Abstract
Aims
The Genous™ Bio-engineered R™ stent (GS) aims to promote vascular healing by capture of circulatory endothelial progenitor cells (EPCs) to the surface of the stent struts, resulting in accelerated re-endothelialization. Here, we assessed the function of the GS in comparison to bare-metal stent (BMS), when exposed to the human and animal circulation.
Methods and results
First, 15 patients undergoing coronary angiography received an extracorporeal femoral arteriovenous (AV) shunt containing BMS and GS. Macroscopical mural thrombi were observed in BMS, whereas GS remained visibly clean. Confocal and scanning electron microscopic (SEM) analysis of GS showed an increase in strut coverage. Quantitative polymerase chain reaction (qPCR) analysis of captured cells on the GS demonstrated increased expression of endothelial markers KDR/VEGFR2 and E-selectin, and a decrease in pro-thrombogenic markers tissue factor pathway inhibitor and plasminogen activator inhibitor-1 compared with BMS. Secondly, a similar primate AV shunt model was used to validate these findings and occlusion of BMS was observed, while GS remained patent, as demonstrated by live imaging of indium-labelled platelets. Thirdly, in an in vitro cell-capture assay, GS struts showed increased coverage by EPCs, whereas monocyte coverage remained similar to BMS. Finally, the assessment of re-endothelialization was studied in a rabbit denudation model. Twenty animals received BMS and GS in the aorta and iliac arteries for 7 days. Scanning electron microscopic analysis showed a trend towards increased strut coverage, confirmed by qPCR analysis revealing increased levels of endothelial markers (Tie2, CD34, PCD31, and P-selectin) in GS.
Conclusion
In this proof-of-concept study, we have demonstrated that the bio-engineered EPC-capture stent, Genous™ R™ stent, is effective in EPC capture, resulting in accelerated re-endothelialization and reduced thrombogenicity.
Keywords: Bio-engineered stent, CD34+-capture stent, Genous stent, Re-endothelialization, Endothelium, Endothelial progenitor cells, In-stent thrombosis, Gene expression, Endothelial markers
Introduction
Vascular homeostasis is maintained by the endothelial cell (EC) layer that is involved in the regulation of platelet adhesion, vasomotor function, and cell cycle quiescence of the cellular constituents of the vascular wall.1 Bone marrow-derived circulating endothelial progenitor cells (EPCs) aid in the regeneration of damaged and dysfunctional endothelium and therefore play a central role in the vascular repair response.2–4 Recruitment of EPCs to the site of vascular injury has been proposed to promote vascular healing and has been shown to inhibit neointimal proliferation and restenosis associated with percutaneous coronary intervention (PCI).5 The Genous™ Bio-engineered R™ stent (GS) (OrbusNeich Medical BV, Hoevelaken, The Netherlands) has been developed to enhance the capture of circulating EPCs to the stent surface using an immobilized antihuman-CD34 monoclonal antibody. CD34 was previously shown to be expressed in circulating haematopoietic cells in humans.4,6 The sequestered EPCs are thought to enhance endothelial healing and thus protect the stented vascular segment against acute thrombosis with minimized neointimal hyperplasia.
The safety, feasibility, and efficacy of the GS in human coronary artery disease (CAD) have been the subject of multiple clinical studies.7–11 Although the long-term effect of the GS on clinical outcome has been investigated, the efficacy of the bio-engineered stent to promote initial endothelial recovery has never been shown in humans before. Here, we studied early cellular interactions of the GS within the circulation of CAD patients.
In the first part of the study, a temporary ex vivo arteriovenous (AV) shunt was established by cannulation of the femoral artery and vein and connection of the two via a synthetic tube comprising the bare-metal stent (BMS) and the GS. The stents were exposed to the human circulation under continuous flow. Endothelial progenitor cell capture and subsequent EPC differentiation were analysed using conventional ultrastructural analysis as well as by quantifying surrogate endothelial markers on the captured stent by quantitative polymerase chain reaction (qPCR) analysis. In the second part of the study, the validation of accelerated endothelialization was further conducted in a well-established primate model for stent-related thrombogenicity. In the third part, CD34+ cell-capture specificity was evaluated in an in vitro capture model. In the final part of the study, long-term effects of the GS on the vascular endothelium were evaluated in a rabbit model of arterial balloon injury and vascular repair.
Methods
Study population
The study was performed in 15 patients undergoing elective heart catheterization, followed in 11 cases by PCI. Informed written consent was obtained prior to the procedure for all patients. The study was reviewed and approved by the institution's ethics review committee. The baseline characteristics of included patients are shown in Table 1.
Table 1.
Characteristics of the patients
| Patients characteristics | n | Per cent |
|---|---|---|
| Male | 10 | 66.67 |
| Age | 69.4 ± 7 | |
| PCI | 11 | 73.33 |
| Нypertension | 8 | 53.33 |
| Diabetes mellitus | 0 | 0 |
| Dyslipidaemia | 5 | 33.33 |
| Smoking | 6 | 40 |
| Clinical pattern | ||
| Stable angina pectoris | 10 | 66.67 |
| Unstable angina pectoris | 4 | 26.67 |
| Syncope | 1 | 6.667 |
| Peripheral vascular disease | 3 | 20 |
| Stroke | 2 | 13.33 |
| Heart failure | 3 | 20 |
| Previous myocardial infarction | 4 | 26.67 |
| Previous PCI | 7 | 46.67 |
| Previous CABG | 2 | 13.33 |
| Use of: | ||
| Statin | 14 | 93.33 |
| Aspirin | 13 | 86.67 |
| Clopidogrel | 13 | 86.67 |
| Warfarin | 2 | 13.33 |
| β-Blockers | 3 | 20 |
PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Ex vivo human arteriovenous shunt
The GS (OrbusNeich) is coated with an immobilized murine monoclonal antibodies directed against human CD34, a known antigen expressed on EPCs. It is designed to capture circulating EPCs to promote vascular healing. Patients received an extracorporeal AV shunt containing two GSs and two BMSs (non-coated, stainless steel R-stent). From each patient, one randomized stent of each group (BMS or GS) was used for qPCR analysis and one was used for scanning electron microscopic (SEM) assessment. The positions of the stents in the shunt were equally alternated in the studied patient group to prevent location bias (see Supplementary material online, Figure S2). For a detailed description of the protocol, see Supplementary material online.
Rabbit model of arterial balloon injury
The early effects of Genous in accelerated re-endothelialization were further assessed in a rabbit endothelial denudation model.12 Stents were implanted in 20 New Zealand white adult male rabbits. One BMS and one GS were implanted per rabbit in the aorta (for qPCR analysis, n = 11) and iliac artery (for SEM analysis, n = 9). For the aorta, the stents were alternated in order. All stents were deployed at nominal pressure (9 atm) for 30 s. Angiography was performed to confirm appropriate stent placement and vessel diameter post-deployment. At 7 days post-stenting, follow-up angiography was performed. To obtain stent samples for SEM analysis, the rabbits were perfusion fixed in 10% formalin and the stented arteries were harvested. For qPCR analysis, the vessels were isolated without in situ fixation, and the stents were removed and incubated in RNA isolation buffer (RLT buffer, Qiagen, The Netherlands) and stored at −80°C until qPCR analysis.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software (version 4.0b). All data are expressed as means ± SEM. Comparisons between the patients groups are performed using a paired or a non-paired two-sided Student's t-test or a linear regression analysis when appropriate after normal distribution was validated for the data set. A P-value of <0.05 is considered statistically significant.
For the SEM protocol, mRNA processing, baboon AV shunt study, and in vitro CD34+-capture stent validation, see Supplementary material online, Material and methods.
Results
Part 1: Human arteriovenous shunt study reveals evidence of accelerated capture of endothelial progenitor cells and protection against in-stent thrombosis and inflammation in Genous vs. bare-metal stent
The GS has been tested extensively in animal models,13 but direct evidence of the in vivo EPC-capture capability of this stent in the human circulation has not been presented. In the first part of the study, we investigated the acute effect of the GS in patients undergoing coronary angiography. In addition, assessment of the GS in a CAD patient setting provides relevant evaluation of bioactivity of the CD34-capture antibody that was raised specifically against human CD34 antigen (Supplementary material online, Figure S1). In this study, the EPC-capture capacity of the GS was compared with that of uncoated, stainless steel R-stents (BMSs).
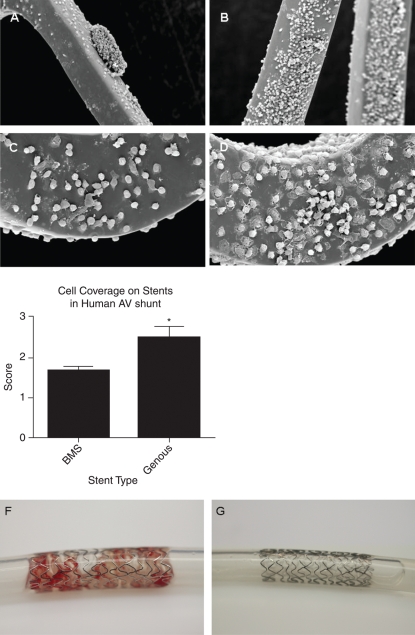
The GS and the BMS that were tested in the human AV shunt model were exposed to the circulation for up to 120 min. Stents selected for SEM analysis revealed marked increase in strut cell coverage in the GS when compared with BMS (Figure 1A and C: BMS, Figure 1B and D: GS). Average stent-strut coverage was visually rated by a blinded (core-lab) technician on a 0–3 scale (corresponding to 0–25, 25–50, 50–75, and 75–100% stent coverage). Cell deposition on the struts was enhanced by 32.5% (P = 0.006, n = 9) in GSs when compared with BMS in paired analysis (Figure 1E). High-resolution assessment of the SEM data revealed cellular deposits present on both stents that could be distinguished into a population of cells with a rounded and flattened morphology, cells with a more monocyte-like appearance, and blood platelets. To further elucidate the identity of this mixed population, we performed by qPCR the cellular substrate. Macroscopical comparison of GS and BMS also revealed the substantial presence of mural thrombi in the two BMSs, whereas all of the GSs remained free of thrombogenic material (Figure 1F and G). This observation led us to further examine markers of thrombogenicity and coagulation by qPCR.
Figure 1.
Scanning electron microscopic analysis of the endothelial progenitor cell capturing stent and bare-metal stent in the human arteriovenous shunt model revealed a marked increase in cell strut coverage compared with bare-metal stent. Scanning electron microscopic inspection of the study stents showed less strut coverage and the presence of thrombus-like structures on bare-metal stent (A) when compared with Genous stent (B). High-magnification scanning electron microscope revealed more adhesion of cells with a flattened polygonal morphology on the struts of the Genous stent (D) vs. bare-metal stent (C). Average stent-strut coverage was visually rated by blinded (core lab) technicians (CV-Path Institute, USA) on a 0–3 virtual scale (corresponding to 0–25, 25–50, 50–75, and 75–100% stent coverage). Bar graph indicates the level of strut coverage as assessed by scanning electron microscope in the two stent study groups. *P< 0.05, n = 9 (E). Macroscopic appearance of bare-metal stent (F) and Genous (G) stents in the human ex vivo shunt model shows mural thrombi in the bare-metal stent, whereas the Genous stent remained free of thrombogenic material.
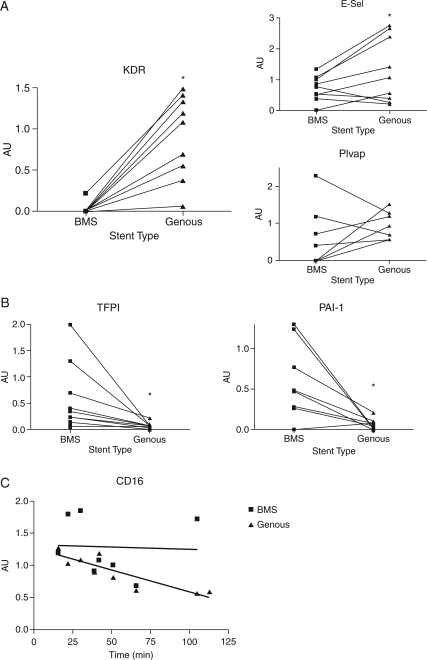
For qPCR analysis, cells were directly lysed from the stents and subsequently analysed by qPCR using the housekeeping genes GAPDH and β-actin to normalize for cell content and quality of total RNA. Quantitative polymerase chain reaction analysis of the attached cells showed no significant difference in CD34 expression between the GS and BMS groups (data not shown). However, evaluation of endothelial markers revealed a marked increase in KDR/VEGFR2 (P< 0.001) and E-selectin RNA expression (P< 0.045) with the GSs, when compared with BMS (Figure 2A). However, the expression of another endothelial specific marker Plvap (Figure 2A) showed no significant increase in the GS group (P = 0.21).
Figure 2.
Quantitative polymerase chain reaction evaluation of cellular markers in cell lysates of captured cells in the human arteriovenous shunt stent model. Paired comparison of the expression levels of the individual genes revealed a marked increase in endothelial markers, including KDR/VEGFR2 (P< 0.001) and E-selectin (P< 0.045) mRNA expression in the stents compared with bare-metal stent (A). Expression of another endothelial specific marker PLVAP (A) showed no significant increased expression in the stent (P = 0.21). Quantitative polymerase chain reaction analysis of markers of thrombosis, coagulation, and inflammation. Paired comparison of the expression levels of the individual genes revealed a marked decrease in tissue factor pathway inhibitor and plasminogen activator inhibitor-1 in the Genous compared with the bare-metal stent (B) (P = 0.04 and 0.02). Quantitative polymerase chain reaction showed a significant decrease in CD16 marker expression in the cells captured by the Genous stent over time, whereas the CD16 mRNA levels on bare-metal stent were maintained (C) (*P< 0.05).
An equivalent CD34 transcript content, with enhanced expression of endothelial markers would suggest accelerated ongoing differentiation into the endothelial lineage concomitant with down-regulation of CD34 expression.
Quantitative polymerase chain reaction evaluation of markers of thrombosis and coagulation (Figure 2B) revealed a significant decrease in expression of tissue factor pathway inhibitor (TFPI) and plasminogen activator inhibitor-1 (PAI-1) in GS compared with BMS (P = 0.04 and 0.02, for TFPI and TF, respectively), suggestive of a less pro-thrombotic state of the cells attached to the GS and thus a reduced risk for stent thrombogenicity.
To assess the potency of the GS to protect the vascular wall against inflammation, inflammatory markers were also included in the study. CD16 is an established neutrophil expression marker. Cells sequestered to the GS presented lower expression levels of CD16 over time (R2 = 0.7641, P< 0.002), whereas the BMS showed persistent CD16+ expression in attached cells (R2 = 0.0013, P< 0.932), suggesting that adhesion of CD16+ inflammatory cells was prevented by accelerated capture of CD34+ endothelial progenitors and subsequent coverage of the stent struts (Figure 2C). Moreover, the inflammatory markers for immune cell subpopulations were not significantly different between the stents, including CD68, CD14, monocyte chemoattractant protein-1, CXCR-1, and VCAM1 (data not shown).
Combined, these qPCR analyses corroborate and extend the ultrastructural analysis by SEM and confirm enhanced attachment of circulating EPCs to the GS surface. In addition, these data suggest that the attached cells could undergo rapid endothelial commitment and differentiation (loss of CD34, increased expression of KDR1/E-selectin), with reduced thrombogenicity and inflammatory response of the injured vascular wall.
Part 2: Genous inhibits in-stent thrombosis in a baboon arteriovenous shunt model
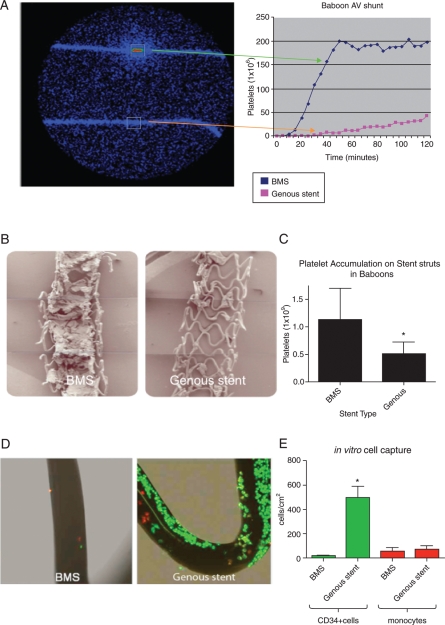
Based on the differences in thrombogenicity by qPCR and the lack of mural thrombi in the GS as observed in the clinical study, we further assessed thrombogenicity of the GS in an established primate model using a similar AV shunt setup with exclusion of anti-platelet treatment in the protocol. Live deposition of platelets and fibrinogen was studied in the AV shunt setup by measuring the accumulation of indium-labelled platelets with a gamma camera for up to 2 h. In line with the human data, the GS had a lower thrombogenic potential than BMS in the baboon shunt model. Within 65 min after initiation of the experiment, the BMS were occluded with a flow-limiting thrombus, whereas the GSs remained patent for at least 2 h (Figure 3A). Further SEM analysis revealed increased platelet deposition and in-stent thrombus formation in BMS vs. GS (Figure 3B). Platelet deposition was significantly higher in BMS compared with GS after flow exposure as quantified by gamma camera (1.13 ± 0.57 × 109 when compared with 0.50 ± 0.22 × 109 platelets, in BMS vs. Genous, respectively, P = 0.04; Figure 3C). Although fibrinogen accumulation seemed less prominent on the GS when compared with BMS, no statistical difference was found between the groups. The values were 0.05 ± 0.02 and 0.18 ± 0.10 mg/stent for the GS and BMS, respectively.
Figure 3.
Live imaging of arteriovenous shunt setup using a gamma camera to measure deposition of indium-labelled platelets on the study stents (A). Line graph shows a typical example of accumulating platelet signal over time. Low-magnification scanning electron microscope images of the bare-metal stent and the endothelial progenitor cell-capture stent in the baboon arteriovenous shunt model revealed a decrease in mural thrombus in the Genous vs. bare-metal stent (B). Bar graph showing the quantified number of platelets accumulated on the bare-metal stent and Genous stent after 2 h of flow exposure (C). Data were acquired from the live imaging of arteriovenous shunt setup, *P< 0.05, n = 3. In vitro assay to test the CD34+ cell-capture specificity of the Genous stent. Genous and BM stents were deployed in silicon tubing and were exposed to a cell mixture of PKH26 red fluorescent-labelled human monocytes (1 × 106 cells/mL) and PKH2 green fluorescent-labelled human CD34+ cells (2 × 105 cells/mL), under a constant rotation speed of 0.3 RPM for 2 h. Micrographs show confocal images of strut coverage of bare-metal stent and Genous stent (D). Bar graph shows the quantified number of CD34+ cells and monocytes per cm2 strut area. *P< 0.05, n = 3 (E).
Part 3: In vitro cell-capture assay demonstrates specific adhesion of human peripheral blood-derived CD34+ cells on Genous CD34-capture stents
To examine whether the CD34 antibody on the GS is also able to bind the more abundant circulatory inflammatory cells such as monocytes, an in vitro assay was performed to test the CD34+ cell-capture specificity of the GS. Genous stent and BMS were deployed in silicon tubing and were exposed to a cell mixture of PKH26 red fluorescent-labelled human monocytes (1 × 106 cells/mL) and PKH2 green fluorescent-labelled human CD34+ cells (2 × 105 cells/mL), under a constant rotation speed of 0.3 RPM for 2 h. Confocal assessment of the stent struts showed a greater number of CD34+ cells to adhere to the GS-strut surface when compared with BMS; cell density for CD34+ cells was 500 ± 158 cells/cm2 strut area on the GS vs. 17 ± 8 cells/cm2 on the BMS (P = 0.0009; Figure 3D and E). In contrast, monocyte adherence was not significantly different between the two stent types, although a trend was observed (79 ± 44 cells/cm2 when compared with 58 ± 39 cells/cm2, GS vs. BMS, respectively, P = 0.07; Figure 3D and E).
Therefore, the specificity of the GS to capture CD34+ cells was significantly higher when compared with BMS, as 86% of the attached cells were CD34+ when compared with only 26% on the BMS.
Part 4: Genous stent promotes re-endothelialization at 7 days in rabbit endothelial denudation model
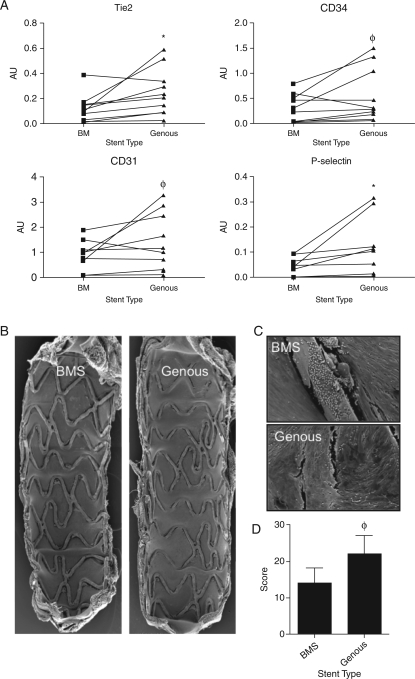
Twenty New Zealand white rabbits received stent placement after endothelial denudation in the aorta, n = 11, and iliac artery, n = 9 (left GS and right BMS). From 11 animals, the aortic stents were harvested after 7 days and the cell lysates were evaluated for EC markers. Quantitative polymerase chain reaction analysis showed significant increased levels of endothelial markers in the GS vs. BMS treated artery including Tie2 (P = 0.02) and P-selectin (P = 0.05), whereas CD34 (P = 0.08) and CD31 (P = 0.07) levels showed positive trends, indicating that the GS promoted long-term endothelialization (Figure 4A).
Figure 4.
Quantitative polymerase chain reaction analysis of the study stents of 11 New Zealand white rabbits was performed to evaluate capture of cells and subsequent expression of endothelial cell markers. Paired quantitative polymerase chain reaction analysis showed increased levels of endothelial markers by the cells captured on the Genous stent vs. bare-metal stent treated arteries, including Tie2 (P= 0.02), CD34 (P = 0.07), CD31 (P = 0.08), and P-selectin (P = 0.05). *P< 0.05, φP< 0.01, n = 11 (A). Scanning electron microscopic analysis of the stents implanted iliac vessels of 9 New Zealand white rabbits: Low (B)- and high (C)-magnification assessment revealed improved cell coverage between and above struts in the Genous stent vs. bare-metal stent. Bar graph shows the level of strut coverage as analysed by scanning electron microscope in the two stent groups. φP < 0.01, n = 9 (D).
These data support our finding of the AV shunt study in CAD patients where we propose earlier endothelialization in GS compare with BMS. In line with these findings, SEM analysis of nine rabbits that received the Genous (left) or BM (right) in bilateral denudated iliac arteries showed a trend of increased strut coverage at 7 days post-implantation (Figure 4B–D). Together, these data indicate that the GS efficiently promotes re-endothelialization in a denudated vessel wall environment as shortly as 7 days after placement.
Discussion
In this study, we have demonstrated for the first time that bio-engineered endothelial progenitor (EPC)-capture stent technology is successful in EPC capture in the human circulation, resulting in effective re-endothelialization and decreased thrombogenicity.
Endothelialization is a critical step in the initiation of vascular repair following stent implantation. Re-endothelialization of the damaged area involves activation and migration of resident EC adjacent to the stent area or by recruitment of blood-derived EPC. Early presence of a functional endothelial lining after vascular injury could improves the process of vascular healing and reduces the risk of restenosis an acute thrombosis.
The stent struts of the bio-engineered R stent (GENOUS®) incorporate an immuno-affinity surface, consisting of covalently bound monoclonal antibodies directed against the human CD34 antigen, a cell surface marker found on circulating EPC. Endothelial progenitor cell capture by the GENOUS® R stent is shown schematically in Figure 1 of Supplementary material online, Figure S1. The efficacy of EPC capture and re-endothelialization of the GS has been extensively evaluated in porcine models13 relying on the cross-reactivity of the monoclonal CD34 antibody against porcine CD34. Although these studies gave a clear indication of stent performance, optimal capture efficacy by the human CD34-directed antibody can only be truly tested under circumstances when the stent is exposed to the human circulation. Here, the performance of the BMS and the bio-engineered GS was studied in an ex vivo AV shunt construction in which stents were exposed to the human circulation for up to 2 h. The data of this study provide for the first time direct evidence of the capture efficiency of a bio-engineered-capture stent in the human circulation.
Previous studies have shown that at least in the porcine models, the efficacy of EPC-capture stent coverage was similar to that of BMS, with a optimal endothelial coverage of total stented area of 99% in both groups.13 However, it has to be taken into account that the lack of difference in response could be due to a low cross-reactivity of the human CD34 antibody on the GS against porcine CD34 antigen, resulting in a suboptimal EPC capture. The ex vivo AV shunt data in the patients and primates in the current study showed that the GS was capable of rapid capture of circulating progenitor cells within the first hours of exposure. Scanning electron microscopic and qPCR analysis have subsequently validated the endothelial phenotype of the adherent population. Taken together, our data indicate that the EPC-capture stent is capable of accelerating the re-endothelialization process in exposure to the human circulation and therefore aid the vascular healing after vascular injury when compared with the BMS.
The combination of the EPC-capture and drug-elution technology has shown thus far to be a promising strategy in the preclinical setting.13 In contrast to that type of study, which focuses on late stent outcome, this study was predominantly designed to provide first-time proof of efficient EPC capture in human patients. Therefore, we have chosen to focus on capture efficiency alone and compare the GS with the BMS. Although we have provided adequate proof for this cell-capture technology, drug elution may compromise the cell-capture efficiency and should be investigated for each of the new generation of combo-devices that are currently being developed.
A second important finding reported in this study is the effect of the GS on thrombogenesis. The rapid coverage of the GS by a protective endothelial lining was hypothesized to protect the stented area from thombogenesis and inflammation, thereby promoting a more efficient healing of the vascular wall. Indeed, macroscopic comparison of the GS and BMS revealed the clear presence of mural thrombi in the BMS of one of the patients, whereas all the EPC-capture stent remained free of visible thrombi. It should be mentioned that this particular patient only received ASA (no clopidogrel therapy) before PCI. Although the patient's blood was exposed to both GS and BMS and the GSs showed no signs of thrombi, the striking difference in thombogenic response between the two stent types could be related to the absence of anti-platelet aggregation surface markers. To further elucidate this, biomarkers of coagulation and thrombosis were further examined in the human shunt material. We observed significant down-regulation in PAI and TFPI expression in the lysates of EPC-capture stent when compared with BMS, pointing to platelet aggregation on the stent struts.14 Similarly, TFPI mRNA enrichment in the attached cells indicates in the BMS a rich platelet environment promoted by recruited inflammatory cells.15,16 Summarized, these data point to active EPC recruitment as playing a putative role in vascular protection against stent thrombosis. These findings were further validated in the baboon shunt experiments. For an extended discussion on this subject, see Supplementary material online.
The early EC lining on the stent could protect against active accumulation of inflammatory cells involved in the innate immune response. However, in line with the in vitro findings, there was no difference in other inflammatory markers or innate immunity markers such as CD68, CD14, MCP1, and CXCR-1. This could be due to the short exposure time, but, more importantly, paracrine stimulation by the injured vessel wall is lacking in the human AV shunt setup. In the absence of cytokine and chemokine release to trigger inflammatory cell activation, the protective effect of the stent re-endothelializing by circulating EPCs on the inflammatory response may only be limited. Previously, Granada et al. performed a comparison study of stents (combining CD34 capture with the sirolimus-eluting strategy) with conventional drug-eluting stents including Xience and Cypher in a porcine experimental model. It was demonstrated that the EPC-capture technology further diminished overall intimal inflammation and giant cell accumulation after 28 days of implantation in the coronary arteries when compared with the Cypher and Xience stents.13 This was associated with a decrease in neointimal growth. This suggests that active re-endothelialization of drug-eluting stents could indeed protect the injured vascular wall from further inflammatory activation, thereby protecting the stented area from further platelet adhesion and restenosis.13 Based on these findings, the GS should provide vascular protection against thrombosis in the patients in short- and long-term follow-up. Recently, supporting data were presented by the e-HEALING (Healthy, Endothelial Accelerated Lining inhibits Neointimal Growth) multicentre registry in which the long-term effect of the GS was followed in 5000 patients. Indeed, low levels of in-stent thrombosis and repeat revascularization of 1.1 and 5.7%, respectively, were observed at 12-month post-intervention.17 New clinical trails are currently under evaluation in which the CD34-capture technology will be combined with sirolimus elution to assess novel combination strategies (REMEDEE: NCT00967902).
In conclusion, we showed in an AV shunt construction in human CAD patients and baboons that the CD34+ EPC recruitment promotes re-endothelialization and inhibited platelet adhesion. This specific aspect of the biological behaviour of the GS is especially promising as it could, combined with a drug-eluting strategy,13 yield safe and efficient therapy against restenosis, while diminishing the need for dual anti-platelet therapy after stent implantation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by OrbusNeich Medical, Fort Lauderdale, FL, USA. Funding to pay the Open Access publication charges for this article was provided by Erasmus MC.
Conflict of interest: S.P., E.L., and S.R. are employed by OrbusNeich Medical.
Supplementary Material
Acknowledgements
Dedicated to K.L., a hardworking and talented colleague, a loving and caring friend, and a kind and wonderful person who will be greatly missed by us all.
References
- 1.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Gulati R, Simari RD. Cell therapy for acute myocardial infarction. Med Clin North Am. 2007;91:769–785. doi: 10.1016/j.mcna.2007.03.003. xiii. [DOI] [PubMed] [Google Scholar]
- 4.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 5.Kipshidze N, Dangas G, Tsapenko M. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Co M, Tay E, Lee CH. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J. 2008;155:128–132. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Klomp M, Beijk MA, de Winter RJ. Genous endothelial progenitor cell-capturing stent system: a novel stent technology. Expert Rev Med Devices. 2009;6:365–375. doi: 10.1586/erd.09.16. [DOI] [PubMed] [Google Scholar]
- 9.Beijk MA, Klomp M, Verouden NJ. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur Heart J. 31:1055–1064. doi: 10.1093/eurheartj/ehp476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckers HJ, Soullie T, den Heijer P. Accelerated vascular repair following percutaneous coronary intervention by capture of endothelial progenitor cells promotes regression of neointimal growth at long term follow-up: final results of the Healing II trial using an endothelial progenitor cell capturing stent (Genous R stent) EuroIntervention. 2007;3:350–358. doi: 10.4244/eijv3i3a64. [DOI] [PubMed] [Google Scholar]
- 11.Duckers HJ, Silber S, de Winter R. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention. 2007;3:67–75. [PubMed] [Google Scholar]
- 12.Segers D, Helderman F, Cheng C. Gelatinolytic activity in atherosclerotic plaques is highly localized and is associated with both macrophages and smooth muscle cells in vivo. Circulation. 2007;115:609–616. doi: 10.1161/CIRCULATIONAHA.106.636415. [DOI] [PubMed] [Google Scholar]
- 13.Granada JF, Inami S, Aboodi MS. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010 doi: 10.1161/CIRCINTERVENTIONS.109.919936. [DOI] [PubMed] [Google Scholar]
- 14.Harrison P, Goodall AH. ‘Message in the platelet’—more than just vestigial mRNA! Platelets. 2008;19:395–404. doi: 10.1080/09537100801990582. [DOI] [PubMed] [Google Scholar]
- 15.Donahue BS, Gailani D, Mast AE. Disposition of tissue factor pathway inhibitor during cardiopulmonary bypass. J Thromb Haemost. 2006;4:1011–1016. doi: 10.1111/j.1538-7836.2006.01896.x. [DOI] [PubMed] [Google Scholar]
- 16.Osterud B, Bajaj MS, Bajaj SP. Sites of tissue factor pathway inhibitor (TFPI) and tissue factor expression under physiologic and pathologic conditions. On behalf of the Subcommittee on Tissue factor Pathway Inhibitor (TFPI) of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;73:873–875. [PubMed] [Google Scholar]
- 17.Silber S, Damman P, Klomp M. Clinical results after coronary stenting with the Genous Bio-engineered R stent: 12-month outcomes of the e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. EuroIntervention. 6:819–825. doi: 10.4244/EIJV6I7A141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.