and the introgression of major-effect QTLs for grain yield under drought conditions may provide a fast-track approach for developing drought-tolerant varieties, and Venuprasad et al. (2009) have reported several QTLs for yield and yield-related traits on different rice chromosomes under drought stress conditions.
Gene enrichment analysis for differentially expressed genes in NILs
The specifically expressed genes in tolerant NILs versus susceptible sister NILs during two drought stress treatments were examined for Gene Ontology (GO, Du et al. 2010) category enrichment using agriGO and KEGG orthology (KO, Kanehisa et al. 2008) analyses. The terms KEGG orthology (KO) and Gene Ontology (GO) are widely applied to understand the biological significance of microarray differential gene expression data (Du et al. 2010). Overall, a global view of the gene expression analysis demonstrated that approximately 85% of genes were differentially expressed in all rice tissues in response to drought stress treatment. The GO and KO enrichment tests and biological pathway analysis indicate that the up-regulated OsAP2/EREBP genes in the drought-tolerant NIL IR77298-14-1-2-B-10 are not involved in other pathways, such as secondary metabolism, amino acid metabolism, response to stimuli, defense response, transcription, photosynthesis, cell wall growth, and signal transduction, and the down-regulated genes were not involved in any biological pathway. An assumption of this analysis is that the OsAP2/EREBP genes that demonstrated up-regulated expression in tolerant NIL contribute indirectly to these categories of gene function; however, further research is required to support this hypothesis. Information like this would contribute to a deeper understanding of gene expression linked to regulatory networks under drought stress.
RT-PCR analysis for confirmation of the transcriptome data
To validate the accuracy of our microarray data, we selected eight up- or down-regulated genes from the different tissues of all genotypes and both DSCs. We found that in most cases, the DEGs that were identified using the microarray were also detected using RT-PCR analysis (Fig. 6). The PCR results suggest that the DEGs observed through microarray analysis represent real differences between drought-stressed and non-stressed rice genotypes. Furthermore, Moumeni et al. (2011) confirmed this microarray data using qRT-PCR analysis on 10 root tissue DEGs selected from different functional pathways.
Fig. 6.
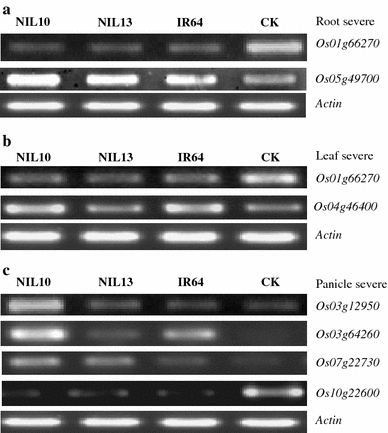
RT-PCR analysis of differential gene expression (up- and down-regulated) in all tissues under both drought treatments in all genotypes. a In the root under severe conditions. b In the leaf under severe conditions. c In the panicle under severe conditions. Actin gene expression was used as the internal control
Discussion
Expression patterns of OsAP2/EREBP genes under normal stress conditions
The objectives of this study were (1) to determine the expression patterns of members of this gene family under normal, hormone, and different drought stress conditions, (2) to assess the number of genes responding to normal conditions or different stress treatments (responses in the drought-tolerant line in particular is a novel contribution), (3) to identify the important subfamily based on gene expression profiles in specific tissues during normal or stress treatments, (4) to identify the drought response cis-element, which may assist the understanding of the function of these novel genes in network systems, and (5) to select the most appropriate candidate genes for further functional analysis. Our studies present genomic information on the OsAP2/EREBP gene family in rice. These results indicate that, to varying extents, normal treatment (no drought stress) induced different expression levels of a number of OsAP2/EREBP genes. The similarity of the expression patterns among the OsAP2/EREBP genes may reflect shared induction mechanisms. However, differences in the accumulation levels of transcripts during normal treatment indicate that OsAP2/EREBP genes may be specifically controlled by specific factors. For example, Os09g11460 was highly expressed in the stamen before flowering, and we can speculate that it plays an important role in pollen tube growth at this stage. In addition, Os03g12950, Os05g25260, and Os08g36920 are mainly transcribed in panicles, suggesting that they participate in the development of young panicles (Fig. 1b). Consequently, pollen is the major site of variations in the expression levels for many genes (Becker et al. 2003; Czechowski et al. 2005). Moreover, Os08g41030 is preliminarily expressed in the endosperm, suggesting that it might be needed for endosperm development (Fig. 1b). Os03g08460/OsEBP-89 and Os02g43970/AP59 exhibited a low expression or down-regulation in Minghui 63, and these plants exhibited a lower grain yield under normal and drought stress conditions (Yang et al. 2002; Oh et al. 2009). A significant percentage of OsAP2/EREBP genes were identified as showing higher expression levels in the reproductive stage compared with the vegetative stage. The expression pattern of a gene can reflect its functional relevance, e.g., the wide expression patterns of some OsAP2/EREBP members suggest that these genes may play regulatory roles at multiple development stages, whereas the unique expression patterns of other genes may indicate that these genes participate in specific biological processes.
Responses of OsAP2/EREBP genes in different treatments
Hormones regulate plant growth and development through a complex set of interactions. OsAP2/EREBP gene expression under hormone treatment requires extensive cross talk between the response pathways, and there are likely substantial physiological connections between AP2/EREBP protein production and hormone treatments. A higher expression pattern of a particular gene under these circumstances suggests that the genes play an important protective role against stressful hormone treatments (Fig. 2). OsAP2/EREBP genes (e.g., CRL5, crownrootless5 and OsAP239/Os04g52090) in plants are affected by auxin and cytokinin (Kitomi et al. 2011), ABA and GA3 (Yaish et al. 2010). Shen et al. (2002) reported that the levels of different types of proteins increase or decrease following GA3 treatment in rice leaf sheaths. Under adverse conditions, abscisic acid (ABA) plays an important role in adapting the plant’s response and can stimulate root growth and increase the ability of the plant to uptake water from the soil during drought stress. In this study, a number of genes (e.g., Os05g41780, Os05g51300, and Os09g11460) were induced in the root, leaf, and panicle under both hormone and drought stresses. Therefore, these genes may be involved in the uptake of water when the soil is dehydrated. The expression of CRL5 (crownrootless5), which encodes a member of the AP2/ERF transcriptional factor family, was observed in the stem region where crown root initiation occurs (Kitomi et al. 2011). ABA triggers the closing of the stomata when soil water is insufficient to keep up with transpiration. Drought-stressed leaves produce large amounts of ABA, which triggers potassium ions to be transported out of the guard cells, causes the stomata to close, and water is held in the leaf. On basis of our results, we speculate that OsAP2/EREBP genes function in ABA signaling pathways and in the defensive response against water deficit. The expression of OsAP239/Os04g52090, which was up-regulated in the leaf in both NILs under severe stress but was higher in IR77298-14-1-2-B-10, was shown to control the ABA/GA3 balance in rice to allow seed production (Yaish et al. 2010). Based on the expression data, it is difficult to configure the complex regulatory network of this gene family, but the data may provide valuable insights for understanding the responses to different hormones. A number of OsAP2/EREBP genes demonstrated a high or low expression under different hormone treatments at the seedling stage (Fig. 2).
AP2/EREBP gene expression profiles under severe stress and mild stress conditions in NILs
During drought stress, plants adapt to the new environment by changing their gene expression profiles after perceiving stress signals (Hazen et al. 2005). Analyses of AP2/EREBP gene family have revealed a wealth of information about the gene expression in this family of genes with fundamental implications for understanding the biological processes underlying growth and development of the plant. Knowing the gene expression profile of OsAP2/EREBP genes may provide clues for selecting the most appropriate candidate genes for functional analyses. Our study focused on the identification and expression profiles of drought stress-responsive genes from rice genotypes at different developmental stages under different stresses, with the aim of identifying important components of the drought-stress response. The analysis of the OsAP2/EREBP genes showed high water-usage efficiency in the drought-tolerant genotype. In the whole rice genome, approximately 7% of the genes are induced by drought or salt stresses (Zhou et al. 2007), whereas in the AP2/EREBP family, at least 85% (140 of 163 rice genes) of the genes were differentially expressed (up- or down-regulated) in one or more tissues with two folds under both DSCs. We considered the DEGs in a tolerant line compared with the sister (susceptible) line and the control and focused on those genes that were exclusively up-regulated in IR77298-14-1-2-B-10. Although the common genetic background of rice NILs, which are the backcross progeny from Aday Se. x IR64, is approximately 97% for the IR77298-14-1-2-B family (Venuprasad et al. 2011), the tolerant NIL showed distinctive differences in its gene expression responses to different drought stress levels. Comparative gene expression profiling is an alternate and efficient way to identify pathways and genes regulating the stress response (Udupa et al. 1999).
Sharoni et al. (2011) identified 13 rice AP2/EREBP genes that were drought-inducible in the seedling stage at different time points; however, our study revealed some common and unique up-regulated genes in different tissues in both NILs under drought stress (Fig. 3) and during hormone treatments (Figs. 1, 2). Complex genetic networks function during the development of every organ in plants, and substantial gene expression overlaps exist between the developmental pathways and the stress-response pathways (Cooper et al. 2003; Shinozaki et al. 2003). More DEGs and higher gene expression intensities were observed in IR77298-14-1-2-B-10 compared with IR77298-14-1-2-B-13 and IR64, and 16 genes were up-regulated in the IR77298-14-1-2-B-10 tolerant line specifically that were not up-regulated in IR77298-14-1-2-B-13 or IR64 (Table 2). For example, Os09g39850, Os02g43940, and Os06g08340 were up-regulated in IR77298-14-1-2-B-10 specifically in the root, leaf, and panicle with higher expression under both DSCs than in IR77298-14-1-2-B-13/IR64. OsDRED2A/Os01g07120, OsDREB1A/Os09g35030, and AP37/Os01g58420 expression intensities were higher in the root, leaf, and panicle in IR77298-14-1-2-B-10 compared with IR77298-14-1-2-B-13 and the control under severe or mild stress, and these genes are tolerant to drought, high salt, and cold stress in the transgenic rice plant (Dubouzet et al. 2003; Oh et al. 2009). TSRF1 improves the osmotic and drought tolerance of rice seedlings without growth retardation and increases the expression of MYB, MYC, and photosynthesis-related genes, probably by binding to dehydration-responsive element and GCC boxes in the promoter regions of the target genes (Quan et al. 2010). A number of genes that are common or overlapped among tissues, for example, Os05g49700 and Os01g07120 (DRED-A1 and DREB-A4 subgroups) were highly expressed in all three tissues under severe and mild DSCs in both NILs. A large fraction of the genes that are regulated by dehydration are also up-regulated by pollination (Lan et al. 2005). In our study, the expression of several OsAP2/EREBP genes (e.g., Os03g08500 and Os05g49010) was highly induced at the pollination stage; thus, these genes may be involved in the drought response in IR77298-14-1-2-B-10 (Table 2), although further research is required to establish this possibility. Similar conclusions have been drawn from the analysis of drought-inducible OsDREB2A/Os01g07120, AP37/Os01g58420, and OsDREB1A/Os09g35030 genes, which regulate plant tolerance in transgenic rice during conditions of drought stress (Dubouzet et al. 2003; Oh et al. 2009). Conversely, Os03g08460/OsEBP-89 and Os02g43970/AP59 genes are expressed at a low level in the root, leaf, panicle, and endosperm, and a decrease in the grain yield by 23 to 43% was observed under normal and drought stress conditions (Yang et al. 2002; Oh et al. 2009). A number of novel genes are candidate reference genes because of their specific/higher expression patterns (Fig. 4; Table 2). Differential gene expression is thought to reflect the drought response. Among the ERF subgroups, the expression of a number of genes from subgroups ERF-B3 and ERF-5 was induced in the panicle under both DSCs, whereas almost all the genes in subgroup DREB-A6 exhibited very little up-regulation under both DSCs in all tissues that were analyzed (Supplementary Table 7). The genes of the RAV subfamily are highly induced in panicle tissue under conditions of severe drought stress compared with normal growth conditions. The N-terminal regions of the RAV subfamily genes are homologous to the AP2 DNA- binding domain but their C-terminal regions are homologous to ABI3 and VP1 transcription factors and the AP-2 and B3-like domains of RAV bind autonomously to CAACA and CACCTG motifs (Kagaya et al. 1999). The protein product of the genus Phaseolus vulgaris ABI3-like factor (PvAlf) is similar to the ABI3 and VP1 proteins and functions to develop seed maturation and seed abscission (Bobb et al. 1995). CAACA and CACCTG motifs were found in the promoter region of RAV subfamily gene members and the genes were induced in the panicle under severe stress conditions in the drought-tolerant NIL, which indicates that these genes may be involved in endosperm development (Table 3).
Defense system of AP2/EREBP genes in rice
We predicted that some gene-specific cis-elements might be important in the regulation of the target gene by other factors and that these cis-elements may influence the introgressed regions of drought QTLs. Transcription factors and cis-elements function in the promoter region of different stress-related genes, and overexpression of these genes may improve the plant’s tolerance to abiotic or biotic stress. In response to drought, the AP2/EREBP TF regulates many target genes by binding to the cis-element (A/GCCGAC) in the promoter region of candidate genes, and this transcriptional regulatory system is known as a regulon. ABA is produced under drought stress conditions and plays a crucial role in plant tolerance to drought (Shinozaki et al. 2003). AP2 and other regulons, such as OsDREB2, respond to dehydration in rice (Dubouzet et al. 2003), and the dehydration-responsive element binding protein (DREB1 and DREB2)/C-repeat binding factor (CBF) regulons function in ABA-independent gene expression. Meanwhile, the ABA-responsive element (ABRE) binding protein (AREB)/ABRE binding factor (ABF) or the ABRE and coupling element (CE) regulon functions in ABA-dependent gene expression. The DREB1/2 homologous genes (OsDERB1A and OsREB2A) were isolated from grasses, such as rice and maize (Dubouzet et al. 2003; Qin et al. 2007b). ABA-activated OSRK1 protein kinases phosphorylate and activate AREB/ABF-type proteins in rice (Chae et al. 2007). ABA-independent and ABA-dependent signal transduction pathways convert the initial stress signal into cellular responses. The members of TF families that are involved in both ABA-independent (AP2/ERF, bHLH, and NAC) and ABA-dependent (MYB, bZIP, and MYC) pathways are up-regulated in rice. TFs belonging to these families interact with specific cis-elements and/or proteins, and their overexpression confers stress tolerance in heterologous systems (Kizis and Pages 2002; Zhu et al. 2003; Lee et al. 2004). It has been shown that OsAP2-39/Os04g52090 is overexpressed in transgenic rice and up-regulates the ABA biosynthetic gene OsNCED-1 (Yaish et al. 2010). Microarray analyses have shown that up-regulated genes in rice plants overexpressing AP37/Os01g58420 were drought stress tolerant and exhibited increased seed setting (16–57% more than controls) in the field (Oh et al. 2009). The homologous genes, wheat TaDREB1, wheat WDREB2, and maize ZmDREB2A are also responsive to cold stress (Shen et al. 2003; Qin et al. 2007b). These results indicate that the AP2 regulon may have additional functions in rice. Therefore, comparative analysis of gene expression profiles under both DSCs to determine the functional role of these genes in the growth of the plant and its response to stress, and the identification of target genes for TFs involved in stress responses are vital. In this study, the regulation of OsAP2/EREBP genes was precise in terms of the spatial and temporal distribution.
This study provides an overview of gene expression patterns of the OsAP2/EREBP gene family under normal and stress conditions. The application of a new comprehensive 44 K oligoarray platform together with rice NILs enabled us to determine gene expression patterns in different tissues of two NILs with contrasting yield performances under drought stress at the reproductive stage of growth. By comparing the gene expression patterns of NILs under drought stress and normal conditions, we identified several putative genes that may be responsible for the drought response in the drought-tolerant line. These genes should be considered novel reference genes, and further research is needed to prove their functions. A number of putative cis-elements were identified, which may help understand the function of these key genes. These data provide a useful reference and establish a starting point for determining the functions of the OsAP2/EREBP family of genes in rice at the reproductive stage of growth. The genes belonging to the ERF-B3 and ERF-B5 subgroups and the RAV subfamily gene members were activated in leaf and panicle tissues but not in the root. The specific subfamily (RAV) or subgroups of this gene family provide a new avenue for investigating appropriate candidate genes for functional analysis under different treatments. Overexpression and knockdown analyses of selected members of this gene family are underway in our laboratory to investigate optimal molecular breeding schemes for the OsAP2/EREBP gene family.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported partially by grants from the Program for Promotion of Basic Research Activities for Innovative Biosciences (BRAIN) to M. Nuruzzaman, A. M. Sharoni, and S. Kikuchi and also partially supported by a grant from the Generation Challenge Programme SP2 4008.08 project.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- NILs
Near-isogenic lines
- GA3
Gibberellic acid
- KT
Kinetin
- NAA
Naphthalene acetic acid
- SA
Salicylic acid
- JA
Jasmonic acid
- ABA
Abscisic acid
- RT-PCR
Reverse transcription polymerase chain reaction
- QTL
Quantitative trait loci
References
- Agarwal PK, Agarwal P, Reddy MK, Sopory SK. Roles of DREB transcription factors in biotic and abiotic stress tolerance in plants. Plant Cell Rep. 2006;25:1263–1274. doi: 10.1007/s00299-006-0204-8. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Agarwal PK, Joshi AJ, Sopory SK, Reddy MK. Overexpression of PgDREB2A transcription factor enhances abiotic stress tolerance and activates downstream stress-responsive genes. Mol Biol Rep. 2010;37:1125–1135. doi: 10.1007/s11033-009-9885-8. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsisthaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar R. NCBI GEO: mining tens of millions of expression profiles–database and tools update. Nucleic Acids Res. 2007;35:D760–D765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijo JA. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol. 2003;133:713–725. doi: 10.1104/pp.103.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J, Kumar A, Ramaiah V, Spaner D, Atlin G. A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci. 2007;47:505–516. doi: 10.2135/cropsci2006.07.0495. [DOI] [Google Scholar]
- Bobb AJ, Eiben HG, Bustos MM. PvAlf, an embryo-specific acidic transcriptional activator enhances gene expression from phaseolin and phytohemagglutinin promoters. Plant J. 1995;8:331–343. doi: 10.1046/j.1365-313X.1995.08030331.x. [DOI] [PubMed] [Google Scholar]
- Chae MJ, Lee JS, Nam MH, Cho K, Hong JY, Yi SA, Suh SC, Yoon IS. A rice dehydration-inducible SNF1 related protein kinase 2 phosphorylates an abscisic acid responsive element binding factor and associates with ABA signaling. Plant Mol Biol. 2007;63:151–169. doi: 10.1007/s11103-006-9079-x. [DOI] [PubMed] [Google Scholar]
- Cooper B, Clarke JD, Budworth P, Kreps J, Hutchison D, Park S, Guimil S, Dunn M, Luginbühl P, Ellero C, Goff SA, Glazebrook J. A network of rice genes associated with stress response and seed development. Proc Natl Acad Sci USA. 2003;100:4945–4950. doi: 10.1073/pnas.0737574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe T, Do PT, Zuther E, Repsilber D, Walther D, Hincha DK, Köhl KI. Expression profiling of rice cultivars differing in their tolerance to long-term drought stress. Plant Mol Biol. 2009;69:133–153. doi: 10.1007/s11103-008-9412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Hosaka A, Nagata T, Satoh K, Suzuki K, Mauleon R, Mendoza MJ, Bruskiewich R, Kikuchi S. Development of a novel data mining tool to find cis-elements in rice gene promoter regions. BMC Plant Biol. 2008;8:20. doi: 10.1186/1471-2229-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought, high-salt- and cold-responsive gene expression. Plant J. 2003;33:751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Thomashow F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators and repressors of GCC box-mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhang H, Tian Y, Li F, Zhang Z, Lu X, et al. Expression of TERF1 in rice regulates expression of stress-responsive genes and enhances tolerance to drought and high-salinity. Plant Cell Rep. 2008;27:1787–1795. doi: 10.1007/s00299-008-0602-1. [DOI] [PubMed] [Google Scholar]
- Guan YS, Serraj R, Liu SH, Xu JL, Ali J, Wang WS, Venus E, Zhu LH, Li ZK. Simultaneously improving yield under drought stress and non-stress conditions: a case study of rice (Oryza sativa L.) J Exp Bot. 2010;61(15):4145–4156. doi: 10.1093/jxb/erq212. [DOI] [PubMed] [Google Scholar]
- Gutterson N, Reuber TL. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol. 2004;7:465–471. doi: 10.1016/j.pbi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hao DY, Ohme-Takagi M, Sarai A. Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plant. J Biol Chem. 1998;273:26857–26861. doi: 10.1074/jbc.273.41.26857. [DOI] [PubMed] [Google Scholar]
- Hazen SP, Pathan MS, Sanchez A, Baxter I, Dunn M, Estes B, Chang HS, Zhu T, Kreps JA, Nguyen HT. Expression profiling of rice segregating for drought tolerance QTLs using a rice genome array. Funct Integr Genomics. 2005;5:104–116. doi: 10.1007/s10142-004-0126-x. [DOI] [PubMed] [Google Scholar]
- Hu YX, Wang YX, Liu XF, Li JY. Arabidopsis RAVI is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res. 2004;14:8–15. doi: 10.1038/sj.cr.7290197. [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya Y, Ohmiya K, Hattori T. RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res. 1999;27:470–478. doi: 10.1093/nar/27.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush GS, Angeles E, Virk PS, Brar DS. Breeding rice for resistance to tungro virus at IRRI. SABRAO J Breed Genet. 2004;36:101–106. [Google Scholar]
- Kitomi Y, Ito H, Hobo T, Aya K, Kitano H, Inukai Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011;67:472–484. doi: 10.1111/j.1365-313X.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- Kizis D, Pages M. Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J. 2002;30:679–689. doi: 10.1046/j.1365-313X.2002.01325.x. [DOI] [PubMed] [Google Scholar]
- Lafitte HR, Li ZK, Vijayakumar CHM, Gao YM, Shi Y, Xu JL, Fu BY, Yu SB, Ali AJ, Domingo J, Maghirang R, Torres R, Mackill D. Improvement of rice drought tolerance through backcross breeding: evaluation of donors and selection in drought nurseries. Field Crops Res. 2006;97:77–86. doi: 10.1016/j.fcr.2005.08.017. [DOI] [Google Scholar]
- Lan L, Li M, Lai Y, Xu W, Kong Z, Ying K, Han B, Xue Y. Microarray analysis reveals similarities and variations in genetic programs controlling pollination/fertilization and stress responses in rice (Oryza sativa L.) Plant Mol Biol. 2005;59:151–164. doi: 10.1007/s11103-005-3958-4. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hong JP, Oh SK, Lee S, Choi D, Kim WT. The ethylene responsive factor like protein 1 (CaERFLP1) of hot pepper (Capsicum annuum L.) interacts in vitro with both GCC and DRE/CRT sequences with different binding affinities: possible biological roles of CaERFLP1 in response to pathogen infection and high salinity conditions in transgenic tobacco plants. Plant Mol Biol. 2004;55:61–81. doi: 10.1007/s11103-004-0417-6. [DOI] [PubMed] [Google Scholar]
- McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, et al. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci USA. 2009;106:12273–12278. doi: 10.1073/pnas.0900992106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumeni A, Satoh K, Kondoh H, Asano T, Hosaka A, Venuprasa R, Serraj R, Kumar A, Leung H, Kikuchi S (2011) Comparative analysis of root transcriptome profiles of two pairs of drought-tolerant and susceptible rice near-isogenic lines under different drought stress. BMC Plant Biol (accepted) [DOI] [PMC free article] [PubMed]
- Nuruzzaman M, Gupta M, Zhang C, Wang L, Xie W, Xiong L, Zhang Q, Lian X. Sequence and expression analysis of the thioredoxin protein gene family in rice. Mol Genet Genomics. 2008;280:139–151. doi: 10.1007/s00438-008-0351-4. [DOI] [PubMed] [Google Scholar]
- Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010;465:30–44. doi: 10.1016/j.gene.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Kim SY, Kwon CW, Park HK, Jeong JS, Kim JK. Overexpression of the transcription factor ap37 in rice improves grain yield under drought conditions. Plant Physiol. 2009;150:1368–1379. doi: 10.1104/pp.109.137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR. The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res. 2007;35:D883–D887. doi: 10.1093/nar/gkl976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent B, Suard B, Serraj R, Tardieu F. Rice leaf growth and water potential are resilient to evaporative demand and soil water deficit once the effects of root system are neutralized. Plant Cell Environ. 2010;33:1256–1267. doi: 10.1111/j.1365-3040.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell. 2001;13:1035–1046. doi: 10.1105/tpc.13.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin QL, Liu JG, Zhang Z, Peng RH, Xiong AS. Isolation, optimization, and functional analysis of the cDNA encoding transcription factor RdreB1 in Oryza Sativa L. Mol Breed. 2007;19:329–340. doi: 10.1007/s11032-006-9065-7. [DOI] [Google Scholar]
- Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Tran LS, Shinozaki K, Yamaguchi-Shinozaki K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007;50:54–69. doi: 10.1111/j.1365-313X.2007.03034.x. [DOI] [PubMed] [Google Scholar]
- Quan R, Hu S, Zhang Z, Zhang H, Zhang Z, Huang R. Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J. 2010;8:1–13. doi: 10.1111/j.1467-7652.2009.00492.x. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM. The AP2/EREBP family of plant transcription factors. Biol Chem. 1998;379:633–646. doi: 10.1515/bchm.1998.379.6.633. [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the AP2/ERF domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Kumar A, McNally KL, Slamet-Loedin I, Bruskiewich R, Mauleon R, Cairns J, Hijimans RJ. Improvement of drought resistance in rice. Adv Agron. 2009;103:41–99. doi: 10.1016/S0065-2113(09)03002-8. [DOI] [Google Scholar]
- Shamir R, Maron-Katz A, Tanay A, Linhart C, Steinfeld I, Sharan R, Shiloh Y, Elkon R. EXPANDER–an integrative program suite for microarray data analysis. BMC Bioinformatics. 2005;6:232. doi: 10.1186/1471-2105-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, Choi IR, Omura T, Kikuchi S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011;52:344–360. doi: 10.1093/pcp/pcq196. [DOI] [PubMed] [Google Scholar]
- Shen S, Sharma A, Komatsu S. Characterization of proteins responsive of gibberellin in the leaf-sheath of rice (Oryza sativa L.) seedling using proteome analysis. Biol Pharm Bull. 2002;26:129–136. doi: 10.1248/bpb.26.129. [DOI] [PubMed] [Google Scholar]
- Shen YG, Zhang WK, He SJ, Zhang JS, Liu Q, Chen SY. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor Appl Genet. 2003;106:923–930. doi: 10.1007/s00122-002-1131-x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6:410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Ludlow M. Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust J Plant Physiol. 1986;13:329–341. doi: 10.1071/PP9860329. [DOI] [Google Scholar]
- Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK. Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol. 2006;61:897–915. doi: 10.1007/s11103-006-0057-0. [DOI] [PubMed] [Google Scholar]
- Spollen WG, Sharp RE, Saab IN, Wu Y (1993) Regulation of cell expansion in roots and shoots at low water potentials. In: Smith JAC, Griffiths H (eds) Water deficits: plant responses from cell to community. BIOS Scientific Publishers, Oxford, pp 37–52
- Udupa SM, Robertson LD, Weigand F, Baum M, Kahl G. Allelic variation at (TAA)n microsatellite loci in a world collection of chickpea (Cicer arietinum L.) germplasm. Mol Gen Genet. 1999;261:354–363. doi: 10.1007/s004380050976. [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Zenna N, Choi IR, Amante M, Virk PS, Kumar A. Identification of marker loci associated with tungro and drought tolerance in near-isogenic rice lines derived from IR64/Aday Sel. IRRN. 2007;32:27–29. [Google Scholar]
- Venuprasad R, Dalid CO, Del-Valle M, Ma-Emeru B, Zhao L, Espiritu M, Sta Cruz MT, Amante M, Atlin G, Kumar A. Identification and characterization of large-effect quantitative trait loci (QTL) for grain yield under lowland drought stress and aerobic conditions in rice using bulk-segregant analysis (BSA) Theor Appl Genet. 2009;120:177–190. doi: 10.1007/s00122-009-1168-1. [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Impa S, Veeresh-Gowda RP, Atlin GN, Serraj R. Rice near-isogenic-lines (NILs) contrasting for grain yield under lowland drought stress. Field Crops Res. 2011;123:38–46. doi: 10.1016/j.fcr.2011.04.009. [DOI] [Google Scholar]
- Yaish MW, El-Kereamy A, Zhu T, Beatty PH, Good AG, Bi YM, Rothstein SJ. The APETALA-2-like transcription factor OsAP2–39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010;6:9. doi: 10.1371/journal.pgen.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Shen H, Chen L, Xing YY, Wang ZY, Zhang JL, Hong MM. The OsEBP-89 gene of rice encodes a putative EREBP transcription factor and is temporally expressed in developing endosperm and intercalary meristem. Plant Mol Biol. 2002;50:379–391. doi: 10.1023/A:1019859612791. [DOI] [PubMed] [Google Scholar]
- Yazaki J, Kishimoto N, Nagata Y, Ishikawa M, Fujii F, et al. Genomics approach to abscisic acid- and gibberellin-responsive genes in rice. DNA Res. 2003;10:249–261. doi: 10.1093/dnares/10.6.249. [DOI] [PubMed] [Google Scholar]
- Ye S, Wang L, Xie W, Wan B, Li X, Lin Y. Expression profile of calcium-dependent protein kinase (CDPKs) genes during the whole lifespan and under phytohormone treatment conditions in rice (Oryza sativa L.) Plant Mol Biol. 2009;70:311–325. doi: 10.1007/s11103-009-9475-0. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang X, Jiao Y, Qin Y, Liu X, He K, Chen C, Ma L, Wang J, Xiong L, Zhang Q, Fan L, Deng XW. Global genome expression analysis of rice in response to drought and high-salinity stresses in shoot, flag leaf, and panicle. Plant Mol Biol. 2007;63:591–608. doi: 10.1007/s11103-006-9111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Cai XL, Wang ZY, Hong MM. An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the rice Wx gene. J Biol Chem. 2003;278:47803–47811. doi: 10.1074/jbc.M302806200. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, Gao X, Tong J, Xiao L, Li W, et al. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene. 2010;457:1–12. doi: 10.1016/j.gene.2010.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


