Abstract
Background
Video-assisted thoracic surgery (VATS) lobectomy has been performed with increasing frequency over the last decade. However, there is still controversy as to its indications, safety, and feasibility. Especially regarding lung cancer surgery, it is not certain whether it can reduce local recurrences and improve overall survival.
Materials and Methods
We retrospectively reviewed 1,067 cases of VATS lobectomy performed between 2003 and 2009, including the indications, postoperative morbidity, mortality, recurrence, and survival rate.
Results
One thousand and sixty seven patients underwent VATS lobectomy for the following indications: non-small cell lung cancer (NSCLC) (n=832), carcinoid tumors (n=12), metastatic lung cancer (n=48), and benign or other diseases (n=175). There were 63 cases (5.9%) of conversion to open thoracotomy during VATS lobectomy. One hundred thirty one (15.7%) of the 832 NSCLC patients experienced pathologic upstaging postoperatively. The hospital mortality rate was 0.84% (9 patients), and all of them died of acute respiratory distress syndrome. One hundred forty-nine patients (14.0%) experienced postoperative complications. The median follow-up was 22.9 months for patients with NSCLC. During follow-up, 120 patients had a recurrence and 55 patients died. For patients with pathologic stage I, the overall survival rate and disease-free survival rate at 3 years was 92.2±1.5% and 86.2±1.9%, respectively. For patients with pathologic stage II disease, the overall survival rate and disease-free survival rate at 3 years was 79.2±6.5% and 61.9±6.6%, respectively.
Conclusion
Our results suggest that VATS lobectomy is a technically feasible and safe operation, which can be applied to various lung diseases. In patients with early-stage lung cancer, excellent survival can be also achieved.
Keywords: Video-assisted thoracic surgery, Lobectomy, Lung neoplasm
INTRODUCTION
Throughout the last decade, the number of video-assisted thoracic surgeries (VATS) has increased rapidly [1-3]. In patients with early stage lung cancer, lobectomy done by using a thoracoscope has been universalized. Technical stability has already been proven and the indication for thoracoscopy is diversifying [4-10]. However, some researchers have questioned the safety and feasibility of thoracoscopy performed in patients with lung cancer [11-14]. There is some debate regarding recurrence and survival rates after thoracoscopic lobectomy in patients with early stage non-small cell lung cancer [8,13,15-19]. According to Rueth's [20] retrospective results in a study about thoracoscopic lobectomies have done since 1994, post-surgical morbidity and inflammation were lower in patients who had gone through thoracoscopic lobectomy than in patients who had gone through thoracotomic lobectomy. In addition, there was no difference in the 5-year survival rate when comparing the results of thoracoscopic lobectomy with the results of thoracotomic lobectomy. The authors of the present study retrospectively analyzed 1,067 cases of thoracoscopic lobectomy and their indications, conversion rate to thoracotomy during surgery, postsurgical complications, hospital mortality rate, and postsurgical recurrence and survival rate in order to suggest ways to widen the surgical application of the thoracoscopic lobectomy in the future.
MATERIALS AND METHODS
This research received approval from the clinic's institutional review board. 1,067 patients who had gone through thoracoscopic lobectomy from December 24, 2003, to December 31, 2009, were retrospectively analyzed based on their medical records. Data regarding age, sex, pre-surgical diagnosis, accompanying disease, date of surgery, name of surgery, conversion to thoracotomy, length of hospitalization, pathological diagnosis, presence of complications, adjuvant chemotherapy, follow-up observation period, recurrence, and survival was collected.
Subjects who underwent surgery had lesions that could be excised anatomically and showed reduced pulmonary ventilation on preoperative pulmonary function tests. The subjects' pulmonary diseases were inflammatory diseases such as pulmonary tuberculosis, empyema, congenital pulmonary disease, primary lung cancer, metastatic lung cancer, and carcinoid tumor. In cases with benign pulmonary disease, lobectomy was performed if the majority of the pulmonary lobe was damaged, or segmentectomy or wedge resection could not be executed due to the proximity of the lesion to the central bronchus or vascular structures. However, thoracotomic lobectomy was performed in cases of CT scan evidence of pleural calcification, severe calcification around pulmonary vessels, or thorax deformation. In cases of non-small cell lung cancer, thoracoscopic lobectomy was executed if the preoperative cancer stage was stage I with no endobronchial lesion, if the tumor was located peripherally, or if the diameter of the tumor was less than 6 cm. In cases with a single metastasis to the brain, gamma knife surgery was performed before lobectomy (5 cases). Pulmonary function testing, CT scanning, and PET CT scanning were done preoperatively. If non-small cell lung cancer was diagnosed pathologically before surgery, mediastinal lymph node biopsies were performed in areas 2R, 4R, 4L, and 7 under mediastinoscopy even if there was no evidence of mediastinal lymph node metastasis. If the patient was incapable of going through neoadjuvant chemoradiotherapy due to underlying disease or old age, lobectomy was executed without mediastinoscopy, even if mediastinal lymph node metastasis was suspected. Patients with no diagnosis of preoperative pathological non-small cell lung cancer underwent thoracoscopic wedge resection, the frozen section was then analyzed, and lobectomy was done if it was diagnosed as cancer. The excised pulmonary lobe was removed from the pleural cavity in a plastic pouch to prevent implantation of cancer cells into the thoracic wall [19]. Mediastinal lymph node dissections were performed after lobectomy in all patients regardless of their clinical stage. In cases involving the right pleural cavity, dissection of the 2R, 3, 4R, 7, 8R, 9R, 10R, and 11R lymph nodes was performed. In cases involving the left pleural cavity, dissection of 4L, 5, 6, 7, 8L, 9L, 10L, and 11L lymph nodes was performed. Adjuvant chemotherapy or radiotherapy was done if the postoperative pathologic stage was more advanced when compared with the preoperative clinical stage. CT scans were done every 3 or 6 months for first 2 years after surgery to check for recurrence. After 2 years, CT scans were done every 6 months or more. All clinical stages or pathological stages of non-small cell lung cancer reported in this research were retrospectively applied according to the 7th lung cancer TNM staging [21] proposed by International Staging Committee (ISC) of the International Association for the Study of Lung Cancer (IASLC).
The authors of this research performed thoracoscopy as follows. After general anesthesia, a 10-mm access port for a thoracoscope was made in the seventh or eighth intercostal space of the mid-axillary line to observe the pleural cavity. Then, a 5 or 10 mm port was made in the seventh intercostal space of the posterior scapular line and a 4 or 5 cm working window was made in the fifth or sixth intercostal space of the mid-clavicular line. Intercostal space was not widened in all cases.
Descriptive statistics were used to collect the statistical data of patients. Continuous variables with a normal distribution are presented with averages and standard deviations. Discrete variables are presented with numbers and ratios. The overall survival period of patients who were still alive was considered to be the period from the date of surgery to either the date of the most recent visit or the last date (March 17, 2010) all living patients were called. If the patient had deceased, the survival period was considered to be from the date of surgery to the date of death. In cases of relapse, the disease free survival period was considered to be from the date of surgery to the date of relapse diagnosis. In cases without relapse, the disease-free survival period was set from the date of surgery to the most recent visit or the date of death. The survival curve was calculated using the Kaplan-Meier method using PASW Statistics 18 (SPSS Inc., IBM, Chicago, Illinois). The log-rank test was used for comparing the survival rates.
RESULTS
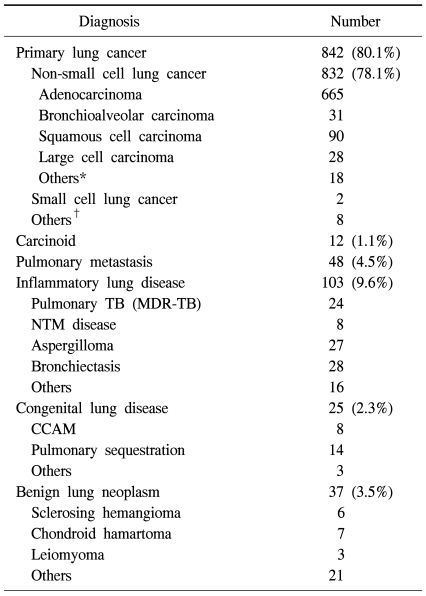
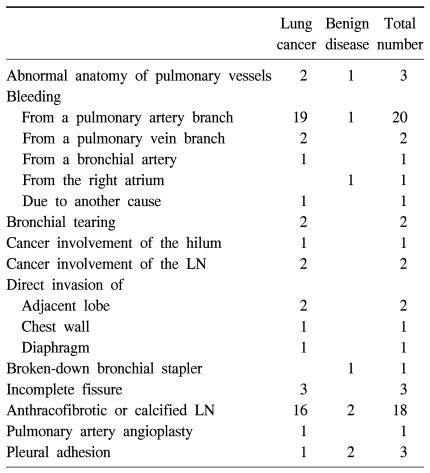
From December 24, 2003 to December 31, 2009, 1,067 patients (542 male, 525 female) underwent thoracoscopic lobectomy. The subjects' diseases included non-small cell cancer (832), carcinoid tumor (12), metastatic lung cancer (48), and other diseases (175) (Table 1). The average age was 56.9 years (12~86 years), and 162 patients (15.1%) were over 70 years old. The median time period from hospitalization to discharge was 6 days (2~110 days). Most patients had one pulmonary lobe excised, 21 patients went through right upper or lower bilobectomy (1.97%, non-small cell cancer, 19 cases), and one patient had a pneumonectomy. Sixty-three cases (5.9%) converted to thoracotomy during thoracoscopic surgery. Common causes for these conversions were hemorrhage from pulmonary artery branches and difficulties in detaching calcified or fibrous lymph nodes (Table 2).
Table 1.
Pathologic diagnoses of 1,067 patients who underwent VATS lobectomy
*=Sarcomatoid carcinoma, adenosquamous cell carcinoma, etc.; †=Lymphoma, sarcoma etc.; TB=Tuberculosis; MDR-TB=Multi-drug resistant tuberculosis; NTM=Nontuberculous mycobacteria; CCAM=Congenital cystic adenomatoid malformation.
Table 2.
Reasons for conversion to thoracotomy
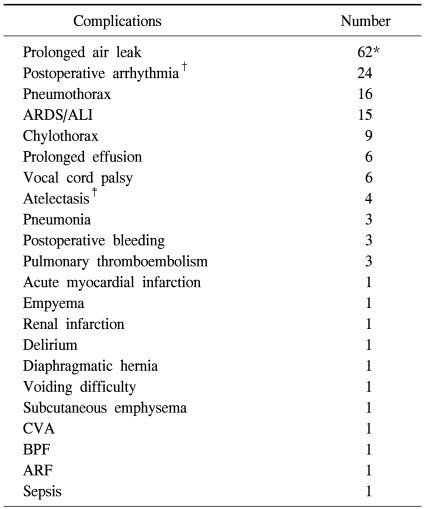
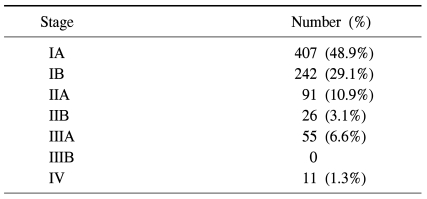
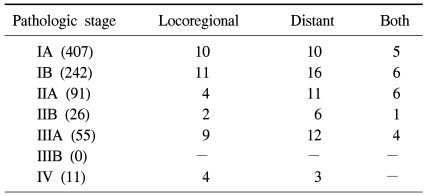
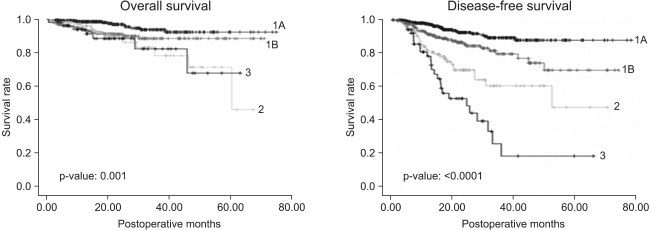
Nine patients (0.84%) died in the clinic after surgery. All of these patients underwent lobectomy because of non-small cell lung cancer and the cause of death was the same for all cases, acute respiratory distress syndrome. Five patients among them had diffuse interstitial pulmonary disease. Complications occurred 162 times in 149 patients (14.0%). The most common complication was continuous air leakage (62 cases) (Table 3). The most common pathological stage of non-small cell lung cancer was stage I with 649 cases (77.6%) (Table 4). The average follow-up observation period of non-small cell lung cancer patients was 22.9 months. The number of patients with relapse during the follow-up observation period was 120, and 55 of them died. Among patients with relapse, 40 patients had local relapse, 58 patients had remote relapse, and 22 patients had both local and remote relapses (Table 5). After surgery, 166 patients underwent adjuvant chemotherapy (106 patients), radiation therapy (19 patients), or chemoradiotherapy (41 patients). The overall 1-year and 3-year survival rates for all non-small cell lung cancer patients were 96.6±7% and 89.5±1.6%, respectively. The disease-free 1-year and 3-year survival rates were 91.8±1.0% and 77.0±2.1%, respectively. The 1-year and 3-year overall survival rates of 407 patients in pathological stage IA were 98.6±6% and 94.1±1.8%, respectively. The disease-free 1-year and 3-year survival rates were 97.5±8% and 89.7±2.2%, respectively. Two hundred and forty-two patients in pathological stage IB showed overall 1-year and 3-year survival rates of 94.3±1.5%, and 89.1±2.5%, respectively. The disease-free survival rates were 91.6±1.9% and 80.3±3.7% for 1 and 3 years, respectively. There were 117 cases where the pathological stage was stage II. Postoperative overall survival rates at 1 and 3 years were 96.2±1.9% and 79.2±6.5%, respectively. The disease-free survival rates at 1 year and 3 years were 81.4±3.9% and 61.9±6.6%. Fifty-five cases were in pathological stage III. Postoperative overall survival rates at 1 and 3 years were 94.2±3.3% and 83.1±7.2%, respectively. The disease-free survival rates at 1 and 3 years were 79.0±5.9% and 21.4±9.6%, respectively (Fig. 1).
Table 3.
Postoperative complications
*=Of those, 58 patients required chemical pleurodesis; †=Atrial fibrillation or sinus tachycardia; ‡=Needed bronchoscopic toileting or reintubation; ARDS=Acute respiratory distress syndrome; ALI=Acute lung injury; CVA=Cerebrovascular accident; BPF=Broncho-pleural fistula; ARF=Acute renal failure.
Table 4.
Pathologic stages of the 832 patients who underwent VATS lobectomy for non-small-cell lung cancer (TNM staging system for lung cancer, 7th Edition)
Table 5.
The pattern of recurrence of the 832 patients who underwent VATS lobectomy for non-small-cell lung cancer according to pathologic stage
Fig. 1.
Overall survival and disease-free survival of patients who underwent VATS lobectomy for non-small cell lung cancer.
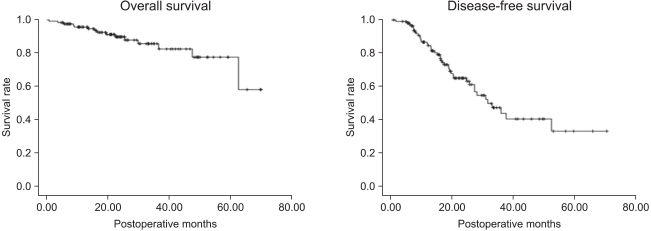
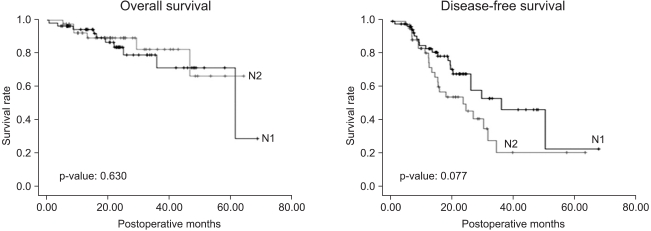
Among the 832 patients with non-small cell lung cancer, 769 patients were in clinical stage I, of which 122 (15.9%) showed increased pathological stage after surgery. The 1-year and 3-year overall survival rates of these patients were 95.7±1.9% and 82.5±5.0%, respectively. The 1-year and 3-year disease-free survival rates were 85.2±3.4% and 45.9±6.8%, respectively (Fig. 2). The most common cause of the pathologic upstaging was pathological diagnosis showing local or mediastinal lymph node metastasis while no lymph node metastasis in the clinical stage. Sixty-four patients out of 813 showed a preoperative clinical stage of N0 and a postoperative stage of N1. Their 1-year and 3-year overall survival rates were 95.0±2.8% and 74.8±8.7%, respectively. The 1-year and 3-year disease-free survival rates were 83.6±5.0% and 53.8±9.3%, respectively. Forty-eight patients showed preoperative clinical stage N0 and postoperative pathological stage N2. Their 1-year and 3-year overall survival rates were 93.3±3% and 84.6±7.2%, respectively. The 1-year and 3-year disease-free survival rates were 81.0±6.1% and 21.4±9.7%, respectively (Fig. 3).
Fig. 2.
Overall survival and disease-free survival of the pathologically upstaged 122 patients among 769 clinical stage I patients.
Fig. 3.
Overall survival and disease-free survival of the pathologic N1 (64) and N2 (48) patients among 813 clinical N0 patients.
DISCUSSION
The application of VATS has been expanding from diseases such as benign pleural disease, lung disease, and mediastinal disease [3] to lung cancer and esophageal cancer [22]. The range of pulmonary resections has also been expanding, currently including pulmonary wedge resection, segmentectomy, lobectomy, bilobectomy, and even pneumectomy [1]. McKenna et al. [23] reported on the largest number of thoracoscopic lobectomy cases in a single institute. Their thoracoscopic indications were primary lung cancer, lymphoma, metastatic lung cancer, or benign pulmonary disease, which were similar to the author's indications.
A low number of postoperative complications and a low hospital mortality rate have been reported in various articles. Hospital mortality rate after thoracotomic lobectomy has been found to be similar to that of thoracoscopic lobectomy, whereas postoperative complications were less common in thoracoscopic lobectomy [4,20,24]. These results are similar to the results of our study in complication occurrence rate (14.0%, 162 cases) and hospital mortality rate (0.84%). Among postoperative complications, continuous air leakage was the most common. In order to minimize leakage, a stapler was used for isolation rather than electrocautery or blunt dissection when severe adhesion was present in the interlobar fissure. Then, denuded visceral pleura was covered with absorbable felt or applied with glue to reduce air leakage. There are numerous risk factors affecting hospital mortality. In this study, 5 out of the 9 patients who died in the clinic had diffuse interstitial lung disease. This implies that patients with diffuse interstitial lung disease must cautiously decide whether to undergo a lobectomy, which may compromise lung function after surgery. Since FEV1 levels are often normal based on pulmonary function testing, diffusing capacity (DLco) must be measured and operation should be avoided when DLco levels are low [25]. In addition, VATS is less painful than thoracotomy [26] and has a shorter hospitalization period.
There have been numerous reports regarding the fact that thoracoscopic lobectomy is not oncologically different from thoracotomic lobectomy. Rueth and Andrade [20] showed that there were no oncological differences between thoracoscopic and thoracotomic lobectomy by analyzing research articles about thoracoscopic lobectomy from 1994 to present [8,17,18]. Yan et al. [27] showed that the 5-year disease-free survival rate of thoracoscopic lobectomy was significantly higher than that of thoracotomic lobectomy (p=0.03).
Sugi et al. [17] and Watanabe et al. [28] showed that there was no difference in the number of collected lymph nodes, survival rate, and recurrence when comparing thoracoscopic mediastinal lymph node dissection and thoracotomic mediastinal lymph node dissection. Common causes for conversion from thoracoscopic surgery to thoracotomic surgery were hemorrhage in pulmonary artery branches and difficulty in detaching a calcified or fiberized lymph node. To reduce the conversion rate, preoperative CT should be analyzed thoroughly to check for the calcification or fibrosis of lymph nodes along with thickening.
On the other hand, there are different views about the feasibility of the thoracoscopic lobectomy for lung cancer. Opponents of insist that thoracoscopic lobectomy results in lower survival rate and higher relapse rate. Thoracoscopic lobectomy may result in more manipulation of the lung, causing cancer cells to disseminate through the pulmonary vein [13]. In addition, excisions with a stapler may leave cancer cells at the resection margin, mediastinal lymph node dissection may be insufficient, and cancer cells may be disseminated while removing the specimen out of the body [19]. In the author's institute, the pulmonary vein was detached before the pulmonary artery, if possible, the resection margin was checked first with the naked eye, and the resection margin went through a frozen section reading when there was a possibility of cancer cells remaining. Mediastinal lymph nodes were completely dissected in all cases. There has been no established definition of complete lymph node dissection. However, the authors defined complete lymph node dissection to mean where no lymph node or fatty tissue remained. To prevent damage to the nerves or thoracic duct, an ultrasonic cutter (Harmonic ACE™, Ethicon Endo-Surgery Inc., Cincinnati, OH) was used. When removing the specimen after lobectomy, the specimen was placed in a plastic bag to prevent dissemination of cancer cells.
The pathological stage I patients' 3-year disease-free survival rate was 86.2±1.9%, whereas pathological stage II patients' 3-year disease-free survival rate was 61.9±6.6%. However, it is too early to affirm that the postoperative relapse rate of stage I non-small cell lung cancer is low or that stages greater than II have greater postoperative relapse rates. In order to draw a more accurate conclusion on the relapse and survival rates of patients treated with thoracoscopic lobectomy for early non-small cell lung cancer, long-term follow-up observation is needed. In the previous articles, an analysis of the 5-year overall survival rate after thoracoscopic lobectomy for early stage non-small cell lung cancer was conducted, whereas most of the previous studies have not reported specifics about 5-year disease-free survival rates [15,18,20,23]. Yamamoto et al. [29] recently reported the patients' 5-year survival and disease-free survival rate and categorized them by each stage.
Some recent studies have focused on whether patients who are diagnosed with clinical stage I and end up with pathological stage II or worse after thoracoscopic lobectomy show lower survival and greater relapse rates than patients treated with thoracotomic lobectomy. In the present study, it was not possible to draw conclusions on this topic, since there were no comparisons made with thoracotomic lobectomy. However, according to the Kim et al's [30] results in the past 4 years, and reports by Watanabe et al. [28] over 9 years, there has been no difference in prognosis, and we have concluded that conversion to thoracotomic surgery is not necessary even if N2 lymph node metastasis is observed during surgery. In order to minimize pathologic upstaging, efforts to improve the accuracy of preoperative diagnosis are necessary.
The consistency of the research results from our clinic was maintained by using the same medical team to perform operations with identical indications and criteria (lymph node biopsy and mediastinal lymph node dissection under mediastinoscopy). Also, with a relatively abundant amount of data (1,067 cases), results such as low morbidity, low hospital mortality, and low conversion rate are acceptable.
In the future, the number of patients with early stage non-small cell lung cancer with an indication of thoracoscopic lobectomy will increase. As diagnostic techniques advance, the number of patients with early stage lung cancer is increasing, adenocarcinoma is becoming more common than squamous cell carcinoma. Adenocarcinoma is usually located peripherally, which facilitates a thoracoscopic approach. In addition, the number of female patients with the disease is increasing, in which the aesthetic benefits of thoracoscopic surgery are more preferable. As more thoracoscopic lobectomy experiences are documented in the future, thoracoscopic lobectomy could be applied to the patients in stage II lung cancer or patients with endobronchial cancer needing a sleeve lobectomy. But more prospective studies are necessary to recognize it as a feasible method of treatment.
Lastly, there are several limitations in this research. First, data was analyzed retrospectively and there were no comparisons made with thoracotomic lobectomy. Second, in spite of the fact that all operations were performed in a single institute with identical indications, it is possible that the surgeons performed thoracoscopic surgery in cases with better expected prognosis. Prospective randomized controlled trials in the future may produce more conclusive results. Finally, since the number of patients within a year increased more than 50% when compared with previous research [31], the overall follow-up observation period was short. Therefore, a longer follow-up observation period of thoracoscopic lobectomy for early stage non-small cell lung cancer would be necessary to verify long-term results.
CONCLUSION
Based on our 6 years of experience, thoracoscopic lobectomy is a safe and useful method for treating various pulmonary diseases including early non-small cell lung cancer, without a high complication occurrence rate or hospital mortality rate. In addition, an excellent survival rate was observed in patients with early non-small cell lung cancer. However, depending on the stage of the disease, continued collection of data may be necessary to observe long-term results.
Footnotes
This article was presented at the 42nd. Annual meeting of Korean Society for Thoracic and Cardiovascular Surgery.
References
- 1.Qu JQ, Gao X, Hou WP, et al. Video-assisted thoracic surgery: clinical experience among 1264 patients. Zhonghua Yi Xue Za Zhi. 2006;86:2309–2311. [PubMed] [Google Scholar]
- 2.Amer K, Khan AZ, Vohra HA. Video-assisted thoracic surgery of major pulmonary resections for lung cancer: the Southampton experience. Eur J Cardiothorac Surg. 2011;39:173–179. doi: 10.1016/j.ejcts.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Yu FL, Wu ZS, Chen MJ. Clinical application of video-assisted thoracoscopic surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:284–287. [PubMed] [Google Scholar]
- 4.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RJ, Caccavale RJ, Bocage JP, Widmann MD. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest. 1999;116:1119–1124. doi: 10.1378/chest.116.4.1119. [DOI] [PubMed] [Google Scholar]
- 6.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg. 1992;104:1679–1685. discussion 1685-7. [PubMed] [Google Scholar]
- 7.Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–647. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg. 2006;132:507–512. doi: 10.1016/j.jtcvs.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 9.Tomaszek SC, Cassivi SD, Shen KR, et al. Clinical outcomes of video-assisted thoracoscopic lobectomy. Mayo Clin Proc. 2009;84:509–513. doi: 10.4065/84.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg. 2000;70:243–247. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 11.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg. 1995;109:997–1001. doi: 10.1016/S0022-5223(95)70326-8. discussion 1001-2. [DOI] [PubMed] [Google Scholar]
- 12.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita JI, Kurusu Y, Fujino N, Saisyoji T, Ogawa M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg. 2000;119:899–905. doi: 10.1016/S0022-5223(00)70084-5. [DOI] [PubMed] [Google Scholar]
- 14.Jancovici R, Lang-Lazdunski L, Pons F, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg. 1996;61:533–537. doi: 10.1016/0003-4975(95)01060-2. [DOI] [PubMed] [Google Scholar]
- 15.Gharagozloo F, Tempesta B, Margolis M, Alexander EP. Video-assisted thoracic surgery lobectomy for stage I lung cancer. Ann Thorac Surg. 2003;76:1009–1014. doi: 10.1016/s0003-4975(03)00267-4. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi T, Shirakusa T, Hiratsuka M, Yamamoto S, Iwasaki A. Video-assisted thoracoscopic surgery lobectomy for c-T1N0M0 primary lung cancer: its impact on locoregional control. Ann Thorac Surg. 2006;82:1021–1026. doi: 10.1016/j.athoracsur.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg. 2000;24:27–30. doi: 10.1007/s002689910006. discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 18.Walker WS, Codispoti M, Soon SY, Stamenkovic S, Carnochan F, Pugh G. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg. 2003;23:397–402. doi: 10.1016/s1010-7940(02)00814-x. [DOI] [PubMed] [Google Scholar]
- 19.Fry WA, Siddiqui A, Pensler JM, Mostafavi H. Thoracoscopic implantation of cancer with a fatal outcome. Ann Thorac Surg. 1995;59:42–45. doi: 10.1016/0003-4975(94)00794-8. [DOI] [PubMed] [Google Scholar]
- 20.Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg. 2010;89:S2107–S2111. doi: 10.1016/j.athoracsur.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2009. [Google Scholar]
- 22.Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2010;89:S2159–S2162. doi: 10.1016/j.athoracsur.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 23.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–425. doi: 10.1016/j.athoracsur.2005.07.078. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 24.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–1970. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–1164. doi: 10.1016/j.athoracsur.2007.12.071. discussion 64-5. [DOI] [PubMed] [Google Scholar]
- 26.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–365. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 27.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur J Cardiothorac Surg. 2008;33:812–818. doi: 10.1016/j.ejcts.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89:353–359. doi: 10.1016/j.athoracsur.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Choi YS, Kim J, Shim YM, Kim K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:1288–1293. doi: 10.1016/j.jtcvs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg. 2010;89:S2118–S2122. doi: 10.1016/j.athoracsur.2010.03.017. [DOI] [PubMed] [Google Scholar]