Abstract
Isolated adult mouse cardiomyocytes are an important tool in cardiovascular research, but are challenging to prepare. Because the energy supply determines cell function and viability, we compared total creatine ([Cr]) and [ATP] in isolated cardiomyocytes with the intact mouse heart. Isolated myocytes suffered severe losses of Cr (−70%) and ATP (−53%). Myocytes were not able to replete [Cr] during a 5 h incubation period in medium supplemented with 1 mM Cr. In contrast, adding 20 mM Cr to the digestion buffers was sufficient to maintain normal [Cr]. Supplementing buffers with 5 mM of inosine (Ino) and adenosine (Ado) to prevent loss of cellular nucleosides partially protected against loss of ATP. To test whether maintaining [ATP] and [Cr] improves contractile function, myocytes were challenged by varying pacing rate from 0.5 to 10 Hz and by adding isoproterenol (Iso) at 5 and 10 Hz. All groups performed well up to 5 Hz, showing a positive cell shortening–frequency relationship; however, only 16% of myocytes isolated under standard conditions were able to sustain pacing with Iso challenge at 10 Hz. In contrast, 30–50% of the myocytes with normal Cr levels were able to contract and maintain low diastolic [Ca2+]. Cell yield also improved in Cr and the Cr/Ino/Ado-treated groups (85–90% vs. 70–75% rod shaped in untreated myocytes). These data suggest that viability and performance of isolated myocytes are improved when they are protected from the severe loss of Cr and ATP during the isolation, making them an even better research tool.
Keywords: Adult mouse cardiomyocytes, Creatine, Nucleosides, Energetics, Positive force–frequency relationship
Introduction
The isolated adult ventricular mouse cardiomyocyte is an important research tool in molecular and cellular cardiology. In contrast to the isolation of rat cardiomyocytes, which reliably yields high cell counts and high percentages of viable myocytes that can be maintained in culture for up to 5 days [1], healthy mouse cardiomyocytes are difficult to isolate and can be maintained in culture for only up to 36–48 h. There are several reports on methods for isolating adult mouse cardiomyocytes [2–4] but it remains difficult to reliably isolate high yields and high percentages of viable cardiomyocytes capable of contracting at high pacing rates and surviving β-adrenergic stimulation.
In this study we tested the hypothesis that the difficulties associated with the isolation and maintenance of adult mouse cardiomyocytes are caused in part by depletion of creatine (Cr) and ATP during the isolation procedure. In the intact heart, the energy supply is a determining factor both for functional recovery from transient ischemia and for long-term survival [5, 6]. It is also well known that the contraction of cardiomyocytes is dependent on the regulation of ion homeostasis [7]. In cardiomyocytes maintenance of low diastolic [Ca2+] is dependent on the proper function of the sarcoplasmic reticulum calcium ATPase (SERCA) and the ATP-dependent plasma membrane calcium transporter [8, 9]. ATP is required for the functioning of these ATPases, and the Cr/phosphocreatine (PCr) energy reserve system of the heart [10] functions in part to maintain a constant [ATP] in the vicinity of these ATPases [11].
Using conditions commonly used to isolate mouse myocytes, we found severe reductions in the Cr and ATP contents of mouse cardiomyocytes. We therefore set out to improve the buffer system used for the enzymatic digestion of myocardium with the goal of preventing the loss of Cr and ATP. We then tested whether this strategy improved the contractile performance of freshly isolated mouse cardiomyocytes. Here we report a strategy that protects the cells from loss of Cr and ATP and yields a larger fraction of viable cells that maintain low diastolic [Ca2+] and have greater contractile reserve.
Materials and Methods
Isolation of Adult Mouse Cardiomyocytes
Cardiac myocytes were isolated from 6 to 8 week-old C57BL6 mice, following Lim et al. [12] with some modifications. In brief, mice were injected with heparin (200 USP units i.p.) and, after cervical dislocation, the heart was quickly excised and arrested in ice-cold Ca2+-Tyrode solution. Hearts were perfused in the Langendorff mode on a gravity flow system at 35–37°C. All solutions used were sterile-filtered using 0.22 μm filters. First, hearts were perfused with a Ca2+-Tyrode solution aerated with 100% O2 (in mM: 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 10 HEPES, 10 glucose, pH 7.4) to free the coronaries of blood. After 5 min hearts were perfused with Ca2+-free Tyrode solution (in mM: 135 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 0.33 NaH2PO4, 10 BDM, 10 glucose, pH 7.2) for not longer than 4 min. Subsequently, hearts were digested by collagenase B (0.6 mg/ml, ROCHE), collagenase D (0.3 mg/ml, ROCHE), and protease XIV (0.08 mg/ml, SIGMA) dissolved in Ca2+-free Tyrode until either the coronary flow rate doubled or a sudden increase in flow rate occurred, indicating effective tissue digestion. Liberase Blendzyme 4 (Roche) instead of collagenases B and D yielded similar results (data not shown).
In our hands, maintaining the perfusion temperature at 35–37°C was crucial for high quality preparations. This was accomplished using a re-circulating water bath to warm perfusion buffers and a lamp to warm the excised heart. In preparations where the perfusion temperature dropped below 34°C levels (due to low coronary flow), the heart turned pale, and these preparations had low cell yields and low cell viability.
Hearts were digested on average for 3–4 min. Then the collagenase solution was flushed from the tissue with Ca2+-free Tyrode solution, the heart was cut away from the perfusion set up, the pericardial sac was pulled away and the tissue was gently pulled apart. High quality cell preparations were marked by myocytes that easily floated away from tissue with very little mechanical separation of the heart tissue. Cells were filtered through a nylon mesh and allowed to settle by gravity for 5 min. [Ca2+] in the buffer was increased stepwise, from 0.6 μM, 0.2 mM, 0.6 mM to 1.2 mM, by suspending the cell pellet in modified Krebs–Henseleit buffer (in mM: 0.5 EDTA, 5.1 KCl, 0.6 MgSO4, 118 NaCl, 1.2 KH2PO4, 10 glucose, 1 NaHCO3, 10 HEPES, 2 mg/ml BSA Fraction V, Sigma). Cells were always allowed to settle by gravity.
All supplements (final concentrations: 1 and 20 mM Cr, 5 mM inosine (Ino) and 5 mM adenosine (Ado), Sigma) were added to Ca2+-free Tyrode solution and the collagenase digestion buffer, creating the following five groups: (1) Control, unsupplemented; (2) Cr 1, 1 mM Cr added; (3) Cr 20, 20 mM Cr added; (4) Cr/Ino/Ado, 20 mM Cr, 5 mM Ino and 5 mM Ado added; (5) Ino/Ado, 5 mM Ino and 5 mM Ado added. Care was taken to readjust the pH of buffers to 7.2 after addition of the supplements.
The relative number of rod shaped and rounded cells were determined by transferring 2 ml of cell suspension to a 35 mm culture dish and counting three fields of view at 10× magnification. This method was preferred over using a hemocytometer, because in our hands, the cardiomyocytes did not easily distribute into the counting area of the hemocytometer due to their size and shape. Cells were either used fresh or incubated for up to 5 h in Dulbecco’s modified Eagles medium (DMEM, Sigma) in a 37°C 95%/5% O2/CO2 incubator supplemented with and without 1 mM Cr.
Biochemical Measurements
Total Cr content was determined according to [13], creatine kinase (CK) activity according to [14], citrate synthase according to [15]. Cr and enzyme activities were normalized to protein determined according to Lowry et al. [16]. CK isoenzyme distribution was determined with an automated electrophoresis system (CardioRep, Helena Laboratories, Beaumont, TX). ATP content was determined by high pressure liquid chromatography of perchloric acid (PCA) extracts (0.6 M PCA, neutralized with 5 M KOH) on a Partisil column with 0.16 M KH2PO4, 0.3 M KCl buffer, pH 6.25, flow rate 1.4 ml/min.
Measurement of Cell Shortening and Ca2+ Transients
Freshly isolated ventricular myocytes were continuously perfused in a heated (35 ± 1°C) chamber mounted on the stage of an inverted microscope (Zeiss Axiovert). The perfusion buffer contained (in mM): 137 NaCl, 5.4 KCl, 0.5 MgCl2, 10 HEPES, 5.5 glucose, 1.2 mM CaCl2 plus 0.5 mM probenecid. Only myocytes that were rod shaped with a clear striation pattern and were quiescent when unstimulated were included in the study reported here. Myocytes were electrically stimulated with a pulsar digital cell stimulator (Cell Microcontrols) at 0.5 Hz for 2 min, following by stepwise increase in pacing frequency to 1, 2, and 5 Hz for 1.5 min each. At 5 Hz, isoproterenol (Iso, 0.1 μM) was added for 2 min, and the pacing rate then increased to 10 Hz. Mechanical properties were assessed by a video edge detection system (IonOptix Corp.) in which contractility information was extracted continuously and in real-time from cell images by first digitizing the images and then analyzing the image intensity information to determine the absolute change in myocyte length. The cell contractility sampling rate was 240 Hz. Contractile performance and cell Ca2+ levels were recorded simultaneously. Ca2+ transients were measured in Fura 2-AM-loaded myocytes (1 mM, 15 min at room temperature in the dark, followed by three washes in perfusion buffer) using a dual excitation Hyperswitch (IonOptix Corp.) that allows for rapid (up to 250 ratio measurements per second) switching between excitation wavelengths of 340 and 380 nm. Emission was captured at 510 nm by a photomultiplier tube. Background fluorescence was recorded at four points adjacent to the myocyte. Using Ionwizard software (IonOptix, Corp.), both steady-state twitches and intracellular transients were averaged (~10 traces) and common kinetic and amplitude parameters determined [17].
Statistics
Data are presented as means ± SE. Biochemical comparisons between untreated and treated groups of cells were made with one-factor ANOVA; functional comparisons between groups were made using two-way ANOVA with repeated measures and Bonferroni posthoc analysis. P < 0.05 was considered significant.
Results
Freshly Prepared Mouse Cardiomyocytes Lost Low Molecular Metabolites but not Macromolecules
The Cr content ([Cr]) of cardiomyocytes freshly isolated using the standard isolation protocol was only ~27% of values found for intact hearts as reported from this laboratory for normal isolated Langendorff perfused mouse hearts [18] and as reported by Weiss and colleagues [19] for normal mouse hearts in vivo (Table 1; Fig. 2).
Table 1.
Cr content and mitochondrial and cytosolic enzyme activities of isolated heart tissue, freshly isolated cardiomyocytes using control conditions and short-term culture of myocytes in the presence and absence of Cr (1 mM)
| Isolated heart tissue | Fresh myocytes | Cultured for 5 h
|
||
|---|---|---|---|---|
| −Cr | +1 mM Cr | |||
| Cr (nmol/mg protein) | 77 ± 3 | 21 ± 2.2 | 22 ± 0.6 | 25 ± 1.1 |
| Total CK (IU/mg protein) | 6.1 ± 0.5 | 4.5 ± 0.2 | 4.3 ± 0.2 | 4.5 ± 0.4 |
| CK isoenzymes | ||||
| sMtCK (% of total CK) | 36 ± 2 | 38 ± 6 | 38 ± 2.6 | 35 ± 3.2 |
| CK-MM (% of total CK) | 58 ± 4 | 56 ± 5 | 56 ± 2.4 | 59 ± 3.1 |
| CS (mIU/mg protein) | 912 ± 44 | 740 ± 98 | 862 ± 32 | 950 ± 52 |
Cr creatine, CK creatine kinase, sMtCK sarcomeric mitochondrial CK, CK-MM main cytosolic CK isozyme, CS citrate synthase. Mean ± SE, n = 5 for freshly isolated myocytes and n = 9 for cultured myocytes without Cr (−Cr) and with 1 mM Cr. Data for intact heart are from [34]
Fig. 2.
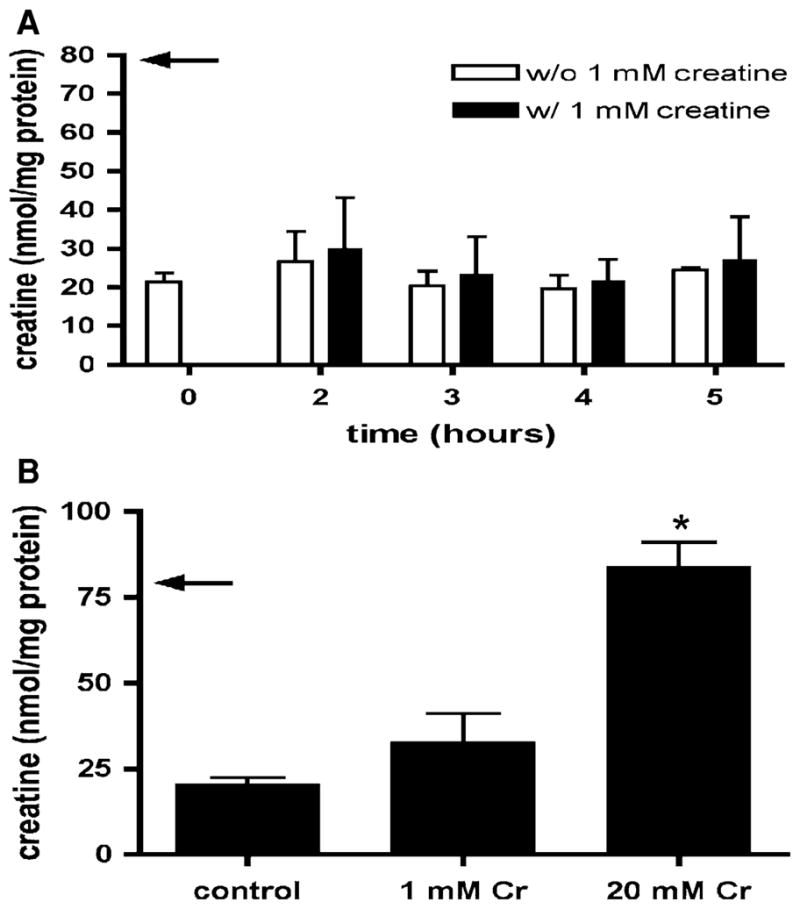
a Cr content of isolated mouse cardiomyocytes during incubation in culture medium with and without 1 mM Cr for up to 5 h. The arrow indicates the value for isolated perfused heart tissue (from [34]). Over the time frame of this experiment isolated cardiomyocytes were unable to increase their intracellular Cr. Means ± SE from three independent experiments. b Cr content of isolated cardiomyocytes from un-supplemented isolation buffers, and buffers supplemented with Cr 1 and Cr 20. Addition of 20 mM Cr is sufficient to maintain normal Cr levels (arrow indicates value for isolated perfused heart tissue (from [34])). Means ± SE from 5 to 7 independent myocyte preparations. *P < 0.001 versus without Cr and 1 mM Cr, one-way ANOVA
To determine whether the standard isolation procedure also led to loss of ATP from cardiomyocytes, we measured the ATP content ([ATP]) in freshly isolated cardiomyocytes. ATP content was 13.3 ± 2.4 nmol/mg protein, which is only 44% of the ATP content known to be present in adult mouse heart tissue [20] (Fig. 3).
Fig. 3.
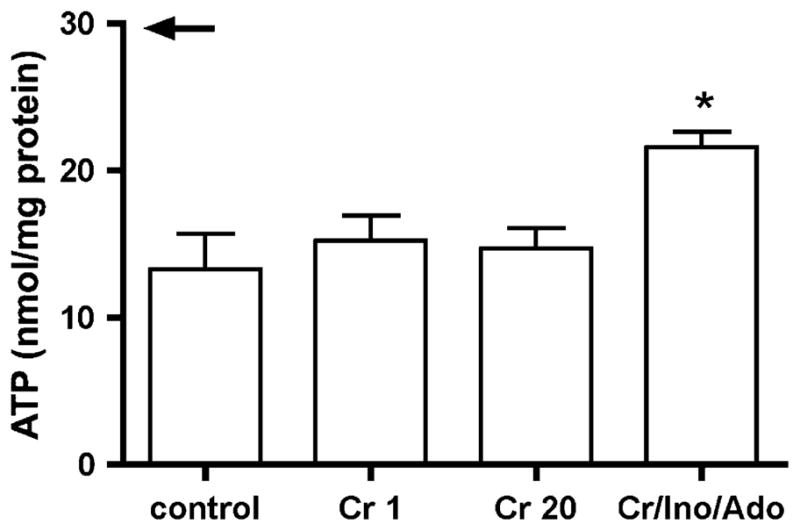
ATP content of cardiomyocytes prepared in the absence or presence of Cr 1, Cr 20 or Cr/Ino/Ado. Supplying Cr/Ino/Ado protects the intracellular ATP pool (arrow indicates the value for isolated perfused heart tissue (from Ref. [22])). Means ± SE from 3 to 4 different cell preparations. *P < 0.05 versus without creatine, one-way ANOVA
In contrast to these large changes in total [Cr] and [ATP], enzyme markers of mitochondria and the cytosol were within 70–75% of values for whole heart tissue (Table 1). This includes the sarcomeric mitochondrial CK, which functions as a channel to transfer the phosphoryl group from ATP made by oxidative phosphorylation to Cr in the inner-membrane space, the citric acid cycle enzyme citrate synthase and the major cytosolic CK isozyme, CK–MM. The relatively moderate decreases in these enzyme activities can be accounted for by the presence of non-viable cells and cell fragments; the percentage of cells that were viable and contained rod-like sarcomeres was 70–75%.
Cr Content was not Replenished in Culture but was Preserved When Using Cr-Supplemented Isolation Buffers
To increase the Cr content, we first cultured isolated cardiomyocytes in medium supplemented with a physiological (plasma) concentration of Cr (1 mM). Over a time period of 5 h [Cr] did not increase (Fig. 2a). We then tested whether adding either 1 or 20 mM Cr to the digestion buffers would prevent the loss of Cr from the cells. Adding 1 mM Cr to the calcium-free Tyrode solution and the collagenase buffer tended to increase [Cr], but adding 20 mM Cr prevented the loss of [Cr], preserving normal [Cr] (Fig. 2b). We chose 20 mM Cr for this test because this approximates the [Cr] lost from the cells (total [Cr] in mouse myocardium is ~25 mM [21]).
Purine Nucleoside Supplementation Protects the Intracellular ATP Pool
It is well known that in myocardial ischemia, loss of ATP occurs as a result of loss of purine nucleosides secondary to increased ATP demand relative to supply. To test whether loss of ATP in mouse cardiomyocytes could be blunted by reducing the outward concentration gradient for diffusible purines during the isolation procedure, we supplemented the Ca2+-free Tyrode solution and the collagenase solution with 5 mM Ino and 5 mM Ado. Ado and Ino were chosen since they are the major purine nucleobases formed in the classic pathway for ATP degradation in the heart (Fig. 1). The concentrations of 5 mM were chosen based on the fact that about half of the ATP pool (10 mM) was lost. Supplementation with nucleosides led to a significant improvement in ATP content, from 44 to 74% of the normal ATP content in intact cardiac tissue (Fig. 3). Supplementing the isolation buffers with Cr (Cr 1, Cr 20) and thereby raising the Cr content in myocytes (Fig. 2b) did not affect ATP content (Fig. 3).
Fig. 1.
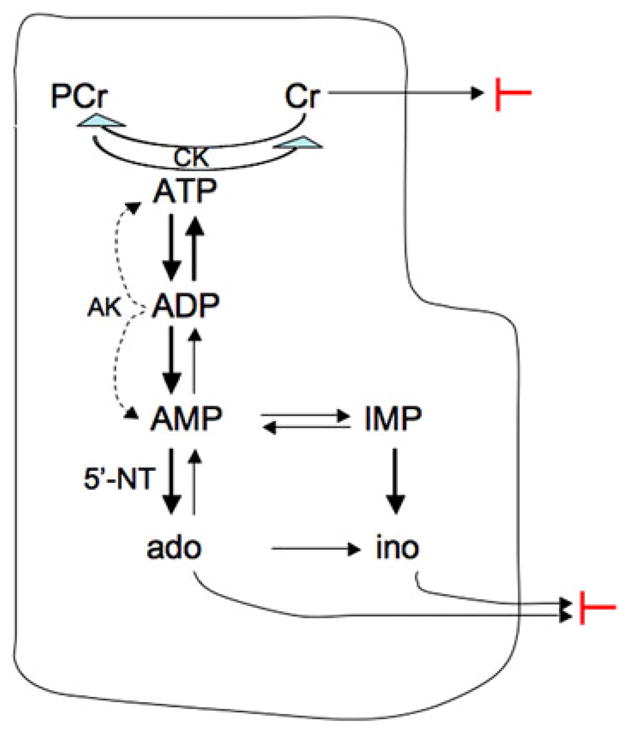
Diagram showing the degradation of ATP illustrating the loss of nucleosides and creatine. The phosphocreatine (PCr) and creatine (Cr) system is the major energetic reserve system of the heart. When the increased utilization of ATP leads to the hydrolysis of PCr to Cr, the uncharged Cr accumulates and may be lost from the myocyte following its concentration gradient. Without adequate ATP replenishment via oxidative phosphorylation and via the creatine kinase (CK) reaction, ATP may be resupplied via the adenylate kinase (AK) reaction: 2ADP → 1ATP + 1AMP. This increase in AMP activates AMP-dependent 5′-nucleotidase (5′-NT) that leads to the conversion of AMP to Ado. The uncharged Ado is rapidly converted to Ino, and both Ado and Ino can cross the plasma membrane following their concentration gradients. A similar mechanism converts IMP to Ino (for detailed description see [18]). The two red blocks indicate where supplementation of the isolation buffers inhibits the loss of Cr and the loss of nucleosides
Functional Consequences of Preserving Cr and ATP
The Cr/CK system is the major energy reserve system of the heart, allowing the cardiomyocyte to survive transient stresses such as acute O2 deprivation as occurs during cardiomyocyte preparation [22]. We tested whether the loss of Cr may be one reason for the low viability of freshly isolated mouse cardiomyocytes. Cell viability was higher for cells isolated using buffers containing supplemental Cr (~90% in Cr 20 and Cr/Ino/Ado groups) than for cells isolated with no Cr (70–75% in control and Ino/Ado groups).
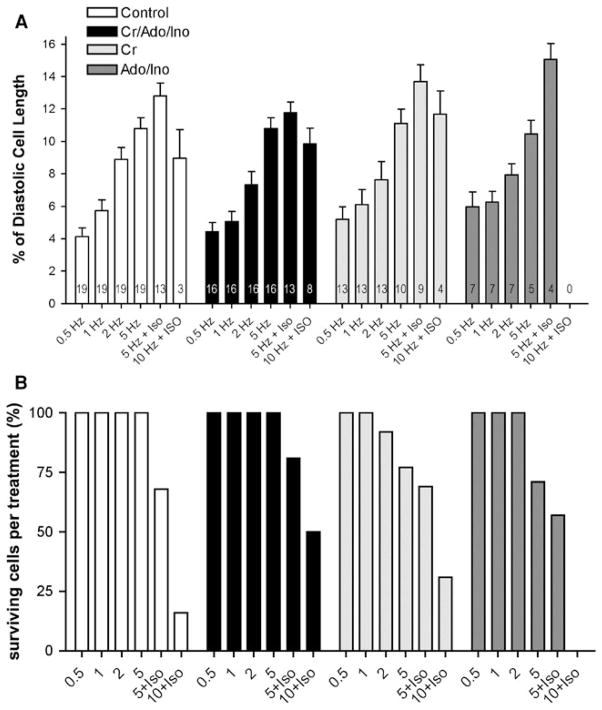
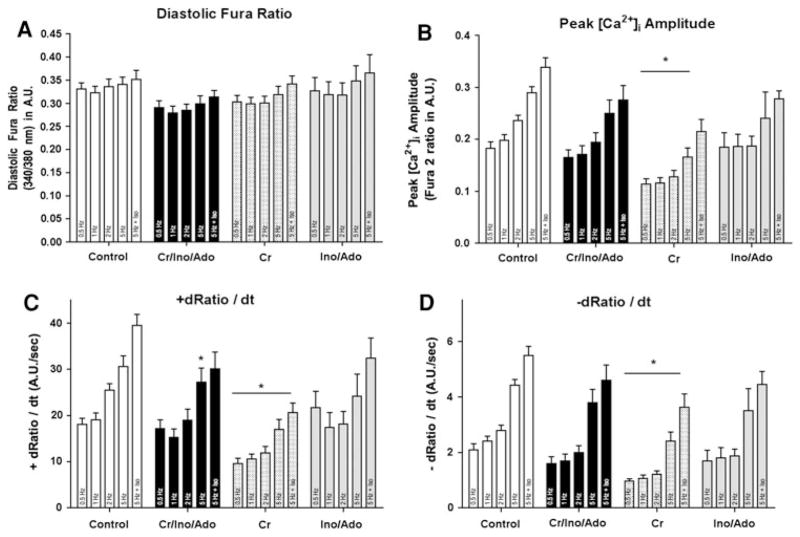
Because the Cr/CK system has been shown to be important in tolerating acute increases in work [23], we then tested whether myocytes with better-preserved Cr levels could sustain being paced at rates approximating normal in vivo heart rates (600 bpm) and to being stimulated with β-adrenergic agents. Myocytes were paced stepwise from 0.5 up to 10 Hz. At 5 and 10 Hz myocytes were additionally challenged with Iso. This approach approximates measuring contractile reserve in the intact heart. Myocytes from all groups responded to a stepwise increase in pacing frequency with a progressive increase in cell shortening. There were no differences in contractile function among the groups at low pacing rates of 0.5, 1, 2, and even 5 Hz. However, only 16% of control myocytes prepared with standard solutions and 0% of the Ino/Ado-treated cells (Fig. 4b) were able to sustain pacing rates of 10 Hz in the presence of Iso. In contrast, 30% of the Cr 20 and 50% of the Cr/Ino/Ado-treated cells could be paced at 10 Hz in the presence of Iso. Myocytes that were included for data analysis at 10 Hz + Iso properly responded to pacing at that frequency and did not show any extraneous contractions or hyper contractions. To test whether the improved performance of Cr 20 and Cr/Ino/Ado-treated cells is based on changes in the Ca2+ transient, cells were loaded with Fura-2 and subjected to the same protocol used to assess contractile function. Figure 5 shows that Cr supplementation is sufficient to maintain low Ca2+ transient amplitude. In addition, maximum rates of the cytosolic Ca2+ transient rise and decline were lower than for cells in the other groups.
Fig. 4.
a Cell shortening (in percent of diastolic length) during the pacing protocol for all groups. Cell shortening changes are comparable between the four groups during the pacing protocol, and all groups show a positive cell shortening–frequency relationship (positive staircase). The number of cells that tolerated the entire pacing protocol and were analyzed is shown in the bars. b Preservation of ATP and Cr pools leads to improved stress endurance. Shown are the % of cells that could be paced at 10 Hz and able to sustain inotropic challenge. 50% of the cells in the Cr/Ino/Ado and 30% in the Cr 20 could be paced at 10 Hz and are able to sustain additional inotropic challenge. Only 16% of the control group and none of the Ino/Ado group were able to sustain this challenge
Fig. 5.
Ca2+ transient data obtained from control myocytes and myocytes prepared with the supplements Cr 20, Cr/Ino/Ado, and Ino/Ado. Pacing frequency was increased stepwise from 0.5 up to 5 Hz. At 5 Hz Iso was added. a Diastolic fura ratio, b Peak [Ca2+]i amplitude, c +dRatio/dt, measures the maximum rate of the Ca2+ transient rise, and d −dRatio/dt, measures the maximum rate of the Ca2+ transient decline. Means ± SE of 2–4 independent myocyte preparations per condition, from which 4–6 myocytes per condition were analyzed. *P < 0.01 two-way ANOVA
Resting sarcomere length was significantly increased only in Cr 20 treated cells (Table 2). Diastolic myocyte length during pacing at 0.5 Hz showed a trend towards an increase in all supplemented groups, but the differences did not reach significance (Table 2). This suggests that maintaining near normal ATP and Cr pools likely has no effect on the actomyosin cross-bridge. Instead, taken together with the data for the Ca2+ transient, maintaining near normal ATP and Cr likely contributes to normal Ca2+ regulation in the cells.
Table 2.
Comparison of cardiomyocyte resting sarcomere length and cell length in 0.5 Hz paced cells in un-supplemented control and supplemented cell preparations
| Resting sarcomere length (lm) | n | Diastolic cell length (lm) | n | |
|---|---|---|---|---|
| Control | 1.776 ± 0.011 | 20 | 121.7 ± 16 | 19 |
| Cr 20 | 1.808 ± 0.007* | 46 | 132.6 ± 16 | 16 |
| Ado/Ino | 1.790 ± 0.008 | 24 | 128.0 ± 16 | 13 |
| Cr/Ado/Ino | 1.768 ± 0.007 | 56 | 127.3 ± 17 | 16 |
Sarcomere and diastolic cell length were determined in separate cell cohorts. Cells from three different preparations except for Ado/Ino group with two preparations (Cr, Ado, and Ino). Means ± SE,
P < 0.01, one-way ANOVA
Discussion
Here we describe an isolation procedure for adult mouse cardiomyocytes that protects against loss of Cr and ATP from the cell. Physiological consequences of maintaining higher Cr and ATP levels in isolated myocytes include: (1) improved viability, (2) ability to maintain a low diastolic Ca2+, and (3) ability to sustain high pacing rates in the presence of β-adrenergic challenge.
On Cr
Our results show that the transient ischemia that occurs during mouse cardiomyocyte isolation leads to net Cr loss from the cells and that supplying high concentrations of Cr to the digestion buffers during the isolation procedure preserved normal [Cr]. Cr, an uncharged low molecular weight β-amino acid, is not synthesized in myocytes but is transported across the sarcolemma by a saturable Cr transporter (CrT). The amount of CrT on the plasma membrane is regulated in two ways. First, it is regulated by the plasma [Cr], with less CrT protein on the membrane when plasma [Cr] is high and vice versa [24]. Second, trafficking of CrT to the plasma membrane is mediated by serum and glucocorticoid-inducible kinase and is regulated by stress, insulin, growth factors and mTOR [25]. Our observations all suggest that the CrT was functionally damaged by cell isolation using standard Cr-free solutions. First, the observation that supplying Cr to cells cultured for 5 h did not increase intracellular [Cr] suggests not only that the CrT was functionally damaged but that the stresses of isolation and culturing did not, as might be expected, lead to repletion of CrT on the plasma membrane. Second, supplying concentrations of Cr approximating plasma levels (1 mM) to the digestion buffers did not lead to preserved [Cr]. Instead, high concentrations were needed to maintain normal [Cr]. The opposite was expected: more CrT should be on the membrane when extracellular Cr is low. Finally, the observation that CK activity and isozyme distribution were preserved even in control myocytes rules out the possibility that loss of Cr occurred because PCr, the chemical trap of Cr, was not made. Taken together, these results are consistent with a simple mass action effect where supplying high concentrations of extracellular Cr diminishes an outward Cr diffusion gradient transiently created during the digestion procedure.
On Consequences of Maintaining Normal Cr in Isolated Adult Cardiomyocytes
Here we present evidence that the preservation of Cr levels in cardiomyocytes not only increases the fraction of cells that are viable but also enhances the fraction of cells able to increase contractile performance due to increased pacing rates and inotropic stimulation. Supplementation of digestion buffers with Cr increased cell viability to 90% compared to 70–75% in cell preparations isolated without Cr. Importantly, 30–50% of cells isolated in the presence of Cr were able to sustain pacing rates of 10 Hz in the presence of Iso whereas only a few Cr-depleted cells (16% of control cells and 0% of Ino/Ado supplemented cells) could be paced at 10 Hz with inotropic challenge. While the improvements in cell viability and contractile reserve are substantial, they are not complete, suggesting that additional manipulations of the isolation procedure are required to further improve the quality of adult mouse cardiomyocyte preparations.
The capacity of the CK system can be estimated by the product of CK activity (Vmax) and [Cr]. Thus, the loss of 75% of Cr with near normal CK activity leads to a decrease in energy reserve via CK of ~75%. Our results suggest that loss of energy reserve via the Cr/CK system contributes to loss of contractile reserve of isolated adult mouse cardiomyocytes as it does in intact hearts [7, 26]. Loss of contractile reserve may be one of the reasons why most published protocols use non-physiologic slow pacing rates in isolated cardiomyocyte experiments. Several mechanisms may contribute to the loss of contractile reserve in standard myocyte preparations. It is known that CK functions as a energy circuit linking mitochondrial ATP production and sites of ATP utilization such as KATP channels and SERCA [27] and also facilitates phosphoryl transfer in the vicinity of these ATPases [11]. Importantly, the Ca2+ transient results presented here also suggest that maintaining normal CK phosphotransfer capacity contributes to maintaining low diastolic [Ca2+].
On Purine Supply
It is well known that during conditions of energy supply imbalance in cardiomyocytes, ATP is degraded as shown in Fig. 1. During the isolation procedure for cardiomyocytes, ATP synthesis falls below ATP demand, leading to increases in ADP and AMP concentrations, which in turn activates 5′-nucleotidase (5′-NT, [28]), leading to Ado and Ino formation. These nucleosides cross the plasma membrane following their concentration gradients, leading to loss of the entire purine pool. Increasing the extracellular concentration of Ino and Ado during the isolation procedure diminishes these concentration gradients, slowing the degradation of AMP and thus protecting the ATP pool. Unlike the nearly complete protection of the Cr pool observed for cells supplied with Cr, however, supplying Ado and Ino did not fully protect against loss of ATP. This may be due to the loss of functional endothelial cells during isolation. Purine metabolism in the heart in complex, and requires intact endothelial cells as well as intact myocytes. Thus, as the endothelial cells are destroyed during the isolation procedure, any salutary effect of Ado and Ino on purine metabolism would diminish and limit preservation of the ATP pool.
Ado and Ino may function in other ways as well. The role of Ado in preconditioning the heart is well known [29]. Part of the protective effect of Ado observed here may also include Ado receptor stimulation which via the activation of protein kinase C leads to mitochondrial KATP channel opening and thus decreased ATP utilization [18, 30, 31]. Although Ino, the major nucleoside that accumulates in the ischemic heart, is not metabolized to IMP in the myocyte, supplying Ino has been shown to delay the loss of ATP during hypoxic insult [18] and ischemic myocardium [32] and to augment the rate of glycolysis in ischemic rabbit myocardium [32].
On a Positive Staircase
There have been only few reports in the literature showing the cell shortening–frequency relationships of isolated mouse cardiomyocytes [3, 12, 33]. Wolska et al. report a negative relationship up to 2 Hz, the maximum frequency used. Lim et al. [12] and Tiemann et al. [33] compared different age groups of mice and found a negative relationship at low frequencies up to 2 Hz as well. However, above 2 Hz both papers show a positive relationship. In contrast to these reports, we have found a positive cell length–frequency relationship in myocytes paced between 0.5 and 5 Hz, which was further augmented by additional β-adrenergic stimulation.
In summary, while we have not yet provided a perfect method to isolate adult mouse cardiomyocytes, the supplementation method described here substantially improves the yield, viability and performance of mouse cardiomyocytes.
Acknowledgments
This project was supported by grants NIH HL52320 to JSI and UM, NIH HL 075619 to JSI, NIH HL 080127 to UM, and 0930260N AHA to IP and 5 P20 RR15555-10 (subproject 6) to IP from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Contributor Information
Ilka Pinz, Email: pinzi@mmc.org, NMR Laboratory for Physiological Chemistry, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Center for Molecular Medicine, Maine Medical Center Research Institute, 81 Research Drive, Scarborough, ME 04074, USA.
Ming Zhu, NMR Laboratory for Physiological Chemistry, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Ulrike Mende, NMR Laboratory for Physiological Chemistry, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Joanne S. Ingwall, NMR Laboratory for Physiological Chemistry, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, et al. Adult rat ventricular myocytes cultured in defined medium: Phenotype and electromechanical function. American Journal of Physiology. 1993;265:H747–H754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods in Molecular Biology. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 3.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. American Journal of Physiology. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: Methods for cellular genetic physiology. American Journal of Physiology Heart and Circulatory Physiology. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer S, Beer M, Landschutz W, Sandstede J, Seyfarth T, Lipke C, et al. Absolute quantification of high energy phosphate metabolites in normal, hypertrophied and failing human myocardium. Magma. 2000;11:73–74. doi: 10.1007/BF02678501. [DOI] [PubMed] [Google Scholar]
- 6.Starling RC, Hammer DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Molecular and Cellular Biochemistry. 1998;180:171–177. [PubMed] [Google Scholar]
- 7.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. American Journal of Physiology. 1996;270:H1207–H1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 9.Del Monte F, Hajjar RJ. Intracellular devastation in heart failure. Heart Failure Reviews. 2008;13:151–162. doi: 10.1007/s10741-007-9071-9. [DOI] [PubMed] [Google Scholar]
- 10.Tian R, Nascimben L, Kaddurah-Daouk R, Ingwall JS. Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. Journal of Molecular and Cellular Cardiology. 1996;28:755–765. doi: 10.1006/jmcc.1996.0070. [DOI] [PubMed] [Google Scholar]
- 11.Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, O’Gorman E, et al. Some new aspects of creatine kinase (CK): Compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors. 1998;8:229–234. doi: 10.1002/biof.5520080310. [DOI] [PubMed] [Google Scholar]
- 12.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. Journal of Molecular and Cellular Cardiology. 2000;32:2075–2082. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- 13.Kammermeier H. Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Analytical Biochemistry. 1973;56:341–345. doi: 10.1016/0003-2697(73)90199-1. [DOI] [PubMed] [Google Scholar]
- 14.Rosalki SB. An improved procedure for serum creatine phosphokinase determination. Journal of Laboratory and Clinical Medicine. 1967;69:696–705. [PubMed] [Google Scholar]
- 15.Srere PA. The enzymology of the formation and breakdown of citrate. Advances in Enzymology and Related Areas of Molecular Biology. 1975;43:57–101. doi: 10.1002/9780470122884.ch2. [DOI] [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Zhu M, Gach AA, Liu G, Xu X, Lim CC, Zhang JX, et al. Enhanced calcium cycling and contractile function in transgenic hearts expressing constitutively active G alpha o* protein. American Journal of Physiology Heart and Circulatory Physiology. 2008;294:H1335–H1347. doi: 10.1152/ajpheart.00584.2007. [DOI] [PubMed] [Google Scholar]
- 18.Ingwall JS. ATP and the heart. Norwell: Kluwer Academic Publisher; 2002. [Google Scholar]
- 19.Gupta A, Chacko VP, Weiss RG. Abnormal energetics and ATP depletion in pressure-overload mouse hearts: In vivo high-energy phosphate concentration measures by non-invasive magnetic resonance. American Journal of Physiology Heart and Circulatory Physiology. 2009;297:H59–H64. doi: 10.1152/ajpheart.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. American Journal of Physiology Heart and Circulatory Physiology. 2000;279:H2218–H2224. doi: 10.1152/ajpheart.2000.279.5.H2218. [DOI] [PubMed] [Google Scholar]
- 21.ten Hove M, Makinen K, Sebag-Montefiore L, Hunyor I, Fischer A, Wallis J, et al. Creatine uptake in mouse hearts with genetically altered creatine levels. Journal of Molecular and Cellular Cardiology. 2008;45:453–459. doi: 10.1016/j.yjmcc.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circulation Research. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 23.Saupe KW, Spindler M, Hopkins JC, Shen W, Ingwall JS. Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. Journal of Biological Chemistry. 2000;275:19742–19746. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 24.Boehm E, Chan S, Monfared M, Wallimann T, Clarke K, Neubauer S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. American Journal of Physiology Endocrinology and Metabolism. 2003;284:E399–E406. doi: 10.1152/ajpendo.00259.2002. [DOI] [PubMed] [Google Scholar]
- 25.Strutz-Seebohm N, Shojaiefard M, Christie D, Tavare J, Seebohm G, Lang F. PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cellular Physiology and Biochemistry. 2007;20:729–734. doi: 10.1159/000110433. [DOI] [PubMed] [Google Scholar]
- 26.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, et al. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- 27.Dzeja PPCS, Terzic A. Integration of adenylate kinase, glycolytic and glycogenolytic circuits in cellular energetics. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- 28.Bak MI, Ingwall JS. Regulation of cardiac AMP-specific 5′-nucleotidase during ischemia mediates ATP resynthesis on reflow. American Journal of Physiology. 1998;274:C992–C1001. doi: 10.1152/ajpcell.1998.274.4.C992. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosio G, Jacobus WE, Mitchell MC, Litt MR, Becker LC. Effects of ATP precursors on ATP and free ADP content and functional recovery of postischemic hearts. American Journal of Physiology. 1989;256:H560–H566. doi: 10.1152/ajpheart.1989.256.2.H560. [DOI] [PubMed] [Google Scholar]
- 30.Jennings RB, Sebbag L, Schwartz LM, Crago MS, Reimer KA. Metabolism of preconditioned myocardium: Effect of loss and reinstatement of cardioprotection. Journal of Molecular and Cellular Cardiology. 2001;33:1571–1588. doi: 10.1006/jmcc.2001.1425. [DOI] [PubMed] [Google Scholar]
- 31.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circulation Research. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 32.Lewandowski ED, Johnston DL, Roberts R. Effects of inosine on glycolysis and contracture during myocardial ischemia. Circulation Research. 1991;68:578–587. doi: 10.1161/01.res.68.2.578. [DOI] [PubMed] [Google Scholar]
- 33.Tiemann K, Weyer D, Djoufack PC, Ghanem A, Lewalter T, Dreiner U, et al. Increasing myocardial contraction and blood pressure in C57BL/6 mice during early postnatal development. American Journal of Physiology Heart and Circulatory Physiology. 2003;284:H464–H474. doi: 10.1152/ajpheart.00540.2002. [DOI] [PubMed] [Google Scholar]
- 34.Pinz I, Ostroy SE, Hoyer K, Osinska H, Robbins J, Molkentin JD, et al. Calcineurin-induced energy wasting in a transgenic mouse model of heart failure. American Journal of Physiology Heart and Circulatory Physiology. 2008;294:H1459–H1466. doi: 10.1152/ajpheart.00911.2007. [DOI] [PubMed] [Google Scholar]