Abstract
Background
Despite aggressive treatment, the mortality rate of cardiogenic shock with acute myocardial infarction (AMI) is high. We performed extracorporeal membrane oxygenation (ECMO) prior to coronary reperfusion, and evaluated the early clinical results and risk factors.
Materials and Methods
From May 2006 to November 2009, we reviewed the medical records of 20 patients in cardiogenic shock with AMI (mean age 67.7±11.7 yrs, M : F 14 : 6). After initially performing ECMO using the CAPIOX emergency bypass system (EBS®Terumo, Tokyo, Japan), patients underwent coronary reperfusion (coronary artery bypass grafting, 13; percutaneous coronary intervention, 7).
Results
All patients were in a cardiogenic shock state, cardiopulmonary resuscitations (CPR) were performed for fourteen patients (mean CPR time 20.8±26.0 min). The mean time from vascular access to the initiation of ECMO was 17.2±9.4 min and mean support time was 3.8±4.0 days. Fourteen patients were able to be weaned from ECMO and ten patients were discharged (mean admission duration 50.1±31.6 days). Patients survived on average 476.6±374.6 days of follow-up. Longer CPR and support time, increased cardiac enzyme, lower ejection fraction, lower albumin, and major complications were the risk factors of mortality (p<0.05).
Conclusion
The early application of ECMO prior to coronary reperfusion and control of risk factors allowed for good clinical results in cardiogenic shock with AMI.
Keywords: Extracorporeal membrane oxygenation (ECMO), Coronary reperfusion, Acute myocardial infarction, Cardiogenic shock
INTRODUCTION
The mortality rate of cardiogenic shock due to acute myocardial infarction (AMI) is as high as 60~80% and may be higher without aggressive treatment. Besides the classical medical treatment, circulatory supportive devices such as the Intra Aortic Balloon Pump (IABP) and Ventricular Assistance Device (VAD) may be helpful [1-5]. Recently ECMO has been adapted to the percutaneous approach and the self-charging system, so that it can support the whole body and coronary circulation fast, and has shown good clinical results in patients experiencing cardiogenic shock due to AMI [6-9]. On the other hand, there is controversy about which procedure should be first, coronary reperfusion or ECMO, for cardiogenic shock due to AMI. Therefore, the authors have reviewed the short-term results of patients who had received ECMO just before coronary reperfusion in cases of cardiogenic shock due to AMI.
MATERIAL AND METHODS
We retrospectively reviewed 20 cases (median age 67.7±11.7, male : female 13 : 7) of coronary reperfusion after application of EBS (Capiox® Emergency Bypass System [Terumo Inc., Tokyo, Japan]) due to postinfarct cardiogenic shock among 106 cases of ECMO from February 2005 to November 2009. We defined a state of cardiac arrest, systolic blood pressure below 70 mmHg for over 30 minutes and clinical symptoms such as oliguria, confusion, and ventricular tachycardia as cardiogenic shock. We applied ECMO to those patients experiencing cardiogenic shock and performed CPR in 14 of the cases (70%) (mean time 20.8±26.0 minutes). In all cases, we underwent coronary artery bypass under the support of ECMO and chose the method of coronary reperfusion according to the results. After using ECMO support, arterial pulse wave was disappeared in 14 cases, and in those cases we additionally inserted an IABP. Immediately after coronary reperfusion, we started Aspirin and Clopidogrel in all patients.
To perform ECMO, we inserted a 17 Fr cannula into the artery and 21 Fr cannula into the vein (Medtronic Inc.®, DLP, MN, USA) percutaneously using the Seldinger method and adjusted the size of cannulae according to the patient's body weight and body surface area. We infused with as much as 5,000 IU heparin before cannulation, and maintained an ACT (Activated Coagulation Time) in the range of 180 to 200 minutes. We maintained cardiopulmonary circulatory blood flow in the range of 3.0~3.5 L/min/m2 and stopped or lowered the vasopressor to the minimum dosage after ECMO support. During ECMO support, we performed echocardiography daily to check heart function and to check for any ventricular dilation or ventricular thrombus. We maintained platelet and hemoglobin counts over 50,000/µ and 10.0 gm/dL, respectively. During ECMO support, we employed continuous renal replacement therapy (CRRT) in cases of acute renal failure (ARF) by connecting CRRT unit to the ECMO circuit directly.
The weaning from ECMO was begun after the initial 48 hours from the application, raising the dosage of vasopressors and decreasing supportive circulatory blood flow. After weaning from ECMO, the cannula insertion sites were sutured after identification of the femoral artery intima in the Intensive Care Unit (ICU), and there were no complications from the cannula insertion site sutures.
We analyzed the risk factors using a 2×2×2 K Pearson's chi-square test and Fisher's exact test. We compared the mean values of the clinical results using a nonparametric Mann-Whitney U-test.
RESULTS
The mean cannular insertion time for ECMO application was 17.2±9.4 minutes, we performed peripheral circulation at the same time to prevent lower extremity from necrosis in two cases. The cannular insertions were performed in the coronary catheterization room in most cases (17 cases). Two cannular insertions were performed in the Emergency Room and one in the ICU.
Coronary angiography showed 11 cases of three-vessel diseases, 6 cases of two-vessel diseases, one case of single coronary disease and 6 cases of left main branch lesions. The main lesion was the left main branch in 5 cases, the left anterior descending artery in 4, the left circumflex artery in 6, and the right coronary artery in 5 cases. We decided the method of coronary reperfusion according to each patient's coronary angiography results and state. We performed 13 cases of coronary bypass graft surgery and 7 cases of Percutaneous Coronary Intervention (PCI). There were surgeries combined with coronary bypass graft surgery in 5 cases (mitral valvuloplasty in 2 cases, repair of a left ventricle rupture in one case, Dor procedure in one case, and repair of postinfarct ventricular septal defect in one case).
Mean ECMO support time was 3.8±4.3 days (0.5 to 19.2 days). Fourteen cases (70%) were weaned from ECMO and 10 patients (50%) were alive and discharged from the hospital. The mean length of hospitalization was 28.8±31.1 days (1 to 58 days) and mean time in ICU was 11.1±6.8 days (1 to 25 days) (Table 1).
Table 1.
Clinical results
*ICU=Intensive care unit.
The cause of death was failure of ECMO weaning in 6 cases, brain injury in 2 cases, restenosis of coronary artery in one case, and sepsis in one case. The causes of ECMO weaning failure were failure of left ventricular decompression in 2 cases, decrease of circulatory blood flow due to bleeding, and sepsis in 3 cases and heart dysfunction before ECMO support in one case. The main complications were sepsis and Disseminated Intravascular Coagulation (DIC) in 9 cases, acute renal failure in 9 cases, bleeding in 8 cases, and brain injury in 4 cases. We applied CRRT to 8 of the 9 cases of ARF. The surviving patients received follow-up care for a mean of 476.6±374.6 days (27 to 1,318 days). There were no deaths after discharge. The mean left ventricular ejection fraction after discharge was 44.4±16.0% (14.5 to 63.1%), and all patients were considered to be in New York Heart Association functional class (NYHA Fc) I or II.
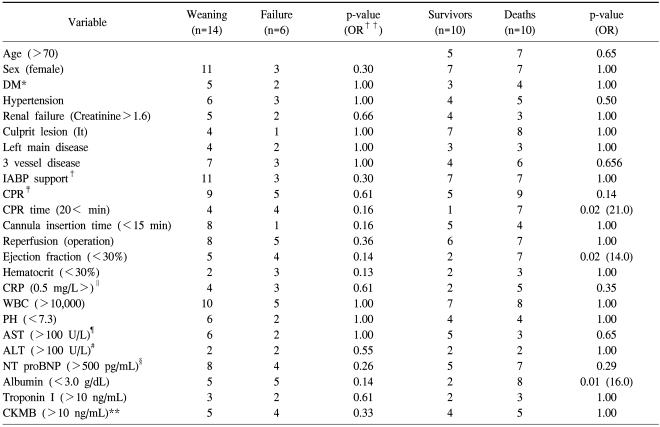
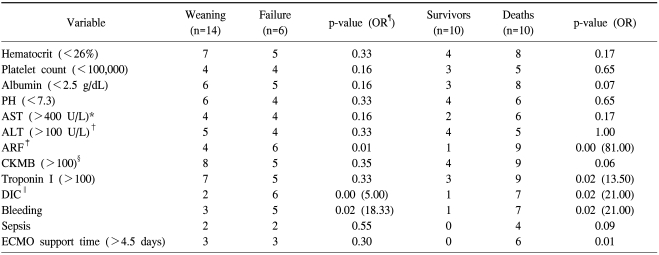
We analyzed the risk factors related to death before ECMO and after ECMO. CPR time before ECMO (>20 min), left ventricular ejection fraction (<40%) before ECMO, and concentration of albumin (<3.0 g/dL) were related to death (p<0.05). ECMO support time (>4.5 days), increase of Troponin I (>100 ng/mL), ARF, DIC, and complications such as bleeding were also related to the death (p<0.05, Table 2, 3).
Table 2.
Pre ECMO risk factors
*=Diabetes mellitus; †=Cardiopulmonary resuscitation; ‡=Intra aortic balloon pump; §=N-Terminal fragment of the prohormone, Brain-Type natriuretic peptide; ∥=C reactive peptide; ¶=Aspartate aminotransferase; #=Alanine aminotransferase; **=Creatin kinase MB; ††=Odd ratio.
Table 3.
Risk factors during ECMO
*=Aspartate aminotransferase; †=Alanine aminotransferase; ‡=Incase creatininin over 50% of normal range; §=Creatin Kinase; ∥=Disseminated intravascular; ¶=Odd ratio.
DISCUSSION
Circulatory supporting devices such as IABP and VAD limit the effect of cardiogenic shock due to AMI. IABP is fast and easy to apply and increases coronary blood flow. However, its effectiveness could be reduced in cases of cardiac arrest or ventricular arrhythmia [1,2]. VAD can also be effective, but requires an additional surgical procedure, more time, and money, and is therefore unsuitable in case of an emergency. Recently, VAD that can be applied percutaneously has become available. However, additional equipment and procedures are required in order to apply a VAD [10].
Since the advent of the percutaneous approach for ECMO, it has shown good results and that it can be applied relatively quickly. Its usage has become popular. CPR with ECMO shows better results than CPR alone, especially in cases of cardiogenic shock. In 2008, Chen et al. reported that CPR with ECMO obtained better results than CPR without ECMO when CPR was required over than 10 minutes. Prodhan et al. reported similar results [11,12].
There has been debate about whether coronary reperfusion or ECMO support should be first applied first at the time of postinfarct cardiogenic shock. In most cases, there has been limited application of ECMO after medical treatment and coronary reperfusion with IABP support at the time of cardiogenic shock due to AMI [13]. However, in the high risk group, maintaining coronary and whole body circulation through aggressive cardiopulmonary circulatory support is helpful in advance.
In 2005, Burkle et al. reported that the results of coronary reperfusion after ECMO support showed a better prognosis in high risk patients who had ischemic heart disease [14]. In 2007, Vanier et al. reported that the weaning rate from ECMO and the survival rate were 100% and 80%, respectively, in patients who underwent coronary reperfusion after ECMO support [15]. In Korea, Rhee et al. [7] and Ryu et al. [8] reported the results of ECMO support before and after coronary reperfusion in ischemic heart disease, however, there has been no report of ECMO support just before coronary reperfusion. In this study, we performed ECMO on high risk patients who had cardiogenic shock and identified an ECMO weaning rate of 70% and a survival rate of 50%, which were similar results to those of several previous studies.
There have been varied results in the weaning and survival rates after ECMO support. Rastan et al. reported a 63.3% weaning rate and a 24.8% survival rate after ECMO support in their study of 517 cases in 2010, and Bakhtiary et al. reported a 56% weaning rate and a 33% survival rate in 45 cases in 2008. There have been studies with weaning rates ranging from 40% to 100% and survival rates ranging from 25% to 80% [16-18]. It is necessary to analyze the risk factors and to reduce them in order to improve the rates of survival and ECMO weaning. Rastan et al. addressed these rates' relationships with age, DM, hepatic failure, Euro score, ARF, and lactate and cardiac enzyme elevation [16]. Zhang et al. reported a noticeable elevation of cardiac enzyme, in weaning failure group [19]. Bakhtiary et al. suggested a relationship with lactate concentration, pulmonary arterial hypertension, DM, and IABP between waning group and weaning failure group [17]. Recently Chen et al. claimed that CPR time was closely related with the results of ECMO support [11]. This study identified that CPR time before ECMO support,, which consistent with the argument of Chen et al., and left ventricular ejection fraction before ECMO support had significant relationship with successful weaning from ECMO. We therefore think that early ECMO application should be considered in high risk patients who have reduced cardiac function. Analysis of cardiac enzyme showed results similar to those reported by Rastan and Zhang et al. Troponin I levels had statistical significance, whereas CKMB levels did not. Continuous monitoring of elevated cardiac enzymes may reveal their relationship to the death rate during or after ECMO support. Previous studies have reported that complications such as bleeding during ECMO support, multiple organ failure, and ARF may contribute to an increased death rate [7,8,20]. In this study, such complications contributed to an increased death rate as well. Decreasing the incidence of major complications would be helpful in improving the survival rate in the future. Nevertheless, factors such as the site of the coronary artery lesion, the method of coronary reperfusion, three-vessel diseases, and the existence of a left main branch lesion did not influence the survival rate. We additionally inserted IABP in the 14 cases which had lost arterial pulse waves, but there was no meaningful difference in survival rates between the group with IABP and the group without IABP. Nevertheless, ECMO support with IABP could help to raise survival rates by recovering the arterial wave, decreasing afterload, increasing coronary blood flow, and weaning ECMO support [17] as has been reported by Bakhtiary et al. [17]. IABP should be applied during ECMO support if there is no contraindication to it.
Short term results are usually satisfactory after discharge. Studies have reported no differences in survival rates between immediately following discharge and one year after discharge. This study also showed no deaths immediately after discharge, and a good clinical course in all cases [11,20].
CONCLUSION
In this study, we analyzed the short-term results and risk factors related to death when ECMO support was applied before coronary reperfusion therapy in cases of postinfarct cardiogenic shock. Study limitations include a small study group, retrospective study, and the possibility of selection bias from nonrandomized study design. Coronary reperfusion therapy after ECMO support showed clinically significant results and good short-term results after discharge. In the high risk group, early ECMO support before coronary reperfusion procedures, correction of risk factors, and minimization of complications would result in improved clinical results for cardiogenic shock due to AMI. Long-term clinical applications of ECMO require further study.
References
- 1.Bhayana JN, Scott SM, Sethi GK, Takaro T. Effects of intraaortic balloon pumping on organ perfusion in cardiogenic shock. J Surg Res. 1979;26:108–113. doi: 10.1016/0022-4804(79)90086-6. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SM, Aufiero TX, Pae WE, Jr, Miller CA, Pierce WS. Mechanical ventricular assistance: an economical and effective means of treating end-stage heart disease. Ann Thorac Surg. 1995;60:284–290. doi: 10.1016/0003-4975(95)00445-q. [DOI] [PubMed] [Google Scholar]
- 3.Hill JG, Bruhn PS, Cohen SE, et al. Emergent applications of cardiopulmonary support: a multiinstitutional experience. Ann Thorac Surg. 1992;54:699–704. doi: 10.1016/0003-4975(92)91014-z. [DOI] [PubMed] [Google Scholar]
- 4.Reichman RT, Joyo CI, Dembitsky WP, et al. Improved patient survival after cardiac arrest using a cardiopulmonary support system. Ann Thorac Surg. 1990;49:101–104. doi: 10.1016/0003-4975(90)90363-b. [DOI] [PubMed] [Google Scholar]
- 5.Matsuwaka R, Sakakibara T, Shintani H, et al. Emergency cardiopulmonary bypass support in patients with severe cardiogenic shock after acute myocardial infarction. Heart Vessels. 1996;11:27–29. doi: 10.1007/BF01744596. [DOI] [PubMed] [Google Scholar]
- 6.Suárez de Lezo J, Pan M, Medina A, et al. Percutaneous cardiopulmonary support in critical patients needing coronary interventions with stents. Catheter Cardiovasc Interv. 2002;57:467–475. doi: 10.1002/ccd.10340. [DOI] [PubMed] [Google Scholar]
- 7.Rhee I, Kwon SU, Sung K, et al. Experiences with emergency percutaneous cardiopulmonary support in in-hospital cardiac arrest or cardiogenic shock due to the ischemic heart disease. Korean J Thorac Cardiovasc Surg. 2006;39:201–207. [Google Scholar]
- 8.Ryu KM, Kim SH, Seo PW, et al. Initial experience of the emergency bypass system (EBS(R)) for the patients with cardiogenic shock due to an acute myocardial infarction. Korean J Thorac Cardiovasc Surg. 2008;41:329–334. [Google Scholar]
- 9.Sung K, Lee YT, Park PW, et al. Improved survival after cardiac arrest using emergent autopriming percutaneous cardiopulmonary support. Ann Thorac Surg. 2006;82:651–656. doi: 10.1016/j.athoracsur.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar K, Kini AS. Percutaneous left ventricular support devices. Cardiol Clin. 2010;28:169–184. doi: 10.1016/j.ccl.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 12.Prodhan P, Fiser RT, Dyamenahalli U, et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009;80:1124–1129. doi: 10.1016/j.resuscitation.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 14.Burkle CM, Nuttall GA, Rihal CS. Cardiopulmonary bypass support for percutaneous coronary interventions: what the anesthesiologist needs to know. J Cardiothorac Vasc Anesth. 2005;19:501–504. doi: 10.1053/j.jvca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Vainer J, van Ommen V, Maessen J, Geskes G, Lamerichs L, Waltenberger J. Elective high-risk percutaneous coronary interventions supported by extracorporeal life support. Am J Cardiol. 2007;99:771–773. doi: 10.1016/j.amjcard.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139:302–311. 311.e1. doi: 10.1016/j.jtcvs.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 17.Bakhtiary F, Keller H, Dogan S, et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008;135:382–388. doi: 10.1016/j.jtcvs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Song SW, Yang HS, Lee S, Youn YN, Yoo KJ. Earlier application of percutaneous cardiopulmonary support rescues patients from severe cardiopulmonary failure using the APACHE III scoring system. J Korean Med Sci. 2009;24:1064–1070. doi: 10.3346/jkms.2009.24.6.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Kofidis T, Kamiya H, et al. Creatine kinase isoenzyme MB relative index as predictor of mortality on extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock in adult patients. Eur J Cardiothorac Surg. 2006;30:617–620. doi: 10.1016/j.ejcts.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Hsu PS, Chen JL, Hong GJ, et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg. 2010;37:328–333. doi: 10.1016/j.ejcts.2009.07.033. [DOI] [PubMed] [Google Scholar]