Abstract
Background
Pulmonary sclerosing hemangioma is a rare thoracic tumor, and pathophysiology or clinical course of this tumor is not yet fully described. Furthermore, there is no consensus on the standard operative procedure for this tumor.
Material and Methods
Medical records of thirty-two patients, who underwent surgical resection of pulmonary sclerosing hemangioma from 1996 to 2007, were retrospectively reviewed.
Results
Nineteen patients underwent lobectomy and thirteen patients underwent limited resection. Video-assisted thoracoscopic surgery was performed in 9 patients in the latter group. Lymph node dissection was done in 21 patients, and one patient was found to have lymph node metastasis of the tumor. There was no postoperative complication, no early death and no tumor-related late mortality. The mean follow-up duration was 39.3 months (2 months~129 months), and all patients were free of local recurrence and distant metastasis during this period. There was no significant difference in patient's characteristics between the two groups, except that the mean hospital stay was shorter in limited resection group than in lobectomy group (p=0.0031).
Conclusion
Pulmonary sclerosing hemangioma usually requires surgical resection for both diagnosis and treatment. Limited resection can decrease hospital stay with a surgical outcome comparable to lobectomy, and may be preferred to lobectomy if sufficient resection margin can be achieved.
Keywords: Lung neoplasms, Surgery, Hemangioma
INTRODUCTION
Pulmonary sclerosing hemangioma (PSH) is a rare benign tumor which was first described by Liebow and Hubbell [1]. PSH usually presents as an asymptomatic solitary peripheral nodule, predominantly in women. PSH is generally considered as benign tumor, but some reports suggested the malignant potential of this tumor. The optimal surgical strategy for treating PSH has not been established. The purpose of this study is to compare the outcome of limited resection with that of lobectomy to determine the optimal surgical strategy of this tumor.
MATERIAL AND METHODS
Between January 1995 and December 2007, 32 patients underwent surgical resection of PSH at out institution. Their medical records were retrospectively reviewed to compare the clinical characteristics and postoperative outcomes of the two groups with different surgical procedures: lobectomy and limited resection, which was defined as lung resection with an extent smaller than that of lobectomy. There were no clear criteria for the selection of the surgical procedure, but limited resection tended to be preferred for peripheral small-sized tumor, and in the recent series after the introduction of video- assisted thoracoscopic surgery (VATS).
Thirty-one patients were female, and the mean age at operation was 47.8 years, ranging from 23 to 73 years. Twelve patients had non-specific subjective symptoms before being diagnosed as having PSH, such as cough, chest discomfort, and mild dyspnea. Five patients had a history of blood-tinged sputum.
All patients were initially diagnosed with solitary pulmonary nodule (SPN) on simple chest X-ray, which prompted us to conduct computed tomography (CT) scan. PSH was suspected in 10 patients, as one of the differential diagnoses in nine cases, and as a single compatible diagnosis in one case. Enhanced spiral CT scan in our hospital improved diagnostic accuracy by indicating PSH in seven out of eight patients. Determination of preoperative pathologic diagnosis was attempted in 12 patients with fine needle aspiration (FNA). Three patients were misdiagnosed preoperatively as adenocarcinoma of the lung, and three were diagnosed with other benign lung tumors such as hemangioma or hamartoma. In two patients, PSH was one of the two differential diagnoses (i.e. PSH and adenocarcinoma). The correct preoperative diagnosis of PSH was made only in 4 cases.
Additionally, intra-operative frozen biopsy failed to diagnose PSH in one case, who was reported to have bronchioloalveolar carcinoma (BAC).
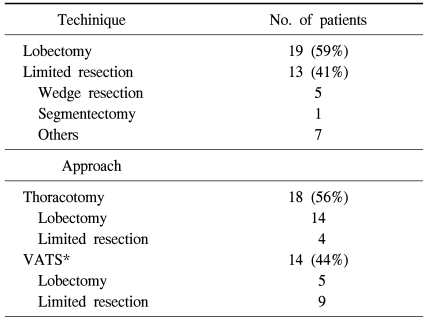
The study population was divided into two groups according to the extent of resection. Lobectomy group included 19 patients, and limited resection group included 13 patients, which consisted of 1 segmentectomy, 5 wedge resections and 7 other minor resections of tumor such as precision excision. Conventional open thoracotomy was done in 19 patients and VATS in 13 patients. Thoracoscopic surgery was preferred in limited resection group (9/13, 69%). The operative technique and approach was summarized in Table 1.
Table 1.
Operative technique and approach
*VATS=Video-assisted thoracoscopic surgery.
All patients were followed up postoperatively. CT scan was done between postoperative 3rd and 6th months in most of the patients. After initial postoperative CT examination CT scan was performed annually for more than 3 years.
Descriptive statistics were used to describe patient characteristics and outcomes. The normally distributed continuous data were expressed as means with standard deviations. Categorical data were expressed as absolute values with percentages. Student t tests and Chi square or Fisher exact tests were used to compare the continuous and categorical variables, respectively. A p-value of less than 0.05 was considered significant.
RESULTS
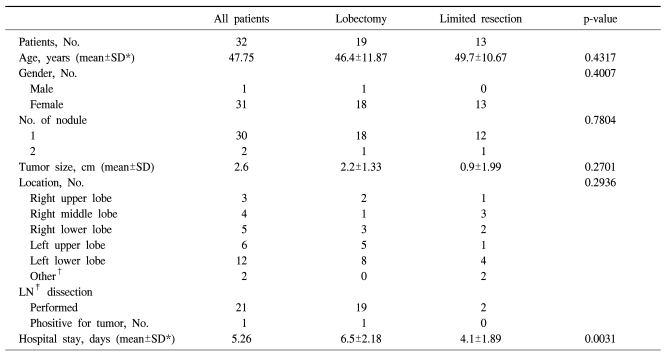
The clinical characteristics and pathologic data of the study population are described in Table 2. Among 32 patients, thirteen patients underwent a limited resection. The lobectomy group consisted of 1 male and 18 female. The limited resection group consisted of only female patients.
Table 2.
Clinical characteristics and pathologic data of the patients
*SD=Standard deviation; †Other=Pleura (1), mediastinum (1); ‡LN=Lymph node.
Two patients had multiple nodules. One patient with two nodules in the right middle lobe (RML) received precision excision of the nodules, and the other patients with two nodules in the right lower lobe received right lower lobectomy. The rest of the patients had a single nodule. In two patients, the masses were found in unusual locations. One tumor was located in the major fissure, and the other one was located in the mediastinum as a visceral pleural-based pedunculated mass. The mean diameter of the tumors was 2.6 cm, varying from 1 cm to 8 cm, and the tumor size was larger than 4 cm in diameter only in four patients.
Lymph node biopsy or dissection was done in 21, and one patient was found to have lymph node metastasis at the left upper pulmonary vein lymph node. In that case, the patient underwent completion lobectomy because of the possible malignant characteristics of PSH with lymph node metastasis. However, there was no clinical evidence of local recurrence or distant metastasis during the 10-year follow-up in this patient.
For tumors embedded in pulmonary parenchyma, at least 0.5 cm of resection margin was achieved. The tumors located in the interlobar fissure and visceral pleura were solid and well-encapsulated mass without invading adjacent tissue.
There was no postoperative complication, and no early or late tumor-related mortality. The mean follow-up period was 39.3 months (from 2 to 129 months), and all patients were free of local recurrence or distant metastasis during the follow-up period.
There was no significant difference in basic patient's characteristics and surgical results between the two groups.
The mean hospital stay was significantly shorter in limited resection group (4.01 days) than in lobectomy group (6.5 days) (p=0.0031).
DISCUSSION
Pulmonary sclerosing hemangioma, first described by Liebow and Hubbell in 1956 [1], is a rare primary pulmonary tumor. It predominantly involves women and is more common among Asians.
Although the biologic behavior of PSH is not clear and its histogenesis is still under debates, it has recently been claimed that PSH originates from primitive respiratory epithelial cells [2,3].
The clinical behavior of PSH has not been clearly elucidated. PSH presents mostly as a single solid mass in the lung, with right-side predominance, but it occasionally exhibits as multiple nodules [2] with an incidence of 4~5%. It can also be found elsewhere (i.e. mediastinum or pleura) in the thorax [4]. We also experienced two cases of multiple nodules and two cases of unusual locations.
PSH is considered to have benign nature. However, there have been several reports on the malignant potential of the tumor. Ever since Tanaka and colleagues reported the first case of PSH with lymph node metastasis [5], there have been sporadic reports on its metastatic nature [6-10]. There was also a case report on the local recurrence of PSH [11]. The prognosis of PSH does not appear to be affected by these malignant potentials, for there has been no documented mortality caused by PSH. Among the 32 patients in our study, one patient with a mass located at the interlobar major fissure was found to have regional lymph node metastasis. This patient had been initially diagnosed as having fibrous tumor of the pleura preoperatively. Postoperative permanent pathology revealed findings compatible with PSH, and the patient received reoperation for completion lobectomy. The lymph node metastasis was found at the pulmonary vein lymph node in the lobectomy specimen. After 10-year follow-up, there was evidence of tumor recurrence in the adjacent lung parenchyma or lymph nodes.
Even though many authors in the literatures pertaining to the surgical strategy for PSH preferred limited resection (i.e. wedge resection or enucleation) to anatomical resection (i.e. lobectomy), there has still been no consensus on the optimal extent of resection for PSH. One of the important reasons for this is the inaccuracy of preoperative diagnosis for PSH. The cytological findings of PSH have been rarely described, and bear a strong resemblance to certain types of pulmonary malignancies. Differential diagnoses of PSH based on cytology include pulmonary adenocarcinoma, BAC, and pulmonary carcinoid [12]. Radiologically, PSH usually presents as a solitary, well-circumscribed mass without cavitation. The tumor typically shows heterogeneous areas of attenuation, marked enhancement, and air trapping, which is forms "air meniscus sign" on plain chest films. However, these findings can also be seen in tuberculoma, aspergilloma, hamartoma, and lung carcinoma. A Chinese group, which favored limited resection in their report of 24 patients with PSH [13], also pointed out the difficulties in the precise preoperative diagnosis of PSH. In this study, the radiologic prediction diagnosis of PSH was only made in 10 out of 32 cases. Preoperative cytological examination with fine needle aspiration was also misleading because eight out of twelve successful diagnostic procedures resulted in misdiagnoses of pulmonary adenocarcinoma (5 cases) or other lung tumors (3 cases). In one case, even the frozen section in the operating room failed to identify PSH, which was misdiagnosed as BAC. As our experience accumulates, pathologists in our institution reported 16 cases of PSH with 5 unusual presentations [14], and from then on, the accuracy of preoperative diagnosis of PSH has considerably improved. Since 2007, PSH was correctly diagnosed in almost all cases (7 cases out of 8 by chest CT scan and all 3 cases by fine needle cytology). Regardless of the extent of surgery, all the patients in our current report are all alive and well at present without the evidence of local recurrence or metastasis.
CONCLUSION
Pulmonary sclerosing hemangioma usually requires surgical resection for both accurate diagnosis and adequate treatment. Limited resection can decrease hospital stay and costs with a surgical outcome comparable to lobectomy. Recent progress in preoperative radiologic and pathologic diagnosis may reduce the probability of unnecessary lobectomy.
References
- 1.Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9:53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::aid-cncr2820090104>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Katzenstein AL, Gmelich JT, Carrington CB. Sclerosing hemangioma of the lung. A clinicopathologic study of 51 cases. Am J Surg Pathol. 1980;4:343–356. doi: 10.1097/00000478-198008000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies. Am J Surg Pathol. 2000;24:906–916. doi: 10.1097/00000478-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto K, Okita M, Kumagiri H, Kawamura S, Takeuchi K, Mikami R. Sclerosing hemangioma isolated to the mediastinum. Ann Thorac Surg. 2003;75:1021–1023. doi: 10.1016/s0003-4975(02)04365-5. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka I, Inoue M, Matsui Y, et al. A case of pneumocytoma (so called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol. 1986;16:77–86. [PubMed] [Google Scholar]
- 6.Yano M, Tamakawa Y, Kiriyama M, Hara M, Murase T. Sclerosing hemangioma with metastases to multiple nodal stations. Ann Thorac Surg. 2002;73:981–983. doi: 10.1016/s0003-4975(01)03122-8. [DOI] [PubMed] [Google Scholar]
- 7.Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases:report of 4 cases. Arch Pathol Lab Med. 2003;127:321–325. doi: 10.5858/2003-127-0321-PSHWLN. [DOI] [PubMed] [Google Scholar]
- 8.Katakura H, Sato M, Tanaka F, et al. Pulmonary sclerosing hemangioma with metastasis to the Mediastinal lymph node. Ann Thorac Surg. 2005;80:2351–2353. doi: 10.1016/j.athoracsur.2004.06.099. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu T, Fukuse T, Wada H, Sakurai T. Pulmonary sclerosing hemangioma with pulmonary metastasis. Thorac Cardiovasc Surg. 2006;54:348–349. doi: 10.1055/s-2005-872976. [DOI] [PubMed] [Google Scholar]
- 10.Jungraithmayr W, Eggeling S, Kudwig C, Kayser G, Passlick B. Sclerosing hemangioma of the lung: a benign tumour with potential for malignancy? Ann Thorac Cardiovasc Surg. 2006;12:352–354. [PubMed] [Google Scholar]
- 11.Wei S, Tian J, Song X, Chen Y. Recurrence of pulmonary sclerosing hemangioma. Thorac Cardiovasc Surg. 2008;56:120–122. doi: 10.1055/s-2007-989280. [DOI] [PubMed] [Google Scholar]
- 12.Ng WK, Fu KH, Wang E, Tang V. Sclerosing hemangioma of lung: a close cytologic mimicker of pulmonary adenocarcinoma. Diagn Cytopathol. 2001;25:316–320. doi: 10.1002/dc.2162. [DOI] [PubMed] [Google Scholar]
- 13.Situ DR, Long H, Ma GW, Lin ZC, Yun JP, Rong TH. Diagnosis and therapeutics of 24 cases of pulmonary sclerosing hemangioma. Ai Zheng. 2008;27:861–865. [PubMed] [Google Scholar]
- 14.Kim GY, Kim J, Choi YS, Kim HJ, Ahn G, Han J. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci. 2004;19:352–358. doi: 10.3346/jkms.2004.19.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]