Abstract
Rationale
A number of cancer chemotherapeutic agents have been associated with a loss of memory in breast cancer patients although little is known of the causality of this effect.
Objectives
To assess the potential cognitive effects of repeated exposure to chemotherapeutic agents, we administered the selective estrogen receptor modulator tamoxifen or the antimetabolite chemotherapy, methotrexate, and 5-fluorouracil, alone and in combination to mice and tested them in a learning and memory assay.
Methods
Swiss-Webster male mice were injected with saline, 32 mg/kg tamoxifen, 3.2 or 32 mg/kg methotrexate, 75 mg/kg 5-fluorouracil, 3.2 or 32 mg/kg methotrexate in combination with 75 mg/kg 5-fluorouracil once per week for 3 weeks. On days 23 and 24, mice were tested for acquisition and retention of a nose-poke response in a learning procedure called autoshaping. In addition, the acute effects of tamoxifen were assessed in additional mice in a similar procedure.
Results
The chemotherapeutic agents alone and in combination reduced body weight relative to saline treatment over the course of 4 weeks. Repeated treatment with tamoxifen produced both acquisition and retention effects relative to the saline-treated group although acute tamoxifen was without effect except at a behaviorally toxic dose. Repeated treatment with methotrexate in combination with 5-fluorouracil produced effects on retention, but the magnitude of these changes depended on the methotrexate dose.
Conclusions
These data demonstrate that repeated administration of tamoxifen or certain combination of methotrexate and 5-fluorouracil may produce deficits in the acquisition or retention of learned responses which suggest potential strategies for prevention or remediation might be considered in vulnerable patient populations.
Keywords: Acquisition, Autoshaping, Chemotherapy, 5-Fluorouracil, Methotrexate, Mice, Tamoxifen, Retention
Many clinical studies demonstrate cognitive deficits during and after the use of common chemotherapeutic agents in the clinical adjuvant setting for early-stage breast cancer (Schagen and van Dam 2006; Schagen et al. 1999). Such symptoms such as reduction in verbal memory, processing speed, and the ability to concentrate are noted in these studies (Ahles et al. 2002; Brezden et al. 2000; Schagen et al. 1999). In contrast, other studies do not find impairments (Donovan et al. 2005; Jenkins et al. 2005). The potential of chemotherapeutic agents to induce early menopause in female cancer patients (Ahles and Saykin 2007) suggests that synergistic impairments might be observed when chemotherapeutic agents are administered to female patients in combination with antiestrogens. Indeed, greater cognitive impairments in attention, mental flexibility, concentration, visual memory, and speed of information processing are reported for women receiving the antiestrogen, tamoxifen (Bender et al. 2006; Castellon et al. 2004; Schilder et al. 2010), or aromatase inhibitors such as anastrozole in their chemotherapy regimen (but see Bender et al. 2007; Schilder et al. 2009; Schilder et al. 2010 for exemestane). These links between chemotherapy, endocrine therapies, and cognitive impairment in the clinical literature are intriguing, especially since the role of endocrine therapy remains an important element of treating hormone-sensitive breast cancer.
Preclinical studies in rodent learning models can directly assess the role of the chemotherapeutic agents on learning, memory, and brain function. For example, three weekly injections of methotrexate and 5-fluorouracil to mice produce deficits in tests of spatial memory, non-matching-to-sample learning, and delayed non-matching-to-sample learning, yet there were no changes noted in cued memory or a discrimination learning test (Winocur et al. 2006). In the Morris Water maze and novel object recognition task, rats treated with methotrexate show deficits that correlate with decreased hippocampal cell proliferation (Seigers et al. 2008). Cyclophosphamide and 5-fluorouracil produce transient memory deficits in a mouse step-down inhibitory avoidance-conditioning task without altering open-field behavior or locomotion (Reiriz et al. 2006). Cognitive deficits after treatment with chemotherapeutic agents in rodents are not always observed. For example, repeated injections of cyclophosphamide or 5-fluorouracil cause a transient enhancement of both memory and hippocampal synaptic plasticity in spatial learning tasks (Lee et al. 2006).
Our laboratory has previously examined the effects of acute injections of multiple doses of methotrexate and 5-fluorouracil and observed reduced retention of newly learned autoshaped responses in mice especially when lower doses of methotrexate were combined with 5-fluorouracil (Foley et al. 2008). These reductions in performance occurred in mice without altering the motivation to respond for the same palatable food in a progressive ratio procedure (Foley et al. 2008) or without producing state-dependent learning in the same autoshaping procedure (Walker 2010). In the present study, we examined tamoxifen, a selective estrogen receptor modulator for its capacity to disrupt learning and memory processes in the same autoshaping-operant procedure used previously for methotrexate and 5-fluorouracil (Foley et al. 2008; Walker 2010). To determine how repeated treatment of these compounds would impact learning and memory in a slight variation on the previous autoshaping procedure, we selected treatment doses for the present study using multiple criteria: individual doses from our dose–response study that produced either no or moderate retention deficits without altering motor or motivational behavior (Foley et al. 2008 Walker 2010), doses similar to treatment cycles of chemotherapy in breast cancer patients (Bonadonna et al. 2005) and estimated from allometric scaling to match these common clinical doses (S. Nagar, personal communication), and finally a previous repeated treatment study examining these agents in the Morris water maze (Winocur et al. 2006).
Methods
Subjects
Male, Swiss-Webster mice (N=153) weighing 20–25 g were obtained from ACE Animals, Inc., (Philadelphia, PA, USA). Mice were group-housed in a vivarium maintained at 70°F and humidity at 30–40% under a 12-h light—dark cycle within polycarbonate cages with Bed O Cob bedding (Andersons Cob Products, Inc., Maumee, OH, USA). This corn-cob-based product fails to alter liver endosomes (Buddaraaju and Van Dyke 2003). Mice had access to food and water ad libitum until behavioral testing. Twenty-four h prior to the first day of testing in the autoshaping procedure, mice were food-restricted for 24 h and weighed and then separated into individual cages with water available ad libitum. In the acute tamoxifen experiments, individual housing occurred 1 week after arrival in the vivarium, and in the chronic treatment experiments, individual housing occurred at the start of the fourth week (day 22) and after all injections had been performed (see below). All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Temple University and the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (Institute of Laboratory Animal Resources 2003). The highest standards of animal welfare were maintained throughout these studies, and the experiments were specifically designed to reduce the number of mice required.
Apparatus
Twelve mouse experimental chambers (21.6× 17.8×12.7 cm, Model ENV-307W, MED Associates, St. Albans, VT, USA) were used, each located within a sound-attenuating enclosure and connected to a computer-driven interface (Model SG-502, MED Associates, St. Albans, VT, USA) which controlled the experimental conditions and collected the data. One wall of the chamber contained three receptacles: one large dipper hole in the center (ENV 313M) and two smaller nose-poke holes on the left and right (ENV 313W). A dipper lever and dipper well were located behind the center dipper hole. The opposite wall featured a house light that illuminated the chamber during the session. Each chamber was also equipped with an audible tone device (Sonalert, 2,900 Hz, Mallory Sonalert, Indianapolis, IN, USA). Nose-poke responses into each hole were detected by photocell head entry detectors (ENV 303HD) and recorded.
Chronic injection schedule
After 1 week in the vivarium, mice (n=10–12/group) were separated into six groups. On day 1, mice were weighed and injected with either saline, tamoxifen, methotrexate, 5-fluorouracil, or a combination of methotrexate and 5-fluorouracil. The injections were repeated weekly for a total of three injections over 3 weeks (days 1, 8, and 15), and the mice were tested for acquisition on day 23 and retention on day 24.
Autoshaping procedure
Twenty-four hours after food restriction, the mice began the acquisition phase of the autoshaping-operant procedure for the tamoxifen (day 1; Foley et al. 2008) and the autoshaping procedure for chronic treatment experiments (day 22; Vanover and Barrett 1998). Mice were weighed and placed inside the experimental chambers for a 15-min habituation period. At the start of the session, the house light illuminated the chamber and the mice were presented a tone on a variable-interval schedule (mean of 45 s, range 4–132 s), with the tone remaining on for 6 s or until a nose-poke response occurred. If the mouse made a dipper-hole, nose-poke response during the tone, a dipper lever with 0.01 ml of a 50:50 vanilla-flavored Ensure/water solution was presented, and the tone was turned off. If no dipper-hole response was made during the tone, there were no consequences in the acute tamoxifen experiments, but in the chronic treatment experiments, the dipper lever of Ensure/water solution was presented at the end of the 6-s tone. This procedural difference was incorporated into the chronic treatment experiments to provide an additional cue to assist the mice while learning the task. Each autoshaping session lasted for 2 h or until 20 reinforced nose pokes were recorded. After the session, mice were fed 4 g of food and returned to their cages until testing again 24 h later. On the second day, all mice were placed back into the chambers for the retention test, either day 2 for the acute tamoxifen experiments or day 24 for the chronic treatment studies, and the autoshaping sessions proceeded as described above.
Drugs
Methotrexate, tamoxifen citrate salt, and 5-fluorouracil (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile water for injection. Different groups of mice were injected i.p. with saline, tamoxifen, methotrexate, 5-fluorouracil, or a combination of methotrexate and 5-fluorouracil in volumes of 0.01 ml/g. In the acute tamoxifen experiments, mice were injected 15 min prior to the 15-min habituation period. In the combination experiments, mice received two injections, one on either side of the peritoneal cavity. All chemotherapeutic agents were stored and handled in accordance with guidelines set forth by the Temple University Department of Environmental Health and Radiation Safety.
Data and statistical analysis
Each response into the center nose-poke hole in concert with the tone produced a 3-s presentation of the dipper of Ensure/water solution and was recorded as a reinforced response. Four different but related measures of acquisition and retention were determined. As a measure of the accuracy of responding, the percentage of responses during the tone (or percentage of correct responses) was determined by dividing the total number of reinforced responses made during the tone by the total number of dipper presentations per session multiplied by 100. This measure was only calculated in the chronic treatment experiments. Another measure of acquisition and retention was the adjusted latency which is determined as the time required in seconds to make the tenth reinforced response minus the time from the first reinforced response (L10−L1). This adjustment corrects for the unequal opportunity to make the first reinforced response for each mouse due to the nature of the variable ratio schedule of the tone stimulus (Vanover and Barrett 1998). The third measure of acquisition and retention was the total number of responses in the dipper nose-poke hole during the session—regardless of the presence or absence of the tone—divided by the total session time (seconds) for each mouse. Finally, the number of responses into the left and right nose-poke holes was also recorded and divided by the total session time (seconds) for each mouse. This response-rate measure was taken as an assessment of general activity and as errors in discrimination. The data from all mice were included in data analysis from the acquisition sessions. However, the data from mice that failed to make at least ten reinforced responses during acquisition were excluded from the adjusted latency measures on the retention. The rationale for this exclusion is that it is inappropriate to evaluate the retention of a response that was insufficiently reinforced, or not reinforced at all, during the acquisition session (Vanover and Barrett 1998).
To ascertain the mice were acquiring and retaining the autoshaped response in this procedure, paired t tests were used to compare acquisition to retention measures in the saline control groups. One-way analysis of variance (ANOVA) was used to evaluate the effects of acute tamoxifen administration on each measure relative to the effects of saline on acquisition or retention. Dunnett's post hoc test was used where significance (p<0.05) was indicated. Two-way ANOVA was used to evaluate the effects of methotrexate treatment (3.2 and 32 mg/kg) on saline or 5-fluorouracil alone on each measure on acquisition or retention. Bonferroni's test was used where significance (p<0.05) was indicated. Finally, one-way ANOVAs were used to determine the effects of chronic treatment of chemotherapeutic agents alone and in combination on body weight relative to the effects of saline administration over the course of the 4-week experiment.
Results
Acute saline control and tamoxifen experiments
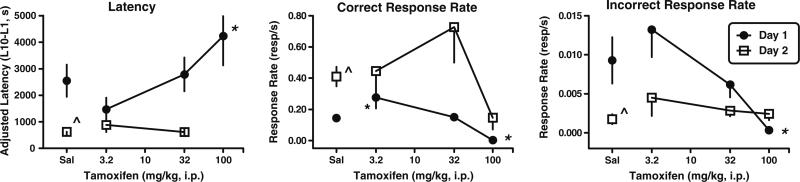
Similar to the data reported in Foley et al. (2008), mice injected with saline on day 1 acquired the nose-poke response and performed this nose-poke response with a shorter mean adjusted latency, a faster rate of responding in the reinforced dipper well, and a slower rate of responding in the non-reinforced left and right nose-poke holes on day 2 (Fig. 1). Overall one-way ANOVA indicated significant effects of tamoxifen on autoshaped-operant responding for both mean adjusted latency (F(3, 34)=4.53; p<0.01) and rate of response in the correct nose-poke hole (F(3, 34)=8.39; p<0.003) on day 1. Although the mean adjusted latency was not different on day 1 (Fig. 1, left panel), mice injected with 3.2 mg/kg tamoxifen responded more quickly in the correct nose-poke hole than the saline control mice (p<0.05; Fig. 1, center panel). Mice injected with 32 mg/kg tamoxifen on day 1 responded similarly to the saline control mice on days 1 and 2 indicating that this dose of tamoxifen does not impact autoshaped-operant responding. However, increasing the tamoxifen dose to 100 mg/kg dramatically increased the mean adjusted latency (p<0.05) and decreased the rate of responding in the correct nose-poke hole (p<0.05) on day 1 during acquisition. Only three mice responded on day 1 after 100 mg/kg tamoxifen, and these three mice each earned less than ten reinforcers so were not included in the data analysis for mean adjusted latency on day 2 (Fig. 1, left panel). Although these mice responded more slowly on day 2 than the saline control mice, these rates of responding were not significantly different (Fig. 1, center panel).
Fig. 1.
Effects of acute injections of tamoxifen on autoshaped-operant responding on acquisition day 1 (closed circles) and retention day 2 (open squares) in male, Swiss-Webster mice. Ordinates: adjusted mean latency (latency to the tenth reinforcer [L10]−latency to the first reinforcer [L1]) in seconds (left); total number of nose pokes in the center, reinforced dipper well per second (middle); and total number of nose pokes in the non-reinforced right or left nose poke holes per second (right). Total number of mice tested on day 1/number of mice completing ten reinforcers on day 1 and therefore included in the day 2 latency measures: saline [12/11] and tamoxifen 3.2 mg/kg [6/6], 32 mg/kg [11/10], and 100 mg/kg [6/0]. Abscissa: dose in milligrams per kilogram administered i.p. Points above Sal are the effects of saline alone. Vertical lines represent SEM. *p<0.05 relative to saline responding.p̂<0.05 for day 1 vs. day 2
Effects of repeated treatment of chemotherapeutic agents on body weight
All groups of mice continued to grow throughout the 4-week course of treatment and testing (Table 1); however, the mice treated with the chemothera peutic agents did not gain weight at the same rate as the mice treated with saline. At the beginning of week 1, prior to any injections, all groups of mice except the 3.2-mg/kg methotrexate and 5-fluorouracil group were the same body weight. By week 3, after two injections of chemotherapeutic agents, all groups of mice weighed significantly less than the saline-treated mice (F(6, 113)=6.67; p<0.0001). This trend continued so that by week 4, 1 week after the last injection and 24 h after food restriction, all groups of mice weighed significantly less than the saline-treated mice (F(6, 113)=10.2; p<0.0001).
Table 1.
Body weights for mice over the 4 weeks of experiments
| Week 1 | Week 2 | Week 3 | Test—week 4e | |
|---|---|---|---|---|
| Saline | 27.6±1.0a | 32.8±0.69b,c | 35.4±0.46c,d | 35.7±0.44c,f |
| 32 mg/kg tamoxifen | 25.2±0.29 | 28.7±0.52c,* | 31.1±0.57c,* | 32.1±0.61c,* |
| 75 mg/kg 5-FU | 27.1±0.48 | 31.0±0.49c | 32.2±0.44c,* | 32.5±0.39c,* |
| 3.2 mg/kg methotrexate | 24.0±0.26 | 29.1±0.31c,* | 32.3±0.45c,* | 31.6±0.27c,* |
| 32 mg/kg methotrexate | 25.5±0.86 | 30.6±0.69c | 32.4±0.64c,* | 32.1±0.37c,* |
| 3.2 mg/kg methotrexate+5-FU | 23.5±0.5* | 28.6±0.65c,* | 31.5±0.79c,* | 31.2±1.04c,* |
| 32 mg/kg methotrexate+5-FU | 27.9±1.1 | 30.0±0.98* | 33.0±0.50c,* | 32.0±0.54c,* |
p<0.05 (significantly smaller than the saline group that week)
Significant differences in body weight between the groups (F(6, 113)=3.22; p<0.01)
Significant differences in body weight between the groups (F(6, 113)=3.74; p<0.002)
Significant growth as compared to week 1
Significant differences in body weight between the groups (F(6, 113)=6.67; p<0.0001)
Mice were food-restricted 24 h prior to this measurement
Significant differences in body weight between the groups (F(6, 113)=10.2; p<0.0001)
Repeated saline control experiments
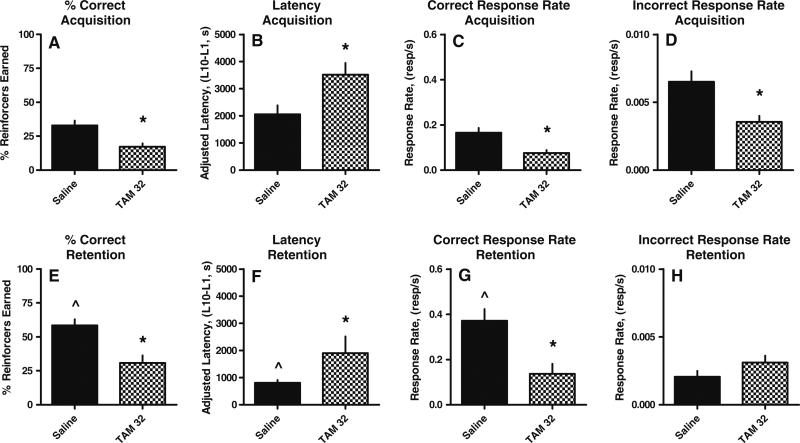
From day 23 to day 24, the saline control mice significantly increased the percentage of correct responses from 32%±3.6% to 59%±4.4% (t=6.1, p≤0.0002; Fig. 2a, e), decreased the mean adjusted latency from 2,052±334 to 810±96 s (t=5.7, p≤0.0004; Fig. 2b, f), and increased the overall rate of responding into the dipper well from 0.17±0.021 to 0.37±0.052 resp/s (t=3.9, p≤0.003; Fig. 2c, g), respectively, indicating that repeated saline treatment does not impair the capacity of the mice to learn and retain autoshaped responses. Although the non-reinforced nose-poke response rate decreased from 0.0065± 0.00077 to 0.0021±0.00042 resp/s (Fig. 2d, h), this response rate was not significantly different than the rate on day 23. One mouse in the saline group (n=22) failed to earn ten reinforcers on day 23 indicating an individual acquisition deficit; therefore, the adjusted latency data from this mouse were not included in the day 24 retention analyses or figures.
Fig. 2.
Effects of the repeated treatment of saline or tamoxifen (TAM) on the acquisition (upper) and retention (lower) of learned responses in mice. Mice were injected i.p. with saline (N=22) or 32 mg/kg TAM (N=10) once per week for 21 days. Ordinate: a, e number of correct center nose-poke responses made during the tone divided by the total number of center nose-poke responses times 100; b, f adjusted latency (latency to the tenth reinforcer [L10] minus the latency to the first reinforcer [L1]) in seconds; c, g total number of nose pokes in the center, reinforced dipper well per second; d, h total number of nose pokes in the non-reinforced right or left nose poke holes per second. Abscissa: drug and dose, in milligrams per kilogram, administered once per week for 3 weeks. *Significantly different than saline control as indicated by unpaired t test; ŝignificantly different than day 1 saline performance as indicated by paired t test. Significance set at p<0.05
Repeated treatment of single chemotherapeutic agents
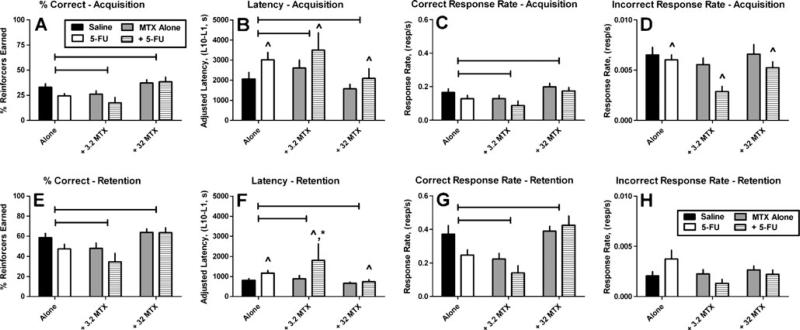
Overall, the effects of repeated administration of tamoxifen on behavior were significantly different than the effects of repeated administration of saline for every performance measure on both days 23 and day 24 with the exception of the non-reinforced response rate on day 24 (Fig. 2). On day 23, prior administration of tamoxifen decreased the percentage of reinforcers earned (p≤0.01; Fig. 2a), increased mean adjusted latency (p≤0.02; Fig. 2b), decreased rate of responding in the dipper well (p≤0.01; Fig. 2c), and decreased the non-reinforced response rate (p≤0.02; Fig. 2d). On day 24, prior administration of repeated doses of tamoxifen decreased the percentage of reinforcers earned (p≤0.001; Fig. 2e), increased mean adjusted latency (p≤ 0.02; Fig. 2f), and decreased overall rate of responding in the dipper well (p≤0.01; Fig. 2g). Repeated administration of methotrexate alone did not significantly alter any day 23 acquisition or day 24 retention measure relative to repeated saline administration (Fig. 3). There were significant overall increases in mean adjusted latency for 5-fluorouracil relative to saline and methotrexate alone for both the acquisition (F(1, 102)=4.48; p<0.04; Fig. 3b) and retention (F(1, 93)=9.36; p<0.003) measures (Fig. 3f) and significant overall decreases in the response rate in the non-reinforced nose-poke holes on the acquisition day (F(1, 102)=5.23; p<0.02; Fig. 3d). One mouse in the 32 mg/kg methotrexate group (n=22) and two mice in the 5-fluorouracil group (n=22) failed to earn ten reinforcers on day 23; therefore, the adjusted latency data from these mice were not included in the day 24 retention analyses or figures. The observation that these three mice did not complete ten reinforcers during the 2-h session demonstrates an acquisition deficit for these mice.
Fig. 3.
Effects of repeated treatments of saline, methotrexate (MTX) or 5-fluorouracil (5FU) alone and in combination on the acquisition (upper) and retention (lower) of learned responses in mice. Mice were injected i.p. with saline (N=22; from Fig. 2), 75 mg/kg 5-FU (N=22), 3.2 mg/kg MTX (N=10), 32 mg/kg MTX (N=22), 3.2 mg/kg MTX in combination with 75 mg/kg 5-FU (N=10) or 32 mg/kg MTX in combination with 75 mg/kg 5-FU (N=22) once per week for 21 days. Horizontal bars over columns indicate significant effects for methotrexate treatment vs. either saline of 5-FU alone as indicated by two-way ANOVA. ^Significantly different than saline or methotrexate alone as indicated by Bonferroni's test; *significantly different than all other treatments as indicated by Bonferroni's test. Significance set at p<0.05
Effects of repeated administration of methotrexate in combination with saline or 5-fluorouracil
Overall, twoway ANOVAs revealed that methotrexate treatment produced significant changes to the percentage of reinforcers earned on day 23 acquisition (F(2, 102)=7.34; p<0.001; Fig. 3a) and day 24 retention (F(2, 102)=8.23; p<0.0005; Fig. 3e), the mean adjusted latencies on day 23 acquisition (F(2, 102)=3.57; p<0.03; Fig. 3b) and day 24 retention (F(2, 93)=5.74; p<0.005; Fig. 3f), and the dipper response rates on day 23 acquisition (F(2, 102)=5.05; p<0.008; Fig. 3c) and day 24 retention (F(2, 102)=9.37; p<0.0002; Fig. 3g). In addition, the dose of 3.2 mg/kg methotrexate in combination with 75 mg/kg 5-fluorouracil produced greater deficits during the retention session for mean adjusted latencies relative to the effects of saline, 3.2 or 32 mg/kg methotrexate alone, 75 mg/kg 5-fluorouracil alone, or the combination of 32 mg/kg methotrexate and 75 mg/kg 5-fluorouracil (p<0.05; Fig. 3f). In the present study, four mice in the 3.2-mg/kg methotrexate and 5-fluorouracil group (n=10) and two mice in the 5-fluorouracil group and 32 mg/kg methotrexate group (n=22) failed to earn ten reinforcers on day 23; therefore, the adjusted latency data from these mice were not included in the day 24 retention analyses or figures. The observation that these six mice did not complete ten reinforcers during the 2-h session demonstrates an acquisition deficit in these mice.
Discussion
In our study, using autoshaped responding as a model for learning and memory, mice treated with saline readily acquired a nose-poke response synchronized with an audible tone to earn palatable food reinforcers. The mice retained this response the following day as indicated by (1) an increase in the percentage of correct responses, (2) a decrease in the latency to earn ten reinforcers, and (3) an increase in the response rate in the reinforced nose-poke hole similar to previous studies (Foley et al. 2008; Barrett and Vanover 2003). Tamoxifen, given acutely 30 min prior to the acquisition session, did not impair acquisition or retention up to doses of 32 mg/kg. The higher dose of 100 mg/kg tamoxifen did, however, completely disrupt responding during the acquisition session. Twenty-four hours later, the mice responded during the retention session, albeit slowly, with four of the six mice earning all 20 reinforcers suggesting that the behaviorally suppressant effects of 100 mg/kg tamoxifen are diminished by 24 h. This dose of 100 mg/kg tamoxifen in mice would be considered a high, but still clinically relevant dose, as converted from the usual regimens used in adjuvant breast cancer chemotherapy (Kisanga et al. 2003; 2004; Robinson et al. 1991). Although 32 mg/kg tamoxifen did not produce any overt effects on autoshaped learning when injected acutely, three weekly repeated injections significantly disrupted all measures of learning and memory. On days 23 and 24, 32 mg/kg tamoxifen produced the greatest effects by a single agent on the acquisition, consolidation, and/or retention of autoshaped responding. The inclusion of tamoxifen in our study is important because such anti-estrogenic drugs are commonly used in breast cancer pharmacotherapy (Jones and Buzdar 2004), and a series of cognitive impairments have been reported for patients receiving tamoxifen and some but not all aromatase inhibitors (Bender 2006; Bender et al. 2001; Castellon et al. 2004; Schilder and Schagen 2007). In mice, 10 mg/kg tamoxifen decreases escape latency in two passive avoidance tasks, delays latencies in an appetitively motivated T-maze, and impairs retrieval but not acquisition of spatial information processing in the Morris water maze (Chen et al. 2002a, b). Similarly, tamoxifen disrupts the consolidation and retrieval of morphine-associated contextual memory in mice (Esmaeili et al. 2009). The observation that tamoxifen disrupts consolidation and retrieval in a range of preclinical rodent models and after repeated treatment in the autoshaping procedure described here supports the clinical findings that tamoxifen can disrupt cognitive function in breast cancer patients.
Methotrexate and 5-fluorouracil produce mixed effects in different rodent learning and memory assays. In the Morris water maze and novel object recognition task, rats treated with methotrexate show deficits suggesting impairments of spatial memory (Seigers et al. 2008) and rats treated with 5-fluorouracil reduce freezing time in a context-dependent conditioned emotional response test and deficits in object location recognition (ElBeltagy et al. 2010). In contrast, methotrexate did not alter learning in a conditioned taste aversion test that included a feature-negative discrimination task (Stock et al. 1995), cued memory or a discrimination task in the Morris water maze (Winocur et al. 2006), and 5-fluorouracil injections transiently enhanced both memory and hippocampal synaptic plasticity in spatial learning tasks (Lee et al. 2006).
In a previous study from our laboratory using the same autoshaped-operant procedure used for the acute tamoxifen experiment, acute doses of methotrexate (1.0–32 mg/kg) and 5-fluorouracil (3–30 mg/kg) were without effect, but an acute injection of 75 mg/kg 5-fluorouracil produced retention deficits without altering motivational responding in a separate progressive ratio assay (Foley et al. 2008). Based on these acute dose–response curves for methotrexate and 5-fluorouracil, we chose the combination dose of 75 mg/kg 5-fluorouracil with a low (3.2 mg/kg) and high (32 mg/kg) dose of methotrexate to repeatedly administer in this study. After repeated treatment, methotrexate alone failed to significantly alter acquisition or retention although there was a trend for the lower dose of 3.2 mg/kg methotrexate to decrease the reinforced response rates and the percentage of correct responses earned during the retention test on the second day of testing. In our previous study, 5-fluorouracil was more effective at disrupting retention than methotrexate (Foley et al. 2008) while in this study, 5-fluorouracil also disrupted acquisition. The number of mice that completed the required number of ten reinforcers during the acquisition session was reduced in any group of mice that received 5-fluorouracil either alone or in combination. This observation indicates that the actual detriments on the retention test may be underestimated for the adjusted latency measure in these groups. Overall, the existence and the extent of a cognitive impairment in rodents after injection of methotrexate or 5-fluorouracil alone appears to be time and task dependent similar to the findings observed in the human clinical studies (Raffa et al. 2006; Vardy et al. 2007).
Mice treated with the lower dose of 3.2 mg/kg methotrexate (but not the higher dose of 32 mg/kg methotrexate) in combination with 75 mg/kg 5-fluorouracil performed less accurately and more slowly than mice treated with saline. This observation systematically replicates previous dose–response findings from our laboratory demonstrating that acute injections of the lower doses of methotrexate in combination with 75 mg/kg 5-fluorouracil produces greater effects than the higher doses of methotrexate (Foley et al. 2008). Other studies reveal deficits in sensory gating, spatial memory, non-matching-to-sample learning, and delayed non-matching-to-sample learning and increased freezing during fear conditioning in mice after three weekly injections of a higher dose of 37.5 mg/kg methotrexate and 75 mg/kg 5-fluorouracil (Gandal et al. 2008; Winocur et al. 2006). These studies did not report lower concentrations of methotrexate in combination with 5-fluorouracil, however, so it is difficult to compare directly with the present study using both a high and low methotrexate dose in combination. Emerging evidence in cancer therapy suggests that drug combinations in which doses of the combination are pushed to a maximal tolerability may not provide optimal clinical efficacy (Mayer and Janoff 2007). For example, certain ratios of cancer chemotherapeutic agents may trigger a collection of pro-apoptotic signals that leads to an amplification of tumor death (synergy) while other ratios lead to the initiation of pro-survival cellular responses (antagonism; Harasym et al. 2007; Mayer et al. 2006). Taken together, these data suggest that certain dose ratio combinations, such as 3.2 mg/kg methotrexate and 5-fluorouracil, may be particularly detrimental to retention, other combinations may produce no effects on learning and memory, or some combinations may reverse learning and memory deficits produced by a single agent. Indeed, the higher dose of 32 mg/kg methotrexate reversed the retention impairments on some measures of autoshaped responding by fluorouracil in our study similar to the findings in cell-based studies (Harasym et al. 2007; Mayer et al. 2006).
Both in vitro and in vivo data suggest a susceptibility of hippocampal regions to chemotherapeutic agents. In vitro immunocytochemistry and immunofluorescence studies reveal that even at standard doses or below, methotrexate and other chemotherapeutic agents increase cell death and decrease cell division in the subventricular zone, in the dentate gyrus of the hippocampus, and in the corpus callosum of mice and rats (Dietrich et al. 2006; Mignone and Weber 2006; Seigers et al. 2008). Similarly, at or below clinically relevant exposure levels, 5-fluorouracil produces toxicity to central nervous system progenitor cells and non-dividing oligodendrocytes. Both acute damage and a delayed syndrome of further damage to myelinated tracts are associated with altered transcriptional regulation in oligodendrocytes and myelin pathology. This occurred with only transient effects on brain vasculature endothelial cell apoptosis and inflammation suggesting that the mechanism of pathology is likely to be oligodendrocyte death and a loss of the progenitor cell population required for replacement of these cells (Han et al. 2008). Chemotherapeutic agents assumed to have minimal penetration into the CNS actually do enter the CNS in small quantities (i.e., sub-clinical concentrations), and these quantities are enough to cause toxicity and damage to neural progenitor cells (Janelsins et al. 2010). The observation that chemotherapeutic agents are more toxic to the cells responsible for hippocampal neurogenesis and neural conductance than to cancer cell lines suggests that at least transient cognitive changes are likely to be found in patients or preclinical studies even with conventional dosing of chemotherapeutic regimens.
The behavioral changes observed in the autoshaping procedure are particularly robust when taking into consid eration the fact that the mice received only three injections, spaced a week apart, and were tested a full 7 days after the last drug injection. These data are consistent with the changes observed in most other preclinical studies (ElBeltagy et al. 2010; Madhyastha et al. 2002; Winocur et al. 2006, but see Lee et al. 2006). For example, a regimen of 3 days of 40 mg/ kg 5-fluorouracil produces progressive changes in auditory brainstem response indicative of myelin damage or myelin loss for up to 56 days after treatment (Han et al. 2008). In humans, the effects of chemotherapeutic treatments on cognitive function can be long-lasting. In breast cancer survivors receiving chemotherapy 5–10 years prior, positron emission tomography revealed significant alterations in both cerebral blood flow in the frontal cortex and cerebellum and in resting glucose metabolism in prefrontal areas and these changes were correlated with impairments in a short-term memory recall task (Silverman et al. 2007). In a longitudinal study, breast cancer patients followed 1 to 12 months after chemotherapy treatment using structural and functional MRI studies revealed reduced activation in frontal brain regions during a working memory task (Saykin et al. 2003; Saykin and Wishart 2003). Therefore, in addition to examining many agents in a number of different ratio combinations, it is important to examine the effects on cognition over time for a complete understanding of the conditions and regimens that may cause chemotherapy-induced cognitive deficits for potential prevention or remediation. Future experiments using additional treatment intervals are required to fully characterize the timeframe for the likelihood of cognitive impairments with these agents.
Although all mice remained healthy throughout the study, repeated treatment of all the chemotherapeutic agents, either alone or in combination, decreased the rate at which the mice grew over the 4-week treatment period relative to the saline-treated mice. Anorexia and emesis are common adverse reactions to a number of chemotherapeutic agents in cancer patients and can also be observed in preclinical studies. In rats, tamoxifen failed to produce a conditioned taste aversion, yet repeated tamoxifen treatment for 5 days caused anorexia via inhibition of fatty acid synthase expression specifically in the ventromedial nucleus of the hypothalamus which reversed after treatment was discontinued (López et al. 2006). As for the cognitive effects of methotrexate and 5-fluorouracil, the magnitude of anorexic effects varies across studies. For example, 5-fluorouracil failed to alter body weight in rats whereas methotrexate temporarily decreased body weights which recovered to baseline values after 6 days. The daily food intake of the rats injected with 5-fluorouracil or methotrexate was significantly reduced for 3 days but then returned to normal on day 4 or day 5, respectively. On day 6, the rats treated with methotrexate actually had an increase in food intake as compared with prechemotherapy values (Le Bricon et al. 1995). Three consecutive injections of 2.5 mg/kg methotrexate reduced food and water intake for approximately 5 days that again recovered and slightly rebounded after day 9 (Sinno et al. 2010) while 35 mg/kg 5-fluorouracil slightly decreased food intake on the day of injection but increased kaolin ingestion behavior “pica” in rats (Yamamoto et al. 2007) which is a model of gastrointestinal discomfort emesis behavior in rats (Takeda et al. 1993). Methotrexate can reverse anorexia and low leptin levels in a rat model of adjuvant-induced arthritis (Jurcovicova et al. 2009), and injections of methotrexate, 5-fluorouracil, tamoxifen, and combinations of methotrexate with 5-fluorouracil in male (Foley et al. 2008; Walker 2010) and female (E.A. Walker, personal observations) mice failed to alter progressive ratio responding for the same Ensure solution used in the present study. These data suggest that while the chemo-therapeutic agents used in this study are causing a degree of anorexia for a few days after injections in the mice, food intake and the motivation to respond for a palatable food reinforcer such as Ensure appears to rebound or resolve within the timeframe of 1 week at testing.
Acknowledgments
This work was supported by R01 CA129092.
Contributor Information
Ellen A. Walker, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA
John J. Foley, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA
Rachel Clark-Vetri, Department of Pharmacy Practice, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA.
Robert B. Raffa, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Vanover KE. Assessment of learning and memory using the autoshaping of operant responding in mice. Curr Protoc Neurosci. 2003;8:8.5 F.1–8.5 F.8. doi: 10.1002/0471142301.ns0805fs25. [DOI] [PubMed] [Google Scholar]
- Bender CM. Chemotherapy may have small to moderate negative effects on cognitive functioning. Cancer Treat Rev. 2006;32:316–319. doi: 10.1016/j.ctrv.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bender CM, Paraska KK, Sereika SM, Ryan CM, Berga SL. Cognitive function and reproductive hormones in adjuvant therapy for breast cancer: a critical review. J Pain Symptom Manage. 2001;21:407–424. doi: 10.1016/s0885-3924(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause. 2007;14:995–998. doi: 10.1097/gme.0b013e318148b28b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni L, Valagussa P. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330:217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Buddaraaju AKV, Van Dyke RW. Effects of animal bedding on rat liver endosome acidification. Comparative Medicine, AALAS. 2003;53(6):616–621. [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol Biochem Behav. 2002a;71:269–276. doi: 10.1016/s0091-3057(01)00656-6. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene impair retrieval, but not acquisition, of spatial information processing in mice. Pharmacol Biochem Behav. 2002b;72:417–421. doi: 10.1016/s0091-3057(01)00782-1. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22.1–22.23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Schmitt FA, Munster P, Jacobsen PB. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBeltagy M, Mustafa S, Umka J, Lyons L, Salman A, Chur-yoe GT, Bhalla N, Bennett G, Wigmore PM. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Esmaeili B, Basseda Z, Gholizadeh S, Javadi Paydar M, Dehpour AR. Tamoxifen disrupts consolidation and retrieval of morphine-associated contextual memory in male mice: interaction with estradiol. Psychopharmacology (Berl) 2009;204:191–201. doi: 10.1007/s00213-008-1448-5. [DOI] [PubMed] [Google Scholar]
- Foley JJ, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berl) 2008;199:527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157:95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16:361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies; Washington, DC: 2003. [PubMed] [Google Scholar]
- Janelsins MC, Roscoe JA, Berg MJ, Thompson BD, Gallagher MJ, Morrow GR, Heckler CE, Jean-Pierre P, Opanashuk LA, Gross RA. IGF-1 partially restores chemotherapy-induced reductions in neural cell proliferation in adult C57BL/6 mice. Cancer Invest. 2010;28(5):544–553. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Buzdar AU. A review of adjuvant hormonal therapy in breast cancer. Endocr Relat Cancer. 2004;11:391–406. doi: 10.1677/erc.1.00594. [DOI] [PubMed] [Google Scholar]
- Jurcovicova J, Svik K, Scsukova S, Bauerova K, Rovensky J, Stancikova M. Methotrexate treatment ameliorated testicular suppression and anorexia related leptin reduction in rats with adjuvant arthritis. Rheumatol Int. 2009;29(10):1187–1191. doi: 10.1007/s00296-009-0838-2. [DOI] [PubMed] [Google Scholar]
- Kisanga ER, Gjerde J, Schjott J, Mellgren G, Lien EA. Tamoxifen administration and metabolism in nude mice and nude rats. J Steroid Biochem Mol Biol. 2003;84:361–367. doi: 10.1016/s0960-0760(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, Serrano D, Pelosi G, Decensi A, Lien EA. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res. 2004;10:2336–2343. doi: 10.1158/1078-0432.ccr-03-0538. [DOI] [PubMed] [Google Scholar]
- Le Bricon T, Gugins S, Cynober L, Baracos VE. Negative impact of cancer chemotherapy on protein metabolism in healthy and tumor-bearing rats. Metabolism. 1995;44(10):1340–1348. doi: 10.1016/0026-0495(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- López M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vázquez MJ, Finer N, Powles TJ, O'Rahilly S, Saha AK, Diéguez C, Vidal-Puig AJ. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55(5):1327–1336. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- Madhyastha S, Somayaji SN, Rao MS, Nalini K, Bairy KL. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can J Physiol Pharmacol. 2002;80:1076–1084. doi: 10.1139/y02-135. [DOI] [PubMed] [Google Scholar]
- Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7:216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Res. 2006;1111:26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Duong PV, Finney J, Garber DA, Lam LM, Mathew SS, Patel NN, Plaskett KC, Shah M, Jen Weng HF. Is ‘chemo-fog’/‘chemo-brain’ caused by cancer chemotherapy? J Clin Pharm Ther. 2006;31:129–138. doi: 10.1111/j.1365-2710.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Reiriz AB, Reolon GK, Preissler T, Rosado JO, Henriques JA, Roesler R, Schwartsmann G. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clin Cancer Res. 2006;12:5000. doi: 10.1158/1078-0432.CCR-06-0138. author reply 5000–1. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA. Mild cognitive impairment: conceptual issues and structural and functional brain correlates. Semin Clin Neuropsychiatry. 2003;8:12–30. doi: 10.1053/scnp.2003.50002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- Schagen SB, van Dam FS. Does cognitive impairment after chemotherapy for breast cancer improve over time or does practice make perfect? J Clin Oncol. 2006;24:5170–5171. doi: 10.1200/JCO.2006.07.8303. author reply 5171–2. [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Schagen SB. Effects of hormonal therapy on cognitive functioning in breast cancer patients: a review of the literature. Minerva Ginecol. 2007;59:387–401. [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, Van Dam FS, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Sinno MH, Coquerel Q, Boukhettala N, Coëffier M, Gallas S, Terashi M, Ibrahim A, Breuillé D, Déchelotte P, Fetissov SO. Chemotherapy-induced anorexia is accompanied by activation of brain pathways signaling dehydration. Physiol Behav. 2010;101(5):639–648. doi: 10.1016/j.physbeh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stock HS, Rosellini RA, Abrahamsen GC, McCaffrey RJ, Ruckdeschel JC. Methotrexate does not interfere with an appetitive Pavlovian conditioning task in Sprague-Dawley rats. Physiol Behav. 1995;58:969–973. doi: 10.1016/0031-9384(95)00147-b. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45(4):817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Barrett JE. An automated learning and memory model in mice: pharmacological and behavioral evaluation of an autoshaped response. Behav Pharmacol. 1998;9:273–283. [PubMed] [Google Scholar]
- Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- Walker EA. Animal models. Adv Exp Med Biol. 2010;678:138–146. doi: 10.1007/978-1-4419-6306-2_18. [DOI] [PubMed] [Google Scholar]
- Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Nakai M, Nohara K, Yamatodani A. The anticancer drug-induced pica in rats is related to their clinical emetogenic potential. Eur J Pharmacol. 2007;554(1):34–39. doi: 10.1016/j.ejphar.2006.09.058. [DOI] [PubMed] [Google Scholar]