Abstract
Background
Surgical treatment of stage I non-small cell lung cancer (NSCLC) can be performed either by thoracotomy or by employing video-assisted thoracic surgery (VATS). The aim of this study was to evaluate the feasibility of VATS lobectomy for pathologic stage I NSCLC.
Material and Methods
Between December 2003 and December 2007, 529 patients with pathologic stage I NSCLC underwent lobectomies (373 thoracotomy, 156 VATS). Patients in both groups were selected after being matched by age, gender and pathologic stage using propensity score method, to create two comparable groups: thoracotomy and VATS groups, and the overall survival, recurrence-free survival, complication and length of hospitalization were compared between these two groups.
Results
After the patients were matched by age, gender and pathologic stage, 272 patients remained eligible for analysis, 136 in each group (mean age of 59.5 years; 70 men, 66 women; 80 stage IA, 56 stage IB). There was no statistical difference in other preoperative clinical characteristics between the two groups. No hospital mortality was observed in both groups. Overall 3-year survival rate was 97.4% in thoracotomy group and 96.6% in VATS groups (p=0.76). During the follow-up, 20 patients (14.7%) developed recurrence in thoracotomy group, including loco- regional recurrence in 7, distant metastasis in 13. In VATS group, 13 patients (9.6%) developed recurrence, including loco-regional recurrence in 4, distant metastasis in 9. Three-year recurrence-free survival rate was 81.8% in thoracotomy group and 85.3% in VATS groups (p=0.43). There was no significant difference in postoperative complications between thoracotomy and VATS groups (30 cases in 22 patients vs. 19 cases in 17 patients, p=0.65, odds ratio=1.19). The mean hospital stay of VATS group was 2 days shorter than that of thoracotomy group (8.8±6.5 days vs. 6.3±3.3 days, p<0.05).
Conclusion
VATS lobectomy for pathologic stage I lung cancer is a feasible operation with shorter hospitalization, while surgical outcome is comparable to thoracotomy lobectomy.
Keywords: Video-assisted thoracic surgery, Lobectomy, Lung neoplasms, Neoplasm staging
INTRODUCTION
Since video-assisted thoracoscopic surgery (VATS) lobectomy was introduced in the early 1990s, its use in the treatment of non-small cell lung cancer (NSCLC) has been gradually increased. Many surgeons demonstrated the technical feasibility and safety of VATS lobectomy. The 'Cancer and Leukemia Group B (CALGB) 39802 prospective multi-institutional feasibility study' revealed that VATS lobectomy could be performed with low morbidity and mortality, and a recent meta-analysis of various trials on safety and efficacy of VATS lobectomy demonstrated that VATS lobectomy is an appropriate procedure for selected patients with early-stage NSCLC when compared with open surgery. However, some surgeons have been concerned about whether VATS lobectomy really can achieve equal, or even superior, surgical and oncological results compared with those of conventional open-thoracotomy lobectomy. This might well be why VATS lobectomy is applied only in less than 20% of lobectomies performed in the United States, even though the detection of early lung cancer has been gradually increasing [1]. Therefore, evidence-based information on VATS lobectomy, regarding not only the safety and technical feasibility but also the oncological outcome, should be accumulated more for this novel approach widely accepted as a standard procedure for early-stage NSCLC.
In our institution, VATS lobectomy was adopted in the early 2000s, and has been performed with a gradual increase, for the treatment of both benign lung disease and early-stage non-small cell lung cancer. The aim of this study is to evaluate the feasibility and surgical outcome of VATS lobectomy compared to conventional open-thoracotomy, based on our experience of pathologic stage I NSCLC.
MATERIAL AND METHODS
Between December 2003 and December 2007, medical records of patients, who underwent surgery for pathologic stage I NSCLC in our institute, were retrospectively reviewed. We determined the cancer stage according to the '6th Edition of TNM staging system of lung cancer', which was suggested by the International Staging Committee (ISC) in the International Association for the Study of Lung Cancer (IASLC). This study was reviewed and approved by the Institutional Review Board of our institute.
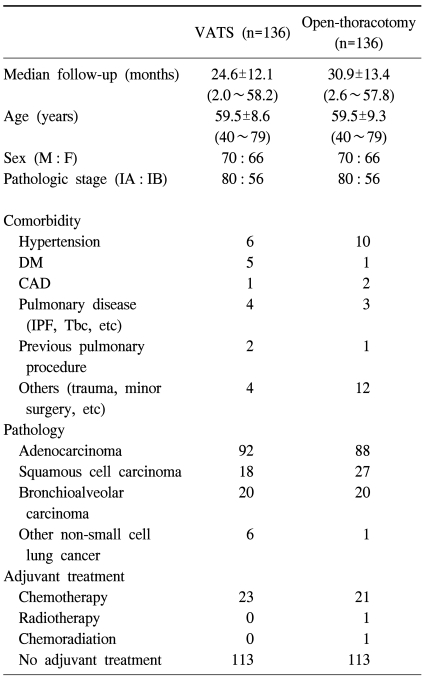
To create two comparable groups, we conducted propensity score analysis using nearest neighbor matching method of covariates without replacement. Verified independent variables are age, gender, and pathologic stage. Preoperative pulmonary function, postoperative adjuvant therapy and pre-existing co-morbidities of patients were compared to confirm that these two groups are nearly identical except for the surgical approaches (i.e. VATS or open-thoracotomy). However, because adenocarcinoma tends to be indicated more frequently for VATS lobectomy, patients could not be exactly matched by tumor pathology. The early postoperative results, such as postoperative complications, hospital stay, recurrence pattern, and overall and recurrence-free survival, were compared between these two groups. Table 1 shows patient characteristics of each group.
Table 1.
Patients' characteristics of each group
VATS=Video-assisted thoracic surgery; DM=Diabetes mellitus; CAD=Coronary artery disease; IPF=Interstitial pulmonary fibrosis; Tbc=Tuberculosis.
The decision to perform either VATS or open-thoracotomy was made by the individual four thoracic surgeons in our institution. Candidates for VATS lobectomy were patients with clinical stage I, peripheral, small-sized (≤5 cm) tumor without endobronchial lesion. The preoperative workup included pulmonary function tests, CT scan, integrated positron emission tomography (PET), and computerized tomography (CT) scan. For patients with preoperatively proven NSCLC, mediastinoscopic lymph node biopsy was routinely performed regardless of CT or PET scan findings.
All patients received operation under a standard general anesthesia protocol, with single lung ventilation by a double-lumen endotracheal tube. VATS lobectomy was performed after making two ports and a utility incision without rib spreading. A 15-mm trocar was introduced through the seventh or eighth intercostal space for the 10-mm, 30-degree thoracoscope. A utility incision of four to five centimeters, which was centered on the anterior axillary line, was made through the fourth or fifth intercostal space. A 5~10 mm additional trocar was placed through the sixth or seventh intercostal space on the posterior scapular line. The target vessels were individually dissected, and divided using endoscopic staplers or surgical clips. The bronchi of target lobes were dissected, and divided using endoscopic staplers. All specimens were put in an impermeable bag and removed through the utility incision. Open-thoracotomy lobectomy was performed via standard posterolateral thoracotomy through the fourth or fifth intercostal space. Bronchial division was carried out using staplers. Mediastinal lymph node dissection was performed in all patients regardless of the surgical approaches, and it consisted of en bloc resection of all nodes at stations of 2R, 4R, 7, 8, 9, 10, and 11R for right-sided tumor, and nodes at stations of 4L, 5, 6, 7, 8, 9, 10 and 11L for left-sided tumor.
Descriptive statistics were used to describe the patients' characteristics and outcomes. The normally distributed continuous data were expressed as means±standard deviations. Categorical data were expressed as counts and proportions. The student t tests and the ×2 test or Fisher exact test were used to compare the continuous and categorical variables, respectively. Overall survival (OS) was defined as the time from the date of surgery to the last date of follow-up for patients who remained alive, or to the date of death. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of recurrence recognition, death, or to the end of observation. Survival curves were plotted using the Kaplan- Meier method and compared univariately using the log-rank test. All statistical testing was done at the two-sided 0.05 level of p-value using SAS® 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
From December 2003 and December 2007, 1,469 patients underwent curative resection for NSCLC in our institution, and among them, 878 were found to have pathologic stage I lung cancer. VATS lobectomy was performed in 268 cases, and 216 of them had pathologic stage I lung cancer. Among the 878 patients, 529 patients were included for analysis, which consisted of 373 open-thoracotomy patients and 156 VATS patients. Patients with co-morbidity which could affect the survival or postoperative courses, such as history of malignancy or co-existing malignancy of other organs, end stage renal disease, heart failure, liver cirrhosis, and pulmonary disease with moderate to severe dysfunction, were excluded from patient enrollment. Among the patients with VATS lobectomy, 17 patients, who underwent surgery with rib spreading in our early experience of VATS practice, were also excluded. There were 3 cases of open-thoracotomy conversion from VATS procedures during the same period. In two of them, the operator decided to open the chest because the intraoperative frozen biopsy of lymph node revealed tumor metastasis. In one patient, there happened the segmental arterial injury during VATS procedure, which forced conversion to thoracotomy. These patients were excluded from analysis.
After the patients were matched by age, gender and pathologic stage, there remained 272 patients eligible for analysis, 136 in each group. The mean age of patients in both groups was 59.5 years, and each groups consisted of 70 men and 66 women, and 80 pathologic stage IA and 56 pathologic stage IB. There was no statistical difference in preoperative clinical characteristics between the two groups. Distribution of tumor pathology, preoperative pulmonary function, postoperative adjuvant therapy and pre-existing co-morbidities of the patients were compared, and there was no significant difference in these variables between the two groups.
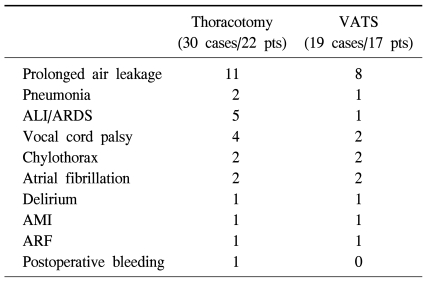
There were 2 operative mortality in the entire VATS lobectomy group during the same period (2/268, 0.74%), and one with pathologic stage I was excluded from the analysis during the matching process. In the two matching groups, there was no in-hospital mortality related to the operation. There were 49 postoperative complications in 39 patients (30 cases/22 patients in open-thoracotomy group, 19 cases/17 patients in VATS group. There was no statistical difference in postoperative complications between the two groups (p=0.65, odds ratio=1.19). Table 2 shows the details of complications in each group.
Table 2.
Postoperative complications
VATS=Video-assisted thoracic surgery; ALI=Acute lung injury; ARDS=Acute respiratory distress syndrome; AMI=Acute myocardial infarction; ARF=Acute renal failure.
The mean length of hospital stay of the patients who underwent VATS lobectomy was significantly shorter, by 2 days, than that of open-thoracotomy group (8.8±6.5 days vs. 6.3±3.3 days, p<0.05).
The median follow-up period was 27.7±13.1 months (2~58.2 months), and median follow-up of open-thoracotomy group and VATS group were 30.9±13.4 months (2.6~57.8 months) and 24.6±12.1 months (2.0~58.2 months), respectively.
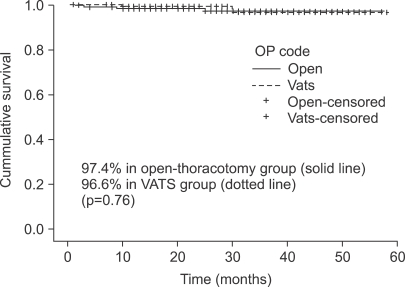
The overall 3-year survival was 97.4% in thoracotomy and 96.6% in VATS groups. There was no significant survival difference between the two groups (p=0.76) (Fig. 1).
Fig. 1.
Three-year overall survival. The 3-year overall survival were 97.4% in open-thoracotomy group (solid line) and 96.6% in VATS (Video-assisted thoracic surgery) group (dotted line) (p=0.76). OP=Operation.
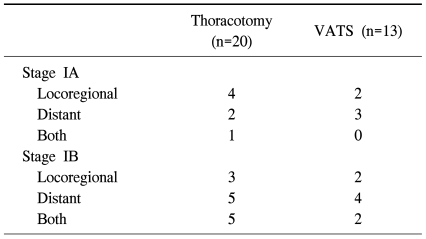
During the follow-up period, twenty patients (20/136, 14.7%) in open-thoracotomy group experienced tumor recurrence, including 7 with loco-regional recurrence and 13 with distant metastasis. In VATS group, 13 patients (13/136, 9.6%) experienced tumor recurrence, including 4 with loco-regional recurrence and 9 with distant metastasis. Table 3 shows the recurrence pattern of each group, according to pathologic stage.
Table 3.
Recurrence patterns according to pathologic stage
VATS=Video-assisted thoracic surgery.
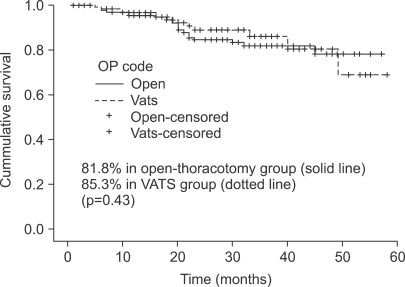
Three-year recurrence-free survival was 81.8% in open-thoracotomy group and 85.3% in VATS group. There was no significant difference in recurrence between the two groups (p=0.43) (Fig. 2).
Fig. 2.
Three-year recurrence-free survival. The 3-year recurrence- free survival were 81.8% in open-thoracotomy group (solid line) and 85.3% in VATS (Video-assisted thoracic surgery) group (dotted line) (p=0.43). OP=Operation.
DISCUSSION
Since VATS was first introduced for pulmonary resection of malignant lung tumors in 1992, surgical technique of this minimally invasive procedure has been markedly refined, and VTAS lobectomy for lung cancer has been increasingly performed. Many large series on the feasibility of VATS approach have been published. Most of these reports have emphasized on the benefit of VATS lobectomy in terms of better cosmesis, less surgical trauma, reduced postoperative pain, lower complication, and shorter hospital stay resulting in reduced cost [2-13]. The 'Cancer and Leukemia Group B (CALGB) 39802' prospective multi-institutional study evaluated the technical feasibility and safety of VATS lobectomy for early-stage non-small-cell lung cancer (NSCLC), and revealed that the procedure can be performed with low morbidity and mortality rates. The failure-free survival after VATS lobectomy for T1N0 lesion was 91% at 1 year and 78% at 2 years [14]. Furthermore, a recent meta-analysis of the reports on the safety and efficacy of VATS lobectomy concluded that it could be a safe alternative to open-thoracotomy lobectomy [15]. However, some surgeons still hesitate to adopt VATS lobectomy for lung cancer, and this is why VATS lobectomy accounts for only less than 20% of all lobectomies performed in the United States [1,15-18]. Main concern is about whether VATS lobectomy can accomplish the oncologic results comparable to conventional open-thoracotomy lobectomy. There certainly are several controversial issues specific to cancer surgery employing VATS, such as 1) under- staging or mis-diagnosis due inadequate lymph node dissection or undetected nodules in other lobes, which could well be manually detected during open-thoracotomy procedure, 2) tumor dissemination during the VATS manipulation, and 3) the risk of leaving residual tumors at the resection margin. Despite these concerns, recent studies on the oncologic outcome of VATS lobectomy in comparison with open-thoracotomy lobectomy have revealed the superiority of VATS, not only in the early postoperative results, such as postoperative pain, morbidity, and hospital stay, but also in terms of long term outcomes, such as quality of life and survival, although statistical power of these studies are somewhat undermined by the small number of patients [19-21].
In this retrospective study, for the valid comparison of two different groups and elimination of possible bias as much as possible, we performed propensity score matching of two groups on the covariates of age, sex, underlying co-morbidity, and pathologic staging. Other variables which can influence the operative results and patient's survival, such as preoperative pulmonary function, postoperative complications, and postoperative adjuvant therapies, were compared to verify that there was no statistical difference between the two groups. By this method, we ascertained that comparable operative results could be achieved by VATS lobectomy in comparison to conventional open-thoracotomy. The occurrence of postoperative complications was not different between the two groups. Furthermore, the hospital stay was significantly shorter in VATS group, which could contribute greatly to lowering the cost and endeavor of postoperative care.
Three-year overall survival and three-year recurrence free survival of VATS group was equivalent to that of open-thoracotomy group. Locoregional recurrence rate was not high either in VATS group. The mean number of dissected lymph nodes in VATS group was 15.0±8.3. The same technique of lymph node dissection with conventional open-thoracotomy was applied to VATS lobectomy, which consisted of en bloc resections of all nodes at stations of 2R, 4R, 7, 8, 9, and 10R for right-sided tumors and nodes at stations of 4 L, 5, 6, 7, 8, 9 and 10 L for left-sided tumors. During the same period, 268 VATS lobectomies were performed for clinical stage I NSCLC, and 52 of them were found to have metastatic regional and/or mediastinal lymph node. This up-staging from a lower preoperative clinical stage to a higher postoperative pathologic stage could be achieved due to complete lymph node dissection.
Our study has several limitations. First, since this is a retrospective study, our results should be interpreted with caution even though we have conducted propensity matching analysis. Prospective randomized trials are needed for more conclusive results. Second, we have chosen to perform VATS lobectomy by individual surgeon's preference. This can cause unrevealed bias and might have predisposed patients undergoing VATS lobectomy to outcomes more favorable than those undergoing open-thoracotomy. It should also be noted that VATS procedures which were converted to open surgery were not included in this study. Third, VATS group, which includes patients with peripheral lesion almost exclusively, consisted of mostly adenocarcinoma patients. On the other hand, open-thoracotomy group had more patients with squamous cell carcinoma patient, because many patients of this group has endobronchial lesion. This difference might have influenced the survival results. Fourth, follow-up duration was relatively short, and we were only able to estimate 3-year survival, which made it difficult to conclude if recurrence-free survival after VATS lobectomy was truly comparable to thoracotomy lobectomy. Longer-term survival data may be necessary to verify our hypothesis.
CONCLUSION
Our results suggest that VATS lobectomy for pathologic stage 1 NSCLC is feasible and safe with shorter hospital stay, low postoperative morbidity and mortality, and favorable survival comparable with conventional open-thoracotomy lobectomy, for the patients with pathologic stage I NSCLC.
References
- 1.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg. 1992;104:1679–1685. discussion 1685-7. [PubMed] [Google Scholar]
- 3.Lewis RJ, Caccavale RJ, Bocage JP, Widmann MD. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest. 1999;116:1119–1124. doi: 10.1378/chest.116.4.1119. [DOI] [PubMed] [Google Scholar]
- 4.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg. 1995;109:997–1001. doi: 10.1016/S0022-5223(95)70326-8. discussion 1001-2. [DOI] [PubMed] [Google Scholar]
- 5.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg. 2000;24:27–30. doi: 10.1007/s002689910006. discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 6.Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg. 2000;70:243–247. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 7.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 8.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–365. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 9.Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg. 2006;132:507–512. doi: 10.1016/j.jtcvs.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 10.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–425. doi: 10.1016/j.athoracsur.2005.07.078. discussion 425-6. [DOI] [PubMed] [Google Scholar]
- 11.Shiraishi T, Shirakusa T, Hiratsuka M, Yamamoto S, Iwasaki A. Video-assisted thoracoscopic surgery lobectomy for c-T1N0M0 primary lung cancer: its impact on locoregional control. Ann Thorac Surg. 2006;82:1021–1026. doi: 10.1016/j.athoracsur.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83:1245–1249. doi: 10.1016/j.athoracsur.2006.12.029. discussion 1250. [DOI] [PubMed] [Google Scholar]
- 13.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–1970. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 14.Swanson SJ, Herndon JE, 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–4997. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 15.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 16.Fry WA, Siddiqui A, Pensler JM, Mostafavi H. Thoracoscopic implantation of cancer with a fatal outcome. Ann Thorac Surg. 1995;59:42–45. doi: 10.1016/0003-4975(94)00794-8. [DOI] [PubMed] [Google Scholar]
- 17.Jancovici R, Lang-Lazdunski L, Pons F, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg. 1996;61:533–537. doi: 10.1016/0003-4975(95)01060-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita JI, Kurusu Y, Fujino N, Saisyoji T, Ogawa M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg. 2000;119:899–905. doi: 10.1016/S0022-5223(00)70084-5. [DOI] [PubMed] [Google Scholar]
- 19.Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–647. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Tomaszek SC, Cassivi SD, Shen KR, et al. Clinical outcomes of video-assisted thoracoscopic lobectomy. Mayo Clin Proc. 2009;84:509–513. doi: 10.4065/84.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]