Abstract
Background
A peripheral extracorporeal membrane oxygenator (p-ECMO) has been developed to support patients who are dying due to a serious cardiopulmonary condition. This analysis was planned to define the clinical situation in which the patient benefits most from a p-ECMO.
Material and Methods
Between June 2007 and Aug 2009, a total of 41 adult patients used the p-ECMO. There were 23 males and 18 females (mean age 54.4±15.1 years). All patients had very unstable vital signs with hypoxia and complex cardiac problems. We divided the patients into 4 groups. In the first group, a p-ECMO was used as a bridge to cardiac operation. In the second group, patients did not have the opportunity to undergo any cardiac procedures; nevertheless, they were treated with a p-ECMO. In the third group, patients mostly had difficulty in weaning from CPB (cardiopulmonary bypass) after cardiac operation. The fourth group suffered from many complications, such as pneumonia, bleeding, infections, and LV dysfunction with underlying cardiac problems. All cannulations were performed by the Seldinger technique or cutting down the femoral vessel. A long venous cannula of DLP® (Medtronic Inc, Minneapolis, MN) or RMI® (Edwards Lifesciences LLC, Irvine, CA) was used together with a 17~21 Fr arterial cannula and a 21 Fr venous cannula. As a bypass pump, a Capiox emergency bypass system (EBS®; Terumo, Tokyo, Japan) was used. We attempted to maintain a flow rate of 2.4~3.0 L/min/m2 and an activated clotting time (ACT) of around 180 seconds.
Results
Nine patients survived by the use of the p-ECMO. Ten patients were weaned from a p-ECMO but they did not survive, and the remainder had no chance to be weaned from the p-ECMO. The best clinical situation to apply the p-ECMO was to use it as a bridge to cardiac operation and for weaning from CPB after cardiac operation.
Conclusion
Various clinical results were derived by p-ECMO according to the clinical situation. For the best results, early adoption of the p-ECMO for anatomical correction appears important.
Keywords: Cardiogenic shock, Extracorporeal membrane oxygenation
INTRODUCTION
Strikingly rapid development in the techniques of cardiothoracic surgery now allows aggressive interventions for patients with high risks for whom previously only conservative management could be applied. However, in such cases, cardiac arrest or cardiogenic shock may easily occur. Proper management of circulation and vital signs postoperatively is crucial to determining the prognosis and outcome of surgery.
Often proper perfusion and vital signs cannot be managed by a sufficient amount of medication such as digitalis or vasoconstrictors. Even an intra-aortic balloon pump (IABP) sometimes may not be able to improve circulation or vital signs. Such an emergent condition may be handled by a ventricular assist device (VAD) or extracorporeal membrane oxygenation (ECMO). However, such conventional mechanical cardiopulmonary support devices are only operated by cardiothoracic surgeons or by a perfusionist. Also, the duration of preparation takes quite a bit of time. Thus, it is very difficult to apply in an emergency in which cardiopulmonary resuscitation is performed. Furthermore, those devices have demonstrated a low survival rate, regardless of their aggressive methods of management [1]. Postoperative complications arising from using a cardiopulmonary bypass system (CPB) include postcardiotomy cardiogenic shock (PCS), which interrupts weaning from the CPB, uncontrolled hemorrhage and arrhythmia, and even cardiac arrest. These complications may develop with a frequency of about 2~8% after open cardiothoracic surgery. Thus, it has been suggested that several supportive devices be developed, which may assist in weaning from CPB postoperatively [2].
Recent supplementation of automated percutaneous cardiopulmonary support (PCPS) has ameliorated management of life support in patients with cardiogenic shock, cardiac arrest, adult respiratory distress, aspiration burns, or drug-induced myocarditis [3,4]. Peripheral extracorporeal membrane oxygenation (p-ECMO) not only complements the weakness of conventional devices but also warrants bedside procedure. It results in faster management of circulation and vital signs than the conventional method does; thus, the p-ECMO has become a very effective device for managing patients with cardiac arrest or cardiogenic shock in-hospital [3,5].
This study investigates the usefulness and clinical application of the p-ECMO, analyzing clinical cases in which p-ECMO was applied to manage conditions and complications caused by cardiogenic shock.
MATERIAL AND METHODS
From June, 2007 to October, 2009, p-ECMO was used for a total of 41 patients who showed cardiogenic shock or cardiac arrest, and their medical records were analyzed retrospectively. For the p-ECMO, an emergent bypass system (CAPIOX EBS; Teramo Inc, Tokyo, Japan) was used. The patients were divided into 4 different groups (Table 1). Group A included patients to whom p-ECMO was applied right after undergoing CPR and before cardiotomy due to cardiogenic shock occurred from coronary, valvular, or aortic diseases. Group B included patients who could not receive cardiotomy because of clinical deterioration in spite of applying p-ECMO. Group C represented patients who had undergone cardiotomy for other clinical conditions, and p-ECMO was applied because they could not be weaned from CPB. Group D consisted of patients who had p-ECMO but were not candidates of open cardiotomy due to complicated underlying cardiac diseases.
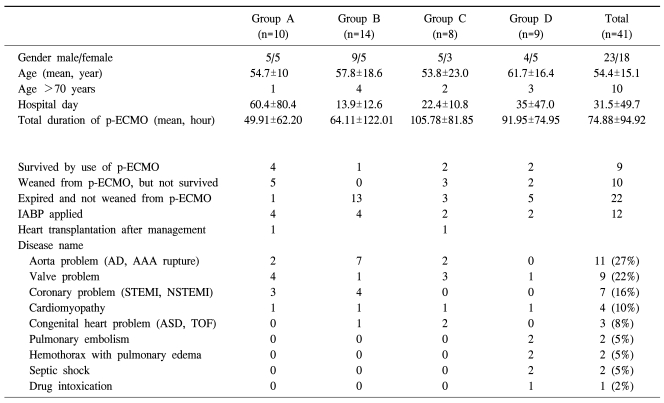
Table 1.
Patients characteristics
Group A=Patients that adopted a peripheral ECMO as a bridge to cardiac operation; Group B=Patients that did not have chance to undergo any cardiac procedures, nevertheless they were applied to peripheral ECMO; Group C=Patients that mostly had difficulty in being weaned from CPB (cardiopulmonary bypass) after cardiac operation; Group D=Patients suffered from many complications, such as pneumonia, bleeding, infections and LV dysfunction. AD=Aortic dissection; AAA=Abdominal aortic aneurysm; STEMI=ST elevation M; NSTEMI=Non ST elevation MI; ASD=Atrial septal defect; TOF=Tetralogy of fallot.
All patients exhibited systolic blood pressure below 80 mmHg for over 30 min along with mental changes, oliguria, or other symptoms and signs related to peripheral vasoconstriction. They did not respond to a full administration of inotropes or vasopressors. Also, blood gas analysis displayed pO2 below 60 mmHg despite having O2 fully administered. CPR was performed for all patients exhibiting cardiac arrest or clinical conditions requiring resuscitation. Most patients fit into the diagnostic criteria for cardiogenic shock, but afterward septic shock was observed in two cases. One case was confirmed in which hypovolemic shock occurred along with cardiogenic shock. For groups A, B, and D, p-ECMO was applied as a bedside procedure, whereas in group C, it was performed in an operating room. To make a percutaneous route for p-ECMO, Seldinger's method was generally used. However, in two cases Seldinger's method had failed to obtain vascular access, so the femoral artery and vein were dissected and exposed. There were no complications associated with the dissection and exposure of the femoral vessels. For optimal drainage, the first 21 Fr percutaneous venous cannula (Medtronic Inc., Minnerapolis, MN, USA or Edwards's Lifescience LLC, Irvine, CA) was inserted through the incurrent canal, reaching the right atrium. Then, a 17 to 21 Fr percutaneous arterial cannula inserted at the femoral artery was located at the common iliac artery for the incurrent canal. While inserting the catheter, auto-priming for the next 10 min began simultaneously. 5,000 IU of heparin was administered just before inserting the catheter, then 3 mg/Kg/min was injected intravenously along with the maintenance of ACT between 180 to 200 sec. After pumping began, the blood volume was maintained between 2.4~3.0 L/min/m2, and the volume was adjusted based on factors such as vital signs and use of inotropes. In the cases of group C, the same method was applied to achieve vascular access, except that the cardiotomy site was closed after obtaining access. Weaning from the p-ECMO was attempted when gradual reduction of blood flow did not provoke any symptoms or signs of cardiogenic shock without increasing doses of digitalis or of inotropes. As the catheter was taken out, the catheter insertion site was compressed in a sterile condition, until either hemorrhage or subcutaneous edema was no longer observed. Vascular suture was performed for the two cases in which vascular dissection was applied. Administration of heparin was stopped after removing the cannula, but neutralization using protamine was not attempted.
The clinical manifestation and study result of applying p-ECMO to the above patients were analyzed. For the statistical methods, analyzing the sex, age, clinical presentation, weaning of EBS and long-term survival, Statistical Package for the Social Sciences (SPSS version 13.0, Chicago, Illinois, USA) was used. Analyzing the risk factors, the continuous variable was examined by the Mann-Whitney U test, where the non-continuous variable was evaluated by Fisher's exact test using a Chi square test. A p-value under 0.05 represented statistical significance. The standard deviation and its range were indicated when necessary.
RESULTS
The mean age of patients in this study group was 54.4±15.1 (19~86), in which 23 male patients and 18 female patients were included. The ages of each group were 54.7 (±10.0) for A, 57.8 (±18.6) for B, 53.8 (±23.0) for C, and 61.7 (±16.4) for D. Distinguished by sex, group A included 5 males and 5 females, B included 9 males and 5 females; C included 5 males and 3 females; and D included 4 males and 5 females. Among them, a total of 10 patients were over 70 years old: one patient in group A, 4 in group B, 2 in group C, and 3 in group D. The mean number of days of hospital stay was 31.5 (±49.7). By each group, it was 60.4 (±80.4) in A, 13.9 (±12.6) in B, 22.4 (±10.8) in C, and 35.0 (±46.7) in D (Table 1).
The underlying diseases of the patients included 11 cases of aortic diseases, such as aortic dissection and rupture (27%); 9 cases of mitral or aortic valvular diseases (22%); 7 cases of acute coronary syndrome, like acute myocardial infarction (16%); 4 cases of myocardial diseases, including hypertrophic cardiomyopathy (10%); 3 cases of congenital heart diseases, such as atrial septal defect or TOF (8%); 2 cases of acute pulmonary embolism (5%); 2 cases of massive hemothorax and pulmonary edema that occurred in the post-cardiotomy state; 2 cases of septic shock following cardiogenic shock in patients with underlying coronary disease or valvular disease; and 1 case of cardiogenic shock due to myocardial damage induced by drug overdose (2%) (Table 1).
Operations used for members of group A included one of ascending aorta and aortic valve replacement; one of ascending and descending aorta replacement; three of coronary bypass surgery; two of aortic valve replacement; two of mitral valve replacement; and one of cardiac transplantation. Failure of weaning from p-ECMO, observed in group C, was due to low cardiac output, shown in 5 cases, and diffuse hemorrhage, exhibited in 3 cases.
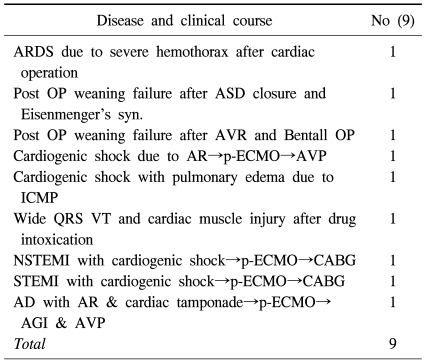
41 patients with cardiogenic shock, who had underlying heart diseases, underwent p-ECMO. The average of the total hours of operation was 74.88 (±94.92), and for each group, -49.91 (±62.20) in group A; 64.11 (±122.01) in group B; 105.78 (±81.85) in group C; and 91.95 (±74.95) in group D (Table 1). Statistical significance based on the hours of operation was not observed (p=0.71). 19 out of 41 patients were able to be weaned from the p-ECMO (46.3%). 9 patients demonstrated long-term survival (21.9%). 10 patients succeeded in being weaned from p-ECMO but did not survive (24.3%). The patients who showed long-term survival in group A included one case of aortic valvuloplasty due to severe aortic regurgitation; two cases of coronary artery bypass graft and CABG due to acute myocardial infarction; one case of ascending aorta replacement, and aortic valvuloplasty due to ascending aortic dissection, aortic regurgitation and cardiac tamponade. In group B, there was one case of cardiogenic shock and severe pulmonary edema due to idiopathic cardiomyopathy. In group C, there were one case of postoperative low cardiac output syndrome due to ventricular septal defect and Eisenmenger's syndrome and one case of Bentall's operation. Group D included a case that had received a coronary arytery bypass graft and succeeded in being weaned from p-ECMO, but developed hemothorax, coronary hypoperfusion, and acute respiratory distress syndrome (ARDS) while receiving postoperative care in the ardiac care unit (Table 2).
Table 2.
Patients characteristics survived by use of peripheral ECMO
ARDS=Acute respiratory distress syndrome; ASD=Atrial septal defect; AVR=Aortic valve replacement; p-ECMO=Peripheral ECMO; AVP=Aortic valvulopasty; VT=Ventricular tachycardia; AR=Aortic regurgitation; ICMP=Idiopathic cardiomyopathy; NSTEMI=Non ST elevation MI; STEMI=ST elevation MI; AD=Aortic dissection; AGI=Aorta graft interposition.
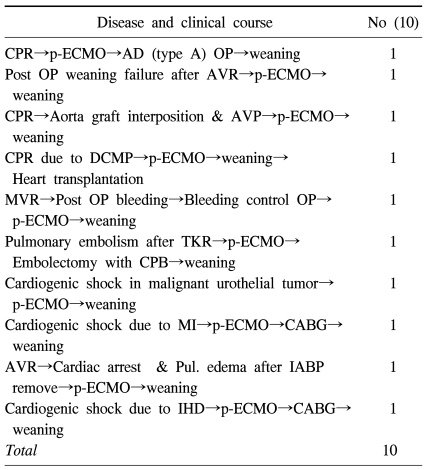
Furthermore, there were a total of 10 cases that were successfully weaned from p-ECMO but expired from unfavorable underlying cardiac disease. In group A, there were five cases: one ascending aorta replacement due to ascending aortic dissection, one cardiac transplantation due to dilated cardiomyopathy, one removal of pulmonary embolus due to acute pulmonary embolism that occurred after total knee replacement, and two coronary artery bypass grafts due to acute myocardial infarction and ischemic heart disease. In group C, there were one case of aortic valvular replacement, one ascending aorta replacement and aortic valvuloplasty, and one cardiogenic shock that occurred during re-operation of a mitral valvular replacement due to postoperative hemorrhage. In group D was observed one case of cardiogenic shock that occurred in a patient with atherosclerotic coronary disease and ureteroepithelioma, and one of pulmonary edema that occurred after aortic valvular replacement (Table 3).
Table 3.
Patients characteristics weaned from peripheral ECMO, but not survived
p-ECMO=Peripheral ECMO; AD=Aortic dissection; AVR=Aortic valve replacement; AVP=Aortic valvulopasty; DCMP=Dilated cardiomyopathy; MVR=Mitral valve replacement; TKR=Total knee replacement; MI=Myocardial infarction; CABG=Coronary artery bypass graft; IHD=Ischemic heart disease.
The major cause of death was due to multiple organ failure due to irreversible cardiac damage resulting low cardiac output syndrome and cardiac failure. In addition, two cases of sepsis were confirmed in the follow-up lab tests. IABP was applied to a total of 12 cases (29.2%). Moreover, 3 cases (7.3%) that simultaneously developed acute renal failure were applied continuous renal replacement therapy (CCRT). Cardiac transplantation was performed in two cases after p-ECMO (4.8%). A long-term follow-up revealed the patient with DCMP in group A had expired, receiving medical care in an outpatient department. On the other hand, the DCMP patient in group C is currently being observed successfully in the outpatient department.
Sex, days of hospital stay, and application of IABP were not statistical factors affecting survival rate or weaning from p-ECMO. However, age over 70 years increased mortality and interfered with weaning from p-ECMO (p=0.037). The survival rate of 41 patients was 21.9% (n=9), and the rate of weaning was 46.3% (n=19). Group A, in which p-ECMO was used preoperatively to correct the underlying heart condition, demonstrated a higher survival rate (n=4, 40%) and weaning rate (n=9, 90%) than the other groups. Group B, which was indicated to receive surgical treatment but it could not be attempted, exhibited the lowest survival rate (n=1, 7%) and weaning rate (n=1, 7%). Also, group C, in which p-ECMO was applied post-cardiotomy to help wean from CPB, showed a survival rate of 25% (n=2) and a weaning rate of 62.5% (n=5). Group D displayed a survival rate of 22.2% (n=2) and a weaning rate of 44.4% (n=4).
DISCUSSION
In the early 1970's, extracorporeal life support (ECLS) using extracorporeal membrane oxygenation (ECMO) was introduced. A large number of patients could receive extensive supportive care, and it is currently known to be the fastest and most cost-effective method of respiratory support for both adults and children [6]. Furthermore, the persistent efforts of cardiothoracic surgeons has resulted in a rapid development in cardiothoracic surgical techniques, allowing surgical treatment for diseases previously considered contraindications for cardiotomy. Recently, even patients exhibiting cardiogenic shock or cardiac arrest are considered eligible for open cardiotomy. Such patients whose condition had not improved by conventional cardiopulmonary resuscitation could benefit from percutaneous cardiopulmonary support (PCPS), which helps proper perfusion in vital organs, such as the brain or kidneys. Recent studies demonstrate that non-postcardiotomy cardiopulmonary failure may receive support from ECLS, leading to a survival rate of 40~50%, but postcardiotomy shock only presents a survival rate of 20~36% despite application of ECLS. Thus, further study on the application of ECLS is necessary [2,7,8].
The most important factor in either ECLS or PCPS applied to patients with unstable cardiopulmonary conditions is the membrane oxygenator of which the ECMO system is composed. Development of bioengineering for oxygentors has increased the survival of patients with cardiopulmonary failure and the uses of ECLS or PCPS, greatly valuable to cardiothoracic surgeons [9]. The improvement of PCPS has enabled the maintenance of the blood supply just adequate for perfusion to vital organs, and it has decreased the myocardial oxygen demand. Several studies have reported that cardiothoracic surgery successfully carried out in very unstable cardiac conditions [10,11].
Such PCPS nowadays was indicated to unresponsive to conventional cardiopulmonary resuscitation [5]. This is found to be particularly useful in patients who require PCI due to ischemic heart disease but in whom cardiogenic shock or cardiac arrest occurred. Previously, a large number of patients with ischemic heart diseases, such as coronary artery disease, had expired before an appropriate intervention, like PCI. Even when re-perfusion was achieved, very severe complications, damage to vital organs, and disturbances in consciousness had resulted in poor prognosis. However, an aggressive approach by PCPS has been noted, for which not only the survival rate has been increased, but also the damage to vital organ has been minimized. Up-to-date research on the application of PCPS in PCI or CABG has shown a 25~76% of success rate [12-15]. The study done in Korea by Rhee, Il in 2006 reported a 55% success rate [16]. Such reports on the efficacy of PCPS have a large deviation in the success rate. Such a deviation resulted from the study design of the patient group, and the timing of application and techniques related to operation and maintenance of PCPS, which differed in among various centers [17]. Our study results show a lower survival rate and weaning rate than studies carried out in other centers. It seems that setting the patient group exhibiting cardiogenic shock or cardiac arrest as the study target would be the most determinant factor affecting the study results. Also, p-ECMO may play a role in enabling surgical treatment for those with very unstable vital conditions. However, it still does have a limited effect on advanced cardiac diseases. Nevertheless, if p-ECMO were applied sooner than expected in spite of extremely unstable vital conditions, such as cardiogenic shock, p-ECMO would be an optimal intermediate, which may serve as a bridge to surgical treatment. Furthermore, it may be another treatment option for stabilizing the fluctuating cardiopulmonary status postcardiotomy.
In this study EBS® (CAPIOX: Terumo Inc., Tokyo, Japan) was used as p-ECMO. This extracorporeal membrane oxygenator has a similar structure and disposition to the conventional extracorporeal membrane oxygenator used in cardiotomy. However, compared to CPB, EBS presents several advantages. It utilizes smaller amounts of anticoagulants. Its lifespan is improved. Also, it is operated as a bedside procedure; it is very mobile, allowing a convenient and easy approach in emergency department or intensive care unit. Moreover, autopriming is feasible, which brings high accessibility, only requiring little knowledge or grasp of the machine and anatomic structure. Therefore, the disadvantage of priming, such as taking more than 30 min and operation only by cardiothoracic surgeon or trained extracorporeal membrane perfusionist, can be resolved. The blood volume required for priming is only 470 cc, giving the least hemodynamic burden on the patient. The small and handy structure of the catheter may reduce operative error, and its structure is designed to easily remove air that enters the circuit. Also, if the vascular condition is optimal, Seldinger's method can be applied for most cases. Even patients with hemodynamic instability, such as cardiogenic shock and cardiac arrest, can benefit from prompt manipulation, which may help to stabilize vital signs and to achieve hemodynamic stability. Such manipulation can serve as a tool utilized for emergent situations and long-term management out of the operating room [3,18]. In spite of the many advantages presented by the EBS system, a few disadvantages are observed. The membranous oxygenator adopts a microporous oxygenating method, leading to frequent plasma leakage. Also, adequate administration of anticoagulants including heparin could not completely prevent thrombus in the oxygenator, and the straight type of pump used in the oxygenator even aggravates the formation of thrombus, resulting in a short lifespan of 2~3 days. Persistent administration of heparin increases the risk of developing cerebral hemorrhage or a bleeding tendency. Pulmonary hypertension developed postoperatively may lead to pulmonary hemorrhage. All such disadvantages augment the postoperative mortality rate; therefore, attentive management is required [8]. Recent advancement of PCPS includes application of diffusion membrane composed of polymethyl pentene (PMP). It supplements the inadequacy of EBS, and some reports indicate better results than EBS [9]. PMP allows fluent blood flow through a kit coated with albumin-heparin, and polymerization of albumin-heparin on the catheter does not wipe out the coating. Therefore, it can be considered a biocompatible model, tolerating long-term use of at least 14 days. Other advantages include: a lower rate of occurrence of hemolysis due to the narrow range of pressure drop and amenable control of body temperature by the heat exchanger, allowing application even on hypothermic patients [9]. In this study, the average duration of use of p-ECMO was 74.88 hours, about 3.12 days. 8 out of 41 patients developed thrombus, which troubled the oxygenator, requiring exchange of the oxygenator. 2 out of those 8 patients had to exchange the oxygenator one more time later in the course.
Vanier et al. [19] reported positive results from performing PCI on patients of high risk ischemic heart disease, who were already inserted with a PCPS. Especially in those presenting technical difficulties due to a diseased lesion in the coronary artery or the artery itself exhibiting a steep angle of approach, p-ECMO was inserted before performing PCI. They could be weaned from p-ECMO after the procedure. 3 of the 15 patients studied expired from cardiac problems Thus, PCPS can support PCI in high-risk patients who are inoperable. Also, Burkle et al. [20] reported the success of PCI in 5 cases of high-risk patients with severe left ventricular failure, severe co-morbid disease, or difficulty receiving CABG, of which PCPS was utilized prior to PCI. Thus, prior utilization of PCPS is suggested when performing PCI. The complications associated with use of PCPS include hemorrhage, ischemia of the lower extremities, renal failure, infection, or multiple organ failure. Such complications may differ greatly with a prolonged duration in maintenance of the machine, skill of the operator, patient condition, and improvement before and after p-ECMO [15,21].
Reichman et al. [12] reported that out of 38 cardiac arrest cases in which PCPS was used due to acute myocardial infarction, 7 survivors received CABG, and only 3 were reported to have survived long-term Early reports by Matsuwaka et al. [13] and Suarez et al. [14] demonstrated the supportive role of PCPS in cardiogenic shock due to acute cardiovascular diseases. Despite the low survival rate in the early reports, a gradual accumulation in clinical reports followed an increase in survival rates, elicited by improved skill of the operator and advancement of PCPS. In regard to the broad gap in the survival rate among different centers, it will be necessary to carry out a multicenter study on the use of PCPS in order to impose specific guidelines for use. However, the most important reason for having a large gap in the survival rate results is probably due to variations in the composition of the patient groups [17].
This study indicated that only 19 out of 41 patients (46.3%) were able to be weaned from p-ECMO, which is not much better than other studies. Also, only a total of 9 patients (21.9%) showed long-term survival. This is probably due to setting up a study group composed of patients with very unstable hemodynamics, such as cardiogenic shock or cardiac arrest. Our study also included patients not only with coronary heart disease but also aortic diseases, valvular diseases, cardiomyopathy, and other diseases that were excluded in other studies. This might have affected the study result of the survival rate. Hill et al. [22] reported that mortality increased such that the point of applying PCPS would move further from the point of occurrence of cardiogenic shock or cardiac arrest. Hence, we made an effort to reduce the duration between the point of shock and PCPS application, by means of bedside procedure. When vital signs were relatively stabilized, surgical treatment for their underlying cardiac diseases were promptly provided to patients as needed. IABP, thought to be helpful in patients for surgical correction as well as postoperative recovery, was readily used. For patients exhibiting signs and symptoms of acute renal failure, such as oliguria, CCRT was utilized (n=3, 7.3%). On the other hand, because blood flow created by PCPS opposes the normal physiologic blood flow, such flow can become a factor increasing the afterload of the left ventricle; consequently, ventricular enlargement and myocardial damage may be induced. Unless decompression of the left atrium is achieved, recovery of the left ventricle may be hindered [23]. If a better clinical manifestation is expected, aggressive use of IABP combined with use of PCPS should be considered for recovery of cardiac function [24].
Usually the vascular connection for p-ECMO is veno-arterial (V-A ECMO) or veno-venous (V-V ECMO), and in our study, V-A p-ECMO was used for all cases. For most cases a venous catheter can be inserted into the internal jugular vein, right atrium, or femoral vein, and an arterial catheter can be inserted into the right carotid artery, axillary artery, femoral artery, or aorta. V-A p-ECMO can help gas exchange and cardiac output, whereas V-V p-ECMO can only support gas exchange [25]. Shown in our study, V-A p-ECMO can aid cardiac function. It also exhibits the advantage of relatively easy access through the carotid artery in both adults and infants. Nonetheless, Ligation of the catotid artery is sometimes necessary during this procedure and it has high risk of developing cerebrovascular complications. Blood flow to the entire body cannot be sent in a pulsatile mode. If pulmonary function is severely deteriorated, the oxygen saturation of the blood running from the left ventricle to the coronary artery would be much lower than the oxygen saturation obtained from peripheral blood gas analysis. This might cause cardiac failure due to inadequate perfusion to the myocardium. If respiratory support is the sole purpose of using PCPS and if there is no abnormality observed in cardiac function, V-V p-ECMO can be applied [26]. Furthermore, if typical V-A p-ECMO cannot provide adequate perfusion to the myocardium, a change to or combined use with V-V p-ECMO may increase not only oxygen saturation of the entire body but also that of the myocardium [27].
CONCLUSION
Despite using a retrospective study with a small sample size, an aggressive approach to surgical treatment, an invasive procedure using PCPS is indispensable for patients with lethal conditions, including cardiogenic shock or cardiac arrest. If p-ECMO is applied in advance to patients exhibiting hemodynamic instability requiring cardiopulmonary resuscitation, it may function as an excellent intermediate stabilizing condition preoperatively. Also, it could work as another treatment option for recovery of cardiac function postoperatively. Lack of analysis on various factors affecting survival rates and small sample size requires further studies to increase greater confidence. It is also necessary to make comparisons of the different types of PCPS and clinical results of using them. Based on persistent efforts by cardiothoracic surgeons, better clinical results will be reported, and a greater number of people may be have their lives extended.
Footnotes
This work was supported by Konkuk University in 2010.
This article was presented at the 41th autumn scientitic meeting of The Korean Society for Thoracic and Cardiovascular Surgery and ATCSA 2009 by poster form.
References
- 1.Reardon MJ, Conklin LD, Letsou GV, Safi HJ, Espada R, Baldwin JC. Methods of acute postcardiotomy left ventricular assistance. J Cardiovasc Surg (Torino) 1999;40:627–631. [PubMed] [Google Scholar]
- 2.Magovern GJ, Simpson KA. Extracorporeal membrane oxygenation for adult cardiac support: the allegheny experience. Ann Thorac Surg. 1999;68:655–661. doi: 10.1016/s0003-4975(99)00581-0. [DOI] [PubMed] [Google Scholar]
- 3.Sung K, Lee YT, Park PW, et al. Improved survival after cardiac arrest using emergent autopriming percutaneous cardiopulmonary support. Ann Thorac Surg. 2006;82:651–656. doi: 10.1016/j.athoracsur.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz B, Mair P, Margreiter J, et al. Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med. 2003;31:758–764. doi: 10.1097/01.CCM.0000053522.55711.E3. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 7.Wu MY, Lin PJ, Tsai FC, Haung YK, Liu KS, Tsai FC. Impact of preexisting organ dysfunction on extracorporeal life support for non-postcardiotomy cardiopulmonary failure. Resuscitation. 2008;79:54–60. doi: 10.1016/j.resuscitation.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Bakhtiary F, Keller H, Dogan S, et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: Clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008;135:382–388. doi: 10.1016/j.jtcvs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Formica F, Avalli L, Martino A, et al. Extracorporeal membrane oxygenation with a poly-methylpentene oxygenator (Quadrox D). The experience of a single italian centre in adult patients with refractory cardiogenic shock. ASAIO J. 2008;54:89–94. doi: 10.1097/MAT.0b013e31815ff27e. [DOI] [PubMed] [Google Scholar]
- 10.Reedy JE, Swartz MT, Raithel SC, et al. Mechanical cardiopulmonary support for refractory cardiogenic shock. Heart Lung. 1990;19:514–523. [PubMed] [Google Scholar]
- 11.Shawl FA, Domanski MJ, Punja A, Hemandez T. Emergency percutaneous cardiopulmonary bypass support in cardiopulmonary shock from acute myocardial infarction. Am J Cardiol. 1989;64:967–970. doi: 10.1016/0002-9149(89)90791-1. [DOI] [PubMed] [Google Scholar]
- 12.Reichman RT, Joyo CI, Dembisky WP, et al. Improved patient survival after cardiac arrest using a cardiopulmonary support system. Ann Thorac Surg. 1990;49:101–105. doi: 10.1016/0003-4975(90)90363-b. [DOI] [PubMed] [Google Scholar]
- 13.Matsuwaka R, Sakakibara T, Shintani H, et al. Emergency cardiopulmonary bypass support in patients with severe cardiogenic shock after acute myocardial infarction. Heart Vessels. 1996;11:27–29. doi: 10.1007/BF01744596. [DOI] [PubMed] [Google Scholar]
- 14.Suárez de Lezo J, Pan M, Medina A, et al. Percutaneous cardiopulmonary support in critical patients needing coronary interventions with stents. Catheter Cardiovasc Interv. 2002;57:467–475. doi: 10.1002/ccd.10340. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto S, Matsubara J, Matsubara T, et al. Clinical effects of percutaneous cardiopulmonary support in severe heart failure: early results and analysis of complications. Ann Thorac Cardiovasc Surg. 2003;9:105–110. [PubMed] [Google Scholar]
- 16.Rhee I, Kwon SU, Sung K, et al. Experience with emergency percutaneous cardiopulmonary support in in-hospital cardiac arrest or cardiogenic shock due to the ischemic heart disease. Korean J Thorac Cardiovasc Surg. 2006;39:201–207. [Google Scholar]
- 17.Willms DC, Atkins PJ, Dembitsky WP, et al. Analysis of clinical trends in a program of emergent ECLS for cardiovascular collapse. ASAIO J. 1997;43:65–68. [PubMed] [Google Scholar]
- 18.Ryu KM, Kim SH, Seo PW, et al. Initial experience of the emergency bypass system (EBS) for the patients with cardiogenic shock due to an acute myocardial infarction. Korean J Thorac Cardiovasc Surg. 2008;41:329–334. [Google Scholar]
- 19.Vainer J, van Ommen V, Maessen J, Geskes G, Lamerichs L, Waltenberger J. Elective high-risk percutaneous coronary interventions supported by extracorporeal life support. Am J Cardiol. 2007;99:771–773. doi: 10.1016/j.amjcard.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Burkle CM, Nuttal GA, Rihal CS. Cardiopulmonary bypass support for percutaneous coronary interventions: what the anesthesiologist needs to know. J Cardiothorac Vasc Anesth. 2005;19:501–504. doi: 10.1053/j.jvca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Orime Y, Shino M, Hata H, et al. Clinical experiences of percutaneous cardiopulmonary support: its effectiveness and limit. Artif Organs. 1998;22:498–501. doi: 10.1046/j.1525-1594.1998.06136.x. [DOI] [PubMed] [Google Scholar]
- 22.Hill JG, Bruhn PS, Cohen SE, et al. Emergent applications of cardiopulmonary support: a multiinstitutional experience. Ann Thorac Surg. 1992;54:699–704. doi: 10.1016/0003-4975(92)91014-z. [DOI] [PubMed] [Google Scholar]
- 23.Scholz KH, Figulla HR, Schroder T, et al. Pulmonary and left ventricular decompression by artificial pulmonary valve incompetence during percutaneous cardiopulmonary bypass support in cardiac arrest. Circulation. 1995;91:2664–2668. doi: 10.1161/01.cir.91.10.2664. [DOI] [PubMed] [Google Scholar]
- 24.Murashita T, Eya K, Miyatake T, et al. Outcome of the perioperative use of percutaneous cardiopulmonary support for adult cardiac surgery: factors affecting hospital mortality. Artif Organs. 2004;28:189–195. doi: 10.1111/j.1525-1594.2003.47255.x. [DOI] [PubMed] [Google Scholar]
- 25.Alpard SK, Zwischenberger JB. Extracorporeal membrane oxygenation for severe respiratory failure. Chest Surg Clin N Am. 2002;12:355–378. doi: 10.1016/s1052-3359(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Meuers KV, Lally KP, Peek G, Zwischenberger JB. ECMO: Extracorporeal cardiopulmonary support in critical care. 3rd ed. Ann Arbor, Michigan: Extracorporeal Life Support Organization; 2005. [Google Scholar]
- 27.Lee SJ, Chee HK, Hwang JJ, et al. Application of veno-venoarterial extracorporeal membrane oxygenation in multitrauma patient with ARDS. Korean J Thorac Cardiovasc Surg. 2010;43:104–107. [Google Scholar]