Abstract
Membrane transporters expressed by the hepatocyte and enterocyte play critical roles in maintaining the enterohepatic circulation of bile acids, an effective recycling and conservation mechanism that largely restricts these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments. In doing so, the hepatic and enterocyte transport systems ensure a continuous supply of bile acids to be used repeatedly during the digestion of multiple meals throughout the day. Absorption of bile acids from the intestinal lumen and export into the portal circulation is mediated by a series of transporters expressed on the enterocyte apical and basolateral membranes. The ileal apical sodium-dependent bile acid cotransporter (abbreviated ASBT; gene symbol, SLC10A2) is responsible for the initial uptake of bile acids across the enterocyte brush border membrane. The bile acids are then efficiently shuttled across the cell and exported across the basolateral membrane by the heteromeric Organic Solute Transporter, OSTα-OSTβ. This chapter briefly reviews the tissue expression, physiology, genetics, pathophysiology, and transport properties of the ASBT and OSTα-OSTα. In addition, the chapter discusses the relationship between the intestinal bile acid transporters and drug metabolism, including development of ASBT inhibitors as novel hypocholesterolemic or hepatoprotective agents, prodrug targeting of the ASBT to increase oral bioavailability, and involvement of the intestinal bile acid transporters in drug absorption and drug-drug interactions.
1. Overview of the Enterohepatic Circulation of Bile Acids
Bile acids are synthesized from cholesterol in the liver, conjugated (N-acyl amidated) to taurine or glycine, secreted into bile, and stored in the gallbladder. During a meal, the gallbladder contracts and bile acids enter the small intestine, where they facilitate absorption of fat-soluble vitamins and cholesterol (Hofmann and Hagey 2008). The majority (> 90%) of bile acids are reabsorbed from the intestine and returned to the liver via the portal venous circulation. The bile acids are then transported across the sinusoidal membrane of the hepatocyte and resecreted across the canalicular membrane into bile (Dawson et al. 2009). Since these processes, i.e. intestinal absorption, return to the liver in the portal circulation, and hepatic extraction of bile acids, are so efficient, the majority of the bile acids secreted across the canalicular membrane into bile are derived from the recirculating pool with less than 10% from new de novo hepatic synthesis. In the small intestine, bile acids are absorbed by passive and active mechanisms, with active transport accounting for the majority of conjugated bile acid uptake (Dietschy 1968; Lewis and Root 1990; Marcus et al. 1991; Aldini et al. 1996). The passive absorption occurs down the length of the intestine, whereas active absorption of bile acids is largely restricted to the distal small intestine (ileum) (Schiff et al. 1972; Krag and Phillips 1974). In man and all other vertebrates examined to date, the ileal epithelium has developed an efficient transport system for active reclamation of bile acids (Hofmann and Hagey 2008; Hofmann et al. 2009). This scheme ensures that the intraluminal concentration of conjugated bile acids will remain sufficiently high in proximal intestine to promote lipid absorption as well as reduce the small intestinal bacterial load. Overall, the enterohepatic circulation maintains a bile acid pool size of approximately 4 mg in mice and 2 to 4 g humans. This pool cycles multiple times per meal (Hofmann et al. 1983; Hulzebos et al. 2001) and intestinal bile acid absorption may be as great as 20 mg/day in mice and 30 g/day in humans. The bile acids that escape intestinal absorption (< 0.5 g/day in humans) are excreted into the feces. The bile acid pool size is carefully maintained by hepatic conversion of cholesterol to bile acid, and this process represents a major route for elimination of cholesterol from the body (Dietschy et al. 1993; Dietschy and Turley 2002). Over the past two decades, investigators have identified all the major hepatic and intestinal transporters that function to maintain the enterohepatic circulation of bile acids (Dawson et al. 2009). The cellular location and properties of these transporters are summarized in Figure 6.1 and Table 6.1, respectively.
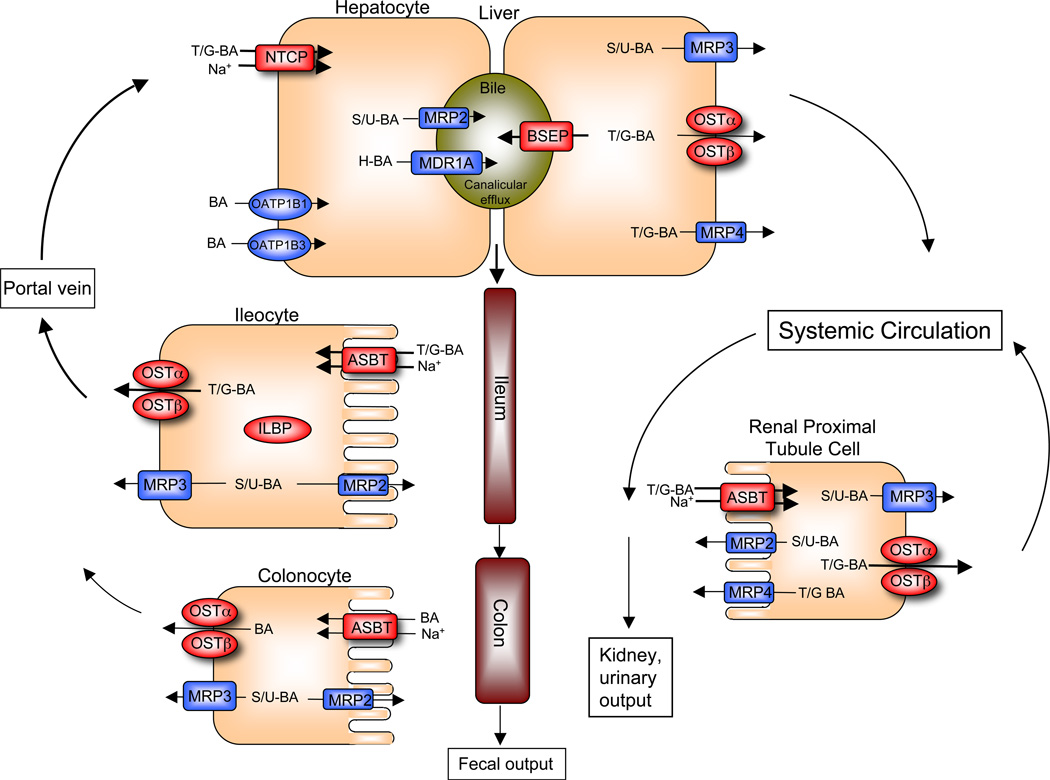
Figure 1. Enterohepatic circulation of bile acids showing the individual transport proteins in hepatocytes, ileocytes (ileal enterocytes), and renal proximal tubule cells.
After their synthesis or reconjugation, taurine and glycine (T/G) conjugated bile acids (BA) are secreted into bile by the canalicular bile salt export pump (BSEP; gene symbol ABCB11). The small amount of bile acids that have been modified by the addition of sulfate or glucuronide (S/U) are secreted by the multidrug resistance-associated protein-2 (MRP2; gene symbol ABCC2), whereas those modified by additional hydroxylation (H) are secreted by P-glycoprotein (MDR1; gene symbol ABCB1A). These divalent (S/G) or tetrahydroxylated (H) bile acids are present in very small quantities under normal physiological conditions, but may accumulate in disease states such as cholestasis. The bile acids are stored in the gallbladder and empty into the intestinal lumen in response to a meal. Bile acids are poorly absorbed in the proximal small intestine, but efficiently taken up by the apical sodium-dependent bile acid transporter (ASBT; gene symbol SLC10A2) in the ileum. The bile acids bind to the ileal lipid binding protein (ILBP; gene symbol FABP6) in the cytosol, and are efficiently exported across the basolateral membrane into the portal circulation by the heteromeric transporter OSTα-OSTβ. The multidrug resistance-associated protein-3 (MRP3; gene symbol ABCC3) is a minor contributor to basolateral export of native bile acids from the enterocyte, but may have a more significant role in export of any modified (glucuronidated or sulfated) bile acids that may be formed. MRP2 may also serve to export modified bile acids, across the apical brush border membrane. While most bile acids are absorbed in the small intestine, colonocytes express very low levels of ASBT and appreciable levels of MRP3 and OSTα-OSTβ; these carriers may serve to absorb a fraction of the unconjugated bile acids from the lumen of the colon. After their absorption from the intestine, bile acids travel back to the liver where that are cleared by the Na+- taurocholate cotransporting polypeptide (NTCP; gene symbol SLC10A1). Members of the Organic Anion Transport Protein family, OATP1B1 (gene symbol SLCO1B1) and OATP1B3 (gene symbol SLCO1B3) also participate, and are particularly important unconjugated bile acids. Under cholestatic conditions, unconjugated, conjugated, or modified bile acids can be effluxed across the basolateral (sinusoidal) membrane of the hepatocyte by OSTα-OSTβ, MRP3, or multidrug resistance-associated protein-4 (MRP4; gene symbol ABBC4) into the systemic circulation. Under normal physiological conditions, a fraction of the bile acid escapes first pass hepatic clearance enters the systemic circulation. The free bile acids are filtered by the renal glomerulus, efficiently reclaimed by the ASBT in the proximal tubules, and exported back into the systemic circulation, thereby minimizes their excretion in the urine. This efficient renal reabsorption occurs even under cholestatic conditions for unconjugated and conjugated bile acids, when serum bile acid concentrations are dramatically elevated. Overall, this integrated transport system minimizes fecal and urinary bile acid loss and functions to largely restrict these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments.
Table 1.
Function of Transport Proteins in the Enterohepatic Circulation of Bile Acids
| Transporter (Gene) | Location | Function |
|---|---|---|
| Hepatocyte | ||
| Process: Hepatocyte Sinusoidal to Canalicular Bile Acid Transport | ||
| NTCP (SLC10A1) | BLM | Na+-dependent uptake of BA |
| OATP1B1 (SLCO1B1) | BLM | Na+-independent uptake of BA |
| OATP1B3 (SLCO1B3) | BLM | Na+-independent uptake of BA |
| BSEP (ABCB11) | CM | ATP-dependent export of BA |
| MRP2 (ABCC2) | CM | ATP-dependent export of BA sulfates/glucuronides |
| MDR1 (ABCB1) | CM | ATP-dependent export of tetrahydroxylated BA |
| Process: Hepatocyte Sinusoidal Bile Acid Export | ||
| MRP3 (ABCC3) | BLM | ATP-dependent export of BA, BA sulfates/glucuronides |
| MRP4 (ABCC4) | BLM | ATP-dependent export of BA |
| OSTα-OSTβ | BLM | BA export |
| Cholangiocyte | ||
| Process: Ductular Secretion and Bile Remodeling | ||
| ASBT (SLC10A2) | APM | Bile acid uptake (cholehepatic shunt) |
| OSTα-OSTβ | BLM | Bile acid export |
| MRP3 (ABCC3) | BLM | Bile acid export of BA, BA sulfates/glucuronides |
| Renal Proximal Tubule Cell | ||
| Process: Reclamation of Bile Acids from the Renal Tubules | ||
| ASBT (SLC10A2) | APM | Bile acid uptake |
| OSTα-OSTβ | BLM | Bile acid export |
| MRP3 (ABCC3) | BLM | ATP-dependent export of BA, BA sulfates/glucuronides |
| MRP4 | BLM | ATP-dependent BA export |
| Ileal Enterocyte | ||
| Process: Reclamation of Bile Acids from the Intestinal Lumen | ||
| ASBT (SLC10A2) | APM | Bile acid uptake |
| OSTα-OSTβ | BLM | Bile acid export |
| MRP3 (ABCC3) | BLM | Bile acid export |
ABC, ATP-binding cassette; ASBT, apical sodium bile acid transporter; BA, bile acid; BLM, basolateral membrane; BSEP, bile salt export pump; CM, canalicular membrane; MDR, multidrug resistance protein; MRP, multidrug resistance–associated protein; NTCP, Na+-taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; OST, organic solute transporter; SLC, solute carrier.
2. Overview of Intestinal Bile Acid Transport
Bile acids are reclaimed through a combination of passive absorption in the proximal small intestine, active transport in the distal ileum, and passive absorption in the colon. Several observations support the concept that the terminal ileum is the major site of bile acid reabsorption in man and experimental animal models. These observations include the finding that there is little decrease in intraluminal bile acid concentration prior to the ileum (Dietschy 1968) and the appearance of bile acid malabsorption after ileal resection (Hofmann and Poley 1972). Subsequent studies using in situ perfused intestinal segments to measure bile acid absorption (Marcus et al. 1991; Aldini et al. 1994; Aldini et al. 1996) demonstrated that ileal bile acid transport is a high capacity system sufficient to account for the hepatobiliary output of bile acids. The general consensus from these studies was that ileal active transport is the major route for conjugated bile acid uptake, whereas the intestinal passive or facilitative absorption may be significant for unconjugated and some glycine-conjugated bile acids. The ileal apical sodium-dependent bile acid cotransporter (abbreviated ASBT; gene symbol, SLC10A2) mediates the initial uptake of bile acids across the ileal enterocyte apical brush border membrane (Dawson et al. 2009). After entering the cytosolic compartment, the bile acids bind to the ileal lipid binding protein (abbreviated ILBP; also called the ileal bile acid binding protein or IBABP; gene symbol, FABP6), an abundant 14 kDa soluble protein (Oelkers and Dawson 1995). ILBP is believed to be involved in the transcellular transport of bile acids (Lin et al. 1990; Kramer et al. 1993; Kramer et al. 1997) or possibly protection of the enterocyte from the cytotoxic properties of bile acids. The Farnesoid X-receptor (FXR) null mouse, which lacks appreciable intestinal ILBP expression, exhibited normal levels of intestinal bile acid absorption. These results suggest that ILBP is not essential for this intestinal bile acid transporter (Kok et al. 2003). Nevertheless, resolving the questions regarding ILBP’s function will require further study, including the generation and analysis of Fabp6-null mice. Regardless of their intracellular route, the bile acids are ultimately shuttled across the ileal enterocyte (Lewis and Root 1990) and exported across the basolateral membrane into the portal circulation by the heteromeric Organic Solute Transporter, OSTα-OSTβ (Ballatori et al. 2005; Dawson et al. 2005).
3. The Apical Sodium-dependent Bile Acid Transporter: ASBT
3.1. ASBT General Properties and Tissue Expression
ASBT (also called IBAT, ISBT, ABAT; gene symbol SLC10A2) was the second member identified of the SLC10 family of solute carrier proteins. The SLC10 family is comprised of six members, SLC10A1 (NTCP), SLC10A2 (ASBT), SLC10A3 (P3), SLC10A4 (P4), SLC10A5 (P5), and SLC10A6 (SOAT), that share between 19 and 42% amino acid sequence identity (Geyer et al. 2006). SLC10A1 (NTCP) and SLC10A2 (ASBT) are the best characterized family members and have important physiological functions as bile acid transporters (Hagenbuch and Dawson 2004). The related SLC10A6 (SOAT) transports steroid sulfates but not bile acids (Geyer et al. 2007), and little is known about the physiological function, substrates, or transport properties of SLC10A3, SLC10A4, and SLC10A5 (Geyer et al. 2006; Splinter et al. 2006).
ASBT is expressed at tissue sites that enable the enterohepatic circulation of bile acids, including the apical membrane of ileal enterocytes, proximal renal convoluted tubule cells, large cholangiocytes, and gallbladder epithelial cells (Wong et al. 1994b; Christie et al. 1996a; Alpini et al. 1997b; Lazaridis et al. 1997a; Chignard et al. 2001). In the intestine, sodium-dependent bile acid transport activity and ASBT expression is found predominantly in villus but not crypt enterocytes (Kapadia and Essandoh 1988; Shneider et al. 1995). ASBT expression in small intestine is restricted to the terminal ileum (distal ~30% of the small intestine) in the mouse, rat, hamster, and monkey, with negligible expression in proximal small intestine (Wong et al. 1994a; Shneider et al. 1995; Dawson et al. 2005). For humans, several lines of evidence suggest that the gradient of expression along the longitudinal axis of the intestine is qualitatively similar with highest levels of ASBT expression in terminal ileum. This evidence includes intestinal perfusion studies demonstrating active bile acid absorption in human ileum but not in proximal small intestine (Krag and Phillips 1974), and studies showing that ASBT mRNA or protein expression is higher in ileum than proximal intestine or colon (Hruz et al. 2006; Meier et al. 2007; Balesaria et al. 2008). Unlike rodents or monkeys, humans express low but readily detectable levels of ASBT in duodenum (Hruz et al. 2006; Balesaria et al. 2008). Nevertheless, the low level of ASBT expression in proximal intestine is insufficient to maintain the enterohepatic circulation of bile acids as evidenced by the clinically significant bile acid malabsorption following ileal resection (Hofmann and Poley 1972). The factors that control ASBT expression along the longitudinal (cephalocaudal) axis of the small intestine are not well understood. But the recent discovery that the transcription factor GATA4 is essential for silencing ASBT expression in proximal small intestine has provided an important new insight to underlying mechanism (Bosse et al. 2006; Battle et al. 2008).
In addition to small intestine, ASBT is expressed in renal proximal tubule cells (Christie et al. 1996b; Craddock et al. 1998) and biliary epithelium (Alpini et al. 1997a; Lazaridis et al. 1997b). In the kidney, the ASBT acts as a salvage mechanism to prevent urinary excretion of bile acids that have undergone glomerular filtration (Wilson et al. 1981). In the ileum and kidney, ASBT functions to absorb the majority of the bile acids available in the adjacent lumen (almost quantitative reclamation). Only a very small fraction escapes absorption and is excreted in the feces or urine. In contrast, almost all the bile acids that are secreted by the liver into bile will ultimately move down the biliary tract and enter the gallbladder or small intestine, indicating that ASBT’s function in this compartment is not quantitative reclamation of bile acids. As such, the physiological significance of active bile acid absorption from the biliary tract is unclear. One possibility is that the ASBT functions in the cholehepatic shunt pathway. The term cholehepatic shunt was originally coined by Alan Hofmann to describe the cycle whereby unconjugated dihydroxy bile acids secreted into bile are passively absorbed by the epithelial cells (cholangiocytes) lining the bile ducts, returned to the hepatocyte via the periductular capillary plexus, and resecreted into bile (Gurantz et al. 1991). Absorption of the protonated unconjugated bile acid molecule generates a bicarbonate anion, resulting in a bicarbonate-rich choleresis. Premature absorption and resecretion of the bile acid also promotes bile formation by increasing bile acid–dependent bile flow. This cycle explains the hypercholeresis (increased bile secretion) observed for unconjugated C-24 dihydroxy bile acids such as UDCA, for unconjugated C-23 bile acid analogs such as nor-ursodeoxycholate (norUDCA) (Halilbasic et al. 2009), and for certain drugs such as the nonsteroidal anti-inflammatory drug sulindac (Hofmann et al. 2005). Since the original description of the cholehepatic shunt pathway included only a passive component, its physiological significance for hepatic secretion was unclear. While exogenously administered UDCA or norUDCA may only be partially conjugated to glycine or taurine, the endogenous bile acids are efficiently conjugated by the liver prior to secretion by the bile salt export pump (BSEP) into bile. As such, the majority of the bile acids in the biliary tract are ionized and unable to diffuse passively across the biliary epithelium. The finding that ASBT and the basolateral bile acid transporter OSTα-OSTβ are expressed by the biliary epithelium provide an important physiological mechanism for cholehepatic shunting of ionized conjugated bile acids (Alpini et al. 1997a; Lazaridis et al. 1997b; Ballatori et al. 2005). In vivo data in support of this pathway for conjugated bile acids has been obtained using a rat model (Alpini et al. 2005), but it’s quantitative significance in humans or under different physiological or pathophysiological conditions still remains to be determined (Xia et al. 2006). Nevertheless, this is an area of increased interest following recent exciting preclinical results obtained for norUDCA as a therapy for Primary Biliary Sclerosis (PSC) (Fickert et al. 2006), and renewed intensive investigation should yield important insights to the physiological role and therapeutic potential of the cholehepatic shunt pathway (Glaser and Alpini 2009).
3.2. ASBT Structure
ASBT is a 348 amino acid membrane glycoprotein with a glycosylated extracellular amino terminus and cytosolic carboxyl terminus, indicating an odd number of transmembrane domains. Early membrane topology models favored seven transmembrane segments for the ASBT and NTCP (Hagenbuch and Meier 1994; Dawson and Oelkers 1995), however subsequent in vitro translation/membrane insertion scanning and alanine-scanning mutagenesis studies carried out by Stefan Hallén and George Sachs yielded conflicting results unable to distinguish between models with seven or nine membrane-spanning regions (Hallen et al. 1999; Hallen et al. 2002; Mareninova et al. 2005). Peter Swaan and colleagues ultimately resolved this question by providing irrefutable evidence supporting a seven transmembrane segment model for the ASBT (Zhang et al. 2004; Banerjee and Swaan 2006). Those studies included the introduction of N-linked glycosylation sites at residues 113–118 (loop 1) and 266–272 (loop 3) (Zhang et al. 2004), and dual epitope insertion scanning mutagenesis (Banerjee and Swaan 2006).
In contrast to its primary structure, the subunit stoichiometry and assembly of the functional ileal bile acid transporter complex is poorly understood. Extensive photoaffinity labeling and SDS-PAGE studies using monomeric and dimeric bile acid analogues labeled a variety of proteins in ileal brush border membrane preparations, but predominantly a 93 kDa protein, a dimer of the ASBT, and a 14 kDa protein identified as the cytosolic ileal lipid binding protein (ILBP; IBABP; FABP6) (Kramer et al. 1993; Kramer et al. 1997; Kramer et al. 1998). Furthermore, radiation inactivation studies found large target sizes for the bile acid transporters in ileal brush border and hepatic sinusoidal membranes, suggesting that the functional units are multimeric complexes. The apparent sizes of the transporters determined by cloning, photoaffinity labeling, and radiation inactivation are summarized in Table 6.2. The molecular mass differences between the cloned transporters and the large functional complexes are difficult to interpret in these crude systems. However, the results are generally consistent with the ASBT functioning as a homomultimer or perhaps even a heteromultimer that includes the ILBP. Alternatively, the large target sizes may reflect interaction of the transporters with specialized regions of plasma membrane such as lipid rafts (Annaba et al. 2008). The requirement for other protein subunits is also unclear and needs to be weighed in light of the widespread observation that expression of the ASBT alone in heterologous systems such as Xenopus oocytes, COS, CHO, and MDCK cells is sufficient to recapitulate membrane targeting and robust sodium-dependent bile acid transport (Craddock et al. 1998; Balakrishnan et al. 2006b). So in contrast to the basolateral bile acid transporter OSTα-OSTβ (discussed below), ASBT can function as a monomer or homomultimer.
Table 2.
Determination of Bile Acid Transporter Size in Ileum and Liver
| Method of Analysis | Ileum (ASBT) |
Reference | Liver (NTCP) |
Reference |
|---|---|---|---|---|
| Cloning: Native protein Glycosylated protein |
38 kDa 40–50 kDa |
(Wong et al. 1994a) (Wong et al. 1995) (Shneider et al. 1995) |
38 kDa 40–50 kDa |
(Hagenbuch et al. 1991) (Hagenbuch and Meier 1994) |
| Photoaffinity Labeling | 93 kDa | (Kramer et al. 1993) | 46 kDa | (Kramer et al. 1982) |
| Radiation Inactivation/Photoaffinity Labeling | 230 kDa | (Kramer et al. 1995) | ND | ND |
| Radiation Inactivation/Bile Acid Transport | 450 kDa | (Kramer et al. 1995) | 170 kDa | (Elsner and Ziegler 1989) |
ND, not determined.
3. ASBT Structure-Function Relationships
A variety of approaches have been taken to identify protein regions and sequences important for transport function within the ASBT and related SLC10 family members, and these structure-activity relationships have been reviewed recently (Balakrishnan and Polli 2006; Geyer et al. 2006; Sievanen 2007). Werner Kramer and coworkers used a combination of affinity labeling with photolabile bile acid analogs, enzymatic fragmentation, and epitope-specific antibodies to identify the terminal 67 amino acids of the rabbit Asbt as a region that strongly interacts with the bile acid 7-hydroxy position (Kramer et al. 2001). Several groups have carried out general mutagenesis studies that targeted cysteine residues, negatively charged amino acids, and threonine residues in the ASBT and NTCP as a first step toward identifying functionally important regions (Hallen et al. 2000; Zahner et al. 2003; Sun et al. 2006). Peter Swaan and coworkers have carried out the most systematic examination of ASBT structure-function relationships. In the absence of a crystal structure for the ASBT, this group used the structure of bacteriorhodopsin (PDB 1AT9) as a scaffold for ASBT modeling studies (Zhang et al. 2002; Zhang et al. 2004). This detailed model served as a guide for the subsequent mutagenesis studies. The experimental strategies included using a combination of charged, exoplasmic specific methanethiosulfonates and site-directed mutagenesis, substitute cysteine accessibility mutagenesis, different bile acid substrates, and high affinity specific inhibitors to finely map the solute binding site and translocation sites in the ASBT (Hallen et al. 2000; Hallen et al. 2002; Banerjee et al. 2005; Hussainzada et al. 2006; Ray et al. 2006; Hussainzada et al. 2008; Khantwal and Swaan 2008).
The interaction of sodium with NTCP and ASBT was studied using similar mutagenesis strategies that focused on the negatively charged amino acids (Zahner et al. 2003; Sun et al. 2006). These studies implicated similar negatively charged residues in extracellular loop 1 (Asp115 in rat Ntcp, Asp122 in rat Asbt) and extracellular loop 3 (Glu257 in rat Ntcp, Glu261 in rat Asbt) as potential extracellular sodium sensors. Again, those observations were extended and refined by Swaan and coworkers for the human ASBT (Banerjee et al. 2008; Hussainzada et al. 2008; Hussainzada et al. 2009). In aggregate, the results are consistent with a model where the larger extracellular loops 1 and 3 and the exofacial half of transmembrane segment 7 participate directly in bile acid binding and substrate entry/translocation. Complementing those results are very recent findings suggesting that the cytosolic half of transmembrane segment 3 forms part of the substrate exit route (Hussainzada et al. 2009).
3.4. ASBT Substrate Specificity and Native Bile Acid Pharmacophore Models
ASBT functions as an electrogenic sodium-solute cotransporter, moving 2 or more sodium ions per molecule of solute (Weinman et al. 1998). In contrast to the strict requirement for sodium (i.e. other cations such potassium, lithium, rubidium, cesium cannot substitute for sodium), there is no apparent anion specificity, arguing against a role for a cotransported anion (Craddock et al. 1998). The driving force for solute transport is provided by the inwardly directed sodium gradient maintained by the basolateral Na+, K+-ATPase as well as the negative intracellular potential. Studies using patch clamped transfected cells demonstrated that ASBT can mediate bidirectional bile acid transport and the directionality was determined by the sodium-gradient and membrane potential; uptake was voltage-dependent and stimulated by a negative intracellular potential (Weinman et al. 1998). While these studies provided important insights regarding the mechanism of transport, it should be noted that transmembrane sodium and electrical gradients under physiological conditions force the ASBT to operate solely as an uptake mechanism for bile acids.
ASBT’s major physiological substrates include the major unconjugated bile acids, cholic acid, deoxycholic acid, chenodeoxycholic acid, and ursodeoxycholic acid, as well as their glycine and taurine-conjugates (Craddock et al. 1998; Kramer et al. 1999). There is limited published information regarding transport of sulfated or glucuronidated bile acid conjugates by the ASBT. However, results from studies using in situ perfused guinea pig ileum (De Witt and Lack 1980) or human ASBT-transfected cells (Craddock et al. 1998) indicated that sulfated di-and trihydroxy bile acids were poor substrates. Based on the known structure-activity relationships for ASBT substrates, it is also predicted that glucuronidated bile acids are not ASBT substrates (Kramer et al. 1999). To date, no non-bile acid transport substrate has been identified for the ASBT. This contrasts with the related liver bile acid transporter NTCP, which also transports estrone-3-sulfate (Craddock et al. 1998; Ho et al. 2004) and the HMG CoA reductase inhibitor, rosuvastatin (Ho et al. 2006).
Leon Lack and colleagues first examined the solute structure characteristic important for the ileal sodium-dependent bile acid transporter using intestinal perfusions and everted gut sac models (Lack and Weiner 1966; Lack et al. 1970; Bundy et al. 1977; Lack 1979). Based on these findings, Lack proposed a hypothetical model for the ASBT substrates that included: 1) a negatively charged side chain for coulombic interaction with a positively charged moiety in the ASBT, 2) at least one axial hydroxyl group on the steroid nucleus at positions 3, 7 or 12, such that trihydroxy bile acids are better transported than dihydoxy bile acids, and 3) a cis configuration of the cyclohexyl rings A and B of the steroid nucleus (Lack 1979). With cloning of the ASBT by Dawson and coworkers (Wong et al. 1994a; Wong et al. 1995), cell-based transport assays became possible using cell lines transfected with human ASBT or Asbt from other species (Craddock et al. 1998; Kramer et al. 1999; Balakrishnan et al. 2006b). This permitted an in depth analyses of ASBT substrate specificity and resulted in significant changes to the original Lack model. The new results confirmed the requirement for the α-hydroxyl groups at the 7 and 12 positions. However, no requirement was found for a cis configuration of the A/B rings of the steroid nucleus or a 3α-hydroxyl group. The new findings included: 1) 6-hydroxylation (a common modification found of rodent bile acids such as α, β, or ω-muricholic acid and hyodeoxycholic acid) dramatically reduced transport, and 2) glycine or taurine conjugation enhanced affinity, as did the presence of fewer hydroxyl groups on the steroid nucleus such that the rank ordering of affinity for the ASBT was monohydroxy > dihydroxy > trihydroxy (Balakrishnan and Polli 2006; Balakrishnan et al. 2006b). Kramer and coworkers went on to develop a detailed 3D pharmacophore (QSAR) conformational model for rabbit Asbt substrates using training sets of various bile acid-based inhibitors and the CATALYST software (Baringhaus et al. 1999). These results extended the predictability of the pharmacophore model generated earlier using comparative molecular field analysis and a more limited set of bile acid analogs (Swaan et al. 1997b). Modification of the bile acid side chain on transport was also explored in these early studies (Lack et al. 1970; Kramer et al. 1999) and more recently in studies by James Polli and colleagues (Tolle-Sander et al. 2004; Balakrishnan et al. 2006a), who generated a conformationally sampled pharmacophore structure activity relationship model to predict these interactions (Zheng et al. 2009). This work indicated that shortening of the side chain by one methylene group (a nor-bile acid) decreased transport. While monoanionic conjugates were favored for ASBT-mediated transport, the presence of a single negative charge at the C24 position was not essential for interaction with the ASBT (Balakrishnan et al. 2006a). Notable with regard to prodrug development, these transport and modeling studies showed that the 3α-hydroxy group in natural bile acids is not essential for interaction with the ASBT or NTCP and that the steroid A-ring, preferably the 3 position, could serve as an attachment site for compounds to target their delivery via the sodium-dependent bile acid transporters (Sievanen 2007). These relationships are discussed further in the later section on targeting the ASBT for prodrug delivery.
A limitation of these earlier modeling studies was that the training sets of compounds included only bile acids, bile acid derivatives, and benzothiazaepine-based ASBT inhibitors rather than larger sets of chemical structures or FDA-approved drugs. This limitation has been addressed by a recent elegant transport and modeling study by James Polli and coworkers that examined the interaction of a large set of FDA approved drugs with the ASBT (Zheng et al. 2009). These findings are discussed in more detail in the later section on the role of ASBT and OSTα-OSTβ in drug absorption and drug interactions.
3.5. ASBT Genomics and Pathophysiology
The SLC10A2 gene is organized in six exons spanning approximately 24 kilobases of DNA sequence on human chromosome 13q33. The first exon encodes the 5’ untranslated region and amino acids 1 to 126. Exons 2 to 6 encompass the remaining coding sequence (amino acids 126 to 348) and exon 6 includes a long 3’ untranslated region (Oelkers et al. 1997). Analysis of the SLC10A2 gene has revealed a variety of single nucleotide polymorphisms (SNPs) and dysfunctional mutations. The coding SNPs previously identified in the ASBT gene are indicated in Figure 6.2. These sequence variations are discussed below in the context of the pathophysiology associated with ASBT defects.
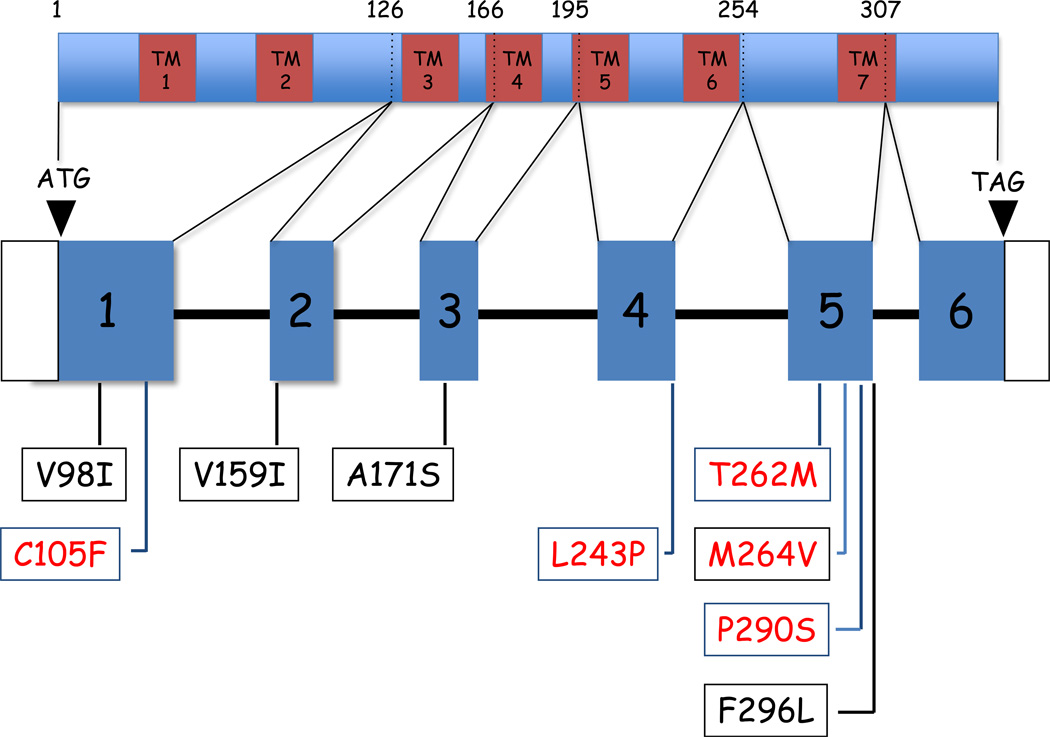
Figure 2. Location of coding region single nucleotide polymorphisms and mutations in the human ileal bile acid transporter gene (SLC10A2).
A schematic diagram depicting the human ASBT protein and gene is shown. The amino acid positions disrupted by the intron/exon junctions are indicated above the schematic of the ASBT protein. The seven predicted transmembrane domains (TM1–TM7) are shown as brown bars. The location of coding region polymorphisms (V98I, V159I, A171S, F296L), and dysfunctional bile acid transporter mutations (C105F, L243P, T262M, M264V, P290S) identified to date is indicated below each exon.
The enterohepatic circulation efficiently conserves and compartmentalizes bile acids, thereby maintaining bile flow and adequate intraluminal bile acid concentrations for micellular solubilization and absorption of lipids. Considering its central role in the enterohepatic circulation, inherited defects or dysfunctional regulation of the ASBT may play a role in the pathogenesis or clinical presentation of a variety of gastrointestinal disorders. Indeed, in the course of cloning and characterizing the SLC10A2 gene, the first dysfunctional mutation (P290S) associated with diminished transporter activity was identified in a single patient diagnosed with Crohn’s disease (Wong et al. 1995). ASBT mutations were later identified as a cause of Primary Bile Acid Malabsorption (PBAM; OMIM:601295), a rare idiopathic disorder associated with interruption of the enterohepatic circulation of bile acids, chronic diarrhea beginning in early infancy, steatorrhea, fat-soluble vitamin malabsorption, intracranial hemorrhage (as a result of the vitamin K deficiency), and life-long reduced plasma cholesterol levels (Heubi et al. 1982; Oelkers et al. 1997). The affected PBAM patient was heterozygous for a splice junction mutation on one allele as well as two dysfunctional missense mutations, L243P and T262M, on the other allele (Oelkers et al. 1997). In contrast to PBAM, ASBT mutations have not been found in patients with adult-onset bile acid malabsorption, chronic diarrhea, and a morphologically functional ileum, a condition known as Idiopathic Bile Acid Malabsorption (IBAM) (Montagnani et al. 2001; Montagnani et al. 2006). For IBAM patients, it is still unclear as to whether altered regulation of the ASBT contributes to the phenotype in a subset of those patients (Balesaria et al. 2008). Indeed, a recent genetic study identified an ASBT haplotype associated with significantly reduced ileal expression of ASBT mRNA and protein (Renner et al. 2009).
Other disorders associated with intestinal bile acid malabsorption that potentially involve the ASBT include Familial Hypertriglyceridemia (Angelin et al. 1987; Duane et al. 2000; Love et al. 2001), Idiopathic Chronic Diarrhea (Schiller et al. 1987), Chronic Ileitis (Meihoff and Kern 1968), Cholesterol and Black Pigment Gallstone disease (Vitek and Carey 2003; Holzer et al. 2008), Postcholecystectomy Diarrhea (80), Crohn’s disease (Krag and Krag 1976; Farivar et al. 1980; Tougaard et al. 1986; Nyhlin et al. 1994; Fujisawa et al. 1998), Irritable Bowel Syndrome (Camilleri et al. 2009), and susceptibility to Colon Cancer (Wang et al. 2001b). In the example of Familial Hypertriglyceridemia (FHTG), a screen of 20 FHTG patients with abnormal bile acid metabolism identified only a single patient with a frame-shift mutation at codon 216 (646insG) (Love et al. 2001). Thus, as with IBAM, dysfunctional mutations of the SLC10A2 gene were largely excluded as a common genetic cause of decreased ASBT expression in hypertriglyceridemia. In the case of gallstone disease, ASBT expression is decreased in non-obese gallstone patients and associated with decreased expression of ILBP and OSTα-OSTβ (Bergheim et al. 2006; Renner et al. 2008). Considering that the ileal transport system is a major determinant of bile acid return in the enterohepatic circulation, reduced ASBT expression could decrease hepatic bile acid secretion, increase the cholesterol saturation of bile and promote cholesterol gallstone formation (Portincasa et al. 2006). Bile acid malabsorption has also been associated with black pigment (bilirubin) gallstones (Brink et al. 1996; Brink et al. 1999; Vitek and Carey 2003). The postulated mechanism is that increased bile acid concentrations in the colon results in solubilization of the precipitated bilirubin glucuronide conjugates, deconjugation by the colonic flora, and passive absorption. Analysis of the ASBT coding region in 39 gallstone patients (mixture of cholesterol and pigment gallstone patients) in a pilot study identified only a single pigment gallstone patient with a dysfunctional missense mutation (C105F) (Montagnani, Carey, and Dawson, unpublished results), suggesting that ASBT coding region SNPs are not a common genetic contributor to black pigment gallstones.
Major limitations of these earlier studies included insufficient patients and the fact that only small portions of the SLC10A2 gene was examined. With the availability of improved SNP maps, high-density SNP arrays, and technical breakthroughs in DNA sequencing technology, genetic analysis of large numbers of subjects is now tractable. These same tools are used in Genome-Wide Association Studies (GWAS), an unbiased powerful approach to identify genetic factors such as transporter genes that influence disease phenotypes, response to therapy, or quantitative traits. In fact, a recent study of genome wide association study designed to identify genetic contributors to variability in serum bilirubin levels showed a weak association with the SLC10A2 locus in addition to the highest scoring loci, UGT1A1 and SLCO1B1 (Johnson et al. 2009). So while the relationship between common polymorphisms in the ASBT and drug metabolism remains to be carefully explored, the tools for such analysis are now readily available.
4. The Basolateral Bile Acid and Organic Solute Transporter: OSTα-OSTβ
4.1. OSTα-OSTβ General Properties and Tissue Expression
The proteins responsible for bile acid export across the basolateral membrane of the ileal enterocyte, cholangiocytes, and renal proximal tubule cell have only recently been identified. The break-through in this area came with the elegant expression cloning of an unusual transporter, OSTα-OSTβ, from the little skate (Raja erinacea) by Ballatori and coworkers (Wang et al. 2001a). Subsequently, the human and mouse orthologs of skate OSTα-OSTβ were cloned and expressed in Xenopus oocytes where they transport bile acids as well as a variety of steroids (Seward et al. 2003). As with the skate, solute transport by the human ortholog required co-expression of two different subunits: OSTα and OSTβ. The human OSTα gene encodes a 340 amino acid polytopic membrane protein with an extracellular amino terminus, 7 predicted transmembrane domains, and a cytoplasmic carboxyl terminus. The human OSTβ, encodes a 128 amino acid predicted type I membrane protein with an extracellular amino terminus and cytoplasmic carboxyl terminus. While the functional role of the individual subunits has not yet been determined, co-expression and assembly of both subunits into a complex is required for their trafficking to the plasma membrane and solute transport (Dawson et al. 2005; Li et al. 2007). In contrast to the ASBT, no systematic studies of OSTα-OSTβ structure-function relationships have been published yet.
OSTα-OSTβ was identified as a candidate ileal basolateral bile acid transporter using a transcriptional profiling approach (Dawson et al. 2005). Support for a role of OSTα-OSTβ in basolateral bile acid transport includes: 1) intestinal expression of OSTα and OSTβ mRNA that generally follows that of the ASBT, with highest levels in ileum (Ballatori et al. 2005; Dawson et al. 2005; Balesaria et al. 2008), 2) appropriate cellular localization on the lateral and basal plasma membranes of ileal enterocyte (Dawson et al. 2005), 3) expression of OSTα-OSTβ on the basolateral plasma membrane of hepatocytes, cholangiocytes, and renal proximal tubule cells, other cells important for bile acid transport (Ballatori et al. 2005), 4) efficient transport of the major bile acid species (Ballatori et al. 2005; Dawson et al. 2005), and 5) positive regulation of OSTα-OSTβ expression by bile acids via activation of farnesoid X-receptor (FXR) (Frankenberg et al. 2006; Landrier et al. 2006), and 6) targeted inactivation of the Ostα gene in mice results in impaired intestinal absorption of bile acids and impaired bile acid homeostasis (Ballatori et al. 2008; Rao et al. 2008).
In contrast to the ASBT, which operates as an electrogenic sodium-cotransporter, the mechanism for OSTα-OSTβ mediated transport has not been fully elucidated (Ballatori 2005; Ballatori et al. 2005). When expressed in Xenopus laevis oocytes, OSTα-OSTβ mediated transport was unaffected by depletion of intracellular ATP, by alterations in transmembrane electrolyte concentration gradients, or by changes in the pH gradient (Ballatori et al. 2005). OSTα-OSTβ exhibits both solute uptake and efflux properties and transport is trans-stimulated by known substrates (Ballatori et al. 2005; Dawson et al. 2005). The general consensus from these studies is that OSTα-OSTβ operates by facilitated diffusion and mediates solute uptake or efflux, depending on the solute’s electrochemical gradient.
Another important difference between the ASBT and OSTα-OSTβ lies in their substrate specificities. While no non-bile acid substrates have been identified for the ASBT, a variety of compounds in addition to the major taurine and glycine-conjugated bile acids have been identified as substrates for OSTα-OSTβ (Ballatori et al. 2005). A systematic screen to identify OSTα-OSTβ transport substrates has not yet been published. Nevertheless, the existing list of OSTα-OSTβ non-bile acid substrates includes estrone-3-sulfate, digoxin, prostaglandin E2, and dehydroepiandrosterone-3-sulfate. The list of inhibitors of OSTα-OSTβ mediated transport of taurocholate or estrone-3-sulfate is also broad and includes a variety of compounds such as spironolactone, bromosulfophthalein, probenecid, and indomethacin (Seward et al. 2003). While preliminary, these results suggest that the substrate specificity for OSTα-OSTβ is relatively broad and is consistent with a direct role of OSTα-OSTβ in drug transport.
In humans, the expression of OSTα and OSTβ generally parallel one another with highest levels in small intestine, liver, kidney, and testis (Seward et al. 2003; Ballatori et al. 2005). Lower levels of OSTα and OSTβ mRNA are also detected by real time PCR in other human tissues including colon, adrenal gland, ovary, with lowest levels in heart, lung, brain, pituitary gland and prostate. Analysis of samples from mouse small intestine, cecum and colon, and human ileum showed that expression of OSTα and OSTβ mRNA are highly correlated (Frankenberg et al. 2006) (Renner et al. 2008); in human ileal biopsy samples, a strong positive correlation was also observed between mRNA expression and protein levels for OSTα and OSTβ (Renner et al. 2008). An important tissue expression difference between rodents and humans is the significantly higher level of OSTα-OSTβ expression in human liver. Under basal conditions, Ostα and Ostβ mRNA expression is almost undetectable in mouse, rat, or hamster liver, but is readily measured in primate and human liver. In humans and rodents, OSTα-OSTβ expression is induced dramatically in response to cholestasis (Boyer et al. 2006; Zollner et al. 2006).
4.2. OSTα-OSTβ Genomics and Pathophysiology
The OSTα and OSTβ genes are encoded on human chromosomes 3q29 and 15q22, respectively. Both are relatively small genes, with OSTα consisting of 9 exons spanning approximately 17 kb and OSTβ consisting of 4 exons spanning approximately 8 kb of DNA. Examination of the most recent Ensembl database for OSTα shows 3 non-synonymous SNPs resulting in the following conservative amino acid changes, V197G, V202I, and R241H; the frequency and functional significance of these SNPs has not been determined. No non-synonymous SNPs have yet been reported for OSTβ.
No inherited defects have been reported for the OSTα or OSTβ genes in humans, however targeted inactivation of the Ostα gene in mice resulted in impaired intestinal bile acid absorption and altered bile acid metabolism (Ballatori et al. 2008; Rao et al. 2008). As predicted for a major intestinal basolateral bile acid transporter, studies using everted gut sacs (Rao et al. 2008) or intra-ileal administration of [3H]taurocholate (Ballatori et al. 2008) demonstrated a significant reduction in trans-ileal transport in Ostα null mice. However fecal bile acid excretion was not increased in Ostα null mice, as had been observed in Asbt (Slc10a2) null mice (Dawson et al. 2003). These results were particularly perplexing since the whole body bile acid pool size was significantly decreased (Rao et al. 2008), a hallmark of intestinal bile acid malabsorption (Oelkers et al. 1997; Jung et al. 2007). Examination of the Fibroblast growth factor (FGF)15/19 signaling pathway (Inagaki et al. 2005) provided a solution to this conundrum. In the Ostα null mice, bile acids are taken up by the ileal enterocyte but their efflux across the basolateral membrane is impaired. As a result, bile acids accumulate within the ileal enterocyte, constitutively activating FXR and inducing greater expression and secretion of FGF15/19 into the portal circulation. FGF15/19 then signals at the hepatocyte to down-regulate hepatic bile acid synthesis. The net result is that hepatic bile acid synthesis is paradoxically repressed rather than induced, the normal physiological response to a block in intestinal bile acid absorption (Davis and Attie 2008; Rao et al. 2008).
5. Development of ASBT Inhibitors
As mentioned in the previous section, disruption of the bile acid enterohepatic circulation normally stimulates de novo synthesis of bile acids in the liver. The resulting demand for cholesterol by the liver is met by increasing the number of hepatic LDL receptors to clear additional plasma LDL, and by increasing hepatic cholesterol synthesis (Brown and Goldstein 1986). If the malabsorption is significant, hepatic bile acid production may be unable to compensate for bile acid loss, leading to decreased bile acid concentrations in the intestinal lumen and a reduced ability to solubilize and absorb biliary and dietary cholesterol. The net result is a decrease in plasma total and LDL cholesterol levels. This is the basis for the reduced morbidity and mortality from cardiovascular disease associated with ileal resection in the POSCH study (Program on the Surgical Control of Hyperlipidemias) (Buchwald et al. 1990) or from the use of polymeric bile acid sequestrants, such as cholestyramine, or colestipol (1984; Bays and Goldberg 2007). The bile acid sequestrants are large polymeric resins, and as such are nonabsorbable and have few systemic side effects. Prior to the development of the HMG CoA reductase inhibitors (Statins), the bile acid sequestrants were one of the mainstays of therapy for hypercholesterolemia (Bays and Goldberg 2007). However, gastrointestinal side effects such as constipation, indigestion, dyspepsia, and flatulence are very common and together with the high doses required (15–30 g/day) adversely affect patient compliance and the efficacy of these agents. The recent development of a higher affinity bile acid binder Colesevelam decreased the required dose (Steinmetz and Schonder 2005), but the efficacy, patience compliance, and side effect profile still favors the use of statins as a first-line therapy. Based on its remarkable substrate specificity and expression on the intestinal brush border membrane, blocking the ASBT using high affinity, nonabsorbable inhibitors represented an attractive strategy and alternative to the sequestrants for treatment of hypercholesterolemia (Kramer and Glombik 2006).
The general properties of the ASBT inhibitors are summarized in Table 6.3. These compounds fall into two general classes, bile acid-derivatives including bile acid dimers, and non-bile acid compounds including benzothiazepine and benzothiepine analogs. The first compounds termed Bile Acid Reabsorption Inhibitors (BARI) were developed by Werner Kramer and colleagues at Hoechst AG (now part of Sanofi-Aventis) in Frankfurt (Wess et al. 1994). This group developed a wide variety of dimeric and trimeric bile acids and extensively characterized their interaction with the ASBT using in situ ileal perfusions, isolated ileal brush border membranes, ASBT-transfected cell lines, and bile acid photoaffinity labeling (Baringhaus et al. 1999; Kramer and Glombik 2006). This mechanistic approach utilized very elegant chemistry, but little was ever published regarding the ability of these compounds to reduce plasma LDL cholesterol levels or prevent the development of atherosclerosis in animal models. Nevertheless, newer bile acid derivatives have been developed as potential ASBT inhibitors by Marin (Vicens et al. 2007) as well as by Polli (Gonzalez et al. 2009), and this general strategy remains an area of interest.
Table 6.3.
Specific ASBT Inhibitors
| Compound | IC50 | Manufacturer | Description (references) |
|---|---|---|---|
| S9060 | ~30 µM | Hoechst AG (Aventis) | Bile acid dimer; (Wess et al. 1994; Baringhaus et al. 1999) |
| R-146224 | ~0.02 µM | Sankyo | Amphiphilic 4-oxo-1-phenyl-1,4-dihydroquinoline derivative; (Kurata et al. 2004; Kitayama et al. 2006) |
| S-8921 | ~66 µM | Shionogi | Substituted napthol derivative; (Hara et al. 1997); glucuronidation significantly increases potency (Sakamoto et al. 2007) |
|
2164U90; 264W94 |
~7 µM ~0.4 µM |
Burroughs Wellcome (GlaxoSmithKline) | Benzothiazepine derivative; (Root et al. 1995; Root et al. 2002) |
| SC-435 | ~2 nM | Monsanto-Searle (Pfizer) | Benzothiepin derivative; (Bhat et al. 2003; Huang et al. 2005; Tremont et al. 2005) |
| PR835 | ~0.15 µM | AstraZeneca | Benzothiazepine derivative; (Galman et al. 2003) |
Researchers working at several Japanese pharmaceutical companies developed a variety of ASBT inhibitors. For example, researchers at Tanabe Seiyaku (Osaka, Japan) described their investigations of a series of arylnapthelene lignans for their hypocholesterolemic properties. The compound TA-7552 [1-(3,4-dimethoxyphenyl)-2,3-bis(methoxycarbonyl)-4-hydroxy-6,7,8-trimethoxynaphthalene] was effective in increasing fecal bile acid excretion, inducing hepatic bile acid synthesis, and decreasing plasma cholesterol levels in a rat model. The agent appeared to inhibit intestinal absorption of both cholesterol and bile acids, but the interaction of TA-7552 with the ASBT was not characterized in those published studies (Takashima et al. 1994). More specific non-systemic ASBT inhibitors, amphiphilic 4-oxo-1-phenyl-1,4-dihydroquinoline derivatives, were developed at Sankyo Co (Tokyo, Japan) (Kurata et al. 2004). The lead compound, R-146224 [1-{7-[(1-(3,5-Diethoxyphenyl)-3-{[(3,5-difluorophenyl)(ethyl) amino]carbonyl}-4-oxo-1,4-dihydroquinolin-7-yl)oxy]heptyl}-1-methylpiperidinium bromide] potently inhibited the human ASBT (IC50 ~23 nM) and significantly reduced plasma non-HDL cholesterol levels in hamster and monkey models (Kitayama et al. 2006). Takauji Mizui, Seijiro Hara and coworkers at Shinogi & Company (Osaka, Japan) developed the ASBT inhibitor S-8921 [methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate]. The interaction of S-8921 with the ASBT was characterized (competitive/noncompetitive inhibitor; IC50 ~66 µM), and S-8921 was shown to reduce serum cholesterol levels in several animal models as well as prevent the development atherosclerosis in rabbits (Hara et al. 1997; Higaki et al. 1998). Interestingly, S-8921 is glucuronidated in vivo and this modification converts the parent S-8921 compound to a 6000-fold more potent inhibitor of the human ASBT (Ki = 18 nM versus 109 µM) (Sakamoto et al. 2007). S-8921 entered phase I trials but does not appear to have progressed beyond that stage (Booker 2001).
Lewis and co-workers at Burroughs Wellcome (now part of GlaxoSmithKline) identified the first series of benzothiazepine-based ASBT inhibitors and published their characterization of 2164U90 [(−)-(3R,5R)-trans-3-butyl-3-ethyl-2,3,4,5-tetra-hydro-5-phenyl-1,4-benzothiazepine1,1-dioxide] and 264W94 [(−)-(3R,5R)-trans-3-butyl-3-ethyl-2,3,4,5-tetrahydro-7,8-dimethoxy-5-phenyl-1,4-benzothiazepine1,1-dioxide]. The compounds were potent competitive inhibitors of the ASBT; in cholesterol-fed rat and mouse models, the compounds inhibited intestinal bile acid absorption, induced hepatic bile acid synthesis, and effectively lowered plasma VLDL and LDL cholesterol levels (Lewis et al. 1995; Root et al. 1995; Root et al. 2002). Additional benzothiepin-based inhibitors such as [1-[4-[4-[(4R,5R)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl]phenoxy]butyl]-4-aza-1-azoniabicyclo[2.2.2] octane methanesulfonate (salt)] SC-435 (IC50 ~ 1.5 nM) and benzothiazepine-based inhibitors such as PR835 (IC50 ~0.15 µM) were developed by Monsanto-Searle (now part of Pfizer) and Astra-Zeneca, respectively (Galman et al. 2003; Tremont et al. 2005). The Monsanto-Searle group initially developed a new series of 2,3-disubstituted-4-phenylquinolines (similar to the Shinogi ester substituted napthol compounds) and benzothiazepine-based compounds (Tollefson et al. 2003) that inhibited the ASBT at micromolar and nanomolar concentrations, respectively. This group later developed on published on a series of potent benzothiepine-based inhibitors, demonstrating their activity as potent competitive inhibitors of the ASBT (Bhat et al. 2003; Huang et al. 2005; Tremont et al. 2005), and characterizing the mechanisms responsible for the LDL-lowering in various animal models (Huff et al. 2002; Bhat et al. 2003; Telford et al. 2003; West et al. 2003). The Monsanto-Searle compound entered the clinical trial phase, but does not appear to have progressed further, potentially due to questions over efficacy and dose-related diarrhea (Hofmann and Hagey 2008).
Development of the ASBT inhibitors focused on their potential for treating hypercholesterolemia. However, since the ASBT is also is expressed by the renal proximal tubule cells, similar inhibitors could be used to block renal reclamation of bile acids and increase urinary bile acid output. This would create a shunt for elimination of hepatotoxic bile acids that can no longer be excreted efficiently via the normal biliary route. The predicted decrease in serum and hepatic bile acid concentrations may relieve cholestasis-associated pruritus and slow the progression of the hepatocellular degeneration. Long before the mechanism for renal reabsorption of bile acids was elucidated, Barbara Billings and colleagues working with Dame Sheila Sherlock at the Royal Free Hospital in London suggested a variation on this therapeutic approach (Summerfield et al. 1977; Corbett et al. 1981). Interestingly, while the use of ASBT inhibitors for eliminating excess bile acids in patients with cholestasis is as yet un-tested, a similar approach in concept is currently being exploited using renal sodium-glucose cotransporter inhibitors to create a shunt for elimination of excess plasma glucose in patients with diabetes (Bakris et al. 2009). Of course, the potential hepatoprotective effects of such an intervention must be balanced against risk of increased bile acid-induced kidney cell injury (Morgan et al. 2008).
6. Targeting the ASBT for Pro-drug Delivery
The specificity and high capacity of the ileal and hepatic bile acid transport systems has stimulated numerous attempts to utilize bile acids or bile acid derivatives as platforms (or “Trojan Horses”) for pro-drug design. This section will provide an overview of the studies targeting the ASBT to enhance oral bioavailability, and the reader is referred to several recent review articles specific to this topic for additional details (Balakrishnan and Polli 2006; Sievanen 2007). Because of their stability and unique structure, bile acids are useful building blocks for synthetic chemistry (Kuhajda et al. 2006; Davis 2007) and drugs and other large molecules have been attached via different chemical bonds or linkers to the side chain and the C-3, C-7, or C-12 positions on the bile acid steroid nucleus. While this general approach dates back to 1948 when Arpad Berczeller published the synthesis of p-aminobenzene sulphonamide conjugated to cholic acid (Unites States Patent 2,441,129), systematic development of bile acid prodrugs did not begin until much later through the efforts of the group led by Werner Kramer and Günther Wess at Hoechst in Frankfurt (Kramer and Wess 1996). Over approximately a decade dating from the early 1990’s, this group published an extensive number of studies detailing their attempts to couple drugs, peptides, and oligonucleotides to bile acids for targeting to the liver and ileal transporters (Kramer et al. 1992; Kramer et al. 1994a; Kramer et al. 1994b; Kullak-Ublick et al. 1997; Starke et al. 2001). Based on their analysis of the structure-activity relationships for NTCP or ASBT substrates, this group avoided modifying the side chain and focused on targeting the C-3 position on the steroid nucleus (Baringhaus et al. 1999). The drugs or large molecules that were coupled to bile acids and analyzed included HMG CoA reductase inhibitors, an oxaprolylpeptide to inhibit hepatic collagen synthesis, the anti-neoplastic alkylating nitogen mustard chlorambucil, small linear peptides up to 10 amino acid residues, and even oligonucleotides. For example, the bile acid-chlorambucil conjugates were absorbed from ileum and secreted by the liver, redirecting chlorambucil away from its normal route of renal clearance (Kramer et al. 1992). The bile acid-HMG CoA reductase inhibitor conjugates exhibited increased liver selectivity in a rat model, however the conjugates were weak reductase inhibitors and it was not clear whether the conjugates were being transported specifically by NTCP and ASBT or by other carriers in vivo (Kramer et al. 1994a). Small oligopeptide-bile acid conjugates were absorbed from the ileum and secreted intact by the liver, albeit with a low efficiency (Kramer et al. 1994b). Overall, the C-3 coupled prodrugs met with mixed success (Kramer and Wess 1996) and none appear to have proceeded beyond early stages of pre-clinical or clinical development. Mark Gallop and colleagues at XenoPort revisited this general approach more recently, synthesizing and evaluating a novel series of C2–C3 annulated bile acid pyrazoles coupled to large molecules such as naproxen. However, the conjugates showed only weak affinity for the ASBT (as compared to the NTCP) and weak to moderate transport activity in ASBT-expressing Xenopus oocytes (Bhat et al. 2005).
Other groups focused on using the native or a modified side chain of the bile acid as a platform for coupling agents. Many of these efforts are focused on targeted the NTCP or OATPs to increase their liver selectivity (Sievanen 2007). For example, Marin and colleagues developed a large series of cisplatin analogs, termed Bamets (a bile acid–metal hybrid) (Macias et al. 1998; Briz et al. 2002), and bile acids conjugated to metals such as gadolinium have been developed as potential contrast agents for magnetic resonance imaging (Anelli et al. 2004). With regard specifically to the ASBT, Swaan and colleagues had some success in targeting the ASBT by coupling small peptides or peptide-based HIV protease inhibitors to the side chain of cholic acid (Kagedahl et al. 1997; Swaan et al. 1997a). But the clearest example of increased oral bioavailability using a prodrug targeted to the ASBT is the study by Polli where acyclovir was conjugated to chenodeoxycholate via a valine side chain linker (acyclovir valyl-chenodeoxycholate), resulting in a two-fold increase in oral bioavailability in rats (Tolle-Sander et al. 2004).
7. Role of the Intestinal Bile Acid Transporters in Drug Absorption and Drug Interactions
7.1. Role of ASBT in Drug Absorption and Drug Interactions
As discussed in the previous sections, a great deal has been learned regarding the substrate specificity of the ASBT in the course of developing potent high affinity inhibitors and pro-drug platforms. In general, ASBT’s substrate specificity is restricted to monovalent unconjugated, glycine-conjugated, and taurine-conjugated bile acids, with little evidence for the active transport of other endobiotics such as steroids or steroid sulfates (Lack 1979; Craddock et al. 1998; Kramer et al. 1999). This narrow substrate specificity agrees with ASBT’s physiological role in the enterohepatic circulation. In the lumen of the ileum or renal proximal tubules, the ASBT is a high capacity system for almost quantitative recovery of specific solutes (bile acids), leaving other metabolites or xenobiotics for elimination in the feces or urine. Nevertheless, the conclusion that few non-bile acid substrates exist for the ASBT does not the exclude the possibility that other compounds, particularly drugs or drug metabolites, may interact as inhibitors. But several events conspired to promote the idea that few compounds unrelated to bile acids interacted with the ASBT. First, early studies comparing the inhibitor profile of NTCP versus ASBT found that the latter had a more restricted ability to interact with cholephilic compounds and drugs. In a study of 28 cholephilic organic compounds and drugs, the majority inhibited NTCP but very few inhibited ASBT-mediated transport (Kramer et al. 1999). The list of compounds that did not affect bile acid transport in rabbit ileal brush border membranes or in ASBT-transfected cells included antibiotics such as benzylpenicillin, cephalexin, cefixime, ofloxacin, rifampicin, tetracyclin, streptomycin, or novobiocin, and compounds such as β-estradiol, digitoxigenine, 6α-methylprednisolone, dexamthasone, cortisol, or reserpine (Kramer et al. 1999). Second, in developing the ASBT inhibitors, the pharmaceutical industry focused on non-absorbable compounds that fell within a few chemical classes, giving the impression that that number of interacting chemical structures may be small. Third, the early substrate molecular modeling studies (reviewed above) focused on bile acids and their derivatives (Swaan et al. 1997b; Baringhaus et al. 1999); as such, the substrate models were difficult to extrapolate to other chemical structures or drugs. Fortunately, this question has recently been revisited in an elegant study carried out by Polli and colleagues (Zheng et al. 2009). The authors initially screened a training set of 30 FDA-approved drugs for their ability to inhibit the ASBT. The most potent inhibitors were used to develop a qualitative pharmacophore, which was then used to search a drug database to identify additional inhibitors. These compounds were used to develop 3D-QSAR and Bayesian models that were further validated by assessing their inhibitory potential in cell-based assays. Ultimately, the authors identified a diverse group of FDA-approved drugs that act as ASBT inhibitors, including the dihydropyridine calcium channel blockers and HMG CoA reductase inhibitors. A list of some of the most potent ASBT inhibitors identified by Polli and colleagues is shown in Table 6.4. These results raise the possibility that inhibition of the ASBT may account for some of the side effects associated with these drugs. As discussed above, ASBT inhibition can result in greater passage of bile acids into the colon, and thus is a possible mechanism for potential drug side effects such as diarrhea, gallstone disease, hypertriglyceridemia, or even susceptibility to colon cancer (Zheng et al. 2009).
Table 6.4.
Drug Inhibitors of the ASBT
| Compound | Ki | Description | Compound | Ki | Description |
|---|---|---|---|---|---|
| Nifedipine | 4 | Dihydropyridine CCB1; antihypertensive | Pioglitazone | 55 | Thiazolidinedione (TZD); hypoglycemic |
| Nisoldipine | 5 | Dihydropyridine CCB; antihypertensive | Propafenone | 62 | Sodium channel blocker; Class 1c anti-arrhythmic |
| Nimodipine | 6 | Dihydropyridine CCB; antihypertensive | Indomethacin | 62 | Non-steroidal anti-inflammatory |
| Simvastatin | 10 | Statin; hypolipidemic | Mevastatin | 65 | Statin; hypolipidemic |
| Latanoprost | 11 | Prostaglandin analog; glaucoma | Manidipine | 73 | Dihydropyridine CCB; antihypertensive |
| Fluvastatin | 12 | Statin; hypolipidemic | Pentamidine | 76 | Antimicrobial |
| Niguldipine | 16 | Dihydropyridine CCB; antihypertensive | Azelnidipine | 86 | Dihydropyridine CCB; antihypertensive |
| Mesoridazine | 18 | Piperidine neuroleptic; schizophrenia | Bendroflumethiazide | 93 | Thiazide diuretic; antihypertensive |
| Isradipine | 19 | Dihydropyridine CCB; antihypertensive | Doxorubicin | 101 | Anthracycline antibiotic; antineoplastic |
| Lovastatin | 22 | Statin; hypolipidemic | Spironolactone | 110 | Aldosterone antagonist; diuretic |
| Nemadipine A | 23 | Dihydropyridine CCB | Atropine | 170 | Tropane alkaloid; muscarine acetylcholine receptor antagonist |
| Cyclosporin A | 24 | Cyclic peptide; immunosuppressant | Ketoprofen | 178 | Non-steroidal anti-inflammatory; propionic acid class |
| Nicardipine | 33 | Dihydropyridine CCB; antihypertensive | Diltiazem | 211 | Benzothiazepine CCB; antihypertensive; class IV anti-arrhythmic |
| Ticonazole | 33 | Imidazole antifungal | Quinine | 223 | Alkaloid; anti-malarial; antipyretic |
| Nitrendipine | 34 | Dihydropyridine CCB; antihypertensive | Bumetanide | 225 | Loop diuretic; anti-edema; antihypertensive |
| Dibucaine | 35 | Amide local anesthetic | Verapamil | 266 | Phenylalkylamine CCB; antihypertensive |
| Thioridazine | 37 | Piperidine neuroleptic; schizophrenia | Torasemide | 292 | Pyridine-sulfonylurea; loop diuretic; anti-edema; antihypertensive |
| Amlodipine | 42 | Dihydropyridine CCB; antihypertensive | Darifenacin | 296 | Muscarinic acetylcholine receptor blocker; urinary incontinence |
| Cilnidipine | 45 | Dihydropyridine CCB; antihypertensive | Trichlormethiazide | 377 | Thiazide diuretic; anti-edema; antihypertensive |
| Felodipine | 50 | Dihydropyridine CCB; antihypertensive | Probenecid | 385 | Uricosuric; gout and hyperuricemia |
CCB, Calcium Channel Blocker; Results from (Zheng et al. 2009).
7.2. Role of OSTα-OSTβ in Drug Absorption and Drug Interactions
Many of the intestinal apical brush border membrane uptake transporters have been identified such as OATP1A2, OATP2B1, PEPT1/2, MCT1, CNT1/2, OCTN1/2, and the role of apical efflux transporters such as Pgp, and MRP4 in limiting drug absorption has been an area of intense investigation, However, less is known about the basolateral membrane transporters important for completing the circuit and exporting various substrates from the enterocyte into the portal circulation (Shugarts and Benet 2009). Its significant expression down the length of the small intestine and colon and potentially broad substrate specificity, raises the possibility that OSTα-OSTβ participates in the absorption of a variety of drugs or other xenobiotics in addition to bile acids. OSTα-OSTβ may play a particularly important role for drugs that fall into the Class 3 (High solubility, low permeability, poor metabolism) or Class 4 (Low solubility, low permeability, poor metabolism) categories in the Biopharmaceutics Drug Disposition Classification System (BDDCS) proposed by Leslie Benet and colleagues (Wu and Benet 2005). A systematic screen to identify OSTα-OSTβ transport substrates has not yet been published, but these drugs, particularly the Class 3 drugs (high solubility, low permeability, poor metabolism), would be excellent candidates to characterize initially. Such studies would be an important first step toward understanding the role of OSTα-OSTβ in drug absorption and potential drug-drug interactions.
In addition to a role in drug absorption from the small intestine, the observation that OSTα-OSTβ operates by facilitated diffusion and can mediate solute uptake or efflux raises the possibility that OSTα-OSTβ may particulate in intestinal drug secretion. In this paradigm, drug or drug metabolites would be taken up from blood across the basolateral membrane by transporters such as OSTα-OSTβ and then efficiently pumped out into the intestinal lumen by the efflux transporters such as Pgp, BCRP, or MRP4. There is growing evidence for an important role of trans-intestinal secretion in the elimination of endobiotics such as cholesterol (van der Velde et al. 2007; Brown et al. 2008; van der Veen et al. 2009) or oxalate (Hatch and Freel 2008). In general, direct intestinal secretion is not thought to be a major route of drug elimination (Lennernas 2007; Fagerholm 2008), in part due to the lower blood flow to the intestinal mucosa as compared to the liver and kidneys (~1/6th to 1/5th) and postulated limited number of enterocytes involved. However, the available data is limited regarding the quantitative contribution of direct intestinal secretion. In light of the potential permeation pathway provided by OSTα-OSTβ working in conjunction with the well-characterized apical brush border efflux transporters (such as Pgp, BCRP), this question deserves greater exploration in the future.
The broad substrate specificity of OSTα-OSTβ raises the concerns regarding drug interactions. Drugs, dietary constituents, or dietary supplements could act as OSTα-OSTβ inhibitors to slow bile acid export from the enterocyte and activate FXR to induce intestinal FGF15/19 expression. This would result in decreased hepatic bile acid synthesis, and is predicted to increase the risk of developing plasma hypercholesterolemia or gallstone disease. In fact, precedence already exists for part of this pathway. Cafestol, the active component in unfiltered coffee that has been described as one of the most potent cholesterol-elevating compounds known in the human diet, is believed to operate by directly activating intestinal FXR to induce FGF19 expression and reduce hepatic Cyp7a1 expression (Ricketts et al. 2007). The phenotype of decreased efficiency for bile acid absorption coupled with an inability of the liver to synthesize additional bile acids to maintain a normal pool was described by the late Z. Reno Vlahcevic in a study of gallstone patients that was published in 1970 (Vlahcevic et al. 1970). Whether the inhibition of OSTα-OSTβ plays any role in the pathophysiology of gallstone disease is an important question that is beginning to be explored (Renner et al. 2008).
Finally, there may be pathophysiological conditions where inhibiting intestinal OSTα-OSTβ is clinically useful. This combination of reduced return of bile acids in the enterohepatic circulation and reduced hepatic bile acid synthesis that would result from inhibiting intestinal OSTα-OSTβ may have therapeutic benefit in various forms of cholestatic liver disease.
Acknowledgements
PAD was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) grant DK047987 and an American Heart Association Mid-Atlantic Affiliate Grant-in-aid. The author thanks Dr. Anuradha Rao for assistance with the figures, valuable suggestions, and critical reading of the manuscript.
References
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- Aldini R, Montagnani M, Roda A, Hrelia S, Biagi PL, Roda E. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 1996;110:459–468. doi: 10.1053/gast.1996.v110.pm8566593. [DOI] [PubMed] [Google Scholar]
- Aldini R, Roda A, Montagnani M, Polimeni C, Lenzi PL, Cerre C, Galletti G, Roda E. Hepatic uptake and intestinal absorption of bile acids in the rabbit. Eur J Clin Invest. 1994;24:691–697. doi: 10.1111/j.1365-2362.1994.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997a;113:1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997b;113:1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- Anelli PL, Lattuada L, Lorusso V, Lux G, Morisetti A, Morosini P, Serleti M, Uggeri F. Conjugates of gadolinium complexes to bile acids as hepatocyte-directed contrast agents for magnetic resonance imaging. J Med Chem. 2004;47:3629–3641. doi: 10.1021/jm0310683. [DOI] [PubMed] [Google Scholar]
- Angelin B, Hershon KS, Brunzell JD. Bile acid metabolism in hereditary forms of hypertriglyceridemia: evidence for an increased synthesis rate in monogenic familial hypertriglyceridemia. Proc Natl Acad Sci U S A. 1987;84:5434–5438. doi: 10.1073/pnas.84.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaba F, Sarwar Z, Kumar P, Saksena S, Turner JR, Dudeja PK, Gill RK, Alrefai WA. Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts. Am J Physiol Gastrointest Liver Physiol. 2008;294:G489–G497. doi: 10.1152/ajpgi.00237.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A, Polli JE. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol Pharm. 2006;3:223–230. doi: 10.1021/mp060022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan A, Wring SA, Coop A, Polli JE. Influence of charge and steric bulk in the C-24 region on the interaction of bile acids with human apical sodium-dependent bile acid transporter. Mol Pharm. 2006a;3:282–292. doi: 10.1021/mp0600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan A, Wring SA, Polli JE. Interaction of native bile acids with human apical sodium-dependent bile acid transporter (hASBT): influence of steroidal hydroxylation pattern and C-24 conjugation. Pharm Res. 2006b;23:1451–1459. doi: 10.1007/s11095-006-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balesaria S, Pell RJ, Abbott LJ, Tasleem A, Chavele KM, Barley NF, Khair U, Simon A, Moriarty KJ, Brydon WG, Walters JR. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur J Gastroenterol Hepatol. 2008;20:413–422. doi: 10.1097/MEG.0b013e3282f41b82. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Biology of a novel organic solute and steroid transporter, OSTalpha-OSTbeta. Exp Biol Med (Maywood) 2005;230:689–698. doi: 10.1177/153537020523001001. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295:G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Hussainzada N, Khandelwal A, Swaan PW. Electrostatic and potential cation-pi forces may guide the interaction of extracellular loop III with Na+ and bile acids for human apical Na+-dependent bile acid transporter. Biochem J. 2008;410:391–400. doi: 10.1042/BJ20071300. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Ray A, Chang C, Swaan PW. Site-directed mutagenesis and use of bile acid-MTS conjugates to probe the role of cysteines in the human apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2005;44:8908–8917. doi: 10.1021/bi050553s. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Swaan PW. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry. 2006;45:943–953. doi: 10.1021/bi052202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na(+)/bile acid cotransporter. J Lipid Res. 1999;40:2158–2168. [PubMed] [Google Scholar]
- Battle MA, Bondow BJ, Iverson MA, Adams SJ, Jandacek RJ, Tso P, Duncan SA. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686. doi: 10.1053/j.gastro.2008.07.074. e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays HE, Goldberg RB. The 'forgotten' bile Acid sequestrants: is now a good time to remember? Am J Ther. 2007;14:567–580. doi: 10.1097/MJT.0b013e31815a69fc. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Harsch S, Mueller O, Schimmel S, Fritz P, Stange EF. Apical sodium bile acid transporter and ileal lipid binding protein in gallstone carriers. J Lipid Res. 2006;47:42–50. doi: 10.1194/jlr.M500215-JLR200. [DOI] [PubMed] [Google Scholar]
- Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J Lipid Res. 2003;44:1614–1621. doi: 10.1194/jlr.M200469-JLR200. [DOI] [PubMed] [Google Scholar]
- Bhat L, Jandeleit B, Dias TM, Moors TL, Gallop MA. Synthesis and biological evaluation of novel steroidal pyrazoles as substrates for bile acid transporters. Bioorg Med Chem Lett. 2005;15:85–87. doi: 10.1016/j.bmcl.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Booker ML. S-8921 (Shionogi) Curr Opin Investig Drugs. 2001;2:393–395. [PubMed] [Google Scholar]
- Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Brink MA, Mendez-Sanchez N, Carey MC. Bilirubin cycles enterohepatically after ileal resection in the rat. Gastroenterology. 1996;110:1945–1957. doi: 10.1053/gast.1996.v110.pm8964422. [DOI] [PubMed] [Google Scholar]
- Brink MA, Slors JF, Keulemans YC, Mok KS, De Waart DR, Carey MC, Groen AK, Tytgat GN. Enterohepatic cycling of bilirubin: a putative mechanism for pigment gallstone formation in ileal Crohn's disease. Gastroenterology. 1999;116:1420–1427. doi: 10.1016/s0016-5085(99)70507-x. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chlorocholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–860. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- Brown JM, Bell TA, 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD, Jr, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH) N Engl J Med. 1990;323:946–955. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- Bundy R, Mauskopf J, Walker JT, Lack L. Interaction of uncharged bile salt derivatives with the ileal bile salt transport system. J Lipid Res. 1977;18:389–395. [PubMed] [Google Scholar]
- Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignard N, Mergey M, Veissiere D, Parc R, Capeau J, Poupon R, Paul A, Housset C. Bile acid transport and regulating functions in the human biliary epithelium. Hepatology. 2001;33:496–503. doi: 10.1053/jhep.2001.22345. [DOI] [PubMed] [Google Scholar]
- Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996a;271:G377–G385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996b;271:G377–G385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- Corbett CL, Bartholomew TC, Billing BH, Summerfield JA. Urinary excretion of bile acids in cholestasis: evidence for renal tubular secretion in man. Clin Sci (Lond) 1981;61:773–780. doi: 10.1042/cs0610773. [DOI] [PubMed] [Google Scholar]
- Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–G169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- Davis AP. Bile acid scaffolds in supramolecular chemistry: the interplay of design and synthesis. Molecules. 2007;12:2106–2122. doi: 10.3390/12082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RA, Attie AD. Deletion of the ileal basolateral bile acid transporter identifies the cellular sentinels that regulate the bile acid pool. Proc Natl Acad Sci U S A. 2008;105:4965–4966. doi: 10.1073/pnas.0801194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009 doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Oelkers P. Bile acid transporters. Curr Opin Lipidol. 1995;6:109–114. doi: 10.1097/00041433-199504000-00009. [DOI] [PubMed] [Google Scholar]
- De Witt EH, Lack L. Effects of sulfation patterns on intestinal transport of bile salt sulfate esters. Am J Physiol. 1980;238:G34–G39. doi: 10.1152/ajpgi.1980.238.1.G34. [DOI] [PubMed] [Google Scholar]
- Dietschy JM. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968;9:297–309. [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- Duane WC, Hartich LA, Bartman AE, Ho SB. Diminished gene expression of ileal apical sodium bile acid transporter explains impaired absorption of bile acid in patients with hypertriglyceridemia. J Lipid Res. 2000;41:1384–1389. [PubMed] [Google Scholar]
- Elsner R, Ziegler K. Determination of the apparent functional molecular mass of the hepatocellular sodium-dependent taurocholate transporter by radiation inactivation. Biochim Biophys Acta. 1989;983:113–117. doi: 10.1016/0005-2736(89)90387-8. [DOI] [PubMed] [Google Scholar]
- Fagerholm U. Prediction of human pharmacokinetics-biliary and intestinal clearance and enterohepatic circulation. J Pharm Pharmacol. 2008;60:535–542. doi: 10.1211/jpp.60.5.0001. [DOI] [PubMed] [Google Scholar]
- Farivar S, Fromm H, Schindler D, McJunkin B, Schmidt F. Tests of bile-acid and vitamin B12 metabolism in ileal crohn's disease. Am. J. Clin. Pathol. 1980;73:69–74. doi: 10.1093/ajcp/73.1.69. [DOI] [PubMed] [Google Scholar]
- Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, Denk H, Hofmann AF, Jaeschke H, Trauner M. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G912–G922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Kimura A, Ushijima K, Nakashima E, Inoue T, Yamashita Y, Kato H. Intestinal absorption of ursodeoxycholic acid in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1998;26:279–285. doi: 10.1097/00005176-199803000-00008. [DOI] [PubMed] [Google Scholar]
- Galman C, Ostlund-Lindqvist AM, Bjorquist A, Schreyer S, Svensson L, Angelin B, Rudling M. Pharmacological interference with intestinal bile acid transport reduces plasma cholesterol in LDL receptor/apoE deficiency. FASEB J. 2003;17:265–267. doi: 10.1096/fj.02-0341fje. [DOI] [PubMed] [Google Scholar]
- Geyer J, Doring B, Meerkamp K, Ugele B, Bakhiya N, Fernandes CF, Godoy JR, Glatt H, Petzinger E. Cloning and functional characterization of human sodium-dependent organic anion transporter (SLC10A6) J Biol Chem. 2007;282:19728–19741. doi: 10.1074/jbc.M702663200. [DOI] [PubMed] [Google Scholar]
- Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:413–431. doi: 10.1007/s00210-006-0043-8. [DOI] [PubMed] [Google Scholar]
- Glaser SS, Alpini G. Activation of the cholehepatic shunt as a potential therapy for primary sclerosing cholangitis. Hepatology. 2009;49:1795–1797. doi: 10.1002/hep.22969. [DOI] [PubMed] [Google Scholar]
- Gonzalez PM, Acharya C, Mackerell AD, Jr, Polli JE. Inhibition requirements of the human apical sodium-dependent bile acid transporter (hASBT) using aminopiperidine conjugates of glutamyl-bile acids. Pharm Res. 2009;26:1665–1678. doi: 10.1007/s11095-009-9877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurantz D, Schteingart CD, Hagey LR, Steinbach JH, Grotmol T, Hofmann AF. Hypercholeresis induced by unconjugated bile acid infusion correlates with recovery in bile of unconjugated bile acids. Hepatology. 1991;13:540–550. [PubMed] [Google Scholar]
- Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B, Foguet M, Lubbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991;88:10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilbasic E, Fiorotto R, Fickert P, Marschall HU, Moustafa T, Spirli C, Fuchsbichler A, Gumhold J, Silbert D, Zatloukal K, Langner C, Maitra U, Denk H, Hofmann AF, Strazzabosco M, Trauner M. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology. 2009;49:1972–1981. doi: 10.1002/hep.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen S, Branden M, Dawson PA, Sachs G. Membrane insertion scanning of the human ileal sodium/bile acid co-transporter. Biochemistry. 1999;38:11379–11388. doi: 10.1021/bi990554i. [DOI] [PubMed] [Google Scholar]
- Hallen S, Fryklund J, Sachs G. Inhibition of the human sodium/bile acid cotransporters by side-specific methanethiosulfonate sulfhydryl reagents: substrate-controlled accessibility of site of inactivation. Biochemistry. 2000;39:6743–6750. doi: 10.1021/bi000577t. [DOI] [PubMed] [Google Scholar]
- Hallen S, Mareninova O, Branden M, Sachs G. Organization of the membrane domain of the human liver sodium/bile acid cotransporter. Biochemistry. 2002;41:7253–7266. doi: 10.1021/bi012152s. [DOI] [PubMed] [Google Scholar]
- Hara S, Higaki J, Higashino K, Iwai M, Takasu N, Miyata K, Tonda K, Nagata K, Goh Y, Mizui T. S-8921, an ileal Na+/bile acid cotransporter inhibitor decreases serum cholesterol in hamsters. Life Sci. 1997;60:365–370. doi: 10.1016/s0024-3205(97)00242-7. PL. [DOI] [PubMed] [Google Scholar]
- Hatch M, Freel RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol. 2008;28:143–151. doi: 10.1016/j.semnephrol.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubi JE, Balistreri WF, Fondacaro JD, Partin JC, Schubert WK. Primary bile acid malabsorption: defective in vitro ileal active bile acid transport. Gastroenterology. 1982;83:804–811. [PubMed] [Google Scholar]
- Higaki J, Hara S, Takasu N, Tonda K, Miyata K, Shike T, Nagata K, Mizui T. Inhibition of ileal Na+/bile acid cotransporter by S-8921 reduces serum cholesterol and prevents atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1304–1311. doi: 10.1161/01.atv.18.8.1304. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279:7213–7222. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res. 2009 doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF, Molino G, Milanese M, Belforte G. Description and simulation of a physiological pharmacokinetic model for the metabolism and enterohepatic circulation of bile acids in man. Cholic acid in healthy man. J Clin Invest. 1983;71:1003–1022. doi: 10.1172/JCI110828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62:918–934. [PubMed] [Google Scholar]
- Hofmann AF, Zakko SF, Lira M, Clerici C, Hagey LR, Lambert KK, Steinbach JH, Schteingart CD, Olinga P, Groothuis GM. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42:1391–1398. doi: 10.1002/hep.20943. [DOI] [PubMed] [Google Scholar]
- Holzer A, Harsch S, Renner O, Strohmeyer A, Schimmel S, Wehkamp J, Fritz P, Stange EF. Diminished expression of apical sodium-dependent bile acid transporter in gallstone disease is independent of ileal inflammation. Digestion. 2008;78:52–59. doi: 10.1159/000159379. [DOI] [PubMed] [Google Scholar]
- Hruz P, Zimmermann C, Gutmann H, Degen L, Beuers U, Terracciano L, Drewe J, Beglinger C. Adaptive regulation of the ileal apical sodium dependent bile acid transporter (ASBT) in patients with obstructive cholestasis. Gut. 2006;55:395–402. doi: 10.1136/gut.2005.067389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Tremont SJ, Lee LF, Keller BT, Carpenter AJ, Wang CC, Banerjee SC, Both SR, Fletcher T, Garland DJ, Huang W, Jones C, Koeller KJ, Kolodziej SA, Li J, Manning RE, Mahoney MW, Miller RE, Mischke DA, Rath NP, Reinhard EJ, Tollefson MB, Vernier WF, Wagner GM, Rapp SR, Beaudry J, Glenn K, Regina K, Schuh JR, Smith ME, Trivedi JS, Reitz DB. Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 2) J Med Chem. 2005;48:5853–5868. doi: 10.1021/jm0402162. [DOI] [PubMed] [Google Scholar]
- Huff MW, Telford DE, Edwards JY, Burnett JR, Barrett PH, Rapp SR, Napawan N, Keller BT. Inhibition of the apical sodium-dependent bile acid transporter reduces LDL cholesterol and apoB by enhanced plasma clearance of LDL apoB. Arterioscler Thromb Vasc Biol. 2002;22:1884–1891. doi: 10.1161/01.atv.0000035390.87288.26. [DOI] [PubMed] [Google Scholar]
- Hulzebos CV, Renfurm L, Bandsma RH, Verkade HJ, Boer T, Boverhof R, Tanaka H, Mierau I, Sauer PJ, Kuipers F, Stellaard F. Measurement of parameters of cholic acid kinetics in plasma using a microscale stable isotope dilution technique: application to rodents and humans. J Lipid Res. 2001;42:1923–1929. [PubMed] [Google Scholar]
- Hussainzada N, Banerjee A, Swaan PW. Transmembrane domain VII of the human apical sodium-dependent bile acid transporter ASBT (SLC10A2) lines the substrate translocation pathway. Mol Pharmacol. 2006;70:1565–1574. doi: 10.1124/mol.106.028647. [DOI] [PubMed] [Google Scholar]
- Hussainzada N, Claro Da Silva T, Swaan PW. The Cytosolic Half of Helix III Forms the Substrate Exit Route during Permeation Events of the Sodium/Bile Acid Cotransporter ASBT. Biochemistry. 2009 doi: 10.1021/bi900616w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussainzada N, Khandewal A, Swaan PW. Conformational flexibility of helix VI is essential for substrate permeation of the human apical sodium-dependent bile acid transporter. Mol Pharmacol. 2008;73:305–313. doi: 10.1124/mol.107.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Kavousi M, Smith AV, Chen MH, Dehghan A, Aspelund T, Lin JP, van Duijn CM, Harris TB, Cupples LA, Uitterlinden AG, Launer L, Hofman A, Rivadeneira F, Stricker B, Yang Q, O'Donnell CJ, Gudnason V, Witteman JC. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet. 2009;18:2700–2710. doi: 10.1093/hmg/ddp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007;48:2693–2700. doi: 10.1194/jlr.M700351-JLR200. [DOI] [PubMed] [Google Scholar]
- Kagedahl M, Swaan PW, Redemann CT, Tang M, Craik CS, Szoka FC, Jr, Oie S. Use of the intestinal bile acid transporter for the uptake of cholic acid conjugates with HIV-1 protease inhibitory activity. Pharm Res. 1997;14:176–180. doi: 10.1023/a:1012044526054. [DOI] [PubMed] [Google Scholar]
- Kapadia CR, Essandoh LK. Active absorption of vitamin B12 and conjugated bile salts by guinea pig ileum occurs in villous and not crypt cells. Dig Dis Sci. 1988;33:1377–1382. doi: 10.1007/BF01536991. [DOI] [PubMed] [Google Scholar]
- Khantwal CM, Swaan PW. Cytosolic half of transmembrane domain IV of the human bile acid transporter hASBT (SLC10A2) forms part of the substrate translocation pathway. Biochemistry. 2008;47:3606–3614. doi: 10.1021/bi702498w. [DOI] [PubMed] [Google Scholar]
- Kitayama K, Nakai D, Kono K, van der Hoop AG, Kurata H, de Wit EC, Cohen LH, Inaba T, Kohama T. Novel non-systemic inhibitor of ileal apical Na+-dependent bile acid transporter reduces serum cholesterol levels in hamsters and monkeys. Eur J Pharmacol. 2006;539:89–98. doi: 10.1016/j.ejphar.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- Krag B, Krag B. Regional ileitis (Crohn's disease): I. Kinetics of bile acid absorption in the perfused ileum. Scand. J. Gastroenter. 1976;11:481–486. [PubMed] [Google Scholar]
- Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Bickel U, Buscher HP, Gerok W, Kurz G. Bile-salt-binding polypeptides in plasma membranes of hepatocytes revealed by photoaffinity labelling. Eur J Biochem. 1982;129:13–24. doi: 10.1111/j.1432-1033.1982.tb07015.x. [DOI] [PubMed] [Google Scholar]
- Kramer W, Corsiero D, Friedrich M, Girbig F, Stengelin S, Weyland C. Intestinal absorption of bile acids: paradoxical behaviour of the 14 kDa ileal lipid-binding protein in differential photoaffinity labelling. Biochem J. 1998;333(Pt 2):335–341. doi: 10.1042/bj3330335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Glombik H, Corsiero D, Stengelin S, Weyland C. Identification of a ligand-binding site in the Na+/bile acid cotransporting protein from rabbit ileum. J Biol Chem. 2001;276:36020–36027. doi: 10.1074/jbc.M104665200. [DOI] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Gutjahr U, Kowalewski S. Radiation-inactivation analysis of the Na+/bile acid co-transport system from rabbit ileum. Biochem J. 1995;306(Pt 1):241–246. doi: 10.1042/bj3060241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Gutjahr U, Kowalewski S, Jouvenal K, Muller G, Tripier D, Wess G. Intestinal bile acid absorption. Na(+)-dependent bile acid transport activity in rabbit small intestine correlates with the coexpression of an integral 93-kDa and a peripheral 14-kDa bile acid-binding membrane protein along the duodenum-ileum axis. J Biol Chem. 1993;268:18035–18046. [PubMed] [Google Scholar]
- Kramer W, Glombik H. Bile acid reabsorption inhibitors (BARI): novel hypolipidemic drugs. Curr Med Chem. 2006;13:997–1016. doi: 10.2174/092986706776361003. [DOI] [PubMed] [Google Scholar]
- Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–1617. [PubMed] [Google Scholar]
- Kramer W, Wess G. Bile acid transport systems as pharmaceutical targets. Eur J Clin Invest. 1996;26:715–732. doi: 10.1111/j.1365-2362.1996.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Kramer W, Wess G, Bewersdorf U, Corsiero D, Girbig F, Weyland C, Stengelin S, Enhsen A, Bock K, Kleine H, Le Dreau MA, Schafer HL. Topological photoaffinity labeling of the rabbit ileal Na+/bile-salt-cotransport system. Eur J Biochem. 1997;249:456–464. doi: 10.1111/j.1432-1033.1997.00456.x. [DOI] [PubMed] [Google Scholar]
- Kramer W, Wess G, Enhsen A, Bock K, Falk E, Hoffmann A, Neckermann G, Gantz D, Schulz S, Nickau L, et al. Bile acid derived HMG-CoA reductase inhibitors. Biochim Biophys Acta. 1994a;1227:137–154. doi: 10.1016/0925-4439(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Kramer W, Wess G, Neckermann G, Schubert G, Fink J, Girbig F, Gutjahr U, Kowalewski S, Baringhaus KH, Boger G, et al. Intestinal absorption of peptides by coupling to bile acids. J Biol Chem. 1994b;269:10621–10627. [PubMed] [Google Scholar]
- Kramer W, Wess G, Schubert G, Bickel M, Girbig F, Gutjahr U, Kowalewski S, Baringhaus KH, Enhsen A, Glombik H, et al. Liver-specific drug targeting by coupling to bile acids. J Biol Chem. 1992;267:18598–18604. [PubMed] [Google Scholar]
- Kuhajda K, Kevresan S, Kandrac J, Fawcett JP, Mikov M. Chemical and metabolic transformations of selected bile acids. Eur J Drug Metab Pharmacokinet. 2006;31:179–235. doi: 10.1007/BF03190713. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Glasa J, Boker C, Oswald M, Grutzner U, Hagenbuch B, Stieger B, Meier PJ, Beuers U, Kramer W, Wess G, Paumgartner G. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology. 1997;113:1295–1305. doi: 10.1053/gast.1997.v113.pm9322525. [DOI] [PubMed] [Google Scholar]
- Kurata H, Suzuki S, Ohhata Y, Ikeda T, Hasegawa T, Kitayama K, Inaba T, Kono K, Kohama T. A novel class of apical sodium-dependent bile acid transporter inhibitors: the amphiphilic 4-oxo-1-phenyl-1,4-dihydroquinoline derivatives. Bioorg Med Chem Lett. 2004;14:1183–1186. doi: 10.1016/j.bmcl.2003.12.063. [DOI] [PubMed] [Google Scholar]
- Lack L. Properties and biological significance of the ileal bile salt transport system. Environ Health Perspect. 1979;33:79–90. doi: 10.1289/ehp.793379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack L, Walker JT, Singletary GD. Ileal bile salt transport: in vivo studies of effect of substrate ionization on activity. Am J Physiol. 1970;219:487–490. doi: 10.1152/ajplegacy.1970.219.2.487. [DOI] [PubMed] [Google Scholar]
- Lack L, Weiner IM. Intestinal bile salt transport: structure-activity relationships and other properties. Am J Physiol. 1966;210:1142–1152. doi: 10.1152/ajplegacy.1966.210.5.1142. [DOI] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids FXR transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Lazaridis K, Tietz Pham L, Marinelli R, deGroen P, Levine S, Dawson P, LaRusso N. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J. Clin. Invest. 1997a;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997b;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennernas H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37:1015–1051. doi: 10.1080/00498250701704819. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Brieaddy LE, Root C. Effects of 2164U90 on ileal bile acid absorption and serum cholesterol in rats and mice. J Lipid Res. 1995;36:1098–1105. [PubMed] [Google Scholar]
- Lewis MC, Root C. In vivo transport kinetics and distribution of taurocholate by rat ileum and jejunum. Am J Physiol. 1990;259:G233–G238. doi: 10.1152/ajpgi.1990.259.2.G233. [DOI] [PubMed] [Google Scholar]
- Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem J. 2007;407:363–372. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MC, Kramer W, Wilson FA. Identification of cytosolic and microsomal bile acid-binding proteins in rat ileal enterocytes. J Biol Chem. 1990;265:14986–14995. [PubMed] [Google Scholar]
- Love MW, Craddock AL, Angelin B, Brunzell JD, Duane WC, Dawson PA. Analysis of the ileal bile acid transporter gene, SLC10A2, in subjects with familial hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2001;21:2039–2045. doi: 10.1161/hq1201.100262. [DOI] [PubMed] [Google Scholar]
- Macias RI, Monte MJ, El-Mir MY, Villanueva GR, Marin JJ. Transport and biotransformation of the new cytostatic complex cis-diammineplatinum(II)-chlorocholylglycinate (Bamet-R2) by the rat liver. J Lipid Res. 1998;39:1792–1798. [PubMed] [Google Scholar]
- Marcus SN, Schteingart CD, Marquez ML, Hofmann AF, Xia Y, Steinbach JH, Ton-Nu HT, Lillienau J, Angellotti MA, Schmassmann A. Active absorption of conjugated bile acids in vivo. Kinetic parameters and molecular specificity of the ileal transport system in the rat. Gastroenterology. 1991;100:212–221. doi: 10.1016/0016-5085(91)90603-i. [DOI] [PubMed] [Google Scholar]
- Mareninova O, Shin JM, Vagin O, Turdikulova S, Hallen S, Sachs G. Topography of the membrane domain of the liver Na+-dependent bile acid transporter. Biochemistry. 2005;44:13702–13712. doi: 10.1021/bi051291x. [DOI] [PubMed] [Google Scholar]
- Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- Meihoff WE, Kern F., Jr Bile salt malabsorption in regional ileitis, ileal resection and mannitol-induced diarrhea. J Clin Invest. 1968;47:261–267. doi: 10.1172/JCI105722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M, Abrahamsson A, Galman C, Eggertsen G, Marschall HU, Ravaioli E, Einarsson C, Dawson PA. Analysis of ileal sodium/bile acid cotransporter and related nuclear receptor genes in a family with multiple cases of idiopathic bile acid malabsorption. World J Gastroenterol. 2006;12:7710–7714. doi: 10.3748/wjg.v12.i47.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M, Love MW, Rossel P, Dawson PA, Qvist P. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand J Gastroenterol. 2001;36:1077–1080. doi: 10.1080/003655201750422693. [DOI] [PubMed] [Google Scholar]
- Morgan WA, Nk T, Ding Y. The use of High Performance Thin-Layer Chromatography to determine the role of membrane lipid composition in bile salt-induced kidney cell damage. J Pharmacol Toxicol Methods. 2008;57:70–73. doi: 10.1016/j.vascn.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Nyhlin H, Merrick M, Eastwood M. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut. 1994;35:90–93. doi: 10.1136/gut.35.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers P, Dawson PA. Cloning and chromosomal localization of the human ileal lipid-binding protein. Biochim Biophys Acta. 1995;1257:199–202. doi: 10.1016/0005-2760(95)00098-w. [DOI] [PubMed] [Google Scholar]
- Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Banerjee A, Chang C, Khantwal CM, Swaan PW. Design of novel synthetic MTS conjugates of bile acids for site-directed sulfhydryl labeling of cysteine residues in bile acid binding and transporting proteins. Bioorg Med Chem Lett. 2006;16:1473–1476. doi: 10.1016/j.bmcl.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Renner O, Harsch S, Schaeffeler E, Schwab M, Klass DM, Kratzer W, Stange EF. Mutation screening of apical sodium-dependent bile acid transporter (SLC10A2): novel haplotype block including six newly identified variants linked to reduced expression. Hum Genet. 2009;125:381–391. doi: 10.1007/s00439-009-0630-0. [DOI] [PubMed] [Google Scholar]
- Renner O, Harsch S, Strohmeyer A, Schimmel S, Stange EF. Reduced ileal expression of OSTalpha-OSTbeta in non-obese gallstone disease. J Lipid Res. 2008;49:2045–2054. doi: 10.1194/jlr.M800162-JLR200. [DOI] [PubMed] [Google Scholar]
- Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Muller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- Root C, Smith CD, Sundseth SS, Pink HM, Wilson JG, Lewis MC. Ileal bile acid transporter inhibition, CYP7A1 induction, and antilipemic action of 264W94. J Lipid Res. 2002;43:1320–1330. [PubMed] [Google Scholar]
- Root C, Smith CD, Winegar DA, Brieaddy LE, Lewis MC. Inhibition of ileal sodium-dependent bile acid transport by 2164U90. J Lipid Res. 1995;36:1106–1115. [PubMed] [Google Scholar]
- Sakamoto S, Kusuhara H, Miyata K, Shimaoka H, Kanazu T, Matsuo Y, Nomura K, Okamura N, Hara S, Horie K, Baba T, Sugiyama Y. Glucuronidation converting methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-na phthoate (S-8921) to a potent apical sodium-dependent bile acid transporter inhibitor, resulting in a hypocholesterolemic action. J Pharmacol Exp Ther. 2007;322:610–618. doi: 10.1124/jpet.106.116426. [DOI] [PubMed] [Google Scholar]
- Schiff ER, Small NC, Dietschy JM. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972;51:1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller LR, Hogan RB, Morawski SG, Santa Ana CA, Bern MJ, Norgaard RP, Bo-Linn GW, Fordtran JS. Studies of the prevalence and significance of radiolabeled bile acid malabsorption in a group of patients with idiopathic chronic diarrhea. Gastroenterology. 1987;92:151–160. doi: 10.1016/0016-5085(87)90852-3. [DOI] [PubMed] [Google Scholar]
- Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278:27473–27482. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievanen E. Exploitation of bile acid transport systems in prodrug design. Molecules. 2007;12:1859–1889. doi: 10.3390/12081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter PL, Lazaridis KN, Dawson PA, LaRusso NF. Cloning and expression of SLC10A4, a putative organic anion transport protein. World J Gastroenterol. 2006;12:6797–6805. doi: 10.3748/wjg.v12.i42.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke D, Lischka K, Pagels P, Uhlmann E, Kramer W, Wess G, Petzinger E. Bile acid-oligodeoxynucleotide conjugates: synthesis and liver excretion in rats. Bioorg Med Chem Lett. 2001;11:945–949. doi: 10.1016/s0960-894x(01)00048-8. [DOI] [PubMed] [Google Scholar]
- Steinmetz KL, Schonder KS. Colesevelam: potential uses for the newest bile resin. Cardiovasc Drug Rev. 2005;23:15–30. doi: 10.1111/j.1527-3466.2005.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Summerfield JA, Cullen J, Barnes S, Billing BH. Evidence for renal control of urinary excretion of bile acids and bile acid sulphates in the cholestatic syndrome. Clin Sci Mol Med. 1977;52:51–65. doi: 10.1042/cs0520051. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Balasubramaniyan N, Chen H, Shahid M, Suchy FJ. Identification of functionally relevant residues of the rat ileal apical sodium-dependent bile acid cotransporter. J Biol Chem. 2006;281:16410–16418. doi: 10.1074/jbc.M600034200. [DOI] [PubMed] [Google Scholar]
- Swaan PW, Hillgren KM, Szoka FC, Jr, Oie S. Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconjug Chem. 1997a;8:520–525. doi: 10.1021/bc970076t. [DOI] [PubMed] [Google Scholar]
- Swaan PW, Szoka FC, Jr, Oie S. Molecular modeling of the intestinal bile acid carrier: a comparative molecular field analysis study. J Comput Aided Mol Des. 1997b;11:581–588. doi: 10.1023/a:1007919704457. [DOI] [PubMed] [Google Scholar]
- Takashima K, Kohno T, Mori T, Ohtani A, Hirakoso K, Takeyama S. The hypocholesterolemic action of TA-7552 and its effects on cholesterol metabolism in the rat. Atherosclerosis. 1994;107:247–257. doi: 10.1016/0021-9150(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Telford DE, Edwards JY, Lipson SM, Sutherland B, Barrett PH, Burnett JR, Krul ES, Keller BT, Huff MW. Inhibition of both the apical sodium-dependent bile acid transporter and HMG-CoA reductase markedly enhances the clearance of LDL apoB. J Lipid Res. 2003;44:943–952. doi: 10.1194/jlr.M200482-JLR200. [DOI] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- Tolle-Sander S, Lentz KA, Maeda DY, Coop A, Polli JE. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol Pharm. 2004;1:40–48. doi: 10.1021/mp034010t. [DOI] [PubMed] [Google Scholar]
- Tollefson MB, Kolodziej SA, Fletcher TR, Vernier WF, Beaudry JA, Keller BT, Reitz DB. A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 1,2-benzothiazepines. Bioorg Med Chem Lett. 2003;13:3727–3730. doi: 10.1016/j.bmcl.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tougaard L, Giese B, Pedersen B, Binder V. Bile acid metabolism in patients with Crohn's disease in terminal ileum. Scand J. Gastroenterol. 1986;21:627–633. doi: 10.3109/00365528609003110. [DOI] [PubMed] [Google Scholar]
- Tremont SJ, Lee LF, Huang HC, Keller BT, Banerjee SC, Both SR, Carpenter AJ, Wang CC, Garland DJ, Huang W, Jones C, Koeller KJ, Kolodziej SA, Li J, Manning RE, Mahoney MW, Miller RE, Mischke DA, Rath NP, Fletcher T, Reinhard EJ, Tollefson MB, Vernier WF, Wagner GM, Rapp SR, Beaudry J, Glenn K, Regina K, Schuh JR, Smith ME, Trivedi JS, Reitz DB. Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 1) J Med Chem. 2005;48:5837–5852. doi: 10.1021/jm040215+. [DOI] [PubMed] [Google Scholar]
- van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Vicens M, Macias RI, Briz O, Rodriguez A, El-Mir MY, Medarde M, Marin JJ. Inhibition of the intestinal absorption of bile acids using cationic derivatives: mechanism and repercussions. Biochem Pharmacol. 2007;73:394–404. doi: 10.1016/j.bcp.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Vitek L, Carey MC. Enterohepatic cycling of bilirubin as a cause of 'black' pigment gallstones in adult life. Eur J Clin Invest. 2003;33:799–810. doi: 10.1046/j.1365-2362.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- Vlahcevic ZR, Bell CC, Jr, Buhac I, Farrar JT, Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970;59:165–173. [PubMed] [Google Scholar]
- Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A. 2001a;98:9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xue S, Ingles SA, Chen Q, Diep AT, Frankl HD, Stolz A, Haile RW. An association between genetic polymorphisms in the ileal sodium-dependent bile acid transporter gene and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001b;10:931–936. [PubMed] [Google Scholar]
- Weinman SA, Carruth MW, Dawson PA. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J Biol Chem. 1998;273:34691–34695. doi: 10.1074/jbc.273.52.34691. [DOI] [PubMed] [Google Scholar]
- Wess G, Kramer W, Enhsen A, Glombik H, Baringhaus KH, Boger G, Urmann M, Bock K, Kleine H, Neckermann G, et al. Specific inhibitors of ileal bile acid transport. J Med Chem. 1994;37:873–875. doi: 10.1021/jm00033a001. [DOI] [PubMed] [Google Scholar]
- West KL, Zern TL, Butteiger DN, Keller BT, Fernandez ML. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis. 2003;171:201–210. doi: 10.1016/j.atherosclerosis.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Burckhardt G, Murer H, Rumrich G, Ullrich KJ. Sodium-coupled taurocholate transport in the proximal convolution of the rat kidney in vivo and in vitro. J Clin Invest. 1981;67:1141–1150. doi: 10.1172/JCI110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994a;269:1340–1347. [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994b;269:1340–1347. [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12:3553–3563. doi: 10.3748/wjg.v12.i22.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner D, Eckhardt U, Petzinger E. Transport of taurocholate by mutants of negatively charged amino acids, cysteines, and threonines of the rat liver sodium-dependent taurocholate cotransporting polypeptide Ntcp. Eur J Biochem. 2003;270:1117–1127. doi: 10.1046/j.1432-1033.2003.03463.x. [DOI] [PubMed] [Google Scholar]
- Zhang EY, Phelps MA, Banerjee A, Khantwal CM, Chang C, Helsper F, Swaan PW. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2004;43:11380–11392. doi: 10.1021/bi049270a. [DOI] [PubMed] [Google Scholar]
- Zhang EY, Phelps MA, Cheng C, Ekins S, Swaan PW. Modeling of active transport systems. Adv Drug Deliv Rev. 2002;54:329–354. doi: 10.1016/s0169-409x(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Zheng X, Ekins S, Raufman JP, Polli JE. Computational Models for Drug Inhibition of the Human Apical Sodium-dependent Bile Acid Transporter. Mol Pharm. 2009 doi: 10.1021/mp900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]