Abstract
Electrochemical DNA (E-DNA) sensors, which are rapid, reagentless and readily integrated into microelectronics and microfluidics, appear a promising alternative to optical methods for the detection of specific nucleic acid sequences. Keeping with this, a large number of distinct E-DNA architectures have been reported to date. Most, however, suffer from one or more drawbacks, including low signal gain (the relative signal change in the presence of complementary target), signal-off behavior (target binding reduces the signaling current, leading to poor gain and raising the possibility that sensor fouling or degradation can lead to false positives) or instability (degradation of the sensor during regeneration or storage). To remedy these problems, we report here the development of a signal-on E-DNA architecture that achieves both high signal gain and good stability. This new sensor employs a commercially synthesized, asymmetric hairpin DNA as its recognition and signaling probe, the shorter arm of which is labeled with a redox reporting methylene blue at its free end. Unlike all prior E-DNA architectures, in which the recognition probe is attached via a terminal functional group to its underlying electrode, the probe employed here is affixed using a thiol group located internally, in the turn region of the hairpin. Hybridization of a target DNA to the longer arm of the hairpin displaces the shorter arm, allowing the reporter to approach the electrode surface and transfer electrons. The resulting device achieves signal increases of ~800% at saturating target, a detection limit of just 50 pM and ready discrimination between perfectly matched sequences and those with single nucleotide polymorphisms. Moreover, because the hairpin probe is a single, fully covalent strand of DNA, it is robust to the high stringency washes necessary to remove the target, and thus these devices are fully reusable.
Keywords: Theranostics, Pathogen Detection, Molecular Beacon, SNP, genotyping, genosensor, microarray
Introduction
Electrochemical DNA biosensors (E-DNA) appear a promising alternative to optical sensors for the specific detection of oligonucleotide sequences1,2,3. These devices are composed of a redox-reporter-modified DNA probe immobilized on a gold electrode. Hybridization-linked changes in the flexibility of this probe (due to specific conformational changes1,4,5 or an increase in the amount of double-helix6) alter the rate with which electrons are transferred from the redox reporter, leading to a readily detectable as a change in Faradaic current upon voltammetric interrogation7,8,. Because E-DNA sensors are driven by electrochemistry, rather than optical detection methods, they can be integrated into microfluidic devices, powered by inexpensive, hand-held electronics, and easily multiplexed9,10,11. Moreover, because their signaling is predicated on a binding-induced change in the physical properties of the probe DNA, rather than to adsorption of analytes to the sensor surface, E-DNA sensors are relatively insensitive to the non-specific adsorption of interferants and are selective enough to deploy directly in complex clinical and environmental samples, such as blood or soil extracts12,13. E-DNA sensors thus appear well suited for point of care medical diagnostics, as well as portable analysis systems for forensics and food quality control12.
Although E-DNA sensors have a wide variety of positive attributes, many of the diverse E-DNA probe architectures described to date operate in a signal-off fashion, meaning that the measured current decreases as the concentration of analyte DNA increases14. For example, first generation E-DNA sensors employ a stem-loop architecture1,14 such that, when a complementary target oligonucleotide is introduced, hybridization causes the stem to open. This moves the redox reporter further from the electrode surface, reducing electron transfer. This signal-off mechanism significantly limits the gain of the sensor: the maximum possible signal change (the change in current between a blank measurement and saturating target concentrations) can never exceed a 100% (i.e., the current cannot decrease below zero). A second limitation of signal-off sensors is that probe degradation can be misinterpreted as a authentic response to target. Signal-on sensors, in contrast, can achieve much improved signaling; as the background current observed in the absence of target is reduced, the gain of such a sensor, at least in theory, increases without limit15. Thus motivated, we and others have explored a number of signal-on E-DNA architectures, including a DNA pseudoknot5, a hybridization-based, double-stranded sensor13, a triblock structure16, an inverted stem-loop17, and a traditional E-DNA sensor probed at new frequencies7. Meanwhile, other groups have developed high-gain electrochemical sandwich assays18,19,20,21. But each of those devices has at least one drawback that would limit their implementation in inexpensive medical diagnostics, including poor stability and lack of reusability13, limited gain4,5 and/or poor signal-to-noise7.
Among the highest gain E-DNA sensors reported to date is a signal-on architecture developed by Xiao et al. that employs a strand-displacement signaling mechanism13. This architecture makes use of a double-stranded probe comprising a capture oligonucleotide affixed to a gold electrode, and a longer MB-labeled signaling DNA strand hybridized to that molecule. Hybridization of the target oligonucleotide displaces the methylene-blue modified terminus of the signaling strand, allowing the redox tag to approach the electrode where it can exchange electrons. This leads to a strongly enhanced signaling current upon target binding. The double-stranded sensor achieves a detection limit in the high femtomolar range13 and more than 700% signal gain. Despite these advantages, this architecture suffers from a potentially important drawback: because its sensing probe is held together by hybridization, the signaling strand is lost when the sensor is washed or stored for long durations. At the time, modifications that could create fully covalent, internally-affixed double-stranded probes were not yet available. Here we describe a new, commercially-available linker that supports the internal attachment of a DNA probe to an electrode. We have used this chemistry to fabricate a fully covalent version of the earlier signal-on E-DNA architecture that, in contrast, is reusable and far more stable than the original design.
Experimental Section
Key Materials
All of the oligonucleotides were synthesized by Biosearch Technologies (Novato, CA), where they were purified by HPLC and used as received. Methylene blue, the redox reporter, was attached to the 5'-end of each probe molecule using either a hydrazone linker or an ester (Fig S1, in Supp. Info.). The probe molecules employed in our sensors were stored dry at −20°C until the day of their use. Supermix iQ PCR mix was obtained from Bio-Rad (Hercules, California) and stored at −20°C until each experiment.
ForkedThiol17
5'MB-AATAAAACGCCGATCCA-3'-T(C12-SH)-5'-TGGATCGGCGTTTTATTCTTGTTCAGATATTCAA-3'
ForkedThiol15
5'MB-TAAAACGCCGATCCA-3'-T(C12-SH)-5'-TGGATCGGCGTTTTATTCTTGTTCAGATATTCAA-3'
Perfectly matched 34 nucleotide target DNA sequence
5’-TTGAATATCTGAACAAGAATAAAACGCCGATCCA-3’
Unlabeled nucleotides were prepared by Sigma Aldrich (St Louis, MO) and isolated by reverse phase cartridge purification. They were dissolved in phosphate buffered saline, pH 7.4, and their concentrations verified with a NanoDrop spectrophotometer. Stock solutions were stored at 100 µM in their original packaging.
Sensor Preparation
Gold disk electrodes were polished using a slurry of 0.05 micron alumina in water on a standard polishing cloth. They were then subjected to a series of electrochemical cleaning steps as described previously2. While the electrochemical cleaning procedure was running, the probe DNA was reduced with four equivalents of 10 mM TCEP and stored in the dark at 4°C for at least one hour. The probe DNA was diluted with phosphate buffered saline (PBS), to either 100 nM, 250 nM, or 1 µM and then, each electrode was rinsed with deionized water, dried, and then immersed in 200 µL of the resulting solution for five minutes. Immediately following this, the electrodes were rinsed, dried and stored overnight in an aqueous solution of 2 mM mercaptohexanol. For further details we recommend several papers previously published by our group2,22,23,24.
In order to achieve good sensor-to-sensor reproducibility, it is imperative to use DNA probes freshly dissolved in buffer. When the probe molecules are dissolved in buffer, they quickly aggregate. Gel electrophoresis showed that these aged probe molecules aggregate into dimers, trimers, and higher order clusters (Figure S2 in Supp. Info.). Sensors made with an aged probe solution, DNA that was reconstituted and then stored in a freezer for two weeks or more before the sensor preparation procedure, exhibit little or no change in signal when target is added.
Electrochemical Measurements
The reported electrochemical experiments were conducted using square wave voltammetry on CH Instruments 630B, 650C, and 660D potentiostats (Austin, TX). Each of those instruments was connected to a CH Instruments 684 multiplexer. Unless otherwise stated, square wave voltammetry measurements were made at 60Hz with an amplitude of 25 mV and a step width of 4mV. All of the experiments were conducted with a platinum wire or platinum disk counter electrode and an Ag/AgCl reference in phosphate buffered saline, pH 7.4 with 200 µM MgCl2 at room temperature.
With our electrochemical instrumentation, the signal to noise ratio was empirically determined to be optimal at 60 Hz, but the signal gain is up to twice as high at higher frequencies. An instrument with better high frequency filters may benefit from making measurements in the vicinity of 200 to 600 Hz.
Sensor Tests with Synthetic Targets
A set of three electrodes was immersed in a shot glass with either 15 mL of phosphate buffered saline or 2 mL of PCR mix. Before data acquisition, several at least six 60 Hz square wave test scans were performed. To acquire data, a macro experiment was set to record 60 Hz square wave measurements every minute for ten minutes. After the first set of data was collected, the target DNA was added, and the solution was gently mixed by pipetting.
Sensor Tests below 5nM Analyte
Three gold disk electrodes were immersed in 750 mL of phosphate buffered saline enriched with 0.2 mM MgCl2. Synthetic target DNA was added after the first measurement, and on the hour every hour thereafter. The mixture was stirred continuously with a magnetic bar throughout the measurement period. For these experiments, the sensors had a particularly low surface coverage of probe DNA molecules. During the sensor preparation procedure, the gold disk electrodes were immersed in a 100 nM probe solution for only five minutes.
Safety Considerations
The primary hazards of this work include concentrated sulfuric acid and dimethyl sulfoxide. All experiments with these reagents were performed in a fume hood. The researchers wore eye protection and gloves when handling both substances.
Results and Discussion
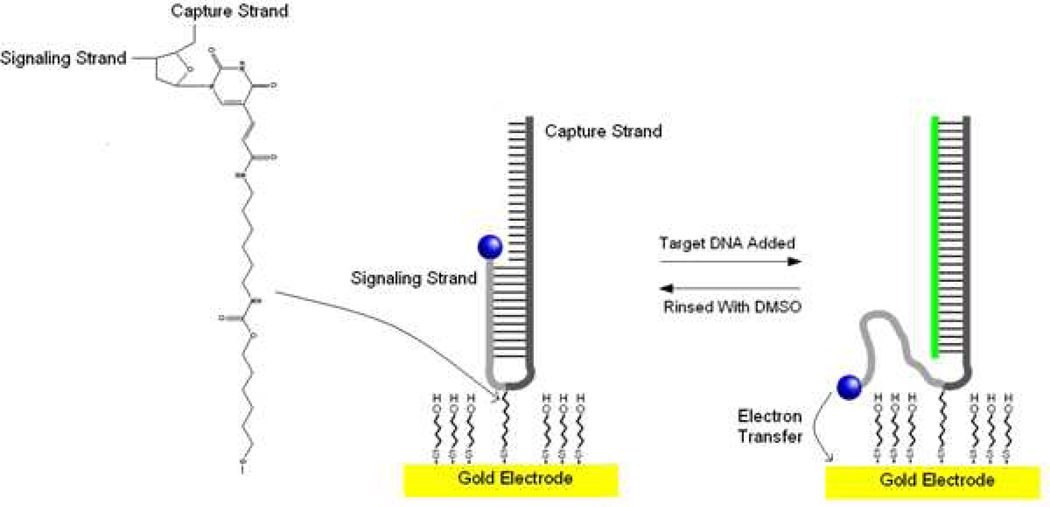
Our objective was to develop a reusable, high signal gain, fully covalent E-DNA sensor. To do so, we designed a commercially available, asymmetric hairpin probe, the shorter arm of which (the “signaling strand” is modified at its 5’-end with a methylene blue redox reporter (Fig. 1a). The two arms of the hairpin are joined together by a “T-linker,” a thymidine nucleotide modified at its C5 position with a 12-atom chain terminating in a thiol (Fig. 1b). This thiol anchors the probe onto a gold electrode via a site on the middle of its turn region via the formation of a mixed monolayer with mercaptohexanol, which is used as a dilutent to control probe packing density and to partially passivate the electrode surface. In the absence of a complementary target, the two strands of the probe form rigid, double-helical DNA, which limits the extent to which the attached methylene blue can approach the electrode and transfer electrons. The hybridization of a target oligonucleotide to the longer “capture” strand displaces part of the signaling strand, producing a much more flexible single-stranded element (Fig. 1b). This increases the efficiency with which the methylene blue approaches the electrode and exchanges electrons.
Figure 1.
We have developed a fully covalent, signal-on E-DNA architecture employing an asymmetric hairpin probe that is attached to its interrogating electrode via a commercially available, internal linkage chemistry (shown at left). The sensor operates via a strand displacement mechanism. When target DNA hybridizes with the longer arm of a probe molecule, the shorter arm is liberated, increasing the efficiency with which the methylene blue redox reporter at its end approaches the electrode surface. This results in a readily measurable, increase Faradaic current. Vacant areas between the probe DNAs are back-filled with mercaptohexanol, producing a stable, continuous self-assembled monolayer.
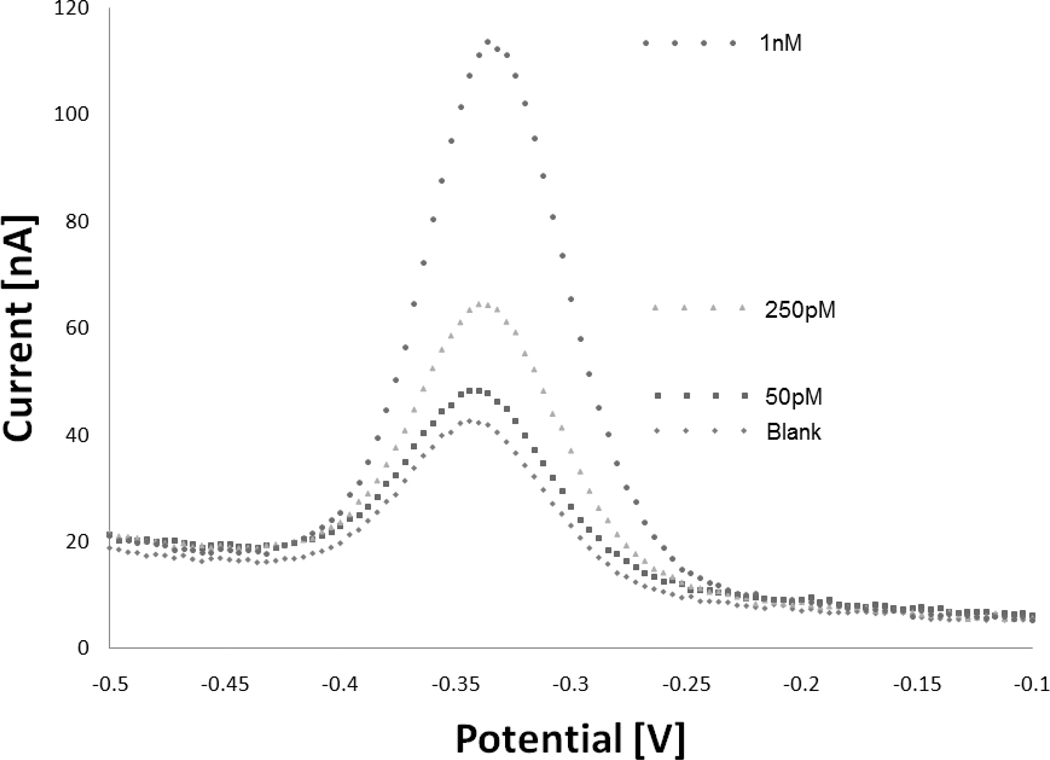
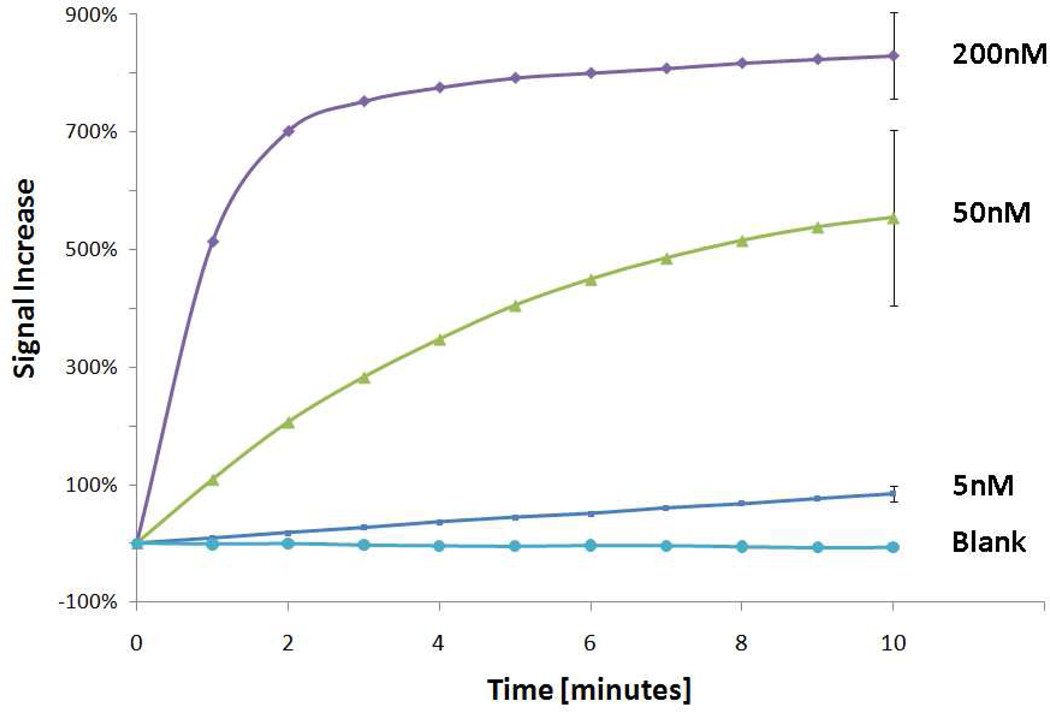
The new sensor architecture achieves high signal gain, and readily detects its target at low picomolar levels (Fig. 2) (and also Fig S3 in Supp. Info.). At saturating target concentrations (200 nM) the observed signal increases nearly eightfold within ten minutes (Fig. 3). Because the two arms of these new sensing elements are covalently linked, rather than held together by hybridization, this new probe architecture can be regenerated and reused by rinsing with a stream of dimethyl sulfoxide, which denatures double-stranded DNA. The regeneration process, which takes less than three minutes, can be repeated multiple times (Fig. S4 in supp. Info.).
Figure 2.
The new signal-on E-DNA architecture achieves 800% gain at saturating target concentrations. With the recognition elements we have employed, this leads to devices sensitive enough to detect low picomolar levels of their target oligonucleotide. The above data were collected one hour after each addition of target.
Figure 3.
The sensor rapidly responds to its complementary target. Here square wave voltammograms were recorded every minute after the addition of the relevant concentration of the target analyte. Error bars represent standard error from triplicate measurements. When blank measurements are made over the course of an hour, the sensor signal decreases slightly.
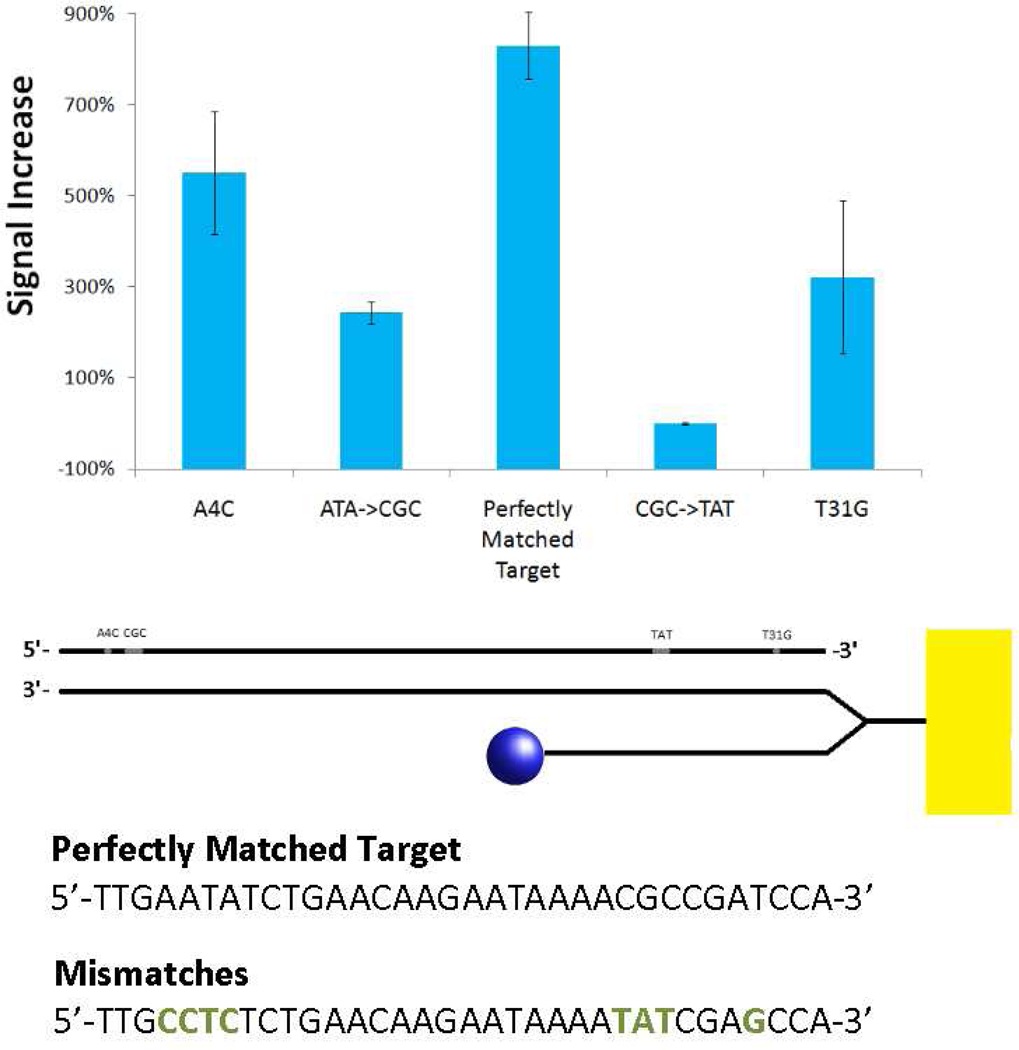
The new architecture is specific, an attribute that is critical for applications such as genotyping. As a test of the sensor's specificity, we challenged it with a perfectly matched target, as well as four sequences with mismatches (at 200 nM) in various locations. Single nucleotide polymorphisms and three-base mismatches that appear in the double-stranded region of the probe can be easily identified. This stands to reason, since an analyte that does not perfectly hybridize with the capture strand should not be able to easily displace the signaling strand. Mismatches that occur in the single-stranded region of the capture probe are also identifiable, although the difference in signaling is less pronounced.
For genotyping purposes, these sensors should be employed in a microarray format. In a sensor with only one probe DNA sequence, mismatched analytes could be misinterpreted as perfectly matched oligonucleotides at low concentrations. For this reason, these sensors should be used in an array format9,10, with related but slightly mismatched capture probes serving as controls.
Given the extremely low limits of detection that are necessary to identify pathogens in biological samples, these sensors will invariably be used in combination with an amplification technique such as LAMP or PCR. Doing so will be straightforward, since these sensors function well when employed directly in PCR mix (Fig. S5 in Supp. Info.). This is a significant advantage over optical DNA detection systems, such as microarrays, which require lengthy incubation and washing steps following the PCR reaction. Furthermore, the cost of amplification systems can be quite low25,26, as is the cost of E-DNA supporting electronics27, suggesting that such a “marriage” could provide an inexpensive and robust system for pathogen detection.
Supplementary Material
Figure 4.
The sensor is specific enough to recognize single nucleotide polymorphisms. Mismatched target DNA can cause an increase in the sensor signal, but that increase is not as great as the changes caused by perfectly matched analytes. The sensor can easily distinguish between mismatches that occur near the T-linker, but has less specificity at its distal 3’-end. Error bars represent standard error from triplicate measurements.
Acknowledgements
This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. It was also supported by the NIH through grants GM062958-01 and 2R01EB002046.
Abbreviations
- PBS
Phosphate Buffered Saline
- E-DNA
Electrochemical DNA Biosensor
- MB
Methylene Blue
Footnotes
Supporting Information Available
Additional material can be found on the Analytical Chemistry website.
References
- 1.Fan C, Plaxco KW, Heeger AJ. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9134–9137. doi: 10.1073/pnas.1633515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao Y, Lai RY, Plaxco KW. Nat. Protocols. 2007;2:2875–2880. doi: 10.1038/nprot.2007.413. [DOI] [PubMed] [Google Scholar]
- 3.Lubin AA, Plaxco KW. Accounts of Chemical Research. 2010;43:496–505. doi: 10.1021/ar900165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Y, Qu X, Plaxco KW, Heeger AJ. Journal of the American Chemical Society. 2007;129:11896–11897. doi: 10.1021/ja074218y. [DOI] [PubMed] [Google Scholar]
- 5.Cash KJ, Heeger AJ, Plaxco KW, Xiao Y. Analytical Chemistry. 2009;81:656–661. doi: 10.1021/ac802011d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci F, Lai RY, Plaxco KW. Chem. Commun. (Camb.) 2007:3768–3770. doi: 10.1039/b708882e. [DOI] [PubMed] [Google Scholar]
- 7.White RJ, Plaxco KW. Analytical Chemistry. 2010;82:73–76. doi: 10.1021/ac902595f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci F, Kevin P. Microchimica Acta. 163:147–236. [Google Scholar]
- 9.Lai RY, Lee S.-ho, Soh HT, Plaxco KW, Heeger AJ. Langmuir. 2006;22:1932–1936. doi: 10.1021/la052132h. [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic E, Lai RY, Wu TT, Ferguson BS, Sun R, Plaxco KW, Soh HT. Langmuir. 2008;24:1102–1107. doi: 10.1021/la702681c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson BS, Buchsbaum SF, Swensen JS, Hsieh K, Lou X, Soh HT. Analytical Chemistry. 2009;81:6503–6508. doi: 10.1021/ac900923e. [DOI] [PubMed] [Google Scholar]
- 12.Lubin AA, Lai RY, Baker BR, Heeger AJ, Plaxco KW. Analytical Chemistry. 2006;78:5671–5677. doi: 10.1021/ac0601819. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Y, Lubin AA, Baker BR, Plaxco KW, Heeger AJ. Proceedings of the National Academy of Sciences. 2006;103:16677–16680. doi: 10.1073/pnas.0607693103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan C. Proceedings of the National Academy of Sciences. 2003;100:9134–9137. doi: 10.1073/pnas.1633515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Y, Piorek BD, Plaxco KW, Heeger AJ. Journal of the American Chemical Society. 2005;127:17990–17991. doi: 10.1021/ja056555h. [DOI] [PubMed] [Google Scholar]
- 16.Immoos CE, Lee SJ, Grinstaff MW. Journal of the American Chemical Society. 2004;126:10814–10815. doi: 10.1021/ja046634d. [DOI] [PubMed] [Google Scholar]
- 17.Farjami E, Clima L, Gothelf K, Ferapontova EE. Analytical Chemistry. doi: 10.1021/ac1032929. 0, 0. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Campuzano S, Halford C, Haake DA, Wang J. Analytical Chemistry. 2010;82:8830–8837. doi: 10.1021/ac101474k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campuzano S, Kuralay F, Lobo-Castañón MJ, Bartosík M, Vyavahare K, Palecek E, Haake DA, Wang J. Biosensors and Bioelectronics. doi: 10.1016/j.bios.2011.02.004. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharuman V, Chang B-Y, Park SM, Hahn JH. Biosensors and Bioelectronics. 2010;25:2129–2134. doi: 10.1016/j.bios.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Drummond TG, Hill MG, Barton JK. Nat Biotech. 2003;21:1192–1199. doi: 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- 22.Ricci F, Lai RY, Heeger AJ, Plaxco KW, Sumner JJ. Langmuir. 2007;23:6827–6834. doi: 10.1021/la700328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubin AA, Vander Stoep Hunt B, White RJ, Plaxco KW. Analytical Chemistry. 2009;81:2150–2158. doi: 10.1021/ac802317k. [DOI] [PubMed] [Google Scholar]
- 24.White RJ, Phares N, Lubin AA, Xiao Y, Plaxco KW. Langmuir. 2008;24:10513–10518. doi: 10.1021/la800801v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal N, Ugaz V. Clinics in Laboratory Medicine. 2007;27:215–223. doi: 10.1016/j.cll.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal N, Hassan YA, Ugaz VM. Angew. Chem. 2007;119:4394–4397. doi: 10.1002/anie.200700306. [DOI] [PubMed] [Google Scholar]
- 27.Rowe AA, Bonham AJ, White RJ, Zimmer MP, Yadgar RJ, Hobza TM, Honea JW, Ben-Yaacov I, Plaxco KW. PLoS ONE. 2011;6:e23783. doi: 10.1371/journal.pone.0023783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.