Abstract
The environment can have a decisive influence on the structure of the genome, changing it in a certain direction. Therefore, the genomic distribution of environmentally sensitive transposable elements may vary measurably across a species area. In the present research, we aimed to detect and evaluate the level of LTR retrotransposon intraspecific variability in Aegilops speltoides (2n = 2x = 14), a wild cross-pollinated relative of cultivated wheat. The interretrotransposon amplified polymorphism (IRAP) protocol was applied to detect and evaluate the level of retrotransposon intraspecific variability in Ae. speltoides and closely related species. IRAP analysis revealed significant diversity in TE distribution. Various genotypes from the 13 explored populations significantly differ with respect to the patterns of the four explored LTR retrotransposons (WIS2, Wilma, Daniela, and Fatima). This diversity points to a constant ongoing process of LTR retrotransposon fraction restructuring in populations of Ae. speltoides throughout the species’ range and within single populations in time. Maximum changes were recorded in genotypes from small stressed populations. Principal component analysis showed that the dynamics of the Fatima element significantly differ from those of WIS2, Wilma, and Daniela. In terms of relationships between Sitopsis species, IRAP analysis revealed a grouping with Ae. sharonensis and Ae. longissima forming a separate unit, Ae. speltoides appearing as a dispersed group, and Ae. bicornis being in an intermediate position. IRAP display data revealed dynamic changes in LTR retrotransposon fractions in the genome of Ae. speltoides. The process is permanent and population specific, ultimately leading to the separation of small stressed populations from the main group.
LARGE cereal genomes are known to consist of an extraordinary number of transposable elements, in particular, LTR retrotransposons, which are highly dynamic (Bennetzen 1996; Wicker et al. 2003). Recent studies have shown that LTR retrotransposons are often found in different densities or copy numbers among individuals of the same species (Baucom et al. 2009; Belyayev et al. 2010), and “bursts” of transposable elements (TEs) in several species of angiosperms over time have been recorded (Vitte and Panaud 2003; Tsukahara et al. 2009; Belyayev et al. 2010). Although there are several known cases of temporal retroelement copy-number change, the important question of the level of current TE intraspecific variability across the area occupied by a particular species is still unclear, especially for a species whose area is declining or shifting under the influence of climate change. It is possible that populations with enhanced TE activity are more likely to survive as new forms, or even new species, during environmental fluctuations due to the production of an extended number of genomic variants for natural selection (Grant 1981; Raskina et al. 2004a; Belyayev et al. 2010). This is one of the key problems in understanding the mechanisms of speciation because, in a certain sense, intraspecific genome diversification, particularly the genesis of differences across eco-geographical gradients, could be regarded as a speciation precursor.
Dobzhansky’s central–marginal model (Da Cunha and Dobzhansky 1954) assumes that populations near the center of a species’ range usually display high levels of genetic and phenotypic variation, while populations on the margin of the range are monomorphic (for review see Eckert et al. 2008). Extrapolating the central–marginal model onto the TE fraction and given the fact that TEs are sensitive to changes in the external environment (Wessler 1996; Kashkush et al. 2003; Grandbastien et al. 2005; Ansari et al. 2007; Martienssen 2008), it is possible to speculate that the TE quantity and structural distribution across the genome may perceptibly vary between populations. Indeed, the environment can have a decisive influence on the structure of the genome, changing it in a certain direction that could be heritable (Martienssen 2008). This is especially true in times of rapid climatic change such as the current period of global warming, when the average temperature of the Earth’s near-surface air and oceans is increasing (http://www.ipcc.ch). Any climate fluctuation causes the movement of plant zones and, consequently, the degradation of peripheral populations (Tchernov 1988; Hofreiter and Stewart 2009). Mesic plant species on the periphery of the distribution area, especially in the Eastern Mediterranean, due to its proximity to the Afro-Arabian desert domain, will be the first to suffer the impact of the current global warming (Kröpelin et al. 2008; Rebernig et al. 2010). Certainly, this could cause a reaction in plant organisms and may lead not only to their extinction or recession but also possibly to the formation of new drought-resistant forms (Raskina et al. 2004b). Moreover, in marginal populations where the influence of the ecologically intensive processes of raciation and speciation may take place, some models suggest that these populations play an important role in the maintenance and generation of biological diversity (Mayr 1963, 1970; Brussard 1984; Kirkpatrick and Barton 1997).
In the present research, we aimed to detect and evaluate the level of LTR retrotransposon intraspecific variability in Aegilops speltoides (2n = 2x = 14), a wild cross-pollinated relative of cultivated wheat. The interretrotransposon amplified polymorphism (IRAP) protocol (Kalendar and Schulman 2006), in which segments between two nearby retrotransposons or LTRs are amplified using outward-facing primers, was applied to determine the diversity of TE elements. We explore IRAP patterns from the Ty1-copia and Ty3-gypsy superfamilies (Kapitonov and Jurka 2008), which predominate in the repetitive fraction in Ae. speltoides (Belyayev et al. 2010).
Materials and Methods
Plant material and DNA extraction
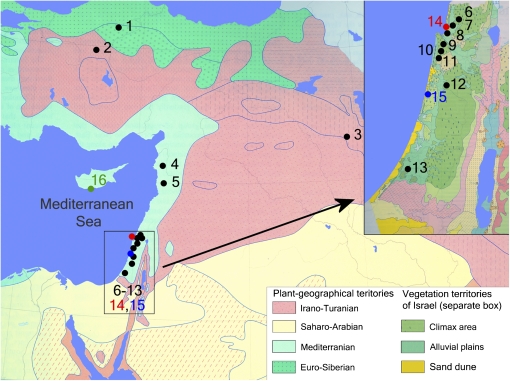
Thirteen populations of Ae. speltoides, throughout the species distribution area, and three populations of related diploid S-genome species, Ae. sharonensis, Ae. longissima, and Ae. bicornis (195 plants totally), were selected for analysis. The geographical distribution of the explored populations is shown in Figure 1. In our experiments we used the continuous sampling, allowing estimation of the studied parameter across the entire species area. The abbreviation of the populations, their origin, and the number of analyzed genotypes are shown in Table 1. Sources of plant material and characteristics of Ae. speltoides populations are shown in supporting information, Table S1. Total DNA was extracted by the CTAB method (Kidwell and Osborn 1992). The purity and quality of the DNA were equivalent among all samples.
Figure 1.
Geographical distribution of explored populations. Populations are numbered according to Table 1. Ae. speltoides populations are shaded in black, Ae. sharonensis in red, Ae. longissima in blue, and Ae. bicornis in green.
Table 1. The accessions numbers and sources of plant material.
| No. | Species, genome | Abbreviation | Origin | No. genotypes analyzed |
|---|---|---|---|---|
| 1 | Ae. speltoides, SS | C | Cankiri, Turkey | 6 |
| 2 | An | Ankara,Turkey | 4 | |
| 3 | Ar | Arbil, Iraq | 2 | |
| 4 | TS-84 | Latakia, Syria | 1 | |
| 5 | Ta | Tartus, Syria | 6 | |
| 6 | A | Achihood, Israel | 12 | |
| 7 | E | En-Efek, Israel | 2 | |
| 8 | Q | Kishon, Israel | 36 | |
| 9 | T0, T2, T5 | Technion 2, Israela | 16, 22, 13 | |
| 10 | R | Ramat Hanadiv, Israel | 23 | |
| 11 | K, TS89 | Katzir, Israel | 27 | |
| 12 | TS43 | Givat Koah, Israel | 2 | |
| 13 | TS01 | Ashkelon, Israel | 4 | |
| 14 | Ae. sharonensis, SshSsh | S | Kishon, Israel | 10 |
| 15 | Ae. longissima, SlSl | L | Wingate, Israel | 8 |
| 16 | Ae. bicornis, SbSb | TB | Cyprus | 2 |
For the Technion 2 population plants collected in three different years were analyzed: T0, collection of 2000; T2, collection of 2002; and T5, collection of 2005.
TE sequence sources and primer design
To determine the interpopulation diversity of four LTR retrotransposons (WIS2, Wilma, Daniela, and Fatima), IRAP analysis of the populations was performed, and the results were compared among themselves and with IRAP data from closely related species of section Sitopsis. This type of data provides insights into the dynamics of LTR retrotransposons in the genome of Ae. speltoides under changing environments. The sequences of transposable elements were taken from the TREP database (Table 2; http://wheat.pw.usda.gov/ggpages/ITMI/Repeats/index.shtml). Different LTRs of certain elements may vary in sequence at specific locations and may have point mutations, but there are places where polymorphism is reduced to a minimum. For each TE family, the sequence accessions were aligned, and the conservation was assessed with the multiple alignment procedure of MULTALIN (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_multalinan.html). The conserved segments of the LTR or internal domain of the retrotransposons were used for the design of PCR primers, which was carried out with the program FastPCR (http://www.biocenter.helsinki.fi/bi/programs/fastpcr.htm). We designed several primer pairs for each of the repeated elements or TEs to compare the efficiency and reproducibility of amplification. None of the primer pairs chosen form dimers, and all showed high PCR efficiency. The chosen primers match motifs sufficiently conserved in the retrotransposons to allow amplification of almost all targets in the genome.
Table 2. Transposable element accessions.
| TE, superfamily | Accessions |
|---|---|
| WIS2, Copia | TREP1723, TREP1724, TREP839, TREP840, TREP841, TREP262, TREP1823, TREP1824, TREP1825, TREP1826, TREP818, TREP819, TREP1325, TREP1439, TREP1440, TREP1441, TREP1442, TREP1443, TREP10, TREP96,TREP105 |
| Wilma, Gypsy | TREP842, TREP820, TREP821, TREP822, TREP1438, TREP2210 |
| Daniela, Gypsy | TREP796, TREP1226, TREP2208, TREP231, TREP1408, TREP1228 |
| Fatima, Gypsy | TREP827, TREP828, TREP252, TREP1229, TREP1230, TREP1804, TREP2209, TREP1231, TREP1232, TREP1306, TREP1413, TREP1414, TREP1415 |
IRAP analysis
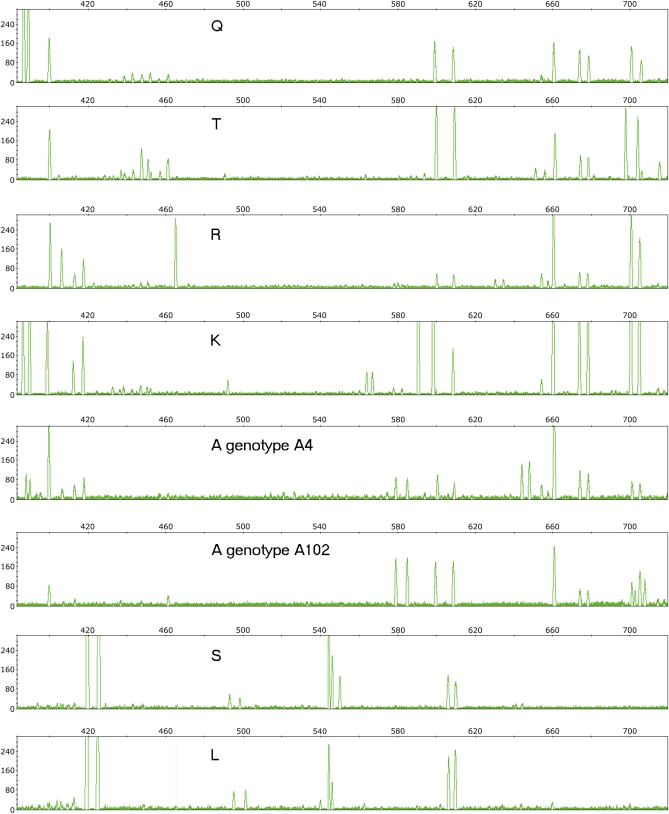
IRAP analysis was conducted according to Kalendar and Schulman (2006). Four additional informative primers were chosen (Table 3) and were labeled with fluorescent tags. The PCR was performed in a 20-µl reaction mixture containing 20 ng DNA, 1× PCR Y buffer [20 mM Tris-HCl (pH 8.55), 2 mM MgCl2, 16 mM (NH4)2SO4, and 0.01% Tween 20], 0.2 µM of each primer, 0.2 mM dNTPs, and 1 unite SAWADY Taq DNA polymerase (PEQLAB Biotechnologie, Erlangen, Germany). The PCR program consisted of (1) 1 cycle at 95° for 5 min; (2) 30 cycles at 95° for 30 sec and at 56°, 58°, or 60° (depending on the primer) for 1 min and at 72° for 30 sec; and (3) a final extension step of 72° for 5 min. Amplification was performed in a PTC-100 Programmable Thermal Controller (Applied Biosystems, Foster City, CA) in 0.2-ml tubes or in 96-well plates. Products were analyzed by gel capillary electrophoresis on an automated 3130xl genetic analyzer (Applied Biosystems) in polyacrylamide gels (Pop 7; Applied Biosystems), using the internal size standard 1200 liz (Applied Biosystems) and standard running protocol 1200 long up. The capillary gel electrophoresis results were analyzed using Gene Mapper version 4.0 (Applied Biosystems) (Figure 2).
Table 3. Primers for IRAP analysis.
| Name | Sequence | TE, region |
|---|---|---|
| 2106 | taatttctgcaacgttccccaaca | WIS2, LTR |
| 2108 | agagccttctgctcctcgttgggt | Wilma, LTR |
| 2109 | tacccctactttagtacaccgaca | Daniela, LTR |
| 2115 | caagcttgccttccacgccaag | Fatima, LTR |
Figure 2.
Example of interretrotransposon amplified polymorphism in the range from 400 to 720 bp for Wilma retrotransposons. Each peak indicates the presence of the amplified DNA fragment of a certain length. Abbreviations of populations are according to Table 1.
Data processing methods
The raw IRAP data for every genotype consisted of logarithms of peak areas across IRAP bands. The purpose of log transformation was to make the signal distribution of every genotype close to the Gaussian distribution. Then, all genotype log profiles were normalized by quantile normalization (Bolstad et al. 2003) to avoid an artificial deviation of some genotypes from their population pools due to a trend in signal measurements of the sample. In the first step of the analysis, the normalized data were studied by the singular value decomposition (SVD) version of principal component analysis (PCA) (Press et al. 2007) (Figure 3). For all four transposons, an obvious clustering of populations was observable. However, the first two components (PC1 and PC2) covered only 11–14% of the total data variance in each IRAP data set. To check how well the populations are separated in PCA spaces of higher dimensions, quadratic discriminant analysis (QDA) (R-package, CRAN; http://www.r-project.org) was performed with the populations as discriminating classes (Figure 4). The QDA is applied to classification of the individual object of interest assuming that there are several populations of objects, to one of which the questionable object belongs, and also assuming that the intrapopulation distribution of objects could be approximated by multidimensional Gaussians. The QDA analysis is based on the hypothesis that the population-associated Gaussians mutually differ in their shapes (the covariation matrices of Gaussians differ both in their diagonal and in their nondiagonal elements). On the first step QDA approximates populations by Gaussians of most appropriate shape and detects the multidimensional subspaces that separate the populations. The following up classification of any individual object of interest is based on a separation of populations by these subspaces. In PCA spaces of three components, we estimated how many QDA misclassifications appear in each population. We also performed QDA for five components (data not shown), but the misclassification in the PCA-3 space was heavier and more informative because the space was more “narrow” (i.e., of fewer dimensions). On the basis of the number of misclassifications and which populations are mutually confounded, one can evaluate an “IRAP distance” between populations or, in other words, detect the groups of similar populations.
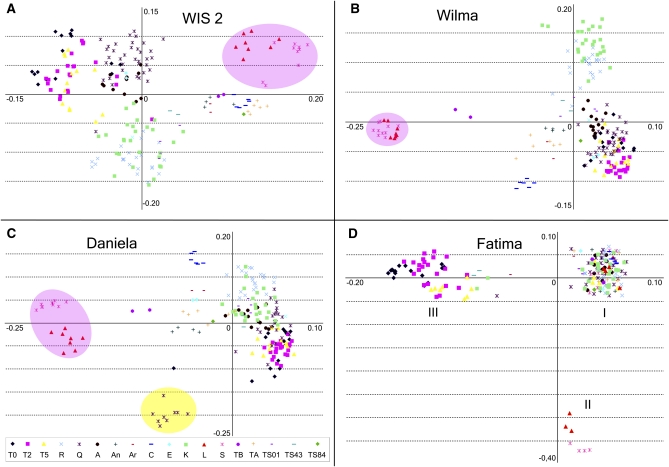
Figure 3.
Distribution of populations in the space of the two first principal components. PC1 is located on x-axes, and PC2 is on y-axes. The raw data for the PCA analysis consist of the appearances of each IRAP band in the individual genotypes. The PCA algorithm was singular value decomposition (SVD). The obvious clustering of populations in the space of the first two PCs (11.4% of total variance) is clearly observed. (A) WIS2 element IRAP pattern variability. (B) Wilma element IRAP pattern variability. (C) Daniela element IRAP pattern variability. Genotypes from the Kishon (Q) population with unusual IRAP patterns are shaded in yellow. (D) Fatima element IRAP pattern variability. Three groups are marked with Roman numerals. (A–C) Ae. sharonensis and Ae. longissima genotypes are shaded in pink.
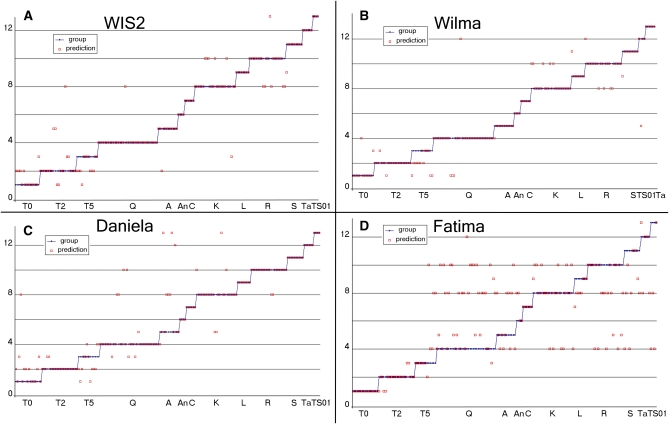
Figure 4.
Prediction for 13 populations by QDA that is based on genotype values of the first three PCs (15% of total data variance). Populations are located on x-axes and clusters on y-axes. The blue dots depict classes of discrimination (i.e., populations) for individual genotypes. The red circles depict what discrimination classes are predicted for individual genotypes by their QDA posterior probabilities. (A) QDA for WIS2 retrotransposons. (B) QDA for Wilma retrotransposons. (C) QDA for Daniela retrotransposons. (D) QDA for Fatima retrotransposons.
Molecular cytogenetical retrotransposon display
The procedure of in situ hybridization retrotransposon display has previously been described (Belyayev et al. 2001). Degenerative oligonucleotide primers were used for PCR amplification of conserved reverse transcriptase regions present in the genomic DNA. The primers and PCR conditions used for the Ty1-copia elements have been previously described by VanderWiel et al. (1993); likewise, those for the Ty3-gypsy elements have been described by Purugganan and Wessler (1994). The gel-isolated amplification products of the RT domain of the two retroelements were labeled with biotin-16-dUTP (Roche) and used as probes for in situ hybridization experiments. Hybridization was carried out at 63° for 3 hr. Biotin was detected with fluorescein isothiocyanate (FITC)-conjugated avidin (Vector Laboratories, Burlingame, CA).
Results
Defining populations
The importance of clearly defining normality/marginality of populations should be emphasized. The main criteria that we used for designating populations as marginal were as follows: (i) the position relative to the center of the species’ range [the present-day center of the Ae. speltoides range is in the middle of the Fertile Crescent (Zohary et al. 1969; Kimber and Feldman 1987) and is limited to the approximate geographic coordinates 36°–38°N, 37°–41°E], (ii) population size (the area inhabited by small populations was <1000 m2), (iii) the degree of population destruction (mainly due to human activity), (iv) local ecology (mainly abiotic components), and (v) elevation (optimum range from 100 to 1000 m above the sea level). The characteristics of the surveyed populations using the criteria listed above can be found in Table S1. We intended to explore three groups of populations that were clustered according to size and eco-geographical conditions: large populations with conducive environments [TS84 (Latakia, Syria), A (Achihood, Israel), R (Ramat Hanadiv, Israel), and K (Katzir, Israel)], small marginal populations [C (Cankiri, Turkey); An (Ankara, Turkey); Ar (Arbil, Iraq); Ta (Tartus, Syria); Q (Kishon, Israel); and T0, T2, and T5 (Technion 2, Israel)], and intermediate populations [E (En-Efek, Israel), TS43 (Givat Koah, Israel), and TS01 (Ashkelon, Israel)]. In our research, emphasis was given to small, marginal, stressed populations on the southern border of the species’ range for several reasons: (i) long-term field observations have shown that modern climate change has led to the degradation of these populations, resulting in at least a threefold reduction in their size over the last decade; and (ii) our previous investigations have shown increased activity of TEs in the marginal populations (Raskina et al. 2004a; Belyayev et al. 2010).
IRAP analysis
IRAP is a novel method in which the insertion of a retrotransposon near another creates a new template for PCR amplification. The PCR products and, therefore, the fingerprint patterns, result from amplification of hundreds to thousands of target sites in the genome (Kalendar and Schulman 2006). Polymorphisms consonant with large changes in TE chromosomal distribution would be expected. All IRAP primers produced multiple fragments from genomic DNA of all Ae. speltoides, Ae. sharonensis, Ae. longissima, and Ae. bicornis accessions (Figure 2 and Table S2). We took into account the fragments ranging from 250 to 1200 bp, which correspond to the maximum accuracy of the assay. The numbers of bands varied for different transposons and populations. WIS2 retrotransposons produced the greatest number of bands, displaying between 41 [L (Wingate, Israel) and TS84 populations] and 159 (A population) bands with an average of 108 bands. Wilma elements produced between 50 (TS43) and 87 [TB (Cyprus)] bands with an average of 62 bands. Daniela elements produced between 32 [S (Kishon, Israel)] and 84 (T5) bands with an average of 77 bands. Fatima elements produced between 32 (K) and 53 (E) bands with an average of 44 bands.
Multivariate analyses
For statistical evaluation of the IRAP data, we used two types of analysis: PCA and QDA.
PCA analysis
The central idea of PCA is “. . . to reduce the dimensionality of a data set consisting of a large number of interrelated variables, while retaining as much as possible of the variation present in the data set. This is achieved by transforming to a new set of variables, the principal components (PCs), which are uncorrelated, and which are ordered so that the first few retain most of the variation present in all of the original variables” (Joliffe 2002, p. 1). We used PCA to project the populations onto a plane with minimal disturbances in distances between individual genotypes and to check visually how good the biologically defined populations are separated on the plane of the first two PCs. The data distribution in two-dimensional PCA spaces (Figure 3) visually demonstrates a clear separation of different populations for each of the explored LTR retrotransposons.
WIS2:
Primers for WIS2 retrotransposons produced a mostly dispersive picture (Figure 3A), although all genotypes in each population occupy sufficiently dense zones, and these zones overlap very often. Ae. sharonensis (S) and Ae. longissima (L) make up a separate group. However, Ae. bicornis (TB) joins the group of peripheral Ae. speltoides populations, which are intermediate between the two Sitopsis species and a majority of Ae. speltoides populations. Israeli populations of Ae. speltoides have specific WIS2 retrotransposon distributions with a significant extent of variability. An analysis of plants collected in three different years in the Technion 2 population (T0, T2, and T5) showed clustering for the years 2000 and 2002 and overlapping with the following year (2005).
Wilma:
IRAP analysis of the Wilma retrotransposon (Figure 3B) classified species in the best way; that is, Ae. sharonensis (S) and Ae. longissima (L) represent a separate compact group. Ae. bicornis (TB) takes an intermediate position between the two Sitopsis species and Ae. speltoides. The Ae. speltoides genotypes represent a dispersed group where the two biggest populations, K and R, grouped together and were distanced from the majority of the other populations. The northernmost peripheral population, C, is also separated.
Daniela:
IRAP analysis of the Daniela retrotransposon (Figure 3C) was similar to that of Wilma, with a separate position for Ae. sharonensis (S) and Ae. longissima (L), an intermediate position for Ae. bicornis (TB), and a separate position for Ae. speltoides. The C population and several genotypes from the Q population (indicated by the yellow circle in Figure 3C) stood out substantially. The K and R populations composed a common core group with other populations.
Fatima:
The IRAP pattern distribution of the Fatima retrotransposon differs significantly from those of the three other retroelements (Figure 3D). We observed complete dissolution of interpopulation and interspecific differences in the IRAP patterns. All genotypes were separated into three distinct groups. The largest group (group I in Figure 3D) includes the majority of populations throughout the Ae. speltoides distribution area, all genotypes of Ae. bicornis (TB), and several of the Ae. sharonensis (S) and Ae. longissima (L) genotypes. The second group (group II in Figure 3D) incorporates a few genotypes of Ae. sharonensis and Ae. longissima. The third group (group III in Figure 3D) consists of the majority of genotypes of the Technion 2 population (T0, T2, and partially T5), one genotype from the Q population, one from TS01, two from TS43, and two from K.
QDA analysis
Statistically, a more accurate study by QDA (Figure 4) identifies differences in LTR retrotransposon-specific approximate IRAP distances between populations (namely, the structure of population clustering). The populations inside of each cluster are mutually misclassified by QDA. Each point represents a single genotype, and each line represents a single population. The QDA algorithm determines in which set (population) a genotype belongs on the basis of its IRAP pattern. If the genotype is similar to others of a given population, the QDA algorithm “puts” it into the same position as the population on the y-axis. If not, the genotype is positioned with respect to other populations that have a similar IRAP pattern while maintaining the genotype position on the x-axis. For example, in Figure 4A, five genotypes from the first population (T0) have IRAP patterns similar to the second population (T2) and one from the third population (T5). These misclassifications represent ∼40% of the observed genotypes. However, 60% of the genotypes in the T0 population are homogeneous; i.e., they form an individual “cloud”. Thus, the QDA method allows for the evaluation of a population’s homogeneity and separation. We applied QDA to classify all genotypes in all populations after approximating each population’s distribution with Gaussian curves of population-specific shapes in the space of the first three principal components. The space of the first principal component was used in the QDA classification because all large populations are well separated in this space. These two types of analyses allowed us to evaluate the similarities and differences in the set of populations.
WIS2:
QDA analysis showed that the number of misclassifications is significant (short IRAP distance) in two groups of populations: (1) Technion 2 (T0, T2, and T5) for all years collected (which was predictable, since it is the same population) and (2) K and R (Figure 4A). The predicted classes in each of these two population groups are not in good correspondence with the initial classes. It must be emphasized that Katzir and Ramat Hanadiv are the largest among all investigated natural populations with minimal destruction. The investigated Sitopsis species S, L, and TB showed no misclassifications.
Wilma:
QDA analysis showed that the number of misclassifications for Wilma is the lowest of all the investigated elements. The same groups of populations were found as for WIS2.
Daniela:
QDA analysis showed that the level of misclassification for Daniela is comparable with that for WIS2 elements. Misclassifications were evenly distributed throughout populations and significant only in one group, which consists of the T0, T2, and T5 and Q populations.
Fatima:
QDA analysis showed a significant level of misclassification across all populations and the investigated Sitopsis species, emphasizing the specific behavior of the Fatima element (Figure 4D). The most spread-out group is the Q population, which shows similarity with the rest of the Ae. speltoides populations and with Ae. longissima (L). All populations show the greatest similarity to the K population.
Summarizing the data from statistical analysis, it should be emphasized that there is a significant difference in IRAP patterns between marginal and relatively normal populations.
Molecular cytogenetic evaluation of transposable element diversity
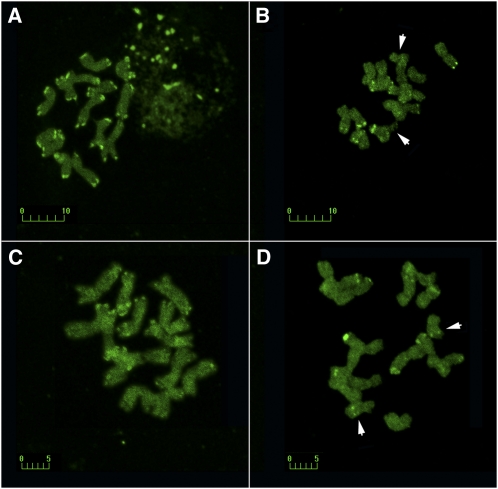
To confirm the IRAP data on retrotransposon diversity, specifically the distribution of the Ty1-copia and Ty3-gypsy retrotransposon superfamilies within the marginal and central populations, an independent evaluation of transposable element diversity was performed. The method of comparative molecular cytogenetics, which allows for an integral evaluation of the genomic distribution of retrotransposons, was used. We applied a categorical sampling approach in which marginal populations were pairwise compared with relatively normal populations. The results are shown in Figure 5. We evaluated the total number of major blocks in the genome. The number of blocks of both the Ty1-copia and Ty3-gypsy retrotransposons was greatly reduced in marginal populations compared with normal populations. In the TS84 population, there are 34 major blocks of Ty1-copia (Figure 5A) and 26 major blocks of Ty3-gypsy (Figure 5C) elements. These blocks mostly coincided with distal/terminal clusters of heterochromatin, which are present on all chromosomes of plants from central populations of Ae. speltoides. In the marginal Q population, the number of blocks for both Ty1-copia (Figure 5B) and Ty3-gypsy (Figure 5D) elements was reduced to 13. The appearance of Ty3-gypsy intercalary block on B chromosomes was also observed. Thus, the data from the molecular cytogenetic analysis were consistent with the IRAP data in terms of the significant reconstitution of the tested retrotransposon patterns in marginal populations.
Figure 5.
Molecular cytogenetic display of transposable element diversity. (A–D) Comparison of FISH signals from the Ty1-copia (A and B) and Ty3-gypsy (C and D) LTR retrotransposons in genotypes of Ae. speltoides from contrasting populations: the central population from Latakia (TS84) (A and C) and the marginal Kishon (Q) population (B and D). The B chromosomes are arrowed. Bars: 10 μm and 5 μm.
Discussion
Variability of the IRAP patterns for the WIS2, Wilma, and Daniela LTR retrotransposons
A high level of intraspecific variability in nuclear DNA LTR retrotransposon fractions of Ae. speltoides was revealed by IRAP analysis. This marker system appears to be an appropriate approach for the evaluation of genetic diversity and evolutionary relationships within and between species (Saeidi et al. 2008). Moreover, part of the changes in IRAP patterns can be attributed to recent proliferation as differences in IRAP patterns between parental genotypes and offspring in Ae. speltoides populations have been previously reported (Belyayev et al. 2010). Various genotypes from the same population differ substantially in the distribution patterns of the four explored LTR retrotransposons. This diversity points to a permanent ongoing process of LTR retrotransposon fractions restructuring in different populations of Ae. speltoides along the species’ range. Despite the observed diversity, PCA of IRAP patterns for three LTR retrotransposons, WIS2, Wilma, and Daniela, revealed an association of the majority of individuals of each population into sufficiently dense groups (less dense for WIS2 and more dense for Wilma) that often overlap (i.e., possessed similar IRAP patterns; Figure 3, A–C). This is particularly emphasized by QDA analysis when misclassifications inside a single population are present, but represent only a small percentage (Figure 4, A–C). It is also essential to note that the peripheral northernmost population, C, and the southern small, marginal populations, Q and T0, T2, and T5, stand out. The most striking case is the very unusual IRAP pattern of the Daniela element in the Kishon population (highlighted with a yellow circle in Figure 3C). This population is extremely small (100 m2) and has been nearly destroyed by anthropogenic factors. In addition, this is the only Israeli population that is located at sea level (2 m above) and is close to the Akko plain terminal of desert plants (Raskina et al. 2004b). It is likely that such an extreme environment has been a crucial factor in the changing pattern of the Daniela element. Although the mechanisms for this phenomenon are unclear, we speculate that it might have resulted from the high recombination rate that is typical of marginal populations (see also Raskina et al. 2011); even so, it is possible that a transposition also contributed to the radical changes in the IRAP pattern.
Further evidence of the permanence of LTR retrotransposon fraction repatterning was provided by time-line IRAP analysis of genotypes from the Technion 2 population, when three sets of plants collected in different years (T0, T2, and T5) were analyzed. This population is extremely small, degraded (i.e., partially destroyed by nearby construction and advancing shrubs), and isolated. Moreover, due to Ae. speltoides cross-pollination, this population could be considered an inbred colony. Therefore, we would expect a rather uniform IRAP pattern. The latter is true for Wilma and Daniela elements, but, for WIS2 retrotransposons, the real data are quite the opposite. Each of the analyzed generations has an individual distribution cloud, and, despite a sufficiently large overlap region, individual genotypes are at a considerable distance from each other (Figure 3A), thus showing a constant restructuring of the WIS2 element pattern over time.
The IRAP retrotransposon display and the following statistical analysis revealed differences between the Ty3-gypsy (Wilma and Daniela) and Ty1-copia (WIS2) retrotransposon superfamilies (Figure 3). Some of these differences result from the structural features of these elements (Suoniemi et al. 1998) and their positions in the genome (Belyayev et al. 2001). However, apart from these differences, our data indicate that there is an important relationship between populations under great stress and high levels of both Ty3-gypsy and Ty1-copia LTR retrotransposon diversity. For example, the majority of the genotypes for the elements of both superfamilies in the Q and T0, T2, and T5 populations form a distinct cluster separated from the main group formed by the rest of the populations. This observation was confirmed by molecular cytogenetic analysis of contrasting populations, which also supported the conclusion that there is a significant difference between the patterns of Ty3-gypsy and Ty1-copia LTR retrotransposons in marginal and normal populations. In both southern and northern marginal populations, we observed a significant decrease in the number of major blocks and detected repatterning. These results are similar to those obtained for species-specific tandem repeats in marginal populations (Raskina et al. 2011).
Variability of the IRAP pattern for the Fatima LTR retrotransposon
Each of the investigated LTR retroelements has a unique IRAP pattern distribution (Figure 3); at the same time, the patterns of Daniela, Wilma, and WIS2 elements resemble one other (Figures 3, A–C, and 4, A–C). Conversely, the PCA distribution of the Fatima element is significantly different from that of the three other LTR retroelements. QDA analysis also confirmed the special status of the Fatima element. The investigated populations fall into three distinct groups (Figures 3D and 4D). Group I (Figure 3D) consists of the majority of Ae. speltoides, Ae. sharonensis, and Ae. longissima genotypes and all genotypes of Ae. bicornis. The occurrence of several species in a dense group points to the stability of the Fatima IRAP pattern over time. This may result from complete silencing of the element for a long period and/or from relatively short LTRs of Fatima, i.e., a poor template for recombination. Anyway, violations of this pattern, such as those observed in groups II and III (Figure 3D), may indicate the movement of elements in the genome. This movement may be a movement of the element itself by a copy and paste mechanism, indicating the activity of Fatima elements in some genotypes, and/or it can be the rebuilding of blocks enriched with Fatima retrotransposons. In any case, this movement can be regarded as a significant microevolutionary event in the genome, especially if inherited.
The next important question is, What is common between genotypes with restructured Fatima element IRAP patterns? Obviously, 96% of these genotypes are from stressed, critically endangered micropopulations. The population of TS43 consisted of just a few plants collected in 1979, which were not found again (M. Feldman, personal communication). This is also the case for the Technion 2 population that was found by the authors in 2000, but was extinct in 2006. Genotypes of Ae. sharonensis (S) are from the northernmost population of Kishon, and Ae. longissima (L) genotypes are from the degraded coastal Wingate population (group II in Figure 3D). Nevertheless, in large populations, a small percentage of genotypes with an unusual IRAP pattern of Fatima elements are also present, as evidenced by Ae. speltoides genotypes from a large population of K. The consequences of Fatima element IRAP pattern rebuilding still need to be determined.
Estimation of the level of IRAP pattern variability
The material examined showed a range of changes in the IRAP pattern, so the next salient question is, How can we estimate the level of LTR retrotransposon IRAP pattern variability? In other words, What kind of information can we get from the differences in the IRAP patterns between genotypes and between populations in terms of microevolution, and how valuable is this information? The answer to these questions can be obtained by interspecific comparisons. The PCA of the three LTR retroelements, namely WIS2, Daniela, and Wilma, showed that Sitopsis species Ae. sharonensis and Ae. longissima usually form a group, while Ae. bicornis stands separately and in an intermediate position between this group and Ae. speltoides (Figure 3, A–C). The group consisting of Ae. sharonensis and Ae. longissima is located some distance from the weighted average of the plot. This distance is >|0.1| for WIS2, >|0.2| for Wilma, and >|0.15| for Daniela. We might ask for which of the three elements can the corresponding distance be taken as characteristic of the species character. In the case of WIS2 retrotransposons, the distance is meaningless because many genotypes are outside the specified values without having any deviations in habitus. Most likely, the large variability of WIS2 IRAP pattern was the result of neutral heterochromatin conversions, as heterochromatin is known to be enriched with elements of the Ty1-copia family (Pearce et al. 1996; Heslop-Harrison et al. 1997; Belyayev et al. 2001; Saunders and Houben 2001; Chang et al. 2008). Thus, the variability of WIS2 element IRAP patterns can only be an indicator of the intensity of genomic rearrangements.
For Wilma retroelements, no genotypes exceed the specified values (Figure 3B); consequently, the data for this element can be taken as a species characteristic. In the case of Daniela elements, nine genotypes from the Q population are at the specified distance from the majority of the Ae. speltoides genotypes (Figure 3C). This population is located on the western banks of the Kishon River (Haifa Bay area, Israel) and is characterized by high heteromorphy, possessing a wide spectrum of chromosomal abnormalities (Raskina et al. 2004a) and enhanced levels of TE proliferation (Belyayev et al. 2010). We assumed that intensive, ongoing intragenomic processes in this population could ultimately create the basis for parapatric speciation (Raskina et al. 2004b). As has been repeatedly shown, the Daniela retrotransposon extensively amplified during the speciation of diploid ancestors of the A and D genomes of cultivated wheat (Liu et al. 2008); therefore, it can be reasonably suggested that significant changes in the Daniela IRAP pattern may accompany the speciation process.
An analysis of relationships between Sitopsis species
IRAP analysis for WIS2, Wilma, and Daniela elements revealed a grouping similar to those determined by other methods, where Ae. sharonensis and Ae. longissima form a separate unit, Ae. speltoides appears as a dispersed group, and Ae. bicornis is in an intermediate position. Similar configurations were revealed by the RFLP method (Giorgi et al. 2002), by α-amylase inhibitor gene analysis (Wang et al. 2007), by sequence-specific amplification polymorphism (Queen et al. 2003), and on the basis of chloroplast DNA changes (Miyashita et al. 1994). These data suggest a very recent divergence of local endemics (i.e., Ae. sharonensis and Ae. longissima) from the common progenitor. Because both species are young, genetic barriers are not yet completely formed, and interspecific hybrids are not only possible but also fertile (Ankory and Zohary 1962).
Taking all of the above lines of evidence together, the IRAP display data revealed dynamic changes of LTR retrotransposon fractions in the genome of Ae. speltoides. The process is permanent and population specific, ultimately leading to the separation of small stressed populations from the main group.
Supplementary Material
Acknowledgments
The authors are most grateful to Jerzy Jurka and anonymous reviewers for helpful comments. A.B. and O.R. designed the project. R.K. designed IRAP primers. E.H. performed the experiments. L.B. performed statistical analysis. A.B. and O.R. wrote the paper. This work is supported by grants from the Israel Science Foundation to A.B. and O.R. (grant 723/07).
Literature Cited
- Ankory H., Zohary D., 1962. Natural hybridization between Aegilops sharonensis and Ae. longissima: a morphological and cytological study. Cytologia (Tokyo) 27: 314–324 [Google Scholar]
- Ansari K. I., Walter S., Brennan J. M., Lemmens M., Kessans S., et al. , 2007. Retrotransposon and gene activation in wheat in response to mycotoxigenic and non-mycotoxigenic-associated Fusarium stress. Theor. Appl. Genet. 114: 927–937 [DOI] [PubMed] [Google Scholar]
- Baucom R. S., Estill J. C., Leebens-Mack J., Bennetzen J. L., 2009. Natural selection on gene function drives the evolution of LTR retrotransposon families in the rice genome. Genome Res. 19: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyayev A., Raskina O., Nevo E., 2001. Chromosomal distribution of reverse transcriptase-containing retroelements in two Triticeae species. Chromosome Res. 9: 129–136 [DOI] [PubMed] [Google Scholar]
- Belyayev A., Kalendar R., Brodsky L., Nevo E., Schulman A. H., et al. , 2010. Transposable elements in a marginal plant population: temporal fluctuations provide new insights into genome evolution of wild diploid wheat. Mobile DNA 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., 1996. The contributions of retroelements to plant genome organization, function and evolution. Trends Microbiol. 4: 347–353 [DOI] [PubMed] [Google Scholar]
- Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P., 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193 [DOI] [PubMed] [Google Scholar]
- Brussard P. F., 1984. Geographic patterns and environmental gradients: the Central-Marginal model in Drosophila revisited. Annu. Rev. Ecol. Syst. 15: 25–64 [Google Scholar]
- Chang S. B., Yang T. J., Datema E., van Vugt J., Vosman B., et al. , 2008. FISH mapping and molecular organization of the major repetitive sequences of tomato. Chromosome Res. 16: 919–933 [DOI] [PubMed] [Google Scholar]
- Da Cunha A. B., Dobzhansky T., 1954. A further study of chromosomal polymorphism in Drosophila willistoni in its relation to the environment. Evolution 8: 119–134 [Google Scholar]
- Eckert C. G., Samis K. E., Lougheed S. C., 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol. Ecol. 17: 1170–1188 [DOI] [PubMed] [Google Scholar]
- Giorgi D., D’Ovidio R., Oronzo A., Tanzarella O. A., Porceddu E., 2002. RFLP analysis of Aegilops species belonging to the Sitopsis section. Genet. Resour. Crop Evol. 49: 145–151 [Google Scholar]
- Grandbastien M. A., Audeon C., Bonnivard E., Casacuberta J. M., Chalhoub B., et al. , 2005. Stress activation and genomic impact of Tnt1 retrotransposons in Solanaceae. Cytogenet. Genome Res. 110: 229–241 [DOI] [PubMed] [Google Scholar]
- Grant V., 1981. Plant Speciation, Ed. 2 Columbia University Press, New York [Google Scholar]
- Heslop-Harrison J. S., Brandes A., Taketa S., Schmidt T., Vershinin V., et al. , 1997. The chromosomal distributions of Ty1-copia group retrotransposable elements in higher plants and their implications for genome evolution. Genetica 100: 197–204 [PubMed] [Google Scholar]
- Hofreiter M., Stewart J., 2009. Ecological change, range fluctuations and population dynamics during the Pleistocene. Curr. Biol. 19: 584–594 [DOI] [PubMed] [Google Scholar]
- Joliffe I. T., 2002. Principal Component Analysis. Springer-Verlag, Berlin/Heidelberg, Germany/New York [Google Scholar]
- Kalendar R., Schulman A. H., 2006. IRAP and REMAP for retrotransposon-based genotyping and fingerprinting. Nat. Protoc. 1: 2478–2484 [DOI] [PubMed] [Google Scholar]
- Kapitonov V. V., Jurka J., 2008. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat. Rev. Genet. 9: 411–412 [DOI] [PubMed] [Google Scholar]
- Kashkush K., Feldman M., Levy A. A., 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat. Genet. 32: 102–106 [DOI] [PubMed] [Google Scholar]
- Kidwell K. K., Osborn T. C., 1992. Simple plant DNA isolation procedures, pp. 1–13 Plant Genomes: Methods for Genetic and Physical Mapping, edited by Beckmann J. S., Osborn T. C. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- Kimber G., Feldman M., 1987. Wild Wheat, an Introduction. College of Agriculture, University Missouri, Columbia, MO [Google Scholar]
- Kirkpatrick M., Barton N. H., 1997. Evolution of a species’ range. Am. Nat. 150: 1–23 [DOI] [PubMed] [Google Scholar]
- Kröpelin S., Verschuren D., Lézine A. M., Eggermont H., Cocquyt C., et al. , 2008. Climate-driven ecosystem succession in the Sahara: the past 6000 years. Science 320: 765–768 [DOI] [PubMed] [Google Scholar]
- Liu Z., Yue W., Li D., Wang R.-C., Kong X., et al. , 2008. Structure and dynamics of retrotransposons at wheat centromeres and pericentromeres. Chromosoma 117: 445–456 [DOI] [PubMed] [Google Scholar]
- Martienssen R., 2008. Great leap forward? Transposable elements, small interfering RNA and adaptive Lamarckian evolution. New Phytol. 179: 572–574 [DOI] [PubMed] [Google Scholar]
- Mayr E., 1963. Animal Species and Evolution. Belknap Press, Cambridge, MA [Google Scholar]
- Mayr E., 1970. Populations Species and Evolution. An Abridgment of Animal Species and Evolution, Belknap Press, Cambridge, MA [Google Scholar]
- Miyashita N. T., Mont N., Tsunewaki K., 1994. Molecular variation in chloroplast DNA regions in ancestral species of wheat. Genetics 137: 883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. R., Pich U., Harrison G., Flavell A. J., Heslop-Harrison J. S., et al. , 1996. The Ty1-copia group retrotransposons of Allium cepa are distributed throughout the chromosomes but are enriched in the terminal heterochromatin. Chromosome Res. 4: 357–364 [DOI] [PubMed] [Google Scholar]
- Press W. H., Flannery B. P., Teukolsky S. A., Vetterling W. T., 2007. Numerical Recipes. Cambridge University Press, Cambridge, UK [Google Scholar]
- Purugganan M. D., Wessler S. R., 1994. Molecular evolution of magellan, a maize Ty3/Ty3-gypsy-like retrotransposon. Proc. Natl. Acad. Sci. USA 91: 11674–11678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen R. A., Gribbon B. M., James C., Jack P., Flavell A. J., 2003. Retrotransposon-based molecular markers for linkage and genetic diversity analysis in wheat. Mol Gen. Genomics 271: 91–97 [DOI] [PubMed] [Google Scholar]
- Raskina O., Belyayev A., Nevo E., 2004a Activity of the En/Spm-like transposons in meiosis as a base for chromosome repatterning in a small, isolated, peripheral population of Aegilops speltoides Tausch. Chromosome Res. 12: 153–161 [DOI] [PubMed] [Google Scholar]
- Raskina O., Belyayev A., Nevo E., 2004b Quantum speciation in Aegilops: molecular cytogenetic evidence from rDNA cluster variability in natural populations. Proc. Natl. Acad. Sci. USA 101: 14818–14823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskina O., Brodsky L., Belyayev A., 2011. Tandem repeats on an eco-geographical scale: outcomes from the genome of Aegilops speltoides. Chromosome Res. 19: 607–623 [DOI] [PubMed] [Google Scholar]
- Rebernig C. A., Weiss-Schneeweiss H., Schneeweiss G. M., Schönswetter P., Obermayer R., et al. , 2010. Quaternary range dynamics and polyploid evolution in an arid brushland plant species (Melampodium cinereum, Asteraceae). Mol. Phylogenet. Evol. 54: 594–606 [DOI] [PubMed] [Google Scholar]
- Saeidi H., Rahiminejad M. R., Heslop-Harrison J. S., 2008. Retroelement insertional polymorphisms, diversity and phylogeography within diploid, D-genome Aegilops tauschii (Triticeae, Poaceae) sub-taxa in Iran. Ann. Bot. (Lond.) 101: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders V. A., Houben A., 2001. The pericentromeric heterochromatin of the grass Zingeria biebersteiniana (2n = 4) is composed of Zbcen1-type tandem repeats that are intermingled with accumulated dispersedly organized sequences. Genome 44: 955–961 [DOI] [PubMed] [Google Scholar]
- Suoniemi A., Tanskanen J., Schulman A. H., 1998. Gypsy-like retrotransposons are widespread in the plant kingdom. Plant J. 13: 699–705 [DOI] [PubMed] [Google Scholar]
- Tchernov E., 1988. The biogeographical history of southern Levant, pp. 159–251 The Zoogeography of Israel: The Distribution and Abundance at a Zoogeographical Crossroad, edited by Yom-Tov Y., Tchernov E. Dr. W. Junk Publishers, Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- Tsukahara S., Kobayashi A., Kawabe A., Mathieu O., Miura A., et al. , 2009. Bursts of retrotransposition reproduced in Arabidopsis. Nature 461: 423–426 [DOI] [PubMed] [Google Scholar]
- VanderWiel P. L., Voytas D. F., Wendel J. F., 1993. Ty1-copia-like retrotransposable element evolution in diploid and polyploid cotton (Gossypium L.). J. Mol. Evol. 36: 429–447 [DOI] [PubMed] [Google Scholar]
- Vitte C., Panaud O., 2003. Formation of solo-LTRs through unequal homologous recombination counterbalances amplifications of LTR retrotransposons in rice Oryza sativa L. Mol. Biol. Evol. 20: 528–540 [DOI] [PubMed] [Google Scholar]
- Wang J.-R., Zhang L., Wei Y.-M., Yan Z.-H., Baum B. R., et al. , 2007. Sequence polymorphisms and relationships of dimeric a-amylase inhibitor genes in the B genomes of Triticum and S genomes of Aegilops. Plant Sci. 173: 1–11 [Google Scholar]
- Wessler S. R., 1996. Turned on by stress. Plant retrotransposons. Curr. Biol. 6: 959–961 [DOI] [PubMed] [Google Scholar]
- Wicker T., Yahiaoui N., Guyot R., Schlagenhauf E., Liu Z. D., et al. , 2003. Rapid genome divergence at orthologous low molecular weight glutenin loci of the A and Am genomes of wheat. Plant Cell 15: 1186–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary D., Harlan J. R., Vardi A., 1969. The wild diploid progenitors of wheat and their breeding value. Euphytica 18: 58–65 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.