Abstract
Background
In cardiac surgery, especially in the reconstruction of vascular structures and intracardiac defects, glutaraldehyde has usually been used as the reagent for fixing porcine or bovine pericardial tissues. But the well-known problem of calcification or cytotoxicity of glutaraldehyde motivates the search for a replacement. The aim of this study is to investigate the physical, mechanical, and biochemical characteristics of bovine pericardial tissues fixed with genipin, which is known to be a less toxic and more natural fixing reagent.
Materials and Methods
Bovine pericardial tissues were fixed with different concentrations and conditions of glutaraldehyde and genipin. To determine the physical, mechanical, and biochemical differences among different concentrations and conditions, we divided the tissue into 18 groups by concentration, the addition of organic solvents, and the timing of adding the organic solvents, and compared the characteristics of each group.
Results
Tensile strength, physical activity, and thermal stability tests revealed that the tissues fixed with glutaraldehyde were better with regard to mechanical strength and biochemical durability. However, the difference was not significant statistically.
Conclusion
Genipin can be used as an alternative crosslinking agent for pericardial tissue, considering given its physical, mechanical, biochemical characteristics and low cytotoxicity comparable to glutaraldehyde. However, further studies are needed on the immune reaction and the long term changes in genipin-fixed tissues in the human body.
Keywords: Xenograft, Glutaraldehyde, Bioprosthesis, Pericardial patch
INTRODUCTION
In the field of cardiovascular surgery, there has been an effort to develop adequate prosthetic materials for cardiovascular reconstruction, such as vascular conduits, valve leaflets or patches for tissue defects. Until now, the biological materials made of bovine or porcine pericardium have been most widely used. These heterograft tissues must be biologically stable and safe in the human body, so they should be chemically modified or fixed to reduce immune or inflammatory reactions and to increase mechanical endurance and tolerance [1]. The ideal condition of heterografts for cardiovascular reconstruction is to be mechanically strong, tolerant of degeneration or calcification, easily manipulated by the surgeon's hand, and also less cytotoxic and immunological [2]. Although several chemicals, including formaldehyde, glutaraldehyde, and epoxy compounds, that can fix biological tissue, glutaraldehyde has usually been used. Because many studies have been performed to evaluate the function, stability, and safety of glutaraldehyde as a fixing crosslinking agent, and to observe its long term use, there is sufficient clinical knowledge and experience on glutaraldehyde. However, glutaraldehyde still exhibits the drawbacks of high cytotoxicity and calicification, and we also lack knowledge on heterotopic immune response to it in the human body. Therefore, development of a new alternative fixation agent is required [3]. For that reason, there have been attempts to use genipin, a natural crosslinking reagent, as a fixation material [4].
Genipin is an agent extracted from gardenia fruit (Gardenia jasminoides ELLIS). In traditional Chinese medicine, genipin has been used as a treatment for DM or jaundice patients. Many studies have reported on genepin's much lower level of cytotoxicity compared to glutaraldehyde [5].
The aim of this study was to evaluate whether genipin could be used as an alternative crosslinking agent to glutaraldehyde for heterograft tissues. Thus, we compared the physical, biochemical, and histological characteristics of heterograft tissues (bovine pericardial tissues) fixed with genipin and those fixed with glutaraldehyde. As is generally known, the fixation of heterograft pericardial tissues with organic solvent causes permanent changes because it suppresses the nucleation of calcium phosphate and decreases the size and amount of collagen, which is assumed to enhance the mechanical durability of heterograft pericardial tissues [6].
Therefore, we divided tissues into groups by concentration of genipin and the addition of organic solvent and observed changes in each tissue's characteristics to prepare clinically useful standards.
MATERIALS AND METHODS
1) Production of bovine pericardial patch
Bovine pericardium removed from cattle in an abattoir was put into cold phosphate buffered solution (PBS, 0.1 M, pH7.4), packed into a cooler and transported to the laboratory. Every bovine pericardium was sterilized in 2% betadine solution for 1 hour before being washed and stored in normal saline.
2) Fixation
To observe differences in the characteristics of a pericardial patch fixed with genipin and one with glutaraldehyde, parts of a pericardial patch were treated with glutaraldehyde only and, separately, glutaraldehyde with organic solvent added.
To identify differences according to the concentration of genipin (0.1, 0.2, 0.3, 0.4, 0.6, and 1.0%) and other conditions, we divided pericardial patchs into 16 groups by the concentration of genipin, the addition of organic solvent, and the timing of adding organic solvent. After fixation, the pericardial patches were washed by PBS solution several times in room temperature and stored in 4℃ PBS solution. In the end, we had prepared 20 pericardial patches for each group and performed various tests with each patch.
All the fixation processes were performed at room temperature.
(1) Fixation with glutaraldehyde (2 groups)
Glutaraldehyde: 0.5% glutaraldehyde solution (PBS buffer, pH 7.4) for 5 days → 0.25% glutaraldehyde solution for 1 week
Glutaraldehyde+Solvent: 0.5% glutaraldehyde solution for 5 days → 1% glutaraldehyde with organic solvent (75% Ethanol+5% Octanol) solution for 2 days → 0.25% glutaraldehyde solution for 1 week
(2) Fixation with genipin (6 groups)
0.1% Genipin: 0.1% genipin solution (PBS buffer, pH 7.4) for 4 days
0.2% Genipin: 0.2% genipin solution for 4 days
0.3% Genipin: 0.3% genipin solution for 4 days
0.4% Genipin: 0.4% genipin solution for 4 days
0.6% Genipin: 0.6% genipin solution for 4 days
1.0% Genipin: 1.0% genipin solution for 4 days
(3) Fixation with genipin and organic solvent (10 groups)
0.1% Genipin+Solvent: 0.1% genipin solution (PBS buffer, pH 7.4) for 2 days → 0.1% genipin with organic solvent (75% Ethanol+5% Octanol) solution for 2 days
0.2% Genipin+Solvent: 0.2% genipin solution for 2 days → 0.2% genipin with organic solvent for 2 days
0.3% Genipin+Solvent: 0.3% genipin solution for 2 days → 0.3% genipin with organic solvent solution for 2 days
0.4% Genipin+Solvent: 0.4% genipin solution for 2 days → 0.4% genipin with organic solvent solution for 2 days
0.6% Genipin+Solvent: 0.6% genipin solution for 2 days → 0.6% genipin with organic solvent solution for 2 days
1.0% Genipin+Solvent: 1.0% genipin solution for 2 days → 1.0% genipin with organic solvent solution for 2 days
Solvent+0.3% Genipin: 0.3% genipin with organic solvent (75% Ethanol+5% Octanol) solution for 2 days → 0.3% genipin solution for 2 days
Solvent+0.4% Genipin: 0.4% genipin with organic solvent (75% Ethanol+5% Octanol) solution for 2 days → 0.4% genipin solution for 2 days
Solvent+0.6% Genipin: 0.6% genipin with organic solvent (75% Ethanol+5% Octanol) solution for 2 days → 0.6% genipin solution for 2 days
Solvent+1.0% Genipin: 1.0% genipin with organic solvent (75% Ethanol+5% Octanol) solution for 2 days → 1.0% genipin solution for 2 days
3) Physical test
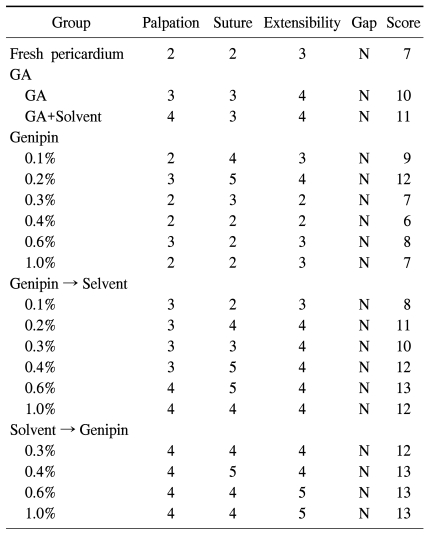
By palpation, extension, and suture, a single thoracic surgeon subjectively evaluated the characteristics of pericardial patches fixed using each method.
Palpation was done by touching the pericardial patches manually and measuring the softness of the pericardial patches. Fresh bovine pericardium was scored 0 for standard, 1 for soft, 2 for slightly soft, 3 for moderately soft, 4 for slightly stiff, and 5 for stiff.
Characteristics of and gaps in patches during suture were described with a score indicating resistance against needling the pericardial patch by a 5-0 Prolene.
The extensibility and gap was also estimated and scored by extending the pericardial patch by pulling the suture needle which had punctured the patch.
Using the sum of those scores, we compared patches fixed with genipin to those with glutaraldehyde, patches fixed with low and high concentrations of genipin, patches fixed with and without organic solvent, and patches to which organic solvent was added first to patches to which genipin was added first.
4) Tensile strength test
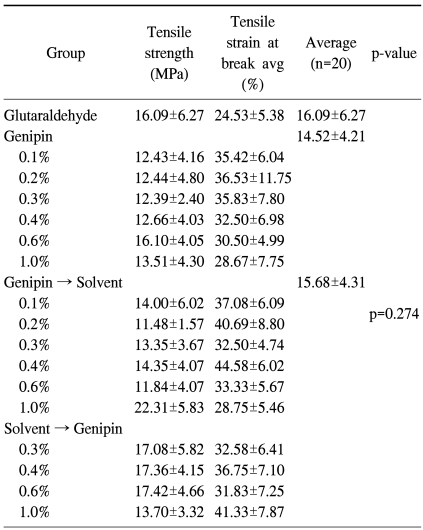
Using tensile strength test equipment and settings developed by our laboratory, we estimated and compared the tensile strength of pericardial patches fixed in various ways [7]. The pericardial patches of each group were made into 5×50 mm patches and the tensile strength of 5-mm-wide patches was estimated. The average of the estimated tensile strength values was set as the representative value of the group. We used a Digital Force Gauge (DS2-50N, Imada, Japan) for estimation of the tensile strength. The load speed was 100 mm/min and measurement unit was N/5 mm2, MPa (megapascals).
5) Thermal stability test
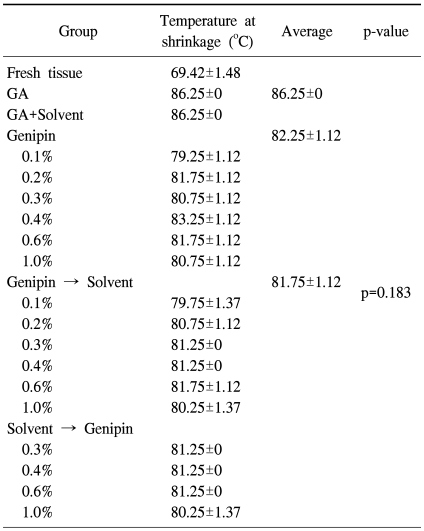
The pericardial patches fixed in various ways were cut to 8×30 mm sections. While applying a continuous tensile strength of 95 g, the patches were put into 55℃ normal saline. Until the point that the patches rapidly contracted, in other words, the point of degeneration of collagen in the tissues, the temperature of normal saline was increased with a velocity of 1~2.5℃/min. Then we measured and compared the temperature of each group when the patched underwent a sudden contraction.
6) Amino acid analysis
Pericardial patches of each group was cut into 10×10 mm sections and put into a heat dryer (80℃, 650 G Fisher Scientific, USA) for 24 hours after washing with normal saline. We then removed the water in the pericardial patches and measured the mass. Then those pericardial patches were moved to 2 mL Effendorf tubes. 5 M HCL 1.5 mL was added to the tube and dried in the dryer for 24 hours. The color of the HCl solution changed to yellow due to dissolution of the specimen. We moved that yellow solution to the Effendorf tube and dried it completely at room temperature. The pellet obtained through the above-mentioned process was sent to the Department of Laboratory Medicine for analysis. They quantified 18 types of amino acids (aspartic acid (Asp), glutamic acid (Glu), histidine (His), proline (Pro), threonine (Thr), arginine (Arg), taurine (Tau), tyrosine (Tyr), methionine (Met), valine (Val), phenylalanine (Phe), isoleucine (Ile), leucine (Leu), and lysine (Lys)) by liquid chromatography/mass spectrometry (LC/MS, Waters, USA). To determine the difference among crosslink characteristics contingent upon concentration of genipin and organic solvent, we separated the pericardial patches fixed with genipin only (0.1, 0.2, 0.3, 0.4, 0.6, and 1.0%), patches fixed with genipin after being treated with organic solvent, and patches fixed with genipin before being treated with organic solvent, and analyzed the residual amino acid in the pellet.
7) Statistical analysis
In the tensile strength test, the unit of tensile strength at break was MPa and the unit of tensile strain at break was 'mean value±standard deviation' (%). In the thermal stability test, the temperature at contraction of the pericardial patch was indicated by ℃, and each value was also indicated as 'mean value±standard deviation'. The statistical difference among the groups was verified by one-way analysis of variance (ANOVA). The results wth a p-value<0.05 were considered statistically significant.
RESULTS
1) Physical test
There was no significant difference in mechanical characteristics between the pericardial patches fixed with genipin and those with glutaraldehyde. Also, there was no clear relationship with the concentration of genipin. The pericardial patches with organic solvent showed a greater tendency to be durable and stiff (Table 1).
Table 1.
Physical characteristics of pericardium determined by a single surgeon's hand
GA=Glutaraldehyde.
2) Tensile strength test
From the result of the tensile strength test, it seemed that pericardial patches fixed with genipin or glutaraldehyde had more tensile strength compared to fresh bovine pericardium. However, there was no significant difference among the groups fixed with glutaraldehyde, fixed with genipin only, and fixed with genipin and treated with organic solvent (p=0.274) (Table 2).
Table 2.
Tensile strength of pericardium (n=20)
3) Thermal stability test
The temperature at which the pericardial patch fixed with genipin started to contract was 82.25℃, higher than the temperature at which the fresh bovine pericardial patch starts to contract (69.42℃), but slightly lower than the temperature for a pericardial patch fixed with glutaraldehyde (86.25℃). However, there was no significant difference among the groups fixed with glutaraldehyde, fixed with genipin only, and treated with organic solvent after being fixed with genipin (p=0.183) (Table 3).
Table 3.
Thermal stability test (n=20)
GA=Glutaraldehyde.
4) Amino acid analysis
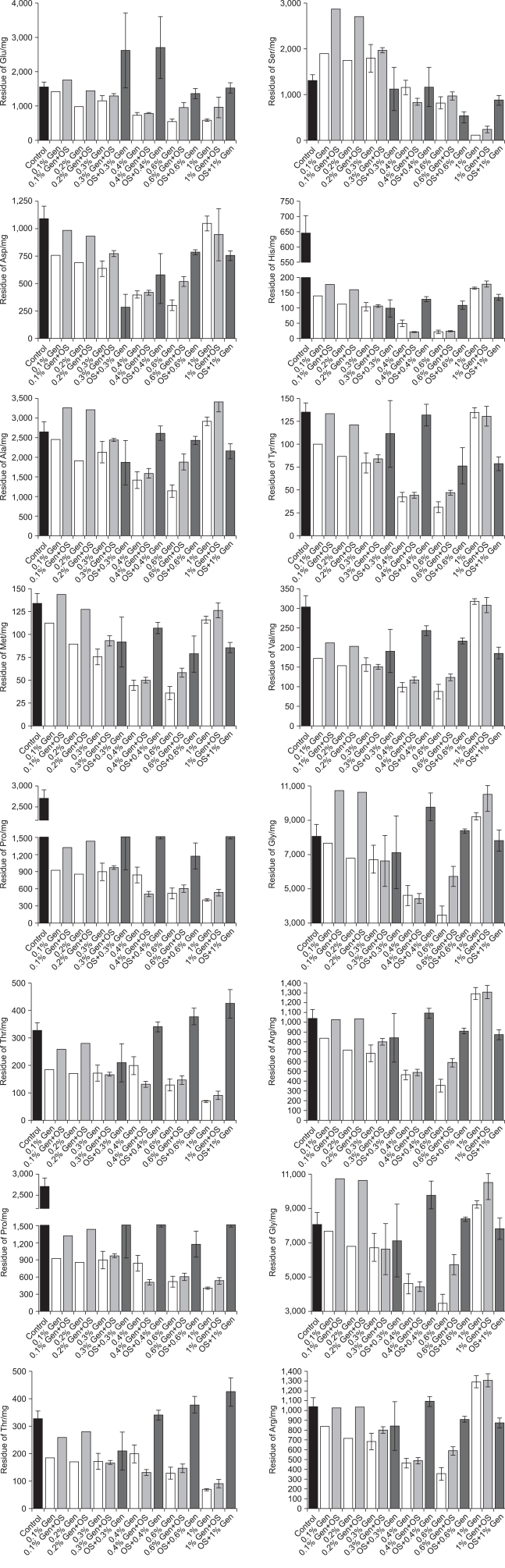
The result of amino acid analysis showed a tendency to decrease in amount of residual amino acid when the concentration of genipin increased from 0.1% to 1.0%. Following the increase in the concentration of genipin from 0.1% to 0.6%, the amount of Glu, Asp, His, Tyr, Met, Val, Gly, Arg, Phe, Ile, Leu, and Lys showed a tendency to constantly decrease. However, there was no significant difference between the 0.6% genipin and 1.0% genipin groups. It seemed that more crosslinks were made with an increase in the concentration of genipin until the concentration of genipin reached 0.6%, but there was no significant difference under high concentrations of genipin, that is, over 0.6% (0.6%, 1.0%). Residual Ala was lowest in 0.4% and 0.6% genipin. Ser showed many crosslinks in over 1% genipin.
In groups fixed with genipin after treatment with organic solvent, there was no remarkable difference in the quantity of residual amino acids according to the concentration of genipin. Only the group fixed with the highest concentration of genipin (1%) after treatement with organic solvent showed a decrease in the amount of residual amino acids (Fig. 1). Seeing that more crosslinking was observed in the group without organic solvent, the crosslink between genipin and amino acids seemed to be hydrophilic. As reported in Fig. 1, groups of pericardial patches fixed with 0.3~0.4% genipin showed the least residual amount of most of the amino acids, so it seems that crosslinking is most actively performed in that range of concentration of genipin.
Fig. 1.
Residue patterns of each amino acid according to each fixation method.
DISCUSSION
Glutaraldehyde is an agent for fixation to suppress the immunogenicity of heterograft tissue and enhance its durability, and is being widely used as an inducer of biochemical crosslinking in tissue [8]. So far, there have been many studies on glutaraldehyde itself, and the biochemical structure and characteristics of crosslinking induced by glutaraldehyde. Because glutaraldehyde is generally used for the fixation of heterograft tissue in the clinical field, clinical outcomes of glutaraldehyde usage are also widely known. However, the cytotoxicity and calcification of glutaraldehyde spurred the search for another fixation agent to replace it. It has been thought that the calcification of glutaraldehyde is caused by a reaction between calcium in the human body and the aldehyde group in glutaraldehyde, which together combine with pericardial tissues. Therefore, there have been various studies on methods for the reduction of calcification, for instance, combining aldehyde groups with other agents in advance and blocking the reaction with calcium [9]. Furthermore, because of the cytotoxicity of glutaraldehyde itself, studies on genipin, which can generate crosslinks naturally and may replace glutaraldehyde, are now being performed.
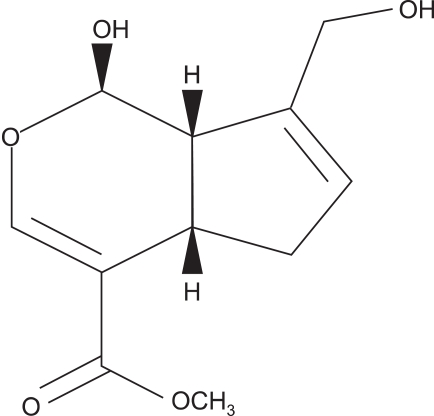
Genipin, which has a C11H14O5 molecular structure (Fig. 2), is iridoid glycoside, which is one of the main ingredients in gardenia fruit (Gardenia jasminoides ELLIS). Genipin generates crosslinks spontaneously with protein, collagen, gelatin, chitosan, etc. For a long time, genipin has been used as a treatment for cholestasis and hepatitis in traditional Chinese medicine. It has even been used as a natural dye because of its tinting properties with proteinic textiles [10]. Genipin itself has no color, but forms blue particles by spontaneous reaction with amino acids and protein. These characteristics of genipin have allowed it to be used as a natural dye for textiles and for food. Many studies have noted the remarkably low cytotoxicity and genotoxicity of genipin compared to glutaraldehyde [11,12].
Fig. 2.
Structure of genipin.
Sung et al. cultured human foreskin fibroblasts with genipin, glutaraldehyde and epoxy compounds added and found out that genipin has markedly low cytotoxicity as evaluated by a difference in cell density [4]. Chang et al. also reported that genipin has much less toxicity than glutaraldehyde and the in vivo biocompatibility of heterograft tissue fixed with genipin is far better [13,14]. Somers et al. cultured chicken embryo fibroblasts in genipin and glutaraldehyde to estimate their cytotoxicity. They found that a high concentration of genipin has less toxicity than a low concentration of glutaraldehyde [15]. Huang et al. injected heterograft tissue fixed with genipin into mice subcutaneously and cultured it. They reported that heterograft tissue fixed with genipin caused less inflammation and contained less calcium compared to tissue fixed with glutaraldehyde [16].
It has not yet understood in detail and studies are in progress concerning the crosslinking mechanism between the gelatin in genipin and the molecule containing the primary amine.
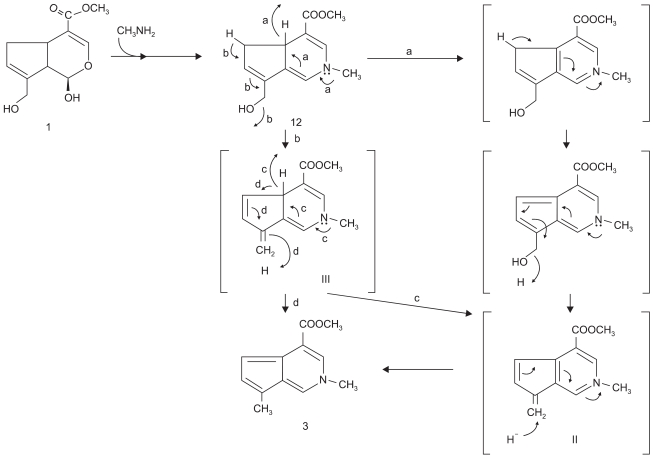
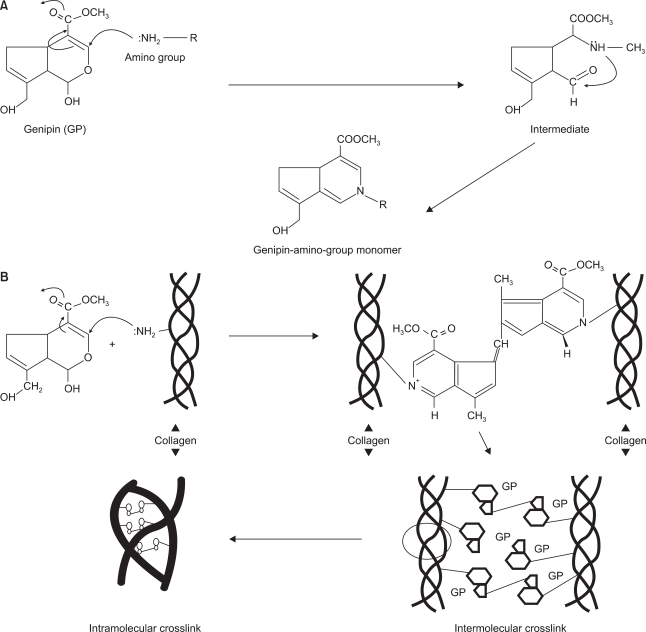
Touyama et al. observed a reaction mechanism between genipin and methylamine. Nucleophilic attack by primary amine occurs at the C3 carbon atom in genipin and the secondary amine is attached to the aldehyde group [17]. Then the collagen fiber is stabilized and the thermal stability is also raised through intra/intermolecular crosslinking as above. Fig. 3 shows a diagram of the reaction mechanism. As it noted in Fig. 4, Chang et al. suggested that there was stable bond among collagen fibers through intra/intermolecular crosslink structure of tissue fixed with genipin and reaction mechanism between genipin and amino acid group [18].
Fig. 3.
Touyama's group's presumed mechanism for the reaction of genipin with methylamine [9].
Fig. 4.
(A) Presumable mechanism of amino group with genipin. (B) Schematic illustration of the presumable intramolecular and intermolecular crosslinking structures of genipin-fixed tissue [18].
The aim of this study is to evaluate the possibility of using genipin, a material for the fixation of heterograft tissue (bovine pericardium) used in cardiac surgery, as a natural crosslink reagent biologically and biochemically modifiable for clinical purposes.
Even on the assumption that genipin has less cytotoxicity, we still need to confirm the physical, and mechanical characteristics and thermal stability are at least equivalent to glutaraldehyde to be able to use genipin for fixation. Sung et al. compared the denaturation temperature of three groups of porcine pericardial tissue, each fixed with glutaraldehyde, genipin, or epoxy compound. They found that all three materials showed a higher denaturation temperature compared to fresh porcine pericardial tissue and all are effective crosslink agents [4]. Although patches fixed with genipin showed a slightly lower temperature than patches fixed with glutaraldehyde in the thermal stability test, there was no statistically significant difference. There was also no significant correlation by concentration of genipin, organic solvent, or timing of adding organic solvent. In addition, the groups fixed with genipin showed an equivalent level of tensile strength to the groups fixed with glutaraldehyde as a result of the tensile strength test.
Therefore, it can be concluded that genipin has potential as an alternative crosslinking reagent to glutaraldehyde, pending clinical studies.
CONCLUSION
By previous studies, genipin has been well known to be less cytotoxic than glutaraldehyde. The present study showed that bovine pericardial tissues fixed with genipin are comparable with those fixed with glutaraldehyde in mechanical characteristics, tensile strength, and thermal stability. Thus, we can consider genipin to be a possible alternative to glutaraldehyde as a crosslinking agent for fixing heterograft tissue. However, the long-term physical and biochemical effects in the human body and the immune response caused by pericardial patches fixed with genipin are not yet known, so further studies and animal experiments are required before application.
Footnotes
This study was supported by a grant of the Korea Health 21 Research and Development Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (Project No.: A040004-006).
References
- 1.Gratzer PF, Pereira CA, Lee JM. Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials. J Biomed Mater Res. 1996;31:533–543. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Kim KC, Choi YK, Kim SH, Kim YJ. Effect of diamine bridges using l-lysine in glutaraldehyde treated porcine pericardium. Korean J Thorac Cardiovasc Surg. 2009;42:157–164. [Google Scholar]
- 3.Pathak CP, Adams AK, Simpson T, Phillips RE, Jr, Moore MA. Treatment of bioprosthetic heart valve tissue with long chain alcohol solution to lower calcification potential. J Biomed Mater Res A. 2004;69:140–144. doi: 10.1002/jbm.a.20129. [DOI] [PubMed] [Google Scholar]
- 4.Sung HW, Huang RN, Huang LL, Tsai CC, Chiu CT. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Xi-xun Y, Fei L, Yuan-ting X, Chang-xiu W. In vitro study in the endothelial cell compatibility and endothelialization of genipin-crosslinked biological tissues for tissue-engineered vascular scaffolds. J Mater Sci Mater Med. 2010;21:777–785. doi: 10.1007/s10856-009-3933-8. [DOI] [PubMed] [Google Scholar]
- 6.Jastrzebska M, Wrzalik R, Kocot A, Zalewska-Rejdak J, Cwalina B. Raman spectroscopic study of glutaraldehyde-stabilized collagen and pericardium tissue. J Biomater Sci Polym Ed. 2003;14:185–197. doi: 10.1163/156856203321142605. [DOI] [PubMed] [Google Scholar]
- 7.Cho S, Kim YJ, Kim SH, Park JE, Kim WH. Comparison of the uniaxial tensile strength, elasticity and thermal stability between glutaraldehyde and glutaraldehyde with solvent fixation in xenograft cardiovascular tissue. Korean J Thorac Cardiovasc Surg. 2009;42:165. [Google Scholar]
- 8.Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987;21:741–771. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 9.Webb CL, Benedict JJ, Schoen FJ, Linden JA, Levy RJ. Inhibition of bioprosthetic heart valve calcification with aminodiphosphonate covalently bound to residual aldehyde groups. Ann Thorac Surg. 1988;46:309–316. doi: 10.1016/s0003-4975(10)65932-2. [DOI] [PubMed] [Google Scholar]
- 10.Akao T, Kobashi K, Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol Pharm Bull. 1994;17:1573–1576. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- 11.Sung HW, Huang RN, Huang LL, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10:63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CC, Huang RN, Sung HW, Liang HC. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52:58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y, Hsu CK, Wei HJ, et al. Cell-free xenogenic vascular grafts fixed with glutaraldehyde or genipin: in vitro and in vivo studies. J Biotechnol. 2005;120:207–219. doi: 10.1016/j.jbiotec.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Tsai CC, Liang HC, Sung HW. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin) Biomaterials. 2002;23:2447–2457. doi: 10.1016/s0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- 15.Somers P, De Somer F, Cornelissen M, et al. Genipin blues: an alternative non-toxic crosslinker for heart valves? J Heart Valve Dis. 2008;17:682–688. [PubMed] [Google Scholar]
- 16.Huang LL, Sung HW, Tsai CC, Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res. 1998;42:568–576. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Touyama R, Inoue K, Takeda Y, et al. Studies on the blue pigments produced from genipin and methylamine II On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem Pharm Bull. 1994;42:1571–1578. [Google Scholar]
- 18.Chang Y, Tsai CC, Liang HC, Sung HW. Reconstruction of the right ventricular outflow tract with a bovine jugular vein graft fixed with a naturally occurring crosslinking agent (genipin) in a canine model. J Thorac Cardiovasc Surg. 2001;122:1208–1218. doi: 10.1067/mtc.2001.117624. [DOI] [PubMed] [Google Scholar]