Abstract
Th1 cells are remarkably more susceptible to activation induced cell death than Th17. Here, we compared cultures of these two cell subpopulations for their expression of apoptosis-related molecules when re-exposed to their specific antigen. We also compared the expression of apoptosis-related molecules in the mouse eye with inflammation induced by Th1 or Th17 cells. Using qPCR we found that the mRNA transcript levels of the majority of tested apoptosis-related molecules were higher in the Th1 cultures, and in eyes with Th1-induced inflammation. Apoptotic intrinsic pathway molecules played minor roles in the processes in vitro or in vivo, whereas extrinsic pathway molecules, as well as PD-1, its ligands and Tim3, were heavily involved.
Keywords: Apoptosis, Apoptosis-related molecules, Activation-induced cell death, Eye, Inflammation, Th1, Th17
1. Introduction
Analysis of the pathogenic processes of autoimmune diseases has established the involvement of the two major T-helper populations, Th1 and Th17[1-4]. The two populations differ in their mechanism of generation, cytokine production and physiological function[1-4]. In addition, we and other authors found that Th1 and Th17 cells also differ in their plasticity[5-7] and more recently, we demonstrated that immunopathogenic processes mediated by Th17 cells are more sustainable than those mediated by Th1 cells [8]. Further, our data [8] and those by Yu et al [9] suggested that the difference in sustainability of inflammation induced by Th1 and Th17 cell lines is related to the latter cell population being more resistant to activation-induced cell death (AICD1, also named “restimulation-induced cell death”). These two studies also suggested that the resistance to AICD by Th17 cells is due to lower production of Fas-ligand (FasL), as compared to Th1 cells [8,9].
Apoptosis, or programmed cell death, plays crucial roles in the immune system, in particular at the selection processes in the thymus [10,11] and during the immune response in the periphery, when the majority of antigen-specific lymphocytes should be eliminated at the end of immunization cycle, by the AICD process [12,13]. AICD has been investigated mainly in vitro, in studies in which pre-sensitized cells undergo apoptosis following re-exposure to their target antigen[12,13]; less is known about this process in vivo.
Data collected in recent years revealed that apoptosis is induced by two mechanisms, the intrinsic (“mitochondrial”) and extrinsic (“death receptor”) pathways, with different families of molecules initiating each of the two pathways [14,15]. The intrinsic pathway, that is triggered by intracellular events, is mediated mainly by molecules that belong to the Bcl-2 family of proteins, that includes both pro-apoptotic molecules such as Bim, Bax, Bak and Bad and anti-apoptotic molecules, represented by Bcl-2 [16,17]. The extrinsic pathway is triggered by extracellular signals that bind to cellular pro-apoptotic (“death”) receptors. Abundant information has been collected in particular concerning the activities of two death-receptor pathways, FasL and its receptor, Fas, and TRAIL and its multiple cellular receptors [18,19]. In addition to the mentioned apoptosis-related molecules of the two pathways, recent studies identified regulatory molecules whose expression leads cells to stages of exhaustion and even death. This group of molecules includes programmed death-1 (PD-1) and its ligands PD-L1 and PD-L2 [20,21], as well as Tim-3 [22].
Here, we compared the expression of various apoptosis-related molecules by Th1 and Th17 cells following antigenic re-stimulation in vitro and the inflammatory process they elicit in their target tissue in vivo.
2. Materials and methods
2.1 Mice
All mice used in this study were (FVB/N × B10.BR) F1 hybrids, transgenically expressing either hen egg lysozyme (HEL) in their eyes (“HEL-Tg”), or HEL-specific TCR by their T-cells (“3A9”); see Refs [1,7] for details. The mice were housed in a pathogen-free facility and all manipulations were performed in compliance with the NIH Resolution on the Use of Animals in Research.
2.2 Reagents
In addition to reagents detailed in Refs [1,7], all tested gene expression assays were obtained from Applied Biosystems.
2.3 Generation of cell lines
Th1 and Th17 cell lines were generated by the procedures detailed in Refs [1,7]. All line cells used in the present study were collected following reactivation, as detailed in the mentioned references.
2.4 AICD assay
The cell death assay procedure detailed in Ref [8] was used with one modification: the CD4 cultures were stimulated here with increasing concentrations of the specific antigen, hen egg lysozyme (HEL), as indicated, in the presence of CD11c+ dendritic cells (DC), serving as antigen presenting cells (APC), from wild type F1 mice at a ratio of 5:1 (CD4:DC).
2.5 Induction of ocular inflammation by adoptive transfer of Th1 or Th17 cells
Activated Th1 or Th17 3A9 cells (5×106) were injected via the tail vein into naïve HEL-transgenic (Tg) mice and the recipients were sacrificed 5 days later. All eyes developed inflammation [1,7,8] and were used to extract mRNA for performing Quantitative real-time PCR (qPCR).
2.6 qPCR
Transcript levels of the tested genes were assessed by qPCR. β-actin was used as an endogenous control in all samples. Relative expression was calculated according to the manufacturer’s instructions (Applied Biosystem), as described elsewhere [7].
2.7 Statistical analysis
Data were shown as the mean ± SEM. The software GraphPad Prism was used to perform the statistical analyses of the data with two-tailed Student t test. Differences were considered significant at p < 0.05. *p < 0.05; **p < 0.01; ***p < 0.001
3. Results
3.1 Th1 cells are more susceptible to AICD than Th17 cells
We have previously shown that Th1 cells are more susceptible than Th17 cells to AICD [8]. The apoptosis-inducing reactivation mechanism in the cited study was by the non-physiological anti-CD3 antibody and we confirmed here the observation by reactivating cells of the two lineages by the physiological mechanism, i.e., exposure to increasing concentrations of the specific antigen, HEL. The data of a representative experiment are summarized in Fig. 1 and show that Th1 cells undergo apoptosis at a rate remarkably faster than that of Th17 cells.
Fig. 1.
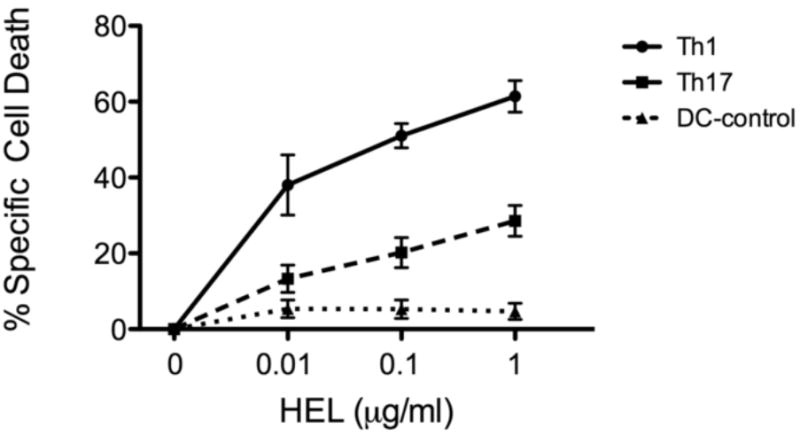
Th1 cells are more susceptible to AICD than Th17 cells. Activated and polarized cells of the two lines were re-exposed to HEL at the indicated concentrations, in the presence of DC, and the % specific cell death was determined by the annexin/propidium iodide staining, as detailed in Ref. 8. The apoptotic levels in control cultures, of DC alone, are also recorded in the Figure.
3.2 Th1 and Th17 cells reactivated in vitro differ in their expression profile of apoptosis-related molecules
To learn about the expression of apoptosis-related molecules by Th1 and Th17 cells that undergo reactivation in culture with the specific antigen, HEL, we extracted RNA from these cells and evaluated by qPCR their expression levels of mRNA transcripts of major molecules involved in the apoptosis process. RNA samples from CD4+ cells of naïve mice were used for controls. The tested molecules belong to several families of molecules, including the intrinsic and extrinsic apoptosis pathways, as well as PD-1, its two ligands and Tim-3. Fig. 2A summarizes combined data of four experiments. Reactivated Th17 cells expressed significantly higher levels of the “pro-life” Bcl-2 transcript than did their Th1 cell counterpart, but no significant differences were noted between cells of the two lineages in their expression levels of pro-apoptotic Bad, Bak and Bax. Unlike the relative similarity between Th1 and Th17 cells in their expression of the three mentioned intrinsic pathway transcripts, cells of the two lineages differed remarkably in their expression of the other tested transcripts (Fig. 2A). Of these other seven tested transcripts, only one, of Fas, was expressed by Th17 at levels higher than those by Th1 cells (the difference was not statistically significant). In contrast, transcripts of Fas-L, TRAIL and Tim3 were expressed by Th1 at levels significantly higher than those expressed by Th17 cells. It is also of note that the expression levels of PD-L1 and PD-L2 transcripts of all three tested RNA samples was marginal.
Fig. 2.
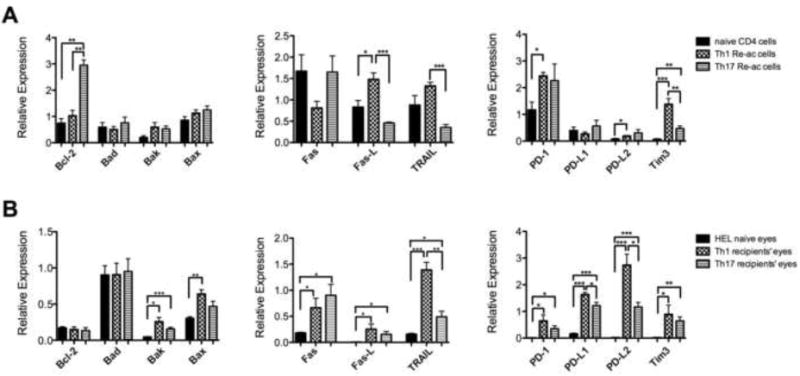
Expression profiles of apoptosis-related molecules are different between reactivated Th1 and Th17 cells in culture and between mouse eyes with inflammation induced by Th1 or Th17 cells. Levels of apoptosis-related molecule transcripts were measured by qPCR in cultures of activated/polarized Th1 or Th17 cells re-exposed to HEL (A), or eyes of recipient mice with inflammation induced by adoptive transfer of reactivated Th1 or Th17 cells (B). Cultures of naïve CD4 cells, with no activation, were used as controls in the experiments with cultured cells (A), whereas eyes of naïve control mice served as controls in the experiments with inflamed eyes, summarized in B. The columns are means ± SEM of four experiments each.
3.3 Differences in transcript expression profile between eyes with inflammation induced by Th1 or Th17 cells
Adoptively transferred activated Th1 or Th17 cells specific toward HEL induce ocular inflammation in syngeneic recipient mice expressing HEL in their eyes (1,7,8). As recorded in detail in Ref. 8, the onset of ocular inflammation induced by Th1 cells is on day 3 post-cell injection, while that of Th17 cells is on day 4. Yet, the inflammatory process is at its peak on day 5 and the inflammatory changes are similar in recipients of cells of the two lineages. As shown by figures in the cited study, the inflammatory process is “panuveitic” and affects essentially all eye components. It mainly includes infiltration of inflammatoty cells into most ocular tissues and is also characterized by proteinaceous exudate in the anterior chamber and vitreous.
Adoptively transferred Th cells activated in vitro undergo re-activation upon exposure to their target antigen in the recipient tissue and initiate an inflammatory response. To examine the transcript expression profile of the inflammatory process induced by adoptively transferred Th1 and Th17 cells in this study we extracted RNA from the inflamed recipient eyes and determined the transcript levels by qPCR. The combined data of four independent experiments are summarized in Fig. 2B. A comparison between the expression levels of the four tested intrinsic death pathway molecule in inflamed and control naïve eyes revealed higher levels of the Bak transcript and moderately higher levels of the Bax transcript in the inflamed eyes, but similar expression of the Bad and Bcl-2 transcripts in the inflamed and naïve eyes. This observation suggests that the molecules of the apoptosis intrinsic pathway were partially or not at all involved in the Th-induced inflammatory process. In contrast, the levels of all other tested transcripts were profoundly higher in the inflamed eyes than in the naïve mouse eyes. Similar to the observation with the cultured Th cells, the Fas transcript level in Th17 recipient eyes was higher (albeit insignificantly) than that in the Th1 recipient eyes, whereas the levels of all other transcripts were higher in the Th1 recipient eyes. Also of interest is the observation that levels of the PD-L1 and PD-L2 transcripts were remarkably higher in the inflamed eyes than in cultures of the reactivated Th1 or Th17 cells (Fig 2B vs Fig. 2A). This finding with the PD-1 ligand transcripts suggests that these transcripts are expressed in the inflamed eyes mainly by cells other than the adoptively transferred lymphocytes. Also of note is the observation that expression level of the Fas-L transcript was much lower in the inflamed eyes, as compared to that in reactivated Th cultures (Fig. 2B).
4. Discussion
Recent publications demonstrated that Th1 and Th17 cells differ in their susceptibility to apoptosis (8, 9). The present study further analyzed the differences between these two cell populations in their susceptibility to apoptosis. Th1 cells are remarkably more susceptible than Th17 to AICD (Fig. 1 and Ref. 8) and were found here to express higher transcript levels of the majority of tested apoptosis-related molecules, with the exception of Fas and, in particular, of Bcl-2 (Fig. 2A). Unlike most of the other members of the Bcl-2 family, Bcl-2 is anti-apoptotic and its high expression by Th-17 cells is thus in accord with the relatively low susceptibility of these cells to apoptosis. Th1 cells were also superior to Th17 cells in their expression of PD-1 and its ligands, PD-L1 and PD-L2, as well as of Tim3; four regulatory molecules that are involved in cell exhaustion and even death [20-22]. We also determined the expression of this battery of transcripts by eyes with inflammation induced by Th1 or Th17 cells. A partial correlation was also observed between the expression profiles by the reactivated Th cultures and the corresponding inflamed eyes. Observations of interest include: (i) molecules of the intrinsic pathway of apoptosis played a mere minor role if any in the apoptotic processes monitored in vitro or in vivo. This finding is in line with the function of this pathway, that is mainly physiological and crucial for processes such as tissue development and homeostasis [23, 24]. (ii) Remarkable differences were noted between the expression profiles of the tested apoptosis-related molecules in Th1 and Th17 cultures exposed to AICD and in recipient mouse eyes in which cells of the two lineages were re-exposed to their specific antigen. The difference is particularly sharp in the expression of BAD, PD-L1, PD-L2, and TRAIL, molecules whose expression in the tested eyes was strikingly higher than in the reactivated cell cultures. This difference is attributed mainly to the high expression of these molecules by ocular resident cells and non-lymphocyte cells that participate in the inflammation process. BAD transcript is strongly expressed constitutively by ocular cells and its expression was not affected by the inflammation. In contrast, the expression of PD-L1 and PD-L2 is selectively enhanced in the inflamed eyes (Fig. 2B) and suggests that cells other than Th lymphocytes contribute to the expression of these two ligands. The high expression of TRAIL transcript in inflamed eyes, in particular of Th1 recipients, could be attributed to its expression by Th cells, as well as by other cells.
It is of note that, unlike Th1, Th17 cells are plastic and a portion of this population switch in the recipient mouse eye and acquire the Th1 phenotype (7). It is assumed, therefore, that the profile of apoptosis-related molecules in recipient eyes of the Th17 cells is affected by this phenomenon and makes it impossible to directly compare between molecules expressed by cultured Th17 cells and those found in eyes with Th17-induced inflammation.
In summary, data recorded here provide new information related to Th1 cells being more susceptible than Th17 cells to apoptotic processes, as indicated by their higher expression of most apoptosis-related molecules. This difference was shown both in vitro, following re-exposure to antigen, as well as in vivo, in an organ in which activated Th cells are exposed to their target antigen and elicit inflammation.
> Th1 cell cultures express apoptosis-related molecules more than Th17 cells do. > Th1-induced inflammation highly involves apoptosis-related molecules. > Extrinsic pathway of apoptosis participates in immune-mediated inflammation.
Acknowledgments
This research was supported by the Intramural Research Program of the National Eye Institute, NIH.
Footnotes
Abbreviations: AICD, activation-induced cell death; FasL, Fas-ligand; Programmed cell death-1, PD-1; HEL, hen egg lysozyme; DC, dendritic cells; Tg, transgenic; APC, antigen presenting cells; qPCR, quantitative real-time PCR.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox CA, Shi G, Yin H, Vistica BP, Wawrousek EF, Chan CC, Gery I. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annunziato F, Romagnani S. The transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3312–3316. doi: 10.1002/eji.201041145. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, Siegel RM, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Iclozan C, Yamazaki T, Yang X, Anasetti C, Dong C, Yu XZ. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohn SJ, Thompson J, Winoto A. Apoptosis during negative selection of autoreactive thymocytes. Curr Opin Immunol. 2007;19:510–515. doi: 10.1016/j.coi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Strasser A, Puthalakath H, O’Reilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 12.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RM, Lenardo MJ. Apoptosis signaling pathways. Curr Protoc Cytom. 2002;Chapter 7(Unit 7):18. doi: 10.1002/0471142956.cy0718s21. [DOI] [PubMed] [Google Scholar]
- 14.Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 15.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 16.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 21.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 23.Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10–15. doi: 10.1177/1073858404269267. [DOI] [PubMed] [Google Scholar]
- 24.Rickman DW, Nacke RE, Bowes Rickman C. Characterization of the cell death promoter, Bad, in the developing rat retina and forebrain. Brain Res Dev Brain Res. 1999;115:41–47. doi: 10.1016/s0165-3806(99)00046-2. [DOI] [PubMed] [Google Scholar]


