Abstract
Electronic cigarettes (e-cigarrettes) were recently introduced and advertised as a smoking cession device in South Korea. As the social norm to quit smoking has gained hold in the country, the number of e-cigarette users is growing rapidly. This phenomenon should be urgently considered, because of the lack of research that has been conducted to examine the safety of e-cigarettes and its efficacy as a smoking cessation aid.
This paper raises several public health concerns on e-cigarettes in South Korea. Uncertain regulations of the government on e-cigarettes are contributing to an increase of e-cigarette users and allowing the e-cigarette industry to circumvent existing regulations. The aggressive marketing activity of this industry is also a core factor that is responsible for the rapid increase of e-cigarette use, in particular among the youth. Following the enforcement of tobacco control, some cigarette smokers may be encouraged to purchase e-cigarettes in order to circumvent the regulations, even though the dual use of e-cigarette and cigarette may be more harmful.
Until there is clear evidence of the e-cigarette's safety, it is recommended that the industry's marketing and promotional activities be banned and closely monitored, and public campaigns be initiated to educate the public regarding e-cigarettes.
Keywords: Electronic cigarettes, Electronic nicotine delivery systems, Tobacco
INTRODUCTION
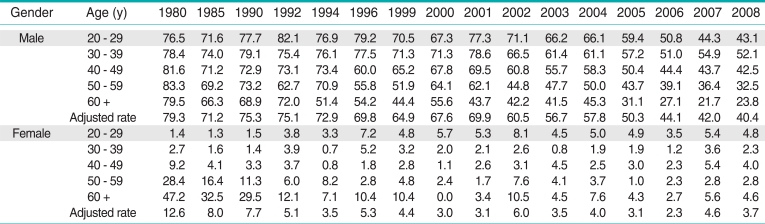
Tobacco use is a leading cause of preventable illness and is responsible for more than 40 000 deaths annually in South Korea [1]. In the early 1980s, it was estimated that 8 out of 10 Korean men smoked cigarettes [2]. Additionally, cigarettes were commonly viewed as something to be enjoyed, similar to an individual's preference for a "favourite food," rather than as a harmful product. However, along with the rapid economic growth during the 1990s, concerns about tobacco use and its harmful effects on health have become hugely widespread throughout the country. This has resulted in a rapid decrease of the overall smoking rate, as shown in Table 1 [2]. Notably, the introduction of the "well-being" life trend in the early 2000s placed a high priority on achieving and maintaining one's health, which accelerated anti-smoking and tobacco control activities. Intensive efforts to reduce tobacco use have also been conducted by the government and public health professionals, leading to the implementation of strong and effective tobacco control policies and measures, such as tobacco tax increases, media campaigns, and ratification of Framework Convention on Tobacco Control [3]. As a result, the number of smokers who want to quit has significantly increased; according to the 2010 National Actual Smoking Survey, approximately 60% of Korean current smokers indicated a desire to quit smoking [4].
Table 1.
Smoking prevalence of Korea's adult over 20 years old, 1980-2008 (%)
Korean Association of Smoking and Health. Actual smoking rate among adults over 19 years old in South Korea [8].
If these smokers were able to quit based purely on their desire to do so, a rapid decrease in the smoking prevalence in Korea would certainly be observed. However, previous studies indicate that given the behaviours or habits driven by nicotine addiction, many smokers who wish to quit can easily relapse and continue their smoking habit [5,6]. This is also true for Korean smokers. Nearly 70% of smokers in South Korea say they are still smoking, because smoking has become their "habit" [4]. Once an individual initiates smoking, nicotine is absorbed into the bloodstream within 10 to 15 seconds, and flows immediately to the brain, where nicotine works to produce a range of gratifying effects [7]. Through this process, smokers experience pleasure, cognitive enhancement, relaxation, and reduction in anxiety [10-12]. As an individual continues to smoke, the amount of nicotine required to experience these effects is increased, leading to an increase in the number of cigarettes smoked in order to achieve the desired effect, ultimately causing nicotine addiction. For this reason, health professionals have focused on counselling, education, and medicinal nicotine replacement products (available as gum and patches) in order to treat nicotine addiction and to encourage cessation among smokers attempting to quit.
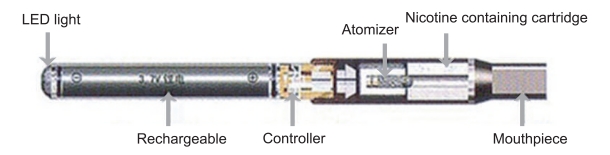
Recently, the electronic cigarette (Figure 1) (referred to as e-cigarettes hereafter) was introduced and advertised as an "incredible smoking cession device" in the South Korean market. On advertisements, e-cigarettes are described as "healthy cigarettes" [13]. Emphasis is also placed on the lack of withdrawal symptoms that e-cigarette users experience, thus making it attractive to current smokers looking to quit [14]. Following the context that quitting smoking may become the social norm as more than half of current smokers want to quit their smoking habit [4], and given that the products are currently advertised as an effective smoking cession device [15], it is not surprising that the amount of e-cigarette imports is growing rapidly [16]. In addition, it is assumed that youth and young people are more likely to be targeted by the e-cigarette industry, and a rapid rise in the number of youth e-cigarette users is also being observed by mass media [16]. This phenomenon should be urgently and intensively considered, because of the lack of research that has been conducted to examine the safety of e-cigarettes and its efficacy, if any, as a smoking cessation aid.
Figure 1.
E-cigarette component diagram.
Based on this background, this commentary paper discusses the public health challenges of e-cigarettes in South Korea and suggests a number of clear and important recommendations for comprehensive control on e-cigarettes.
E-CIGARETTES: BRIEF INTRODUCTION AND CONTROVERSIAL ISSUES
In recent years, e-cigarettes have been introduced to the global market. This device was invented by Ruyan Group (Holdings) Limited, China in 2003, and the company patented e-cigarettes in Canada in 2004 [17]. E-cigarettes look like real cigarettes, but do not burn and combust tobacco leaves; thus, the e-cigarette manufacturers and distributors insist that it is safer than other tobacco products. The device consists of a plastic tube, an electronic heating element, a liquid nicotine cartridge, a lithium battery, and atomization chamber with a membrane to suspend ingredients [18]. It also has an LED-generated red light to simulate the burning end of a cigarette. Because of the lack of combustion involved in an e-cigarette, the device does not deliver tar or chemical elements that are present in regular cigarettes that are known to cause and increase the risk of developing cardiovascular and pulmonary diseases. Instead, there is a replaceable cartridge containing nicotine (with the ability of the user to adjust the nicotine concentration), chemical additives, and flavours (e.g. chocolate, coffee, mint, fruits). As the user exhales, some visible vapour is released, but no tobacco smoke. With these features, the e-cigarette industry insists that the device is safe, can be used in non-smoking areas, and is free from second-hand smoke concerns.
Are e-cigarettes truly safe? Can they be used as a smoking cessation aid as the industry advertises? There is a lack of scientific evidence offering clear answers to these questions. There are a few short-term studies that have investigated e-cigarettes [19,20], but the evidence is not sufficient to conclusively end the controversial debates on the safety of e-cigarettes. Despite the lack of scientific evidence to confirm the e-cigarette industry's claims of safety, e-cigarettes are being sold and distributed throughout South Korea and worldwide. The World Health Organisation (WHO) and the US Food and Drug Administration (US FDA) have warned against the widespread use of e-cigarettes as a nicotine replacement product. Both bodies have recognised that e-cigarettes may be less harmful than tobacco smoking, given the lack of tar in e-cigarettes, but they emphasise that e-cigarettes are almost certainly more dangerous than medicinal nicotine replacement products [19,20].
The WHO defines that the e-cigarette as an electronic nicotine delivery system (ENDS). The Organisation not only recommends that ENDS be treated differently from traditional tobacco products, but also recommends that until conclusive evidence shows that e-cigarettes are safe, they should not be marketed as a nicotine replacement product, and that marketing activities of the e-cigarette manufacturers and distributors must be strictly controlled [19].
The US FDA has attempted to treat e-cigarettes as a drug-delivery device under the Food, Drug, and Cosmetic Act since January 2010. As a result, e-cigarette manufacturers were required to submit an application for evaluation and approval of their device before they could be marketed to the public. This decision was based on US FDA's research showing that e-cigarettes contained carcinogens, including nitrosamines, toxic chemicals, such as diethylene glycol, and tobacco-specific components suspected of being harmful to humans, such as anabasine, myosmine, and beta-nicotyrine [20]. However, the US Court of Appeals ruled that the FDA lacked the authority to regulate e-cigarettes as drugs or devices on 6th December 2010. In response, the FDA announced that e-cigarettes would be regulated as a tobacco product [21]. Meanwhile, given the lack of evidence regarding e-cigarettes' safety or efficacy as a smoking cessation aid, Australia, Canada, Singapore, and Brazil have banned the sale of e-cigarettes [19].
There is some research to support that e-cigarettes are effective in reducing the desire to smoke; however, this research was funded by Ruyan Group (Holdings) Limited. In this study, 40 participants were randomised to use e-cigarettes containing 16 mg nicotine, placebo e-cigarettes, or medicinal nicotine inhalators for four days. The results indicated that there was no difference in reducing desire to smoke between a 16 mg e-cigarette and an inhalator, which can be interpreted to mean that e-cigarettes probably work similarly to medicinal nicotine replacement inhalators. In addition, the 16 mg e-cigarettes were found to be more pleasant to use than a nicotine inhalator [22]. Not surprisingly, these findings support the e-cigarette industry in advertising their device as a smoking cessation aid.
Although a few studies are available, given the absence of evaluation of e-cigarettes for longer-term safety, potential effects of long-term use and efficacy as a smoking cessation aid, uncertainty on e-cigarettes still remain. As such, more careful and comprehensive control polices and measures on e-cigarettes are urgently needed to prevent unexpected public health impacts.
PUBLIC HEALTH CONCERNS OF E-CIGARETTES IN SOUTH KOREA
I. Uncertainty in the Regulations
E-cigarettes have been imported to South Korea since 2007, and the total volume of imports have rapidly increased to more than three times in mid-2011, compared to 2008 [15]. Following the recent upward trend of e-cigarette sales, the Korean government urgently considered how to regulate the new device and the decision was made to place e-cigarettes under the jurisdiction of two separate government bodies. On the one hand, e-cigarettes which contain nicotine have been registered by the Ministry of Finance (MOF) as same as a cigarette product since 2010. The MOF has revised the Tobacco Business Act (enacted in 1989), added e-cigarettes as a type of cigarette, and also levied health promotion tax on e-cigarettes. However, e-cigarettes containing no nicotine were placed under the jurisdiction of the Korea Food and Drug Administration (KFDA) as a health supplement, and were named "electronic smoking desire reducers."
Up until 2002, the MOF ran the domestic mono-polised tobacco company, Korea Tobacco and Ginseng Corporation, and still maintains a good relationship with the tobacco industry to this present day. In 1989, the Tobacco Business Act was legislated and enacted to foster tobacco business, which gave the MOF the opportunity to encourage tobacco business, due to the tax revenue. Because of this relationship and potential conflict of interest, e-cigarette surveillance via the MOF and the Tobacco Business Act may not be the most reliable or effective.
Meanwhile, the KFDA authorised the sales of e-cigarettes containing no nicotine as an "electronic smoking desire reducer." Although the KFDA officially stated that the safety of e-cigarettes and its efficacy as a smoking cessation aid have not yet been scientifically proven, the e-cigarette industry currently advertises their products with a message claiming that "E-cigarettes are authorised by the KFDA, thus it is safe" [23]. In fact, the KFDA's authorisation of the sales of e-cigarettes is misleading to the public and has potential threats to public health.
Uncertain regulations and policies of the Korean government on e-cigarettes are contributing to an increase of e-cigarette users in the country and also allow the e-cigarette manufacturers and distributors to circumvent existing regulations.
II. E-cigarette Use Among Youths
At present, no research studies have been conducted to examine the actual e-cigarette prevalence in South Korea, but it has already been widely assumed by the media that the number of e-cigarette users is rapidly increasing, particularly among youth. One such report indicates that 20% of the total e-cigarette sales in South Korea are contributed by youth users [16]. In addition, the report describes youth e-cigarette users often enjoying the device in the classroom and during class time, and that youth users will frequently exchange cartridges with other youth users in order to experience and enjoy the different flavours of e-cigarettes [16]. In the beginning of e-cigarette sales in Korea, expensive price of the device (about US$ 200-300) took a role as a barrier to youth access, however, following the competitive market environment, the industry has introduced a cheaper price range device (US$ 100 -150) to the market. Thus, price itself can no longer be relied on as a barrier to youth access.
Evidence in the literature indicates that the most important factors for youth smoking initiation are having friends who smoke and being exposed to smoking offers from close friends [24-26]. Because of the increasing popularity of e-cigarettes among Korean youth, non-smoking youth who have friends who use e-cigarettes are likely to be susceptible to initiate smoking e-cigarettes. In addition, the e-cigarette packaging may be attractive and fashionable to youth, who may be further tempted to try it. Compared to adult users who may decide to use e-cigarettes for the purpose of quitting, youth users are more likely to use e-cigarettes for the novelty factor. Of particular concern is the ability of the e-cigarette user to adjust the nicotine concentration, as youth users may not be aware that they can experience symptoms of nicotine addiction much more rapidly than adults [27,28]. Therefore, it is important to understand youth users' behaviours with e-cigarettes, and the impact of e-cigarettes of e-cigarette on youth should be carefully and quickly considered by policy makers and public health experts.
III. Aggressive Marketing Activities
The aggressive marketing activity of e-cigarette manufacturers and distributors is one of the core factors that may be responsible for the rapid increase of e-cigarette use in South Korea. The WHO and US FDA have already addressed their concerns about the aggressive marketing activities of e-cigarette companies in their reports. Until conclusive scientific evidence of safety and efficacy of e-cigarettes is released, all marketing activities of the e-cigarette industry should be banned [19,20]. Despite the viewpoints of the WHO and US FDA, the industry is quick to advertise that e-cigarettes can be an effective smoking cessation aid by employing "above-the-line" and "below-the-line" marketing tactics in South Korea. As previously mentioned, given that e-cigarettes with nicotine are regulated in the same way as a cigarette by the Tobacco Business Act in South Korea, the e-cigarette industry's marketing activities should be limited as follows: (1) E-cigarette promotions, such as free samples or the displaying of posters, stickers, and signboards promoting e-cigarettes, should only be available in e-cigarette retail shops; (2) advertisements should only be permitted at point-of-sale locations; (3) any kind of e-cigarette advertising using broadcast media, such as TV and radio, should be prohibited, but placement of 120 advertisements per e-cigarette brand family in magazines, except magazines specifically targeted to women or youth, would still be possible under the Tobacco Business Act; and (4) sponsorship of social, cultural, musical, athletic, and other specific events, except events targeted to women or youth, would still be possible for the e-cigarette industry.
The most common and powerful marketing tactics of the e-cigarette industry include the use of internet websites and blogs to sell and promote their products. Importantly, internet blogs allow the industry to achieve two-way communication between themselves and existing / future consumers. These strategies of e-cigarette companies can be seen to be contributing to the rapid increase of e-cigarette use in South Korea. Internet blogs are a familiar, easily accessible, and unrestricted media for youth to learn about what e-cigarettes are, where to buy cheaper e-cigarettes, and how to buy e-cigarettes without national ID cards. In addition, the use of the internet to locate information is commonplace; for example, a simple internet search within NAVER, the nation's largest internet portal site, with the keyword searching term, "electronic cigarette" indentified almost 850 legitimate retail websites (accessed at 18th July 2011), in which the majority of the websites were run by e-cigarette manufacturers, distributors, and local retailers. The same keyword search on NAVER's blog search engine identified 40 422 blog sites (access at 18th July 2011). To date, no investigation has been conducted to examine whether these seemingly independent internet websites and blogs are being used by the e-cigarette industry as one of their aggressive marketing tactics. It should also be noted that all of the information in the e-cigarette internet websites and blogs are open and accessible to youth under the age of 18.
IV. Dual Use of Cigarettes and E-cigarettes: Undermining Tobacco Control Policies and Regulations
Dual use of e-cigarettes and traditional cigarettes should be examined, as smokers who do not wish to quit can easily use an e-cigarette as an alternative in non-smoking areas. Recently, through the nationwide enforcement of tobacco control policies and regulations in South Korea, areas designated as non-smoking are expanding. In Seoul, the capital city of South Korea, some major squares (public outdoor places) are designated as non-smoking areas. As trends are beginning to indicate that smoking is increasingly becoming socially unacceptable in South Korea, it is anticipated that the next step in expanding non-smoking areas would include prohibiting smoking in every indoor places, including restaurants and bars. Given the restrictions on public smoking, some cigarette smokers may be encouraged to purchase e-cigarettes in order to circumvent the tobacco control regulations, as the e-cigarette industry encourages dual use by advertising that the public use of e-cigarettes is not restricted and is therefore legal to use in restaurants, bars, and other indoor areas where cigarette smoking is strictly prohibited.
V. Recommendations
Further research investigating the effects of e-cigarettes must be conducted in order to conclusively determine the safety of this product. In addition, these studies should be conducted independently and should not be sponsored or funded by the e-cigarette industry. Descriptive studies to investigate the current prevalence of e-cigarette use among adults and youths and to explore current e-cigarette users' behaviours and attitudes should be performed. In order to determine the safety of long-term use and efficacy as a smoking cessation aid, longitudinal cohort studies should also be conducted. E-cigarette initiation among non-smokers and their behaviour changes are an important topic to be examined. In addition, the industry's retail websites and internet blogs which include e-cigarette information should be also examined and regulated in terms of what health messages they provide and how this information affects potential users.
Until there is clear evidence of the e-cigarette's safety, it is recommended that the industry's marketing and promotional activities be banned, in agreement with the recommendations of the WHO and the US FDA. However, a complete banning and regulation of these activities may take a long length of time to enact; as such, restrictions, particularly on youth access and youth-centred marketing of e-cigarettes, should be in place until such a ban can be put into effect. With no restrictions in place concerning the e-cigarette industry's marketing activities, the prevalence of e-cigarette users continues to rapidly grow in South Korea. This is cause for concern, as the e-cigarette industry may employ tactics used by transnational tobacco companies in order to exert influence on government policies and for legislation and regulations to be largely made in their favour [29,30]. It is possible that the e-cigarette industry currently lobbies policy makers, and uses third parties, such as consumer groups, in order to undermine regulations that are not favourable in order to continue their business. Therefore, the e-cigarette industry should be closely observed and monitored. In recent years, it has been discovered that Philip Morris International (PMI), the one of the largest tobacco manufacturers in the world, attempted to negotiate with Ruyan Group, which manufactured the original e-cigarettes [31]. The motives of PMI are not clear, but it is suspected that the transnational tobacco companies have a great deal of interest in for the acquisition of e-cigarette markets.
Furthermore, educational campaigns should be initiated in order to educate the public regarding e-cigarettes and to provide correct information. Not only does the industry's marketing tactics and misleading health information on their websites (for example, e-cigarette is a "healthy cigarette" and it helps to quit smoking without withdrawal symptoms, as mentioned above) raise serious public health concerns, but users who describe their experiences with e-cigarettes on their internet blogs may unknowingly be providing misinformation, which also impacts public health by potentially attracting new e-cigarette users. Therefore, the government, public health professionals, tobacco control advocates, and non-government organisations must conduct educational campaigns to inform the public regarding the use of e-cigarettes. In addition, the National Quit Line, physicians in smoking cessation clinics, school teachers, and other individuals who have opportunities to meet and counsel current smokers who want to quit should discourage the use of e-cigarettes as a smoking cessation aid until there is enough scientific evidence to support this claim.
ACKNOWLEDGEMENTS
The authors are grateful to Professor Il Soon Kim, Dr. Joann Lee, and Professor Hong Kwan Seo for reviewing the manuscript.
Funding: This study was supported by a grant of the Seoul R&BD program (10526), Korea.
Footnotes
The authors have no conflicts of interest with the material presented in this paper.
This article is available at http://jpmph.org/.
References
- 1.Jee SH, Lee JK, Kim IS. Smoking-attributable mortality among Korean adults: 1981-2003. Korean J Epidemiol. 2006;28(1):92–99. doi: 10.4178/epih.e2024011. (Korean) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korean Association of Smoking and Health. Actual smoking rates in South Korea. Seoul: Korean Association of Smoking and Health; 2008. (Korean) [Google Scholar]
- 3.Levy DT, Cho SI, Kim YM, Park S, Suh MK, Kam S. SimSmoke model evaluation of the effect of tobacco control policies in Korea: the unknown success story. Am J Public Health. 2010;100(7):1267–1273. doi: 10.2105/AJPH.2009.166900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health and Welfare. Actual smoking rates survey. Seoul: Ministry of Health and Welfare; 2010. (Korean) [Google Scholar]
- 5.Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- 6.Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193(1):1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C] nicotine in human brain. Psychopharmacology (Berl) 2010;209(4):383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- 8.Actual smoking rate among adults over 19 years old in South Korea. Korean Association of Smoking and Health. [cited 2011 Jun 20]. Available from: http://www.kash.or.kr/user_new/pds_view.asp.
- 9.E-cigarette. Ruyan Korea. [cited 2011 Jul 10]. Available from: http://ruyankorea.co.kr/sub02/index.htm.
- 10.Cosci F, Knuts IJ, Abrams K, Griez EJ, Schruers KR. Cigarette smoking and panic: a critical review of the literature. J Clin Psychiatry. 2010;71(5):606–615. doi: 10.4088/JCP.08r04523blu. [DOI] [PubMed] [Google Scholar]
- 11.Metz CN, Gregersen PK, Malhotra AK. Metabolism and biochemical effects of nicotine for primary care providers. Med Clin North Am. 2004;88(6):1399–1413. ix. doi: 10.1016/j.mcna.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and smoking cessation -- the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201(2):225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 13.H-Plus. [cited 2011 Jul 10]. Available from http://hsmoking.com/index.php?spage=page_company.
- 14.Cigacare. [cited 2011 Jul 10]. Available from http://cigacare.com/m_mall_detail.php?ps_ctid=43040600&ps_goid=342674520&menu=
- 15.Media Team. Successful quit smoking with e-cigarettes? No ways! Sege-ilbo. 2011. Jun 23, [cited 2011 July 6]. Available from: http://www.segye.com/Articles/NEWS/ECONOMY/Article.asp?aid=20110616002450&subctg1=&subctg2=
- 16.Kim HK. "It has no smell"... Students smoke e-cigarette at schools. Nocutnews. 2011. May 27, [cited 2011 July 6]. Available from: http://www.nocutnews.co.kr/show.asp?idx=1814232.
- 17.Hon L. A non-smokable electronic spray cigarette (CA 2518174) [cited 2011 July 6]. Available from: http://www.wikipatents.com/CA-Patent-2518174/a-non-smokable-electronic-spray-cigarette.
- 18.Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med. 2010;153(9):607–609. doi: 10.7326/0003-4819-153-9-201011020-00011. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization, Study Group on Tobacco Regulation. Report on the scientific basis of tobacco product regulation: third report of a WHO study group. Geneva: World Health Organization; 2010. [Google Scholar]
- 20.Westenberger BJ. US Food and Drug Administration evaluation of e-cigarettes. [cited 2011 July 5]. Available from: http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf.
- 21.Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365(3):193–195. doi: 10.1056/NEJMp1105249. [DOI] [PubMed] [Google Scholar]
- 22.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 23.Nokingdays. [cited 2011 Jul 10]. Available from http://www.nokingdaysmall.com/
- 24.Lee S, Yun JE, Lee JK, Kim IS, Jee SH. The Korean prediction model for adolescents' future smoking intentions. J Prev Med Public Health. 2010;43(4):283–291. doi: 10.3961/jpmph.2010.43.4.283. [DOI] [PubMed] [Google Scholar]
- 25.Bricker JB, Peterson AV, Robyn Andersen M, Leroux BG, Bharat Rajan K, Sarason IG. Close friends', parents', and older siblings' smoking: reevaluating their influence on children's smoking. Nicotine Tob Res. 2006;8(2):217–226. doi: 10.1080/14622200600576339. [DOI] [PubMed] [Google Scholar]
- 26.de Vries H, Engels R, Kremers S, Wetzels J, Mudde A. Parents' and friends' smoking status as predictors of smoking onset: findings from six European countries. Health Educ Res. 2003;18(5):627–636. doi: 10.1093/her/cyg032. [DOI] [PubMed] [Google Scholar]
- 27.DiFranza JR, Wellman RJ, Mermelstein R, Pbert L, Klein JD, Sargent JD, et al. The natural history and diagnosis of nicotine addiction. Curr Pediatr Rev. 2011;7(2):88–96. doi: 10.2174/1573396311666150501002703. [DOI] [PubMed] [Google Scholar]
- 28.Scragg R, Wellman RJ, Laugesen M, DiFranza JR. Diminished autonomy over tobacco can appear with the first cigarettes. Addict Behav. 2008;33(5):689–698. doi: 10.1016/j.addbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Apollonio DE, Bero LA. The creation of industry front groups: the tobacco industry and "get government off our back". Am J Public Health. 2007;97(3):419–427. doi: 10.2105/AJPH.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley CP, Cummings KM, Hyland A, Giovino GA, Horan JK. Tobacco Institute lobbying at the state and local levels of government in the 1990s. Tob Control. 2002;11(Suppl 1):i102–i109. doi: 10.1136/tc.11.suppl_1.i102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phiip Morris International (PMI) enters into a patent purchase agreement of new technology with the potential to reduce the harm of smoking. PhilipMorris International Inc. [cited 2011 July 20]. Available from: http://www.pmi.com/eng/media_center/press_releases/pages/201105261249.aspx.