Abstract
The taxane chemotherapeutic paclitaxel frequently produces peripheral neuropathy in humans. Rodent models to investigate mechanisms and treatments are largely restricted to male rats, whereas female mouse studies are lacking. We characterized a range of paclitaxel doses on cold and mechanical allodynia in male and female C57Bl/6 mice. Because the nonpsycho-active phytocannabinoid cannabidiol attenuates other forms of neuropathic pain, we assessed its effect on paclitaxel-induced allodynia. Paclitaxel produced allodynia that was largely dose independent and more robust in female mice, and this effect was prevented by treatment with cannabidiol. Our preliminary findings therefore indicate that cannabidiol may prevent the development of paclitaxel-induced allodynia in mice and therefore be effective at preventing dose-limiting paclitaxel-induced peripheral neuropathy in humans.
Dose-limiting, poorly managed paclitaxel-induced peripheral neuropathy is observed in the setting of advanced breast or ovarian cancer, and although mice are the primary species used in genetic animal models for biomedical research, male rats are almost exclusively the subjects for rodent models of paclitaxel-induced allodynia and hyperalgesia.1,2 Therefore, we have initiated studies to characterize the effect of a range of paclitaxel doses on cold and mechanical allodynia in female and male C57Bl/6 mice.
The phytocannabinoid cannabidiol (CBD) is gaining increasing attention for the treatment of neuropathic pain because it lacks affinity for cannabinoid receptors and therefore their associated side effects, while exerting both anti-inflammatory and analgesic actions.3 Therefore, we assessed the effect of CBD on paclitaxel-induced allodynia in female mice.
METHODS
This study was approved by the Temple University Animal Care and Use Committee and was conducted in a manner that does not inflict unnecessary pain or discomfort upon the animal, as outlined by the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Twenty-six male and 120 female C57Bl/6 mice (ACE Animals, Boyertown, PA) were group housed under a reverse 12-hour light/dark cycle with ad libitum access to food and water. Mice were treated with paclitaxel (1.0 to 8.0 mg/kg IP), Cremophor vehicle (ETOH: Cremophor: saline in a 1:1:18 ratio), or saline on a standard dosing regimen of days 1, 3, 5, and 7. CBD-treated mice (5.0 or 10.0 mg/kg IP) or controls (Cremophor vehicle) were also injected once daily on days 1 to 14. The acetone drop test4 and Von Frey filament assay5 were used to assess cold and mechanical allodynia, respectively. Baseline responses in each assay were determined on separate days before administration of paclitaxel. Subsequent behavioral testing was performed every 3 to 10 days after the start of drug dosing. Briefly, mice were placed inside ventilated polycarbonate chambers on an aluminum mesh table. For assessment of cold allodynia, 0.05 mL of acetone was projected via a needle and syringe onto the plantar surface of the right hindpaw, and time spent attending to the paw was recorded for 60 seconds. For assessment of mechanical allodynia, von Frey filaments ranging from 0.16 to 6 g bending force were applied to the plantar skin of the right hindpaw, and each application was held for 6 seconds, using the up–down method to determine threshold sensitivity. Two-way (GraphPad Prism 4.0) analysis of variance (ANOVA) with the factors of Treatment and Time were used to examine the effect of paclitaxel and CBD treatment within a sex. Three-way (PASW Statistics 18.0) ANOVAs with factors of Treatment, Time, and Sex were used to examine the effect of paclitaxel between sexes.
RESULTS
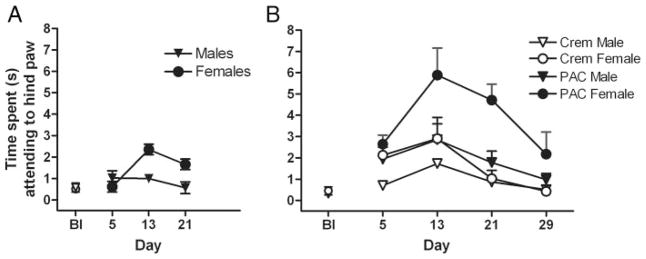
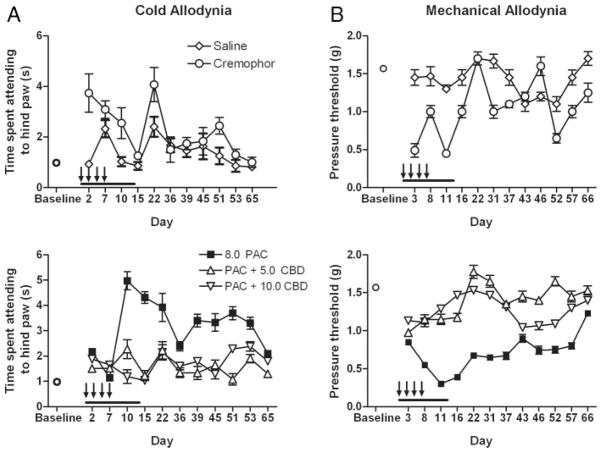
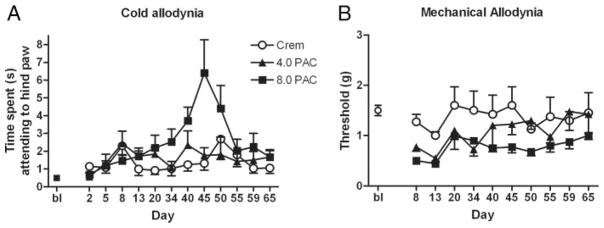
Paclitaxel produced allodynia in male and female C57Bl/6 mice (Figs. 1 to 3). In the present report, as well as in other ongoing studies in our laboratory, these effects were largely dose independent and more robust in female mice, and peaked in general between days 10 and 12, with the exception of the effect on cold allodynia depicted in Figure 2A. The 4 injections of 1.0 mg/kg paclitaxel dosing regimen only produced cold allodynia in female C57Bl/6 mice (Fig. 1A). A 2-way ANOVA revealed significant main effects of sex [F(1,36) = 10.3, P = 0.003] and time [F(2,36) = 5.62, P = 0.008] and a significant interaction [F(2,36) = 6.61, P = 0.004]. Four injections of 2.0 mg/kg paclitaxel produced a more robust cold allodynia, and this effect was significant in female mice (Fig. 1B). A 3-way ANOVA revealed a significant effect of sex (female) [F(1,48) = 7.51, P = 0.009], treatment (paclitaxel) [F(1,48) = 10.8, P = 0.002], and time (day 13) [F(1,48) = 5.71, P = 0.02] but no interactions [Fs<1.0]. These data also reveal that treatment with the Cremophor vehicle alone increases cold allodynia in relation to baseline. On the basis of these findings, we increased the paclitaxel doses to 4.0 and 8.0 mg/kg, proceeded with only females, and added a mechanical allodynia measurement. In this study, 4 injections of paclitaxel produced significant cold allodynia (Fig. 2A) and mechanical allodynia (Fig. 2B) in female mice. For cold allodynia, a 2-way ANOVA revealed a significant effect of treatment [F2203 = 7.62, P = 0.0002] and time [F(6203) = 2.75, P = 0.0008], and a significant interaction [F(12,203) = 2.22, P = 0.0261]. For mechanical allodynia, a 2-way ANOVA revealed a significant effect of treatment [F(2126) = 9.35, P <0.0001] and time [F(2126) = 2.51, P = 0.046], but no significant interaction [F<1.0]. Lastly, we assessed the effect of 5.0 and 10.0 mg/kg CBD on paclitaxel-induced allodynia (8.0 mg/kg), and we also added a saline control group given the behavioral effects observed with the Cremophor vehicle (Fig. 3). Control and drug data are depicted separately for visual clarity, but all subjects were run in cohorts together, and data were analyzed together for each allodynia measurement by 2-way ANOVAs. Both doses of CBD prevented the development of paclitaxel-induced cold and mechanical allodynia. For cold allodynia (Fig. 3A), a 2-way ANOVA revealed significant effects of treatment [F(4345) = 56.0, P <0.0001] and time [F(10,345) = 8.16, P <0.0001] and a significant interaction [F(39,345) = 5.51, P <0.0001]. For mechanical allodynia (Fig. 3B), a 2-way ANOVA revealed significant effects of treatment [F(4392) = 236, P <0.0001] and time [F(11,392) = 32.4, P <0.0001] and a significant interaction [F(44,392) = 11.0, P <0.0001]. Bonferroni post-tests revealed significant differences between saline and Cremophor, Cremophor and paclitaxel, and paclitaxel and 5.0 and 10.0 CBD treatments on both measures.
Figure 1.
Effect of 4 1.0 mg/kg (A) and 2.0 mg/kg (B) paclitaxel injections on cold allodynia in male and female C57Bl/6 mice. Paclitaxel 1.0 mg/kg × 4 injections produced significant main effects of sex (P = 0.003) and time (P = 0.008) (P = 0.004). Paclitaxel 2.0 mg/kg × 4 injections produced significant effect of sex (female: P = 0.009), treatment (paclitaxel: P = 0.002), and time (day 13: P = 0.02) but no interactions. X-axes: Timepoints pre- or postday 1 injection (Bl = baseline). Paclitaxel (PAC) or Cremophor (Crem) was injected on days 1, 3, 5, and 7. Y-axes: Time spent by mice lifting, fluttering, or licking hindpaw after administration of 10 μL acetone. Data points represent the mean time spent attending to the hindpaw in seconds and SEM (vertical bars); n = 6 to 10 per group.
Figure 3.
Effect of repeated cannabidiol (5.0 to 10.0 mg/kg) on paclitaxel-induced cold (A) and mechanical (B) allodynia in female C57Bl/6 mice. For cold and mechanical allodynia, significant effects of treatment (Ps<0.0001) and time (Ps<0.0001) and a significant interaction (Ps<0.0001) were observed. Bonferroni posttests revealed significant differences between saline and Cremophor, Cremophor and paclitaxel, and paclitaxel and 5.0 and 10.0 cannabidiol (CBD) treatments on both measures. X-axes: Time points pre- or postday 1 injection. Paclitaxel (PAC), Cremophor, or saline was injected on days 1, 3, 5, and 7 (indicated by arrows). CBD or Cremophor was injected on days 1 to 14 (indicated by black line). Y-axes: Time spent by mice lifting, fluttering, or licking hindpaw after administration of 10 μL acetone (A) or threshold pressure, which evoked a paw lift (B). Data points represent mean time spent attending to the hindpaw in seconds (A) or mean pressure required to evoke a lift of the hindpaw in grams (B) and SEM (vertical bars); n = 8 per group. Bonferroni posttests revealed significant differences between saline and Cremophor, Cremophor and paclitaxel, and paclitaxel and 5.0 and 10.0 CBD treatments on both measures.
Figure 2.
Effect of 4 4.0 mg/kg and 8.0 mg/kg paclitaxel on cold (A) and mechanical (B) allodynia in female C57Bl/6 mice. Paclitaxel produced a significant effect of treatment (P = 0.0002) and time (P = 0.0008) and a significant interaction (P = 0.0261) on cold allodynia. X-axes: Paclitaxel produced a significant effect of treatment (P < 0.0001) and time (P = 0.046), but no significant interaction on mechanical allodynia. Timepoints pre- or postday 1 injection (Bl = baseline). Paclitaxel (PAC) or Cremophor (Crem) was injected on days 1, 3, 5, and 7. Y-axes: Time spent by mice lifting, fluttering, or licking hindpaw after administration of 10 μL acetone (A) or threshold pressure, which evoked a paw lift (B). Data points represent mean time spent attending to the hindpaw in seconds (A) or mean pressure required to evoke a lift of the hindpaw in grams (B) and SEM (vertical bars); n = 8 to 12 per group.
DISCUSSION
The important and novel aspects of the present study include (1) a comprehensive allodynia assessment in a mouse strain that is widely used as a genetic animal model; (2) the side-by-side comparison of the allodynic effects of paclitaxel in female and male rodents; and (3) a characterization of the effects of CBD and Cremophor vehicle on these behaviors. Our results are in agreement with a previous mouse strain comparison1 regarding a lack of strong dose dependency of paclitaxel’s allodynic effects in mice. However, unlike the previous report, we found paclitaxel to be relatively ineffective at producing cold allodynia in male C57Bl/6 mice while showing efficacy at producing both cold and mechanical allodynia in females. We recognize that some inconsistencies within this preliminary data set and in comparison with other lab’s findings are present, such as differences in peak effects across time courses or the observed lack of cold allodynia in male paclitaxel-treated mice. We attribute some of these inconsistencies to the natural variability of this form of testing; however, our statistical analyses indicate that the reported main effects are significant. Also, we have recently replicated the lack of cold allodynia effect in another set of male mice as well as the most commonly observed time course of peak allodynia effects between days 10 and 12 in several additional groups of female mice, and we are aware of a study in rats that also reported increased allodynia in females in comparison with males after sciatic nerve ligation-induced neuropathy.6
These data also revealed that in comparison with baseline, treatment with the Cremophor vehicle alone increases cold allodynia measurements, an important experimental control group that is not always run alongside paclitaxel-treated animals in the literature.7 It is important to note that Cremophor produces other, well-characterized adverse events in chemotherapy patients,8 and these data suggest that investigation into the relative contribution of Cremophor to paclitaxel-induced neuropathy in humans is needed. Concomitant treatment with CBD completely prevented the development of paclitaxel-induced cold and mechanical allodynia, with no latent neuropathy emerging after the cessation of CBD treatment. These results support a larger literature demonstrating the ability of CBD to ameliorate allodynia and hyperalgesia associated with other neuropathic pain states, both in animal models and human patients.9 Further work is warranted to investigate potential mechanisms underlying CBD’s effectiveness, beginning with its potent effects at reversing key proinflammatory cytokine levels,10 because paclitaxel has been shown to increase their expression.11 Adjunct treatment with CBD may be effective in the prevention or attenuation of paclitaxel-induced neuropathic pain and improve the outcomes for patients administered this chemotherapeutic drug.
Acknowledgments
Funding: Supported by R01 CA129092 (to E.A.W.) and Peter F. McManus Charitable Trust (to E.A.W.).
The authors greatly appreciate the time and expertise provided by Dr. Peter McLaughlin regarding data analysis using the 3-way analysis of variance.
Footnotes
The authors declare no conflict of interest.
Reprints will not be available from the authors.
DISCLOSURES
Name: Sara Jane Ward, PhD.
Contribution: Sara Jane Ward was responsible for study design, data analysis, and manuscript preparation and participated in conduct of the study.
Attestation: Sara Jane Ward is the archival author and attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Ward also attests to approving the final manuscript.
Name: Michael David Ramirez, BS.
Contribution: Michael David Ramirez was responsible for conduct of the study and participated in manuscript preparation.
Attestation: Michael David Ramirez attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Ramirez also attests to approving the final manuscript.
Name: Harshini Neelakantan, MS.
Contribution: Harshini Neelakantan was responsible for study design and conduct of the study.
Attestation: Harshini Neelakantan attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Neelakantan also attests to approving the final manuscript.
Name: Ellen Ann Walker, PhD.
Contribution: Ellen Ann Walker participated in manuscript preparation and study design and provided the funding for this project.
Attestation: Ellen Ann Walker attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Walker also attests to approving the final manuscript.
References
- 1.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Golden JP, Johnson EM. Models of chemotherapy drug-induced peripheral neuropathy. Drug Discovery Today: Disease Models. 2004;1:186–91. [Google Scholar]
- 3.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol—recent advances. Chem Biodivers. 2007;4:1678–92. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–76. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yakshm TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.DeLeo JA, Rutkowski MD. Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neurosci Lett. 2000;282:197–9. doi: 10.1016/s0304-3940(00)00880-6. [DOI] [PubMed] [Google Scholar]
- 7.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–49. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from Taxol. J Clin Oncol. 1990;8:1263–8. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 9.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;7:607–15. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 10.Toth CC, Jedrzejewski NM, Ellis CL, Frey WH., 2nd Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. doi: 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–98. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]