Abstract
In the CNS, lipocalin-type prostaglandin D synthase (L-PGDS) is predominantly a non-neuronal enzyme responsible for the production of PGD2, an endogenous sleep promoting substance. We have previously demonstrated that estradiol differentially regulates L-PGDS transcript levels in the rodent brain. In hypothalamic nuclei, estradiol increases L-PGDS transcript expression, whereas in the ventrolateral preoptic area L-PGDS gene expression is reduced after estradiol treatment. In the present study, we have used an immortalized glioma cell line transfected with a L-PGDS reporter construct and estrogen receptor (ER) α and ERβ expression plasmids to further elucidate the mechanisms underlying estradiol regulation of L-PGDS gene expression. We found that physiologically relevant concentrations of estradiol evoked an inverted U response in cells expressing ERα. The most effective concentration of estradiol (10-11M) increased the promoter activity 3-fold over baseline. Expression of ERβ did not increase activity over control and when ERβ was co-expressed with ERα there was a significant attenuation of the promoter activity. While ERα significantly increased L-PGDS promoter activity, our previous in vivo studies demonstrate a greater magnitude of change in L-PGDS gene expression in the presences of estradiol. This led us to ask whether estradiol is signaling via a paracrine factor released by the neighboring neurons. Conditioned media from estradiol treated neurons applied to the glioma cell line resulted in a significant 7-fold increase in L-PGDS promoter activity supporting the possibility that neuronal-glial interaction are involved in estradiol regulation of L-PGDS.
Lipocalin-Prostaglandin D2 Synthase (L-PGDS) catalyzes the conversion of Prostaglandin (PG)H2 to PGD2. In the adult brain, PGD2 is the most abundant prostanoid and has been implicated in a variety of neurological functions including olfaction, nociception, thermoregulation and sleep (for review [13]). In fact, PGD2 is one of the most potent endogenous somnogen identified to date [14]. L-PGDS is predominantly expressed in non-neuronal cells that include the leptomeninges, choroids plexus and parenchymal oligodendrocytes [1, 19, 28]. Numerous organs outside of the CNS also express the functional protein [7, 25, 27, 31] suggesting that L-PGDS gene expression may be regulated by tissue and cell-specific factors. Notwithstanding this fact, regulators of L-PGDS gene expression in the CNS are not well understood.
We have previously demonstrated region-specific alterations in L-PGDS gene expression and protein levels in the CNS of ovariectomized female rodents receiving estradiol replacement [11, 19, 20]. Estradiol increases expression in MBH nuclei (arcuate nucleus and the ventromedial nucleus of the hypothalamus) while markedly down-regulating its expression in the ventrolateral preoptic area (VLPO) [11, 19].
In the present study, we are interested in further investigating the regulation of L-PGDS gene expression by estradiol. Classically, estradiol effects are mediated by two nuclear hormone receptors, estrogen receptor alpha (ERα and estrogen receptor beta (ERβ. Both receptor proteins belong to a class of ligand-activated proteins that when bound to specific sequences of DNA (i.e. estrogen response element; ERE) either activate or repress transcription within the cell nucleus. Thus, the aim of this study was to address whether ERα and/or ERβ are capable of mediating transcriptional activity of the L-PGDS gene. We used the immortalized U251 human glioma cell line that was transfected with a L-PGDS reporter construct and ERα and ERβ expression plasmids. We chose the U251 cell line as our model because of its non-neuronal compliment of cells and ease of transfection. Additionally, low protein expression of estrogen receptors (supplemental figure 1 a-c) was advantageous in testing the individual contributions of ER α and β via transfection of the specific expression constructs.
The U251 cell line was maintained in DMEM (Invitrogen, Carlsbad, California) containing 10% FBS (Bioreclamation, New York), 100units/ml penicillin and 50μg/ml streptomycin. For transfection experiments, the cells were plated in 6-well plates at a density of 0.2×106 cells/well. For transfection, the cells was cultured in steroid and phenol-red free media supplemented with 5% charcoal dextran-stripped FBS (Gemini Biotech, Alachua, FL) plus antibiotics. Cells were transfected at 50-80% confluence, approximately 48 hours after plating, via Effectene (Qiagen, Valencia, CA) according to the manufacture’s instructions. In addition to the reporter constructs described below, all cells were transfected with pSV-βgal (80ng/well; Promega; Madison, WI) and pBSSKII+ (to a final concentration of 400ng/well). The pSV-βgal plasmid was used as an internal control for the normalization of transfection efficiency and lysate preparation. The pBSSKII+ plasmid was used as a means of standardizing the total amount of DNA transfected. Following transfection, the cells were washed, replaced with fresh phenol-red free media and 17β-estradiol (10-7M dissolved in ethanol; Sigma, St. Louis, MO) was added directly to the culture well. Following 24h of exposure, the cells were lysed with Glo Lysis Buffer (Promega) and lysate from each well was used for both luciferase and β-gal assays according to the manufacture’s instructions. Luciferase activity was normalized per total protein and reported as activity (fold induction over vehicle).
We first tested whether the U251 glioma cell line was supported ER mediated transcriptional activity. The cells were transfected with 150ng/well of a 3xERE Reporter Plasmid only (non-transfected; NT) or co-transfected with the expression plasmids encoding ERα (pSG-hERα; 80ng) or ERβ (pCMV-hERβ; 80ng) or both together (160ng total). The 3xERE reporter construct (pGL2-TATA-Inr-Luc plasmid construct) was a kind gift from Donald McDonnell and has three-tandem consensus EREs upstream form the luciferase reporter gene [12]. The plasmids encoding human ERα (pSG-hERα) and human ERβ (pCMV-hERβ) were kind gifts from Dr. P. Chambon (Strasbourg, France), and Dr. J.-Å. Gustafsson (Huddinge, Sweden), respectively.
Enhanced immunocytochemical detection of ERα and ERβ protein in the transfected cell lines compared to non-transfected cells was seen following transfection (supplemental Figure 1a-d). The transfected cells were incubated with 10-10M estradiol for either 2 or 8 h. Previous work has shown this time course is sufficient to elicited transcriptional activity from the 3xERE reporter [30]. Following hormone treatment the cells were lysed and luciferase activity measured as described above.
The next set of experiments tested whether ERα or ERβ or both initiated L-PGDS promoter activity in the U251 cells. The cells were transfected with the L-PGDS -1250/+77 luciferase-reporter construct alone (NT) or co-transfected with either the ERα or ERβ expression plasmids or both simultaneously as described above. Construction of the L-PGDS promoter/luciferase gene plasmid was previously described [8]. Following transfection, individual wells were treated with increasing concentrations of estradiol and incubated for 24 h. The cells were then lysed and luciferase activity measured as described above.
Finally, neuron-glia interactions have been implicated in the modulation of glial function. To test whether an estrogen-induced, neuron-derived factor was sufficient to initiate L-PGDS promoter activity, SKN cells were treated with 17β-estradiol (10-7M) for either 0 or 2 hr. Following 17β-estradiol exposure, the cells were washed and placed in fresh media for approximately 16 hours. U251 cells transfected with the L-PGDS -1250/+77 luciferase-reporter construct were then treated with the neuronally conditioned media for 24 h. Following hormone treatment the cells were lysed and luciferase activity measured as described above.
Results from all replicate experiments (n=8 per treatment group in each experiment) were plotted as fold induction over vehicle for that group. All values represent the mean ± SEM of samples in a treatment group from the replicate experiments. Data gathered from experiments 1 and 2, were analyzed using a two-way ANOVA with independent variables estrogen receptor expression and estradiol concentration or time. Bonferroni post hoc analysis was used to determine significant differences between groups. The alpha level was set at 0.05. For experiment 3, statistical analysis was performed with a two-tailed t-test. Statistical tests were conducted using GraphPad Prism 4 (San Diego, CA) on a Macintosh computer.
The U251 glioma cell line was demonstrated ER mediated transcription from the 3xERE reporter supporting its use as a potential model system for investigating ER action at the L-PGDS promoter. Following 2 and 8 hr of estradiol treatment, U251 cells expressing ERα resulted in an approximate 17-fold and 7.5-fold induction of activity, respectively, (Figure 1; two-way ANOVA; F(6, 36)=5.40, p<0.0005). The ERα induced activity was significantly greater than the activity induced by ERβ alone or ERα and Erβ together (Figure 1; two-way ANOVA; F(8, 27)=8.056, p<0.0002). Independent one-way ANOVAs for the ERβ and ERα and β transfected groups revealed that the 2 and 8 h treatments of estradiol significantly increased the promoter activity over their respective controls albeit to a lesser degree than ERα (ERβ; F(2,11)=18.93; p<0.0006, Erα/β; F(2,11)=31.64; p<0.0001). While some U251 cells endogenously expressed ERα and ERβ protein (Supplemental Figure 1a-c), estradiol treatment did not significantly increase transcriptional activity over the control in the NT group (NT; F(2,11)=0.2438) suggesting that endogenous expression of ERs may not to be sufficient for estradiol-initiated transcription.
Figure 1. Estrogen response element (ERE) mediated promoter activity in U251 glial cell line.
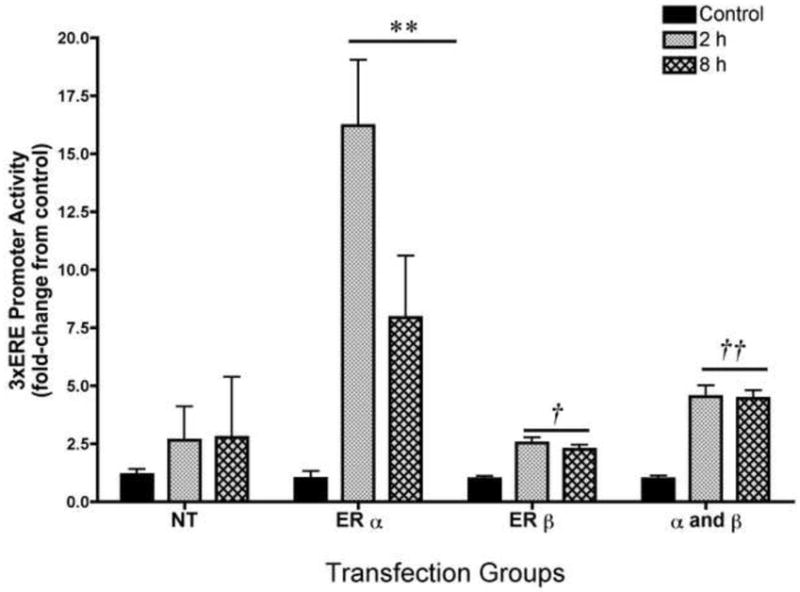
The addition of 10-10M estradiol for 2 and 8 h to U251 cell co-transfected with the 3xERE reporter construct and the ERα expression plasmid resulted in an approximate 17-fold and 7.5-fold induction of the 3xERE promoter activity, respectively **p<0.0001, compared to NT, ERβ alone, and ERα/ERβ together; n=8 for all groups). Independent one-way ANOVAs for the ERβ and ERα and β transfected groups revealed that the 2 and 8 h treatments of estradiol significantly increased the promoter activity over their respective controls albeit to a lesser degree than ERα (†p<0.0006 and ††p<0.0001). Estradiol treatment did not induce ER activity in the U251 cell not transfected with ER expression plasmids (NT). Basal expression of the pGL3-Enhancer vector was unchanged in all groups (data not shown). Data are the mean± SEM.
Since the previous experiment suggested that U251 cells were capable of supporting ER mediated transcription, we next investigated whether ERs were capable of initiating L-PGDS promoter activity in U251 cells. ERα and ERβ expression plasmids were transiently co-transfected, separately and together, with the L-PGDS reporter construct in the U251 cell line. The transfected cells were incubated with a range of concentrations of estradiol for 24 h. In cells over expressing ERα only, a maximum 3-fold stimulation was produced by 10-11M of estradiol and the induction in this group was significantly greater than all other transfection groups except for the ERα cells treated with 10-10M estradiol (Figure 2; two-way ANOVA; F=(16,112)=2.945; p<0.004). The highest concentration of estradiol had no effect. Curiously, this inverted U response curve was also seen in the NT group where 10-10M estradiol produced a maximum 1.7-fold stimulation that was significantly greater than the control, but the highest concentration of estradiol (10-9M) was not (Figure 2; NT group one-way ANOVA; F(3,31)=3.07, p<0.05). Expression of ERβ did not significantly change L-PGDS promoter activity over control for the respective groups. However, co-expression of ERα and ERβ led to a significant attenuation of the activity at the highest concentrations of estradiol (Figure 2; α and β group; one-way ANOVA; F(3,31)=6.51, p<0.05).
Figure 2. Exogenous estrogen receptor mediated L-PGDS promoter activity in U251 glial cell line.
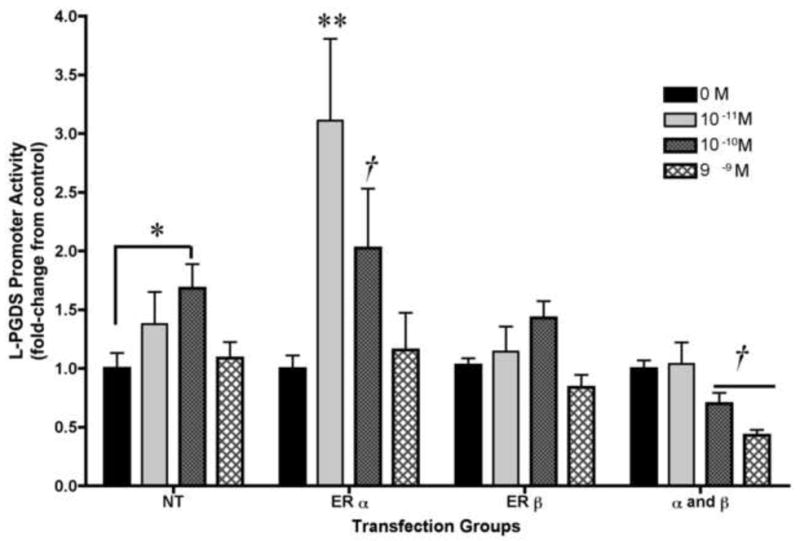
In cells over-expressing ERα-only, 10-11M and 10-10M of estradiol produced 3-fold and 2-fold stimulation over control (two-way ANOVA; **p<0.0001, and *p<0.05, respectively). In the non-transfected cells (NT), produced 1.7 fold stimulation over control (*p<0.05). Co-expression of ERα and ERβ led to a significant attenuation of the activity at the highest concentration of estradiol (one-way ANOVA; †p<0.05). Basal expression of the pGL3-Enhancer vector was unchanged in all groups (data not shown). Data are the mean± SEM (n=8 for all groups).
The results from the above experiments suggested that, while estradiol acting primarily through ERα resulted in significant changes in L-PGDS promoter activity, these changes were of relatively small magnitude that were not consistent with our previous results found in vivo [19]. One explanation is that estradiol may also mediate L-PGDS promoter activity through indirect signaling mechanism. Evidence for estradiol mediated neuronal-glial interaction exists [18]. To test whether the L-PGDS promoter activity is affected by an estradiol mediated factor secreted from neurons, we treated the SKN neuroblastoma cell line with 10-7M estradiol for either 0 or 2 h, removed the treatment media, and allowed the SKN cells to condition fresh media for approximately 16 hr. The addition of neuronally conditioned media to U251 cell transfected with the L-PGDS reporter construct resulted in a 6-fold induction of the promoter activity that was significantly different from the control (Figure 3; two tailed t-test; t(14)=3.37, p<0.005).
Figure 3. Neuron-conditioned medium.
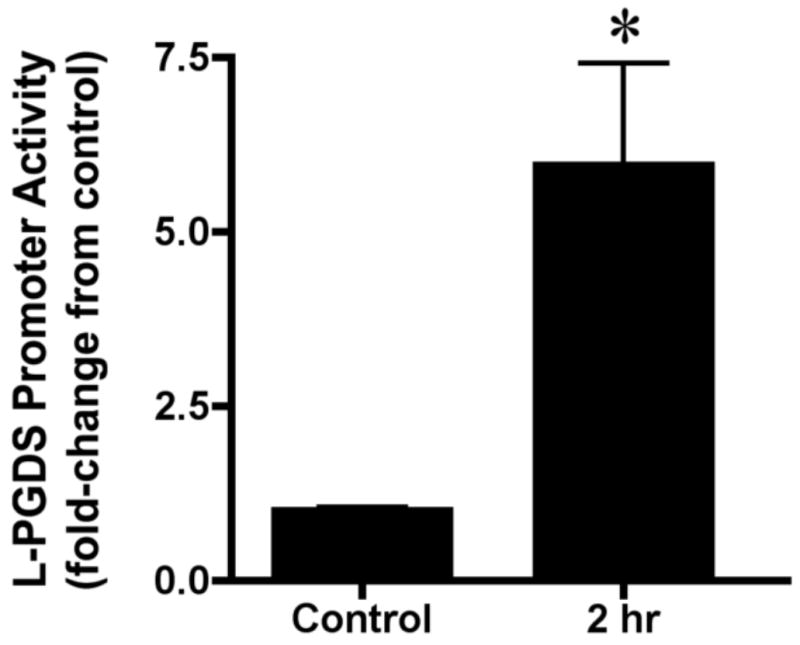
The SKN neuroblastoma cell line was treated with 17β-estradiol (10-7M) for either 0 (control) or 2 hours (n=8 for both conditions). Following 17β-estradiol exposure, the cells were washed and placed in fresh media for approximately 16 hours. The resulting conditioned media was used to treat the U251 cells transfected with the L-PGDS -1250/+77 luciferase-reporter construct for 24 hours. The neuronally conditioned media induced L-PGDS reporter activity approximate 6-fold over control, *p<0.005. Basal expression of the pGL3-Enhancer vector was unchanged in all groups (data not shown). Data are the mean± SEM.
The purpose of this study was to investigate the role of ERα and ERβ in the regulation of L-PGDS gene expression by estradiol. In a glial cell line expressing ERα, physiologically relevant concentrations of estradiol evoked an inverted U response where the most effective concentration, 10-11M, increased the promoter activity approximately 3-fold over baseline. Expression of ERβ did not increase activity over control for the respective groups and when ERβ was co-expressed with ERα there was a significant attenuation of promoter activity. While Erα significantly increased L-PGDS promoter activity, the level of activity was weak in comparison to the level of activity initiated at the 3xERE construct in the same cell line. In fact, our previous in vivo studies demonstrate a greater magnitude of change in L-PGDS gene expression in the presences of estradiol [19]. Taken together, this led us to posit estradiol via its cognate receptors may influence L-PGDS promoter activity by direct and indirect interactions. In support of this assertion, conditioned media from estradiol treated neurons applied to the glioma cell line resulted in a significant 6-fold increase in L-PGDS promoter activity supporting the possibility that neuronal-glial interaction are involved in estradiol regulation of L-PGDS.
Our study suggests two potential mechanisms through which estradiol may be regulating L-PGDS gene expression in vivo. First, ERα induced L-PGDS promoter activity whereas ERβ, when co-expressed with ERα, blocked this action and even further decreased L-PGDS promoter activity. Second, estradiol may be coordinating the communication via a releasable signaling factor, between neighboring cells such as the neurons and the non-neuronal cells that express L-PDGS (oligodendroctyes and leptomeninges).
Glial cells, in general, are remarkably sensitive to changes in their extracellular environment and are capable of responding to neuronally derived factors leading to responses that include but are not limited to changes in gene and protein expression[26, 29]. While the suspected estradiol-induced paracrine factor in this study is unknown, interleukin-1β(IL-1β) may be a potential candidate. Mounting evidence has implicated cytokines and in particular IL-1β in the production and release of PGD2 [5, 8, 10, 15]. In fact, IL-1β upregulates L-PGDS genes expression in cultured leptomeningeal cells to maximum levels by 24hr via activation of two NF-κB response elements on the L-PGDS promoter [8]. In the CNS, estrogens have been implicated in the regulation of proinflammatory cytokine expression including as IL-1β, but thus far only, in the context of an immune challenge [2-4, 16]. At present, it is unclear what role estrogens may have in the normal expression and release of cytokines in the CNS.
Another exciting finding in this study was that ERβ acted as a suppressor of L-PGDS promoter activity. ERα and ERβ are separate gene products that most likely evolved from a gene duplication event and thus share some similarities in amino acid sequences especially in the ligand binding and DNA binding domains. However, there is a significant divergence in the amino acid sequence of the activation domains (A/B and F domain) and hinge region [6, 21]. Consequently, ERα exhibits greater transcriptional activity than ERβ at certain target genes [6, 21]. When both receptors are co-expressed, ERβ has been reported to attenuate the transcriptional ability of ERα presumably through actions as a dominant negative when the two receptors heterodimerize [12, 17]. At present, it is not known whether these heterodimers are present in our in vitro model system or in vivo.
Interestingly, the preoptic area is one of the few regions that demonstrate similar numbers and distribution of ERα and ERβ expressing cells [23] as well as co-expression of ERα and ERβ [24]. This is in contrast to mediobasal hypothalamic (MBH) nuclei, like the arcuate nucleus and the ventromedial nucleus of the hypothalamus, where ERα is expressed in higher levels, while there is little ERβ expression [23, 24]. We have previously reported, in vivo, a dual regulation of L-PGDS mRNA expression by estradiol. In the arcuate nucleus and the ventromedial nucleus of the hypothalamus, estradiol significantly increases L-PGDS mRNA expression, whereas, there is a highly specific down-regulation in the presence of estradiol in the VLPO [11, 19]. This raises an interesting possibility that the limited distribution of cells that co-express ERα and ERβ may enable that population to respond differently to estradiol when compared to cells expressing ERα or ERβ alone.
In light of our current data, the co-expression of ERα and ERβ in preoptic area nuclei may have a broader implication on sleep-wake behavior. Suppression of L-PGDS protein by estradiol in the VLPO of female rodents is one potential molecular mechanism through which estradiol may be acting to reduce sleep via a concomitant decrease in PGD2 levels leading to a reduction in VLPO neuronal activity [11]. In a recent study, estradiol regulation of L-PGDS gene expression in preoptic area extracts follows a U-shaped response curve where physiologically relevant doses of estradiol decrease L-PGDS expression. More interestingly, the estradiol-mediated changes in L-PGDS expression are correlated with increases in running wheel behavior [22]. In light of our current findings, it is tempting to speculate that, in the VLPO, ERβ in the presences of estradiol may be acting as a repressor at the L-PGDS promoter resulting in a more aroused state.
The current study suggests two potential mechanisms through which estradiol may be regulating L-PGDS gene expression in vivo. However, because glioma cell lines are typically heterogeneous in their glial compliment, it is difficult to know whether oligodendrocytes, the main cell type expressing L-PGDS in the brain parenchyma, respond in a similar manner as the gliomas. Future studies will begin to explore whether one or both of these mechanisms affect in vivo L-PGDS expression.
Supplementary Material
Immunocytochemical detection of ER〈 and ® protein in U241 cells before (A, B, C) and after transfection (D, E) with expression plasmids. Arrows represent immunopositive cells. Coverslips with adhered U251 cells were immersed in 4% paraformaldehyde in 0.1 M PBS, pH 7.5, warmed to 37°C for 10 min, washed three times in PBS, and permeabilized with 50% ethanol for 60 mi n at 4°C. The cells were then washed three times in PBS and incubated in 10% normal goat serum in 0.4% Triton X-100 PBS (PBST) for 60 mi n at room temperature with agitation. After blocking, the primary antibody for either ERβ (Z8P; 1:4000; Zymed Laboratories, San Francisco, CA), or ERα (C1355; 1:10,000; Upstate Biotechnology, Lake Placid, NY) was applied in 10% normal goat serum PBST, and the coverslips were incubated overnight at 4°C. The coverslips were washed three times in PBST and incubated in biotinylated secondary antibody (1:1000; goat anti-rabbit; Vector Laboratories, Burlingame, CA) for 60 min at room temperature with agitation, followed by three washes in PBST. The coverslips were then incubated with an avidin –biotin horseradish-peroxidase complex (Vectastain ABC, Elite K it; Vector Laboratories) for 60 min at room temperature with agitation, washed twice with PBST, and visualized with 0.05% 3,3’-diaminobenzidine tertrahydrochloride (Polysciences, Warrington, PA) and 0.005% H2O2. After visualization, the coverslips were mounted on glass slides, viewed through a Zeiss Axioscope and photographed. On a technical note, the specificity of the ER antibodies had been determined by the respective companies and in our own immunocytochemical analysis in the ER α and β knockout mice.
Acknowledgments
This research was supported by HL-085037awarded to JAM and HD-05751 and MH-38273 awarded to DWP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beuckmann CT, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, Urade Y. Cellular localization of lipocalin-type prostaglandin D synthase (beta- trace) in the central nervous system of the adult rat. J Comp Neurol. 2000;428:62–78. doi: 10.1002/1096-9861(20001204)428:1<62::aid-cne6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, Bagetta G, Corasaniti MT. Evidence to implicate early modulation of interleukin-1beta expression in the neuroprotection afforded by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]
- 3.Choi JS, Kim SJ, Shin JA, Lee KE, Park EM. Effects of estrogen on temporal expressions of IL-1beta and IL-1ra in rat organotypic hippocampal slices exposed to oxygen-glucose deprivation. Neurosci Lett. 2008;438:233–237. doi: 10.1016/j.neulet.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Corasaniti MT, Amantea D, Russo R, Piccirilli S, Leta A, Corazzari M, Nappi G, Bagetta G. 17beta-estradiol reduces neuronal apoptosis induced by HIV-1 gp120 in the neocortex of rat. Neurotoxicology. 2005;26:893–903. doi: 10.1016/j.neuro.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Dayton ET, Major EO. Recombinant human interleukin 1 beta induces production of prostaglandins in primary human fetal astrocytes and immortalized human fetal astrocyte cultures. J Neuroimmunol. 1996;71:11–18. doi: 10.1016/s0165-5728(96)00111-7. [DOI] [PubMed] [Google Scholar]
- 6.Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–590. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 7.Eguchi Y, Eguchi N, Oda H, Seiki K, Kijima Y, Matsu-ura Y, Urade Y, Hayaishi O. Expression of lipocalin-type prostaglandin D synthase (beta-trace) in human heart and its accumulation in the coronary circulation of angina patients. Proc Natl Acad Sci U S A. 1997;94:14689–14694. doi: 10.1073/pnas.94.26.14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimori K, Fujitani Y, Kadoyama K, Kumanogoh H, Ishikawa K, Urade Y. Regulation of lipocalin-type prostaglandin D synthase gene expression by Hes-1 through E-box and interleukin-1 beta via two NF-kappa B elements in rat leptomeningeal cells. J Biol Chem. 2003;278:6018–6026. doi: 10.1074/jbc.M208288200. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Fernandez LF, Iniguez MA, Rodriguez-Pena A, Munoz A, Bernal J. Brain-specific prostaglandin D2 synthetase mRNA is dependent on thyroid hormone during rat brain development. Biochem Biophys Res Commun. 1993;196:396–401. doi: 10.1006/bbrc.1993.2262. [DOI] [PubMed] [Google Scholar]
- 10.Grill M, Heinemann A, Hoefler G, Peskar BA, Schuligoi R. Effect of endotoxin treatment on the expression and localization of spinal cyclooxygenase, prostaglandin synthases, and PGD2 receptors. J Neurochem. 2008;104:1345–1357. doi: 10.1111/j.1471-4159.2007.05078.x. [DOI] [PubMed] [Google Scholar]
- 11.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 13.Hayaishi O. Molecular mechanisms of sleep-wake regulation: roles of prostaglandins D2 and E2. Faseb J. 1991;5:2575–2581. [PubMed] [Google Scholar]
- 14.Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaka M, Ohe Y, Senbongi T, Wakabayashi K, Ishikawa K. Inflammatory stimuli increase prostaglandin D synthase levels in cerebrospinal fluid of rats. Neuroreport. 2001;12:1161–1165. doi: 10.1097/00001756-200105080-00022. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AB, Bake S, Lewis DK, Sohrabji F. Temporal expression of IL-1beta protein and mRNA in the brain after systemic LPS injection is affected by age and estrogen. J Neuroimmunol. 2006;174:82–91. doi: 10.1016/j.jneuroim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 18.Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138:967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Mong JA, Devidze N, Frail DE, O’Connor LT, Samuel M, Choleris E, Ogawa S, Pfaff DW. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: Evidence from high-density oligonucleotide arrays and in situ hybridization. PNAS. 2003;100:318–323. doi: 10.1073/pnas.262663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mong JA, Devidze N, Goodwillie A, Pfaff DW. Reduction of lipocalin-type prostaglandin D synthase in the preoptic area of female mice mimics estradiol effects on arousal and sex behavior. Proc Natl Acad Sci U S A. 2003;100:15206–15211. doi: 10.1073/pnas.2436540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 23.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana M, Fex J, Urade Y, Hayaishi O. Brain-type prostaglandin D synthetase occurs in the rat cochlea. Proc Natl Acad Sci U S A. 1987;84:7677–7680. doi: 10.1073/pnas.84.21.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 27.Ujihara M, Urade Y, Eguchi N, Hayashi H, Ikai K, Hayaishi O. Prostaglandin D2 formation and characterization of its synthetases in various tissues of adult rats. Arch Biochem Biophys. 1988;260:521–531. doi: 10.1016/0003-9861(88)90477-8. [DOI] [PubMed] [Google Scholar]
- 28.Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci U S A. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volterra A, Magistretti PJ, Haydon PG, editors. The Tripartite Synapse. Oxford Press; New York: 2002. p. 272. [Google Scholar]
- 30.Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Ma H, Ni H, Ma X-H, Mills N, Yang Z-M. Expression and Regulation of Lipocalin-Type Prostaglandin D Synthase in Rat Testis and Epididymis. Biol Reprod. 2003 doi: 10.1095/biolreprod.103.022079. biolreprod.103.022079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunocytochemical detection of ER〈 and ® protein in U241 cells before (A, B, C) and after transfection (D, E) with expression plasmids. Arrows represent immunopositive cells. Coverslips with adhered U251 cells were immersed in 4% paraformaldehyde in 0.1 M PBS, pH 7.5, warmed to 37°C for 10 min, washed three times in PBS, and permeabilized with 50% ethanol for 60 mi n at 4°C. The cells were then washed three times in PBS and incubated in 10% normal goat serum in 0.4% Triton X-100 PBS (PBST) for 60 mi n at room temperature with agitation. After blocking, the primary antibody for either ERβ (Z8P; 1:4000; Zymed Laboratories, San Francisco, CA), or ERα (C1355; 1:10,000; Upstate Biotechnology, Lake Placid, NY) was applied in 10% normal goat serum PBST, and the coverslips were incubated overnight at 4°C. The coverslips were washed three times in PBST and incubated in biotinylated secondary antibody (1:1000; goat anti-rabbit; Vector Laboratories, Burlingame, CA) for 60 min at room temperature with agitation, followed by three washes in PBST. The coverslips were then incubated with an avidin –biotin horseradish-peroxidase complex (Vectastain ABC, Elite K it; Vector Laboratories) for 60 min at room temperature with agitation, washed twice with PBST, and visualized with 0.05% 3,3’-diaminobenzidine tertrahydrochloride (Polysciences, Warrington, PA) and 0.005% H2O2. After visualization, the coverslips were mounted on glass slides, viewed through a Zeiss Axioscope and photographed. On a technical note, the specificity of the ER antibodies had been determined by the respective companies and in our own immunocytochemical analysis in the ER α and β knockout mice.


