Abstract
Both Th1 and Th17 cells have been implicated in the pathogenesis of inflammatory bowel disease (IBD) and experimental colitis. However, the complex relationship between Th1 and Th17 cells and their relative contributions to the pathogenesis of IBD have not been completely analyzed. Although it has been recently shown that Th17 cells can convert into Th1 cells, the underlying in vivo mechanisms and the role of Th1 cells converted from Th17 cells in the pathogenesis of colitis are still largely unknown. We report here that Th17 cells from CBir1 TCR transgenic mice, which are specific for an immunodominant microbiota antigen, are more potent than Th1 cells in the induction of colitis, as Th17 cells induced severe colitis, whereas Th1 cells induced mild colitis when transferred into TCRβxδ−/− mice. High levels of IL-12 and IL-23, and substantial numbers of IFNγ+ Th1 cells emerged in the colons of Th17 cell recipients. Administration of anti-IL-17 monoclonal antibody abrogated Th17 cell-induced colitis development, blocked colonic IL-12 and IL-23 production, and inhibited IFNγ+ Th1 cell induction/conversion. IL-17 promoted dendritic cell production of IL-12 and IL-23. Furthermore, conditioned media from colonic tissues of colitic Th17 cell recipients induced IFNγ production by Th17 cells, which was inhibited by blockade of IL-12 and IL-23. Collectively, these data indicate that Th17 cells convert to Th1 cells through IL-17 induction of mucosal innate IL-12 and IL-23 production.
Introduction
Both Th1 cells, which produce IFNγ, and Th17 cells, which produce IL-17 (IL-17A), IL-17F, IL-21 and IL-22, have been implicated as important mediators of inflammatory bowel disease (IBD) (1–5). It has been shown that IL-12 stimulates Th1 cell differentiation (6), while IL-6, TGFβ and IL-23 promote Th17 cell development (7). There is increased production of IL-12 and IL-23 in the lesions of Crohn’s disease, and mesenteric lymph node (MLN) dendritic cells (DC) from patients with Crohn’s disease induce both Th1 and Th17 immune responses (8–12). T cells from Crohn’s disease lesions express high levels of activated STAT4 and T-bet, the Th1-associated transcription factors indicative of IL-12 signaling (13–14). The important role of Th17 cells, which express the IL-23 receptor (IL-23R) on their surface, in the pathogenesis of IBD is supported by recent genome-wide association studies indicating that IL-23R and other genes involved in Th17 cell differentiation are associated with susceptibility to Crohn’s disease and ulcerative colitis (15–18). Anti-IL-12/IL-23p40 antibody therapy, which targets both Th1 and Th17 cells, is effective in Crohn’s disease (19–20). Data from our own studies demonstrate that anti-IL-23p19 monoclonal antibody (mAb) prevents, as well as treats, colitis in an experimental model induced by adoptive transfer of microbiota antigen-specific T cells, further confirming a role for the IL-23/Th17 pathway in the pathogenesis of chronic intestinal inflammation (5). However, in patients with Crohn’s disease, a unique subset of CD14+ macrophages have been identified that contribute to the pathogenesis of Crohn’s disease by promoting IL-23-dependent IFNγ production rather than IL-17 production by lamina propria (LP) mononuclear cells (21). Significant IL-17 mRNA upregulation is found in LP CD4+ T cells from patients with ulcerative colitis, while IFNγ levels are increased in Crohn’s disease. These data argue somewhat against the concept that IL-23 contributes only to Th17 cytokine production (10), and demonstrate that IL-23 can promote Th1 cell IFNγ production as well. A number of reports have identified a subset of Th17 cells that co-produce the Th1 cytokine IFNγ (22–23). This is particularly prominent at sites of inflammation such as active Crohn’s disease (22). Those reports suggest that the complex relationship between Th1 and Th17 cells in IBD remains unclear. However, it is important to delineate the specific contributions of these cells to chronic intestinal inflammation, especially in regard to the persistence and progression of colitis.
Recently, substantial developmental plasticity of the Th17 lineage has been observed in human Th17 clones derived from intestinal isolates of patients with Crohn’s disease (22). There is also considerable plasticity late in the mouse Th17 program, which allows committed Th17 cells to transition from effectors that produce predominantly IL-17 to effectors that produce predominantly IFNγ in a process driven by IL-12 and IL-23 via a STAT4- and T-bet-dependent manner (24–27). These elegant studies reveal a mechanism for the latent Th1-like responsiveness of Th17 cells, and provide a basis for understanding the relationship between Th17- and Th1-mediated pathophysiology. However, much of the data defining Th17 cell conversion to Th1 cells is derived from in vitro studies. Whether IL-12 and IL-23 mediate Th17 cell conversion to Th1 cells in vivo, and if so, where and how IL-12 and IL-23 are induced in vivo in the first place, remain unknown. In this report, we demonstrate that Th17 cells from CBir1 TCR transgenic (CBir1 Tg) mice, which are specific for an immunodominant microbiota flagellin, induced colitis in TCRβxδ−/− recipient mice. Furthermore, Th17 cells promoted Th1 cell development through IL-17 induction of mucosal IL-12 and IL-23 in inflamed colonic tissues.
Materials and Methods
Mice
C57BL/6 (B6) and B6.TCRβxδ−/− (TCRβxδ−/−) mice were obtained from the Jackson Laboratory. B6.CBir1 TCR transgenic (CBir1 Tg) mice (28) were generated and bred in the Animal Facility at the University of Alabama at Birmingham. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Antibody and reagents
Recombinant IL-6, TGFβ1, and IL-12 were from R&D Systems. Antibodies against IL-17 and IFNγ, and anti-CD4-magnetic beads were from BD Biosciences.
Generation of bone marrow-derived DC (BMDCs)
Bone marrow cells were isolated as described previously (29). Briefly, bone marrow cells were resuspended in complete RPMI 1640 media containing 10% heat-inactivated FBS (Atlanta Biologicals), 25 mM HEPES buffer, 2 mM sodium pyruvate, 50 mM 2-mercaptoethanol, 100 IU/ml Penicillin, and 100 µg/ml Streptomycin (Cellgro Mediatech), and cultured in the presence of 20 ng/ml GM-CSF (R&D Systems) in 6-well plates at 37°C in humid air with 5% CO2. On day 8, BMDCs were harvested and plated at 1 × 106/ml per well in 24-well plates in the presence or absence of 100 ng/ml recombinant IL-17 (R&D Systems).
Isolation of CD4+ T cells
CD4+ T cells were isolated using anti-mouse CD4-magnetic beads as previously described (30). Briefly, splenic cells were washed twice and incubated with anti-CD4 magnetic beads at 4°C for 30 min, and then separated by magnetic field. When checked by flow cytometry, >95% of the cells were CD4+ T cells.
Preparation of lamina propria (LP) lymphocytes
LP lymphocytes were isolated as previously described (31). Briefly, for removal of epithelial cells and intraepithelial lymphocytes, the intestines were washed, cut into small pieces, and then the pieces were incubated with calcium- and magnesium-free HBSS supplemented with 2% FBS and 5 mM EDTA (Sigma-Aldrich) on a magnetic stirrer at 37°C for 30 min. The tissues were then incubated with RPMI 1640 containing 5% FBS and 0.5 mg/ml collagenase type IV (Sigma) for 30 min at 37°C with stirring. The liberated cells were collected by passage through a stainless steel sieve. The isolated cells were pooled together and separated on a 40/75% discontinuous Percoll gradient (Pharmacia). The cell yield was typically ~2 × 106 lymphocytes per mouse with >90% cell viability.
Flow cytometry
As described previously (28), cells were stimulated for 5 h with PMA (50 ng/ml) and ionomycin (750 ng/ml), with monensin added for the last 3 h of culture. The cells were fixed and permeabilized using Cytofix/cytoperm solution (BD Pharmingen). Staining was performed for IL-17 and IFNγ using fluorescence-conjugated antibodies, and the cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson) using FlowJo software (Tree star).
Ex vivo organ culture
Ex vivo organ fragment cultures of colon and small intestine were performed by removing and longitudinally opening the small and large intestines. After washing with cold RPMI three times, three 3-mm circular full thickness pieces of small intestine and colon were obtained using a 3-mm dermal punch (Baker-Cummins). Each fragment was then placed in 0.5 ml of complete RPMI in separate wells of a 48- well plate and incubated for 24 h at 37°C in humid air with 5% CO2. Culture supernatants from each biopsy were collected and stored at −80°C before analysis for cytokine content.
Statistical analysis
For comparisons between samples, levels of significance were determined by Student’s t test in Prism 5.0 (Graphpad). Where appropriate, mean ± SEM is represented on graphs. *p < 0.05; **p < 0.01.
Results
Naïve CBir1 Tg T cells differentiate into both Th1 and Th17 cells in the intestine during the induction of colitis in TCRβxδ−/− mice
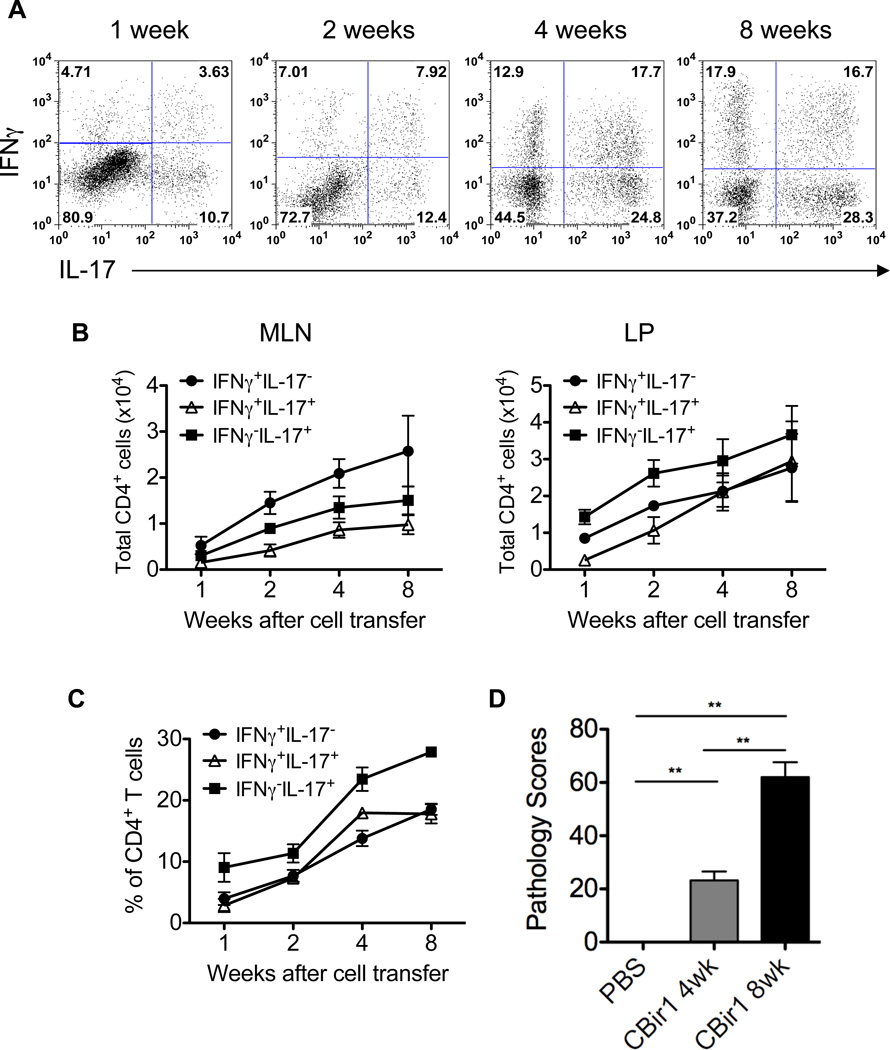
We have previously shown that microbiota antigen-specific CD4+ T cells from CBir1 Tg mice (28) that are specific for CBir1 flagellin, an immunodominant commensal antigen (32), induced colitis in immunodeficient mice (33–34). With the progression of colitis, IFN-γ+ T cells, IL-17+ T cells, IFN-γ+IL-17+ T cells, as well as Foxp3+ T cells, Foxp3+IFN-γ+ T cells, Foxp3+IL-17+ T cells and Foxp3+IFN-γ+IL-17+ T cells emerged in the intestinal lamina propria of recipient mice (33). To investigate Th1 and Th17 cell differentiation of microbiota antigen-specific CD4+ T cells in the intestine and their role in the pathogenesis of colitis, 1 × 106 naïve CD4+ T cells from CBir1 Tg mice were transferred intravenously into TCRβxδ−/− mice. Unlike SCID mice or RAG deficient mice which lack both T cells and B cells, TCRβxδ−/− mice only lack T cells but have a fully functional innate immune system, B cell repertoire and NK cells. Using TCRβxδ−/− mice as recipients allows us to dissect the role of transferred T cells in colitis development in mice with a relatively intact immune system. A group of TCRβxδ−/− mice injected with PBS served as control. Five recipient mice/group were sacrificed 1, 2, 4, or 8 weeks after cell transfer, and IFN-γ and IL-17 production by LP T cells were assessed. One week after adoptive transfer, 4.7% of transferred CBir1 Tg CD4+ T cells in the LP were IFNγ single positive (Th1) and 10.7% were IL-17 single positive (Th17). Interestingly, a significant number of transferred CBir1 Tg CD4+ T cells (3.6%) expressed both IFNγ and IL-17 (“Th1+17” cells) (Figure 1A). The percentages and absolute numbers of these populations increased over time (Figures 1B and 1C). Eight weeks after cell transfer, 17.9% were IFNγ single positive, 28.3% were IL-17 single positive, and 16.7% were IFNγ and IL-17 double positive cells (Figures 1A and 1C). As shown previously, the recipients developed mild colitis as early as 4 weeks and severe colitis 8 weeks after cell transfer (Figure 1D). Collectively, these data demonstrate that CD4+ T cells reactive to a single dominant microbiota antigen can differentiate into both Th1 and Th17 cells in the intestine and induce colitis. Large numbers of IFNγ and IL-17 double positive CD4+ T cells present in the LP of colitic mice may play a role in the colitis development, as they are not found in the LP of normal control mice (data not shown).
Figure 1. Naïve CBir1 Tg T cells differentiate into both Th1 and Th17 cells in the intestine and induce colitis in TCRβxδ−/− mice.
1 × 106 CBir1 Tg CD4+ T cells were transferred into TCRβxδ−/− mice. A groups of 5 TCRβxδ−/− mice were injected with PBS as control. The recipients were sacrificed at different time points. A, Lamina propria (LP) CD4+ T cell intracellular IFNγ and IL-17 production was determined by flow cytometry. Plot numbers represent the percentage of CD4 positive T cells in the respective quadrants. B, Absolute numbers of IFNγ+ and IL-17+ CD4+ T cells in MLN and LP. C, Aggregate data of percentages of LP IFNγ+ and IL-17+ T cells from three independent experiments. D, Colonic histopathology was assessed at different time points after cell transfer. Pathology scores are shown. **p < 0.01. One representative of three independent experiments is shown.
CBir1-specific Th17 cells are more potent than Th1 cells in induction of colitis
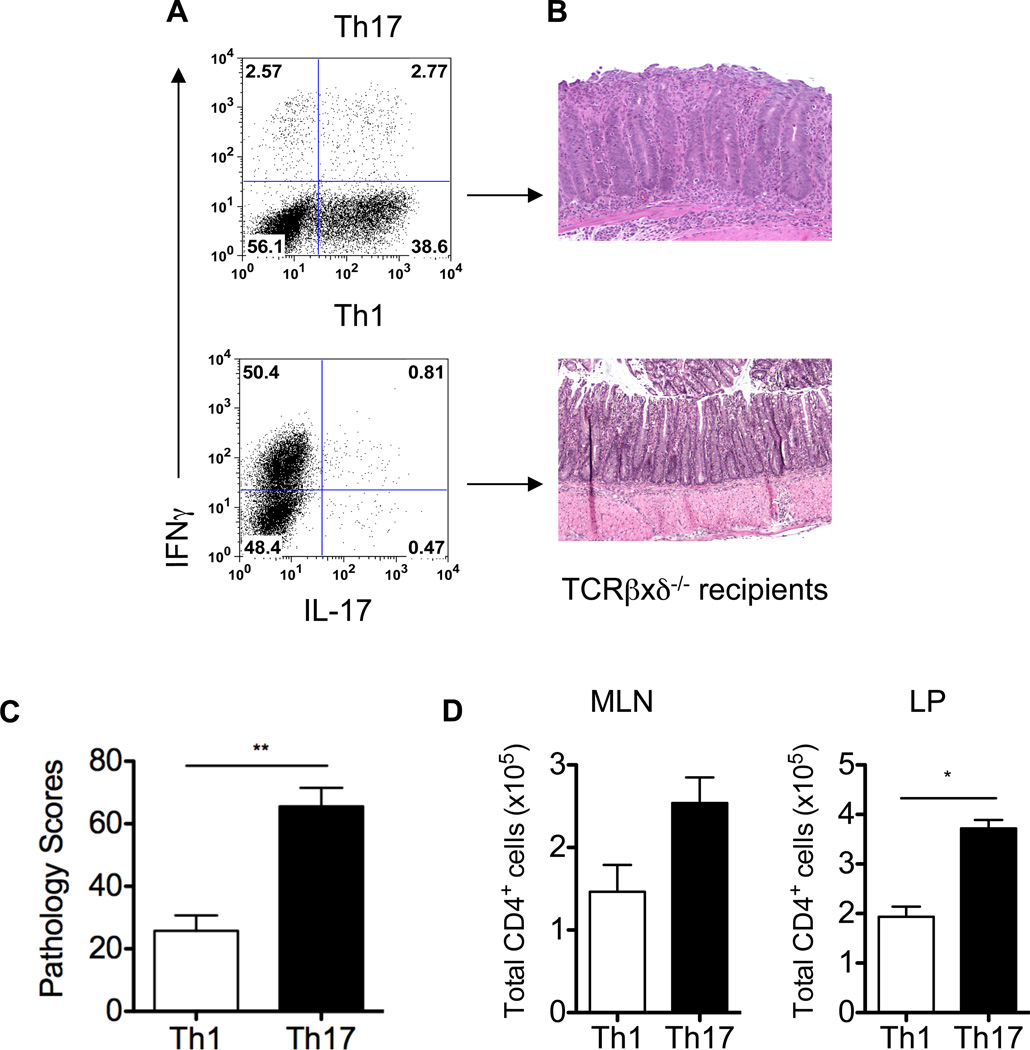
Both Th1 and Th17 cells have been implicated in the pathogenesis of colitis. To determine the role of CBir1 flagellin-specific Th1 and Th17 cells in the induction of colitis in this model, CBir1-specific Th1 and Th17 cells were polarized in vitro by culture of CD4+ T cells from CBir1 Tg mice with CBir1-pulsed antigen-presenting cells (APC) under standard Th1 conditions (IL-12 and anti-IL-4 mAb) or Th17 conditions (IL-6, TGFβ, anti-IL-4 mAb, and anti-IFNγ mAb), respectively. Seven days later, T cell cytokine production was analyzed by intracellular staining. As expected, activation of naïve CBir1 Tg CD4+ T cells under Th1 conditions induced the development of a polarized Th1 phenotype with high levels of IFNγ and little IL-17. Activation of naïve CBir1 Tg CD4+ T cells under Th17 conditions resulted in the development of Th17 effector cells with high levels of IL-17 and low levels of IFNγ (Figure 2A). These polarized CBir1-specific Th1 and Th17 cells were then transferred into TCRβxδ−/− mice separately. Eight weeks after cell transfer, recipient mice were sacrificed and intestinal histopathology was assessed. The recipient mice of CBir1-specific Th1 cells developed mild colitis, whereas the recipients of CBir1-specific Th17 cells developed severe colitis (Figures 2B and 2C). There were more CD4+ T cells in MLN and LP of Th17 recipient mice compared to Th1 recipients (Figure 2D). Collectively, these data indicate that CBir1-specific Th1 and Th17 cells may function differently in the pathogenesis of colitis, and specifically, Th17 cells are more potent than Th1 cells in the induction of colitis. Currently we still do not know whether the difference in pathology is related to the differences in proliferative potential of these two different types of T cells or to the pathogenicity of their signature cytokines. Of note, despite the presence of CBir1 Tg T cells in both small and large intestinal LP, no inflammation was observed in the small intestines of recipient mice that received either Th1 or Th17 cells (data not shown). Interestingly, A4 bacteria, which produce CBir1-like flagellins (35) and activate CBir1 Tg T cells, were only detected in the cecum and colon but not in the small intestine (data not shown), indicating that the lack of inflammation in the small intestine is due to the absence of antigen-induced TCR activation, which is required for the induction of colitis.
Figure 2. CBir1 Tg Th17 cells are more potent than Th1 cells in the induction of colitis.
To determine the ability of Th17 and Th1 cells to induce colitis, CBir1 Tg Th17 and Th1 cells were polarized in vitro under standard Th17 and Th1 conditions (A), and 1 × 106 Th17 and Th1 cells were transferred into groups of 5 TCRβxδ−/− mice separately. Eight weeks after transfer, the recipient mice were sacrificed and assessed for histopathology. Colonic histopathology (B) and pathology scores (C) are shown. **p < 0.01. D, Absolute numbers of CD4+ T cells in MLN and LP are shown. *p < 0.01. Data are one representative of three independent experiments.
CBir1-specific Th17 cells convert into Th1 cells in TCRβxδ−/− recipient mice
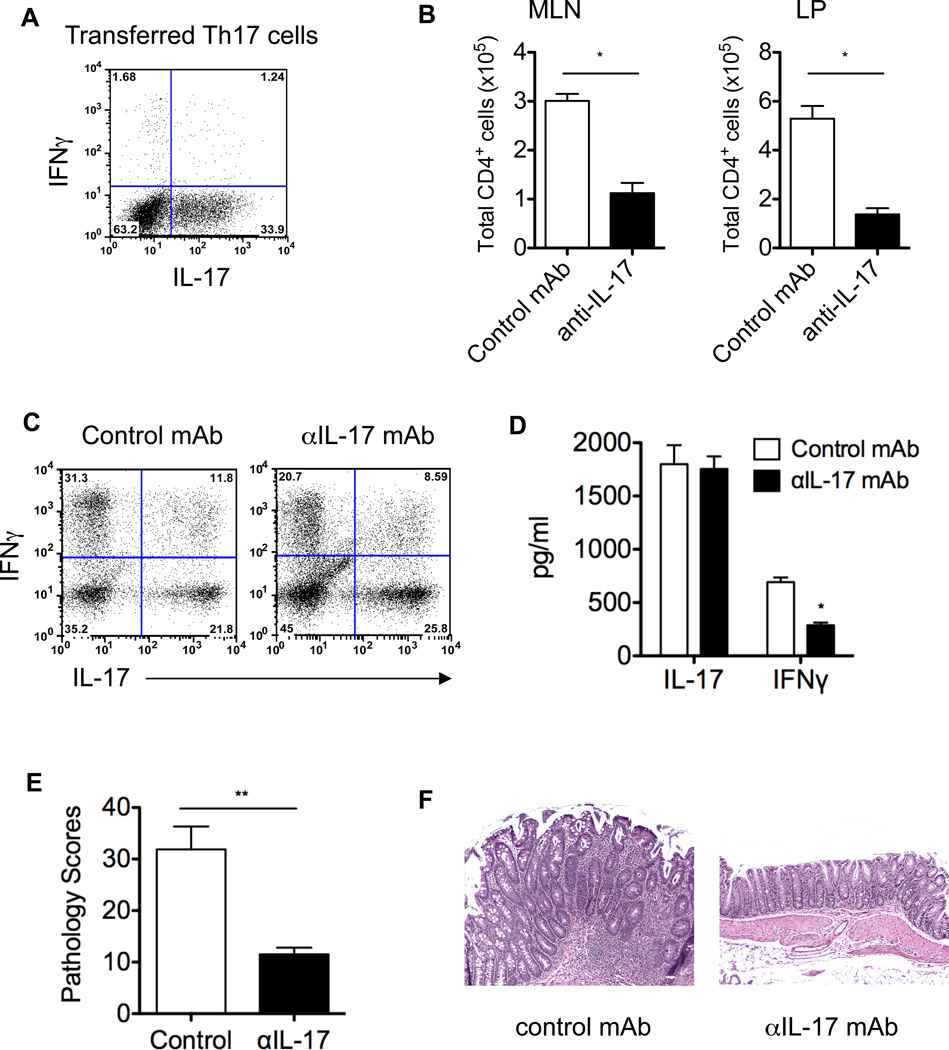
Th17 cells have demonstrated substantial plasticity. However, whether their signature cytokine IL-17 regulates Th17 cell stability has not been defined, and its role in the pathogenesis of colitis is also controversial as both protective and pathogenic functions of IL-17 have been reported in different experimental models of colitis (36–38). To determine whether microbiota antigen-specific Th17 cells convert to Th1 cells in the intestine and the role of IL-17 in Th17 cell conversion and induction of colitis, Th17 cells were generated from CBir1 Tg mice in vitro (Figure 3A) and transferred into TCRβxδ−/− mice. Groups of five recipient mice were treated with anti-IL-17 or control mAb. Eight weeks later, total CD4+ T cells in the MLN and LP, and T cell cytokine production in the LP and colonic histopathology were examined. As shown in Figure 3B, substantial CD4+ T cells accumulated in the LP of Th17 recipient mice treated with control mAb, and treatment with anti-IL-17 mAb decreased CD4+ T cell accumulation in the LP. Adoptively transferred Th17 cells in the recipient mice treated with control mAb expressed IFNγ, and a large proportion of T cells coexpressed both IL-17 and IFNγ in the LP. Treatment with anti-IL-17 mAb did not affect IL-17 expression, but reduced the expression of IFNγ in T cells (Figure 3C). Consistently, colonic tissue of colitic Th17 recipient mice that were treated with control mAb produced high levels of IL-17 and IFNγ (Figure 3D). Although production of IFNγ was significantly inhibited by treatment with anti-IL-17 mAb, it is interesting to note that while there were fewer CD4+ T cells in the LP of anti-IL-17 mAb-treated recipient mice, the colonic tissues produced similar levels of IL-17 ex vivo in both groups of recipient mice (Figure 3D). The colonic tissues contain not only Th17 cells but also many different types of cells that could produce IL-17, including recently identified IL-17-producing innate cells (39–41). This raises the possibility that transferred Th17 cells could promote IL-17 production by such innate cells. Collectively, these data indicate that IL-17 induces T cell IFNγ production, probably through promoting Th17 cell conversion to Th1 cells. Although high levels of IL-17 have been detected in patients with Crohn’s disease, its role in the pathogenesis of IBD is still controversial. As shown in Figures 3E and 3F, Th17 cell recipient mice treated with control mAb developed severe colitis. In contrast, anti-IL-17 mAb treatment abrogated colitis development, demonstrating a pathogenic role of IL-17 in the development of colitis, at least in this model.
Figure 3. IL-17 mediates CBir1-specific Th17 cell conversion to Th1 cells and induction of colitis in TCRβxδ−/− mice.
1 × 106 in vitro-polarized CBir1 Tg Th17 cells (A) were transferred into TCRβxδ−/− mice. Groups of 5 recipient mice were administered with 100 µg/mouse of anti-IL-17 mAb or control mAb at the time of transfer and weekly thereafter. Eight weeks after transfer, the mice were sacrificed. Absolute numbers of CD4+ T cells were counted in MLN and LP (B), and LP CD4+ T cell IFNγ and IL-17 production was determined by flow cytometry (C). Plot numbers represent the percentage of CD4 positive T cells in the respective quadrants. *p < 0.01 compared to control mAb-treated group. To determine local mucosal cytokine production, colonic tissues of Th17 recipient mice were cultured for 24 h, and production of IFNγ and IL-17 in the supernatants was determined by ELISA (D). *p < 0.01 compared to control mAb-treated group. The colonic histopathology was assessed. Pathology scores (E) and colonic histopathology (F) are shown. **p < 0.01 compared to control mAb-treated group. Data are one representative of two independent experiments.
IL-17 stimulates dendritic cell IL-12 and IL-23 production, and anti-IL-17 mAb inhibits colonic IL-12 and IL-23 production in colitic mice
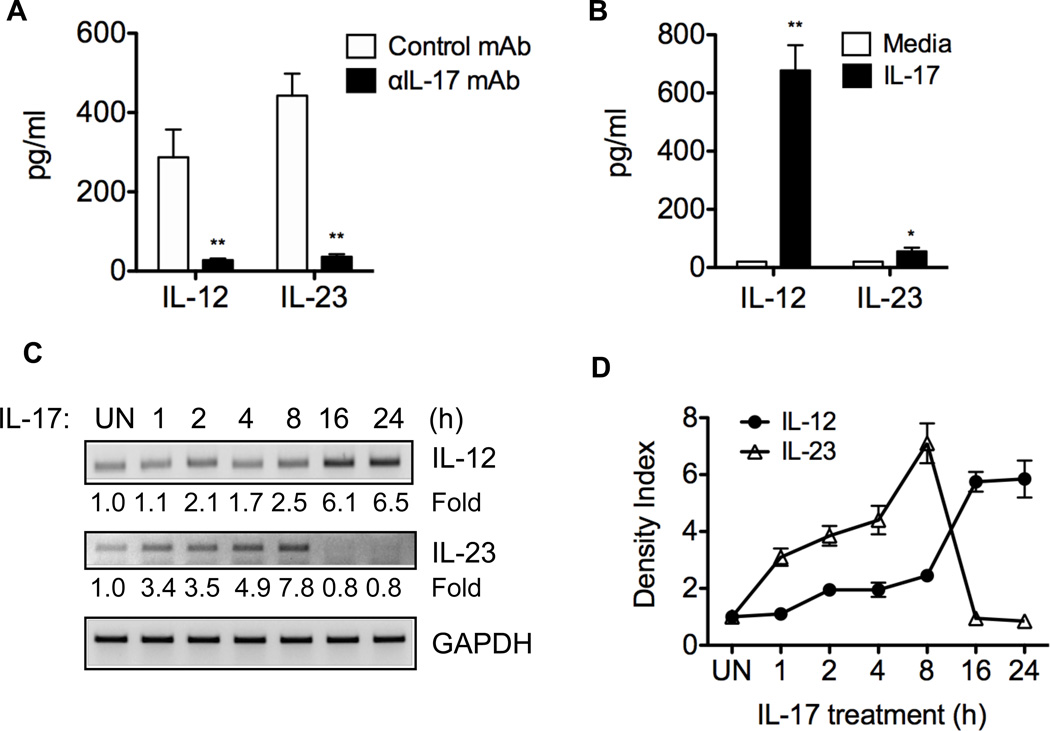
It has been shown that IL-12 and IL-23 mediate Th17 cell conversion to Th1 cells in vitro (24). To determine the role of IL-12 and IL-23 in Th17 cell conversion to Th1 cells in the intestines of CBir1-specific Th17 recipient mice, colonic IL-12 and IL-23 production was determined by ex vivo organ culture. High levels of IL-12 and IL-23 were detected in colonic tissue of control mAb-treated Th17 recipients but not of control TCRβxδ−/− mice that received PBS, and treatment with anti-IL-17 mAb decreased colonic IL-12 and IL-23 production (Figure 4A and data not shown), indicating that Th17 cells induce mucosal IL-12 and IL-23 production, likely through IL-17.
Figure 4. IL-17 mediates Th17 cell induction of colonic IL-12 and IL-23 in vivo and stimulates DC IL-12 and IL-23 production in vitro.
A, 1 × 106 in vitro-polarized CBir1 Tg Th17 cells were transferred into TCRβxδ−/− mice that were administered with anti-IL-17 mAb or control mAb at the time of transfer and weekly thereafter for eight weeks. Colonic tissues of Th17 recipients were cultured for 24 h, and IL-23 and IL-12 production in the supernatants was measured by ELISA. **p < 0.01 compared to control mAb-treated group. One representative of two independent experiments is shown. B, To determine the effect of IL-17 on BMDC IL-12 and IL-23 production, 1 × 106 cells/ml of BMDC were stimulated with IL-17 (100 ng/ml). Supernatants were collected 24 h later, and IL-12 and IL-23 levels were determined by ELISA. *p < 0.05; **p < 0.01 compared to IL-17-treated BMDC. C, BMDC were also harvested at different time points after treatment, and IL-12 and IL-23 mRNA expression was determined by RT-PCR. Data are one representative of two independent experiments with similar results. D, Aggregate data of density from two independent experiments.
IL-12 and IL-23 are produced mainly by activated APC including dendritic cells (DC). To determine the effect of IL-17 on DC IL-12 and IL-23 production, bone marrow-derived DC (BMDC) were treated with IL-17. Supernatant was collected after 24 h of culture, and the cells were harvested at different time points. IL-12 and IL-23 protein and mRNA expression were determined by ELISA and RT-PCR, respectively. As shown in Figure 4B, IL-17 induced BMDC IL-12 and IL-23 protein production. Interestingly, IL-17 differentially stimulated IL-12 and IL-23 mRNA expression: IL-23 mRNA was induced at early time points, peaked at 8 h, and diminished at later time points, whereas expression of IL-12 mRNA occurred later, peaked at 16 – 24 h (Figures 4C and 4D). These data are consistent with a recent report showing that IL-17 induced DC IL-12 production, and promoted T cell IFNγ production (42).
Conditioned media from colons of colitic Th17 recipients induce Th17 cell conversion to Th1 cells
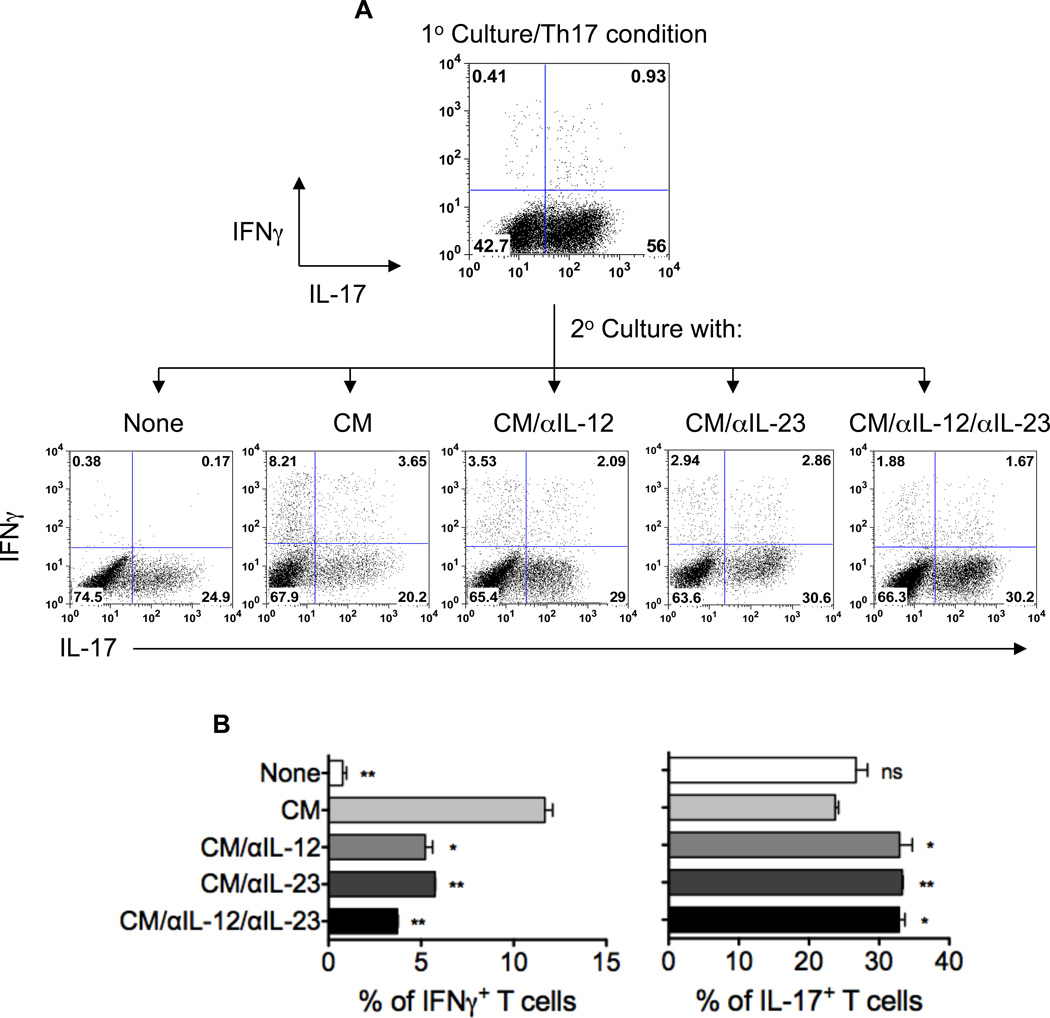
To determine the role of colonic IL-12 and IL-23 from colitic Th17 recipients in promoting Th17 cell conversion to Th1 cells in the intestine, colonic tissues of Th17 cell recipient mice were cultured with complete RPMI, and the culture supernatants were collected after 24 h to serve as “conditioned media” (CM). Culture supernatants from colonic tissues of TCRβxδ−/− mice that were given PBS were also collected and served as control. In vitro-polarized CBir1-specific Th17 cells were restimulated with CBir1 flagellin-pulsed APC in the absence or presence of 20% colonic CM. Five days later, CD4+ T cell IL-17 and IFNγ production was assessed by intracellular staining. As shown in Figure 5, addition of colonic CM from colitic Th17 recipients promoted in vitro-polarized Th17 cell IFNγ production, whereas control CM did not affect Th17 cell IFNγ production (data not shown). Blockade of IL-12 or IL-23 greatly reduced IFNγ production by Th17 cells, and IFNγ production was further inhibited by blocking both IL-12 and IL-23 (Figures 5A and 5B). Together, these data demonstrate that IL-12 and IL-23 are required in order for colonic CM from colitic Th17 recipients to promote IFNγ production by Th17 cells.
Figure 5. Conditioned media from colonic tissues of colitic Th17 recipients induce Th17 cell conversion to Th1 cells through IL-12 and IL-23 production.
A, Colonic tissues of colitic Th17 cell recipient mice were cultured in complete RPMI, and the culture supernatants were collected after 24 h to serve as “conditioned media” (CM). In vitro-polarized CBir1 Th17 cells were restimulated with CBir1 flagellin-pulsed APC in the absence or presence of 20% colonic CM with anti-IL-12, anti-IL-23, or both. Five days later, IL-17 and IFNγ production was assessed by intracellular staining. One representative of three independent experiments is shown. B, Aggregate data of percentages of cytokine-expressing T cells from three independent experiments. *p < 0.05; **p < 0.01 compared to CM-treated group.
Discussion
Although accumulating evidence indicates important roles of Th17 cells in chronic intestinal inflammatory conditions, there is considerable controversy as to whether their signature cytokine IL-17 is essential in the pathogenesis of colitis. Our data show that Th17 cells, specific for a single microbiota antigen, induce colitis. IL-17 plays a crucial role in the pathogenesis of chronic intestinal inflammation. Importantly, IL-17 promoted IFNγ production by T cells in the lamina propria of colitic Th17 cell recipient mice through induction of colonic DC IL-12 and IL-23 production.
Both protective and pathogenic functions of IL-17 have been reported in different experimental models of colitis (36–38). IL-17 and/or IL-17F deficiency did not prevent colitis mediated by transfer of Treg-depleted CD4+ T cells (43–44). Adoptive transfer of IL-17−/− CD45RBhi T cells, compared to wild type counterparts, induced a more severe wasting disease when transferred into RAG−/− mice (37). In contrast, IL-17 deficiency resulted in resistance to DSS-induced colitis, indicating a pathogenic role of IL-17 in intestinal inflammation (38). Moreover, recent genetic studies have shown that IL-23 and its receptor (IL-23R), as well as RORγt, which are essential for the maintenance and differentiation of Th17 cells, are required for IBD (45–46). Interestingly, it has also been shown that polymorphisms in the IL-23R gene are strongly associated with either protection from or susceptibility to Crohn’s disease (18–47). In our current study, transfer of CBir1-specific Th17 cells induced colitis in TCRβxδ−/− mice, and neutralizing IL-17 inhibited colitis development (Figures 2 and 3), indicating a proinflammatory role of Th17 cells and their signature cytokine IL-17 at least in this model. These data, although conflicting, may actually indicate a dual role of Th17/IL-17 in homeostatic and inflammatory settings: Th17/IL-17 is normally involved in the maintenance of intestinal homeostasis by protecting the host from inflammation through induction of antimicrobial peptides (48–49), however, under certain circumstances, Th17/IL-17 is involved in the pathogenesis of intestinal inflammation by recruiting neutrophils and stimulating production of various proinflammatory cytokines (50, 51).
IL-12 and IL-23 have been shown to promote Th17 cell conversion to Th1 cells via a STAT4- and T-bet-dependent manner in vitro (24–27). However, where and how IL-12 and IL-23 are induced in vivo in the first place is still undefined. Our data demonstrate that colonic tissues of colitic Th17 recipient mice produced high levels of IL-12 and IL-23 (Figure 4). Conditioned media (CM) of such colonic tissues promoted Th17 cell conversion to Th1 cells through IL-12 and IL-23, as blockade of CM IL-12 and IL-23 inhibited IFNγ production by Th17 cells (Figure 5). Intriguingly, IL-17 produced by Th17 cells stimulated DC IL-12 and IL-23 production, and neutralizing IL-17 by administration of anti-IL-17 mAb inhibited colonic IL-12 and IL-23 production as well as IFNγ production by T cells in Th17 recipient mice (Figure 4). These data provide direct in vivo evidence that Th17 cells can promote their own conversion to IFNγ-producing T cells by induction of local DC IL-12 and IL-23 production. This is consistent with a recent report that IL-17 is required for Th1 immunity against the intracellular pathogen Francisella tularensis (42). Notably, anti-IL-17 reduced the absolute numbers of single IFNγ-producing cells (Figure 3C), however, the reduction of IFNγ was not complete. This suggests that other mechanisms by which Th17 cells convert to Th1 cells may exist. Among many possibilities, IL-17F, IL-21 or IL-22 could be most likely involved in such process. Although it is still unclear whether Th17 cells act similarly in patients with IBD, recent reports support the notion that Th17 cells in IBD patients may convert to IFNγ-producing T cells via induction of mucosal DC IL-12 and IL-23 production, as there is substantial developmental plasticity in human Th17 clones derived from intestinal isolates of patients with Crohn’s disease (22), and MLN DC from Crohn’s disease patients induce both Th1 and Th17 immune responses, probably through production of IL-12 and IL-23 (8–12). Th17 cell conversion to Th1 cells could provide the pathogenic effect of both T cell subsets for the induction of intestinal inflammation. This can be at least one mechanism for Th17 cells to be more potent than Th1 cells in the induction of colitis.
Acknowledgments
This work was supported by research grants from NIH DK079918, AI083484, DK071176, Digestive Diseases Research Development Center (grant DK064400), RR-20136, NS57563, NS50655, NMSS grant RG3892-A-12, and a start-up fund from University of Texas Medical Branch.
Abbreviations used
- LP
lamina propria
- BMDC
bone marrow derived DC
- TGFβ
transforming growth factor β
- IL
interleukin
- TCR
T cell receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 2.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 4.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn#x00027;s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, Hibi T. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 11.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, Neurath MF, Strober W, Mannon PJ. Both IL-12p70 and IL-23 are synthesized during active Crohn's disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006;12:9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 13.Wirtz S, Finotto S, Kanzler S, Lohse AW, Blessing M, Lehr HA, Galle PR, Neurath MF. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- 14.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H, Lees CW, Onnie CM, Hanson C, Blaszczyk K, Ravindrarajah R, Hunt S, Varma D, Hammond N, Lewis G, Attlesey H, Watkins N, Ouwehand W, Strachan D, McArdle W, Lewis CM, Lobo A, Sanderson J, Jewell DP, Deloukas P, Mansfield JC, Mathew CG, Satsangi J, Parkes M. Investigation of Crohn's disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523–529. e523. doi: 10.1053/j.gastro.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Pare P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Yang Z, Neurath MF, Salfeld J, Veldman GM, Schwertschlag U, Strober W. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 24.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cong Y, Konrad A, Iqbal N, Hatton RD, Weaver CT, Elson CO. Generation of antigen-specific, Foxp3-expressing CD4+ regulatory T cells by inhibition of APC proteosome function. J Immunol. 2005;174:2787–2795. doi: 10.4049/jimmunol.174.5.2787. [DOI] [PubMed] [Google Scholar]
- 30.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, Benveniste EN, Cong Y. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 32.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng T, Cao AT, Waever CT, Elson CO, Cong Y. IL-12 Converts Foxp3+ Regulatory T Cells to Foxp3+IFN-g+ T Cells with Inhibitory Functions During Induction of Colitis. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.03.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duck LW, Walter MR, Novak J, Kelly D, Tomasi M, Cong Y, Elson CO. Isolation of flagellated bacteria implicated in Crohn's disease. Inflamm Bowel Dis. 2007;13:1191–1201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 41.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat(+) innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011 doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leppkes M, C Becker Ivanov, II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 48.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 49.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]