Abstract
Monophosphoryl lipid A (MLA), a nontoxic derivative of the endotoxin lipopolysaccharide (LPS), has been approved in the United States for use as a vaccine adjuvant. LPS and MLA are ligands of Toll-like receptor 4 (TLR4), and it has been unclear why LPS triggers toxic inflammation, whereas MLA generates safe and effective immunostimulation. Signaling downstream of TLR4 is mediated by the adaptor proteins TRIF [Toll–interleukin-1 (IL-1) receptor (TIR) domain–containing adaptor-inducing interferon-β], which is required for adaptive immune outcomes, and MyD88 (myeloid differentiation marker 88), which is responsible for many proinflammatory effects. Two models have provided nonexclusive explanations for the differential effects of LPS and MLA. According to the first model, MLA fails to induce maturation of the proinflammatory cytokine IL-1β because it fails to activate caspase-1, which is required for the conversion of pro–IL-1β into its bioactive form. The second model suggests that MLA triggers unequal engagement of both of the signaling adaptor pathways of TLR4, such that signaling mediated by TRIF is largely intact, whereas signaling mediated by MyD88 is incomplete. We show that the TRIF-biased signaling that is characteristic of low-toxicity MLA explains its failure to activate caspase-1. Defective induction of NLRP3, which depends on MyD88, led to decreased assembly of components of the IL-1β–activating inflammasome required for the activation of preformed, inactive procaspase-1. In addition, we elucidated the contributions of MyD88 and TRIF to priming of the NLRP3 inflammasome and demonstrated that TRIF-biased TLR4 activation by MLA was responsible for the defective production of mature IL-1β.
INTRODUCTION
Toll-like receptor 4 (TLR4) recognizes a wide range of pathogen-associated molecular patterns (PAMPs) and endogenously released damage-associated molecular patterns (DAMPs), which alert the immune system to invading pathogenic organisms and cellular damage, respectively. Lipopolysaccharide (LPS) from Gram-negative bacteria is the canonical PAMP ligand for TLR4, and it induces a proinflammatory cytokine response and an increase in the abundance of factors that are involved in antigen presentation and the activation of T cells. These outcomes are mediated by the two signaling pathways downstream of TLR4, which are named for the adaptor proteins myeloid differentiation marker 88 (MyD88) and Toll–interleukin-1 (IL-1) receptor (TIR) domain–containing adaptor-inducing interferon-β (TRIF), respectively (1). The immunostimulatory properties of TLR4 ligands make them potentially useful as vaccine adjuvants (2); however, their therapeutic exploitation requires careful consideration of the potentially toxic inflammatory side effects of TLR4 signaling. One derivative of LPS, monophosphoryl lipid A (MLA), is potently immunostimulatory and exhibits only 0.1 to 1% of the toxicity of its parent molecule (3), which has led to its approval for use as a vaccine adjuvant in its clinical-grade form (4). Clinical use of this compound for prophylactic immunization has demonstrated that low-toxicity stimulation of TLR4 for therapeutic purposes is feasible; however, the intracellular signaling activities that control whether a given TLR4 ligand is likely to trigger inflammatory side effects are not fully defined.
Two explanations for the low toxicity of MLA have emerged in recent years. First, Okemoto et al. found that MLA fails to stimulate secretion of the highly inflammatory cytokine IL-1β to the same extent as does LPS, and they traced this failure to the defective activation of caspase-1, which is needed for the proteolytic maturation of IL-1β from its precursor form pro–IL-1β (5). Subsequently, we reported that MLA signals through TLR4 in a TRIF-biased manner, which is characterized by impaired stimulation of MyD88-dependent signaling events, relative to the extent of stimulation by lipid A or LPS (6, 7). In this second model, weak MyD88 signaling is proposed to account for reductions in a broad range of inflammatory activities, with little impairment of TRIF-dependent promotion of adaptive immune responses. Currently, it is unclear whether these two low-toxicity mechanisms operate independently of one another or whether one is induced by the other such that either (i) impaired IL-1β maturation generates the appearance of a TRIF bias through a loss of autocrine-paracrine signaling through the IL-1 receptor (IL-1R), which requires MyD88 as a signaling adaptor, or (ii) TRIF-biased TLR4 signaling directly results in decreased IL-1β maturation through a previously uncharacterized mechanism. Here, we sought to determine whether IL-1RI was required for MLA to appear to be a TRIF-biased ligand relative to its proinflammatory structural counterpart, lipid A, as well as to systematically examine which steps in the IL-1β production pathway required MyD88 compared to TRIF-specific signaling downstream of TLR4.
The production of IL-1β is organized into two regulated phases: an initial priming period that involves de novo protein synthesis of the 31-kD protein pro–IL-1β, followed by the assembly and activation of a multiprotein complex, termed the inflammasome, which is responsible for the proteolytic maturation of pro–IL-1β into its 17-kD active form. In response to TLR4 activation, priming includes increases in the abundance of both pro–IL-1β and NLRP3 (NACHT, LRR, and PYD domain–containing protein 3, also known as Nalp3, Pypaf1, and cryopyrin), a regulatory component of the inflammasome (8, 9). Several events can then provide a secondary stimulus for the activation of NLRP3, including the ligation of the purinergic receptor P2X7, bacterial pore-forming toxin, and particulate matter (10). Treatment of primed cells with exogenous adenosine triphosphate (ATP) induces the cytosolic protein Asc [apoptosis-inducing speck-like protein containing a caspase recruitment domain (CARD)] to form large aggregates, roughly 2 µm in diameter, that act as nucleation sites for the assembly of the NLRP3 inflammasome through PYD (pyrin domain) interactions (11, 12). Procaspase-1 is then recruited to the inflammasome, where it is cleaved to form functional heterodimeric complexes of 10- and 20-kD subunits that can mediate IL-1β maturation (13).
TLR4 signaling alone is sufficient for the production of substantial amounts of IL-1β (5), and although the amounts of IL-1β produced by this mechanism are lower than those in response to the addition of ATP, they may be relevant in situations of chronic inflammation. For example, DAMPs recognized by TLR4 are implicated in the pathogenesis of several inflammatory conditions, including diabetes, arthritis, systemic lupus erythematosus, septic shock, stroke, and myocardial infarct, as well as Alzheimer’s and Crohn’s diseases, each of which has been linked to aberrant production of IL-1β, activation of NLRP3, or both (14–20). In addition, MLA prevents ischemic reperfusion injury if given before organ transplant or cardiac tissue manipulation in several animal models (21–26), suggesting a possible mechanism whereby MLA exerts a protective effect in tissues by interfering with inflammasome assembly in response to TLR4 signaling or DAMPs. A more precise understanding of the means by which TLR4 signaling results in IL-1β maturation is needed for the development of targeted signaling therapies and to generate next-generation vaccine adjuvants that remain immunostimulatory with as little risk of adverse inflammatory effects as is possible.
Despite their obvious clinical importance, the contributions of MyD88 and TRIF to TLR4-dependent priming and activation of the inflammasome remain undefined. A previous study attempted to define the roles of TLR4 signaling adaptor molecules in terms of caspase activation; however, this study used a preparation of LPS of low purity that was likely to contain microbial contaminants, such as peptidoglycan (PGN) and muramyl dipeptide (MDP), which are themselves capable of priming cells and inducing the production of IL-1β through TLR2 and nucleotide-binding oligomerization domain (NOD) receptors (27–31). Indeed, this study concluded that caspase-1 activation is independent of MyD88 and TRIF (31), whereas it was shown elsewhere that purified LPS does not induce the production of IL-1β from cells doubly deficient in MyD88 and TRIF (8, 9, 32–34). Use of highly pure, synthetic TLR4 agonists enabled us to more accurately define the relative contributions of the MyD88 and TRIF signaling pathways to various phases of IL-1β production, without the confounding activation of other TLRs or NOD receptors. Our results demonstrate that the TRIF-biased TLR4 signaling activity of MLA was a cause, and not a consequence, of impaired IL-1β production, because TLR4-induced priming at the level of NLRP3 induction was an MyD88-dependent event.
RESULTS
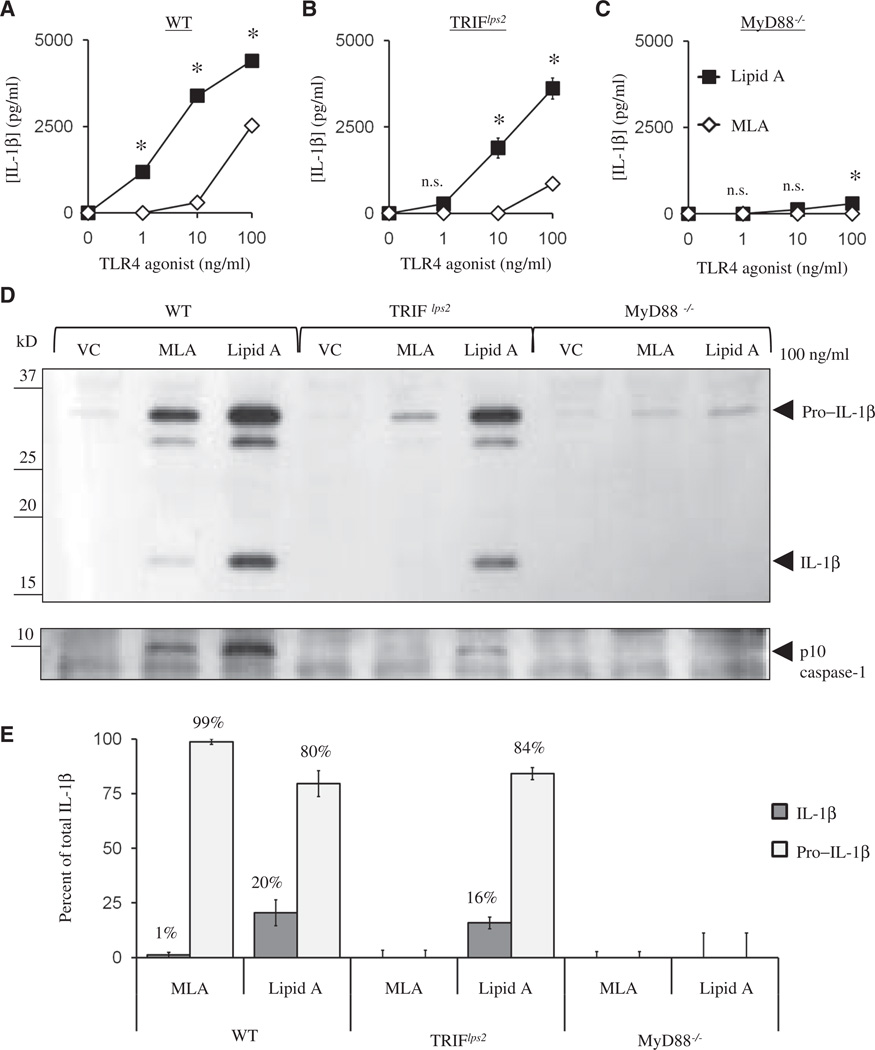
Lipid A, but not MLA, induces substantial IL-1β production from myeloid dendritic cells in a caspase-1–dependent manner in the absence of ATP
To confirm the previous findings that MLA fails to stimulate IL-1β production in our cell culture system and to establish that the absence of one phosphate from the diglucosamine head group of lipid A was sufficient for this effect, we treated myeloid dendritic cells (DCs) with structurally defined, synthetic TLR4 agonists in the absence of exogenous ATP and analyzed culture supernatants by enzyme-linked immunosorbent assay (ELISA) for the production of cytokines. Myeloid DCs activated with lipid A secreted substantially more IL-1β relative to DCs activated with MLA (Fig. 1A). This secretion of IL-1β was dependent on caspase activity, because pretreatment of DCs with the caspase inhibitor peptide Z-YVAD-FMK reduced IL-1β production to undetectable amounts. Production of IL-6, a caspase-1–independent cytokine (35), was not affected by Z-YVAD-FMK (Fig. 1B). These data suggested that synthetic lipid A induced IL-1β production in a caspase-dependent manner, that MLA failed to promote IL-1β production from myeloid DCs, and that removal of one phosphate from the head group of lipid A was sufficient for this effect.
Fig. 1.
TRIF-biased TLR4 signaling proceeds independently of IL-1RI. (A and B) ELISA analysis of (A) IL-1β and (B) IL-6 in culture supernatants from 2 × 105 wild-type (WT) myeloid DCs activated for 6 hours with MLA or lipid A (100 ng/ml) after a 30-min treatment with Z-YVAD-FMK, where indicated. (C) Real-time RT-PCR analysis of Cox2 and Ifit2 mRNA abundance from 1 × 106 WT, TRIFlps2, or MyD88−/− myeloid DCs activated for 1 hour with MLA or lipid A (100 ng/ml) and of Cox2 and Ifit2 mRNA abundance from 1 × 106 (D) WT or (E) IL-1RI−/− myeloid DCs activated with MLA or lipid A (100 ng/ml). Vehicle control (VC) is represented as the zero-hour time point. Results are the average of triplicate wells ± SEM from four (A and B) or three (C to E) independent experiments. n.s., not significant; **P < 0.01; ***P < 0.001.
TRIF-biased TLR4 signaling is independent of IL-1RI
The receptor for IL-1β, IL-1RI, requires the adaptor molecule MyD88; thus, it is possible that the greater amounts of IL-1β released by lipid A–stimulated cells (Fig. 1) supported the greater extent of MyD88-associated outcomes, relative to TRIF-biased MLA, that we previously observed (6, 7). Here, we could not detect IL-1β in culture supernatants within 1 hour of treatment with lipid A or MLA, which is the time frame during which TRIF-biased signaling by MLA was evident previously (6, 7). Nevertheless, small amounts of IL-1β produced early could potentiate MyD88 signaling through autocrine or paracrine activation of its receptor, IL-1RI. Thus, we tested whether the presence of a functional IL-1RI was required for lipid A to induce the higher expression of an MyD88-dependent gene product relative to that of MLA (Fig. 1, C to E). First, tests of the ability of lipid A to increase the abundances of Cox2 and Ifit2 mRNAs in wild-type, TRIF mutant (TRIFlps2), and MyD88−/− myeloid DCs confirmed that these transcripts were MyD88- and TRIF-dependent, respectively (Fig. 1C). TRIFlps2 mice contain a frameshift mutation that truncates the C terminus of TRIF such that it is no longer able to function as a signaling adaptor protein. As reported previously (7), the MyD88-dependent increase in the abundance of Cox2 mRNA over time was lower in response to MLA than in response to lipid A, whereas the increased abundance of TRIF-dependent Ifit2 mRNA was similar with both agonists (Fig. 1D). We reasoned that if the differential increase in the abundance of MyD88-dependent transcripts by MLA and lipid A was dependent on IL-1β production and subsequent IL-1RI autocrine signaling, then the lipid A–induced amount of Cox2 mRNA would be less than that induced by MLA in cells that lack IL-1RI signaling. Instead, we found that the differential increase in the extent of MyD88-dependent Cox2 expression was retained in lipid A–treated IL-1RI−/− cells (Fig. 1E), indicating that TRIF-biased TLR4 signaling by MLA was independent of IL-RI signaling.
Both MyD88 and TRIF contribute to the priming of myeloid DCs at the levels of Il1b mRNA and pro–IL-1β protein production
We next investigated several of the steps involved in the maturation of IL-1β to determine whether signaling by MLA could directly lead to impaired IL-1β production. Okemoto et al. and subsequently our group showed that the extent of expression of Il1b by biological preparations of MLA was similar to that by LPS (5, 6). With synthetic agonists, we first confirmed that DCs treated with either MLA or lipid A produced similar amounts of Il1b mRNA, with induction peaking 1 hour after activation (Fig. 2A). When we next measured the amounts of pro–IL-1β protein in activated DCs, we found slight differences in protein abundance in response to stimulation by either MLA or lipid A (Fig. 2B). To test further for any differences in pro–IL-1β priming, we performed experiments in the presence of the caspase inhibitor Z-YVAD-FMK, which normalizes the translation of pro– IL-1β by preventing its loss as a result of proteolytic cleavage. Caspase inhibition to block IL-1β maturation also prevents any potentiation of pro–IL-1β synthesis that can occur as a result of autocrine or paracrine exposure to mature IL-1β. Production of pro– IL-1β in response to MLA or lipid A was similar in cells pretreated with Z-YVAD-FMK, indicating that the agonists had similar potencies in inducing the transcription and translation of pro–IL-1β (Fig. 2, C and D). We confirmed that caspase activity was indeed inhibited under these conditions, because the 17-kD band corresponding to mature IL-1β in lipid A–stimulated DCs was absent after pretreatment with the inhibitor (Fig. 2C).
Fig. 2.
Induction of Il1b mRNA and pro–IL-1β protein production in WT, TRIFlps2, and MyD88−/− DCs activated with MLA and lipid A. (A) Real-time RT-PCR analysis of Il1b mRNA abundance in 1 × 106 WT myeloid DCs activated for 1 hour with MLA or lipid A (100 ng/ml). Vehicle control (VC) is represented as the zero-hour time point. (B) Western blotting analysis of pro–IL-1β from 2 × 106 WT myeloid DCs activated for 5 hours with MLA or lipid A (100 ng/ml). (C and D) Western blotting analysis and quantification of the amounts of pro–IL-1β and mature IL-1β. WT myeloid DCs (2 × 106) were pretreated with Z-YVAD-FMK (50 µM) for 30 min before undergoing activation with MLA or lipid A (100 ng/ml) for 5 hours. (E) Real-time RT-PCR analysis of Il1b mRNA abundance from 1 × 106 WT, TRIFlps2, or MyD88−/− myeloid DCs activated with MLA or lipid A (100 ng/ml). (F) Western blotting analysis of pro–IL-1β from 2 × 106 WT, TRIFlps2, and MyD88−/− myeloid DCs activated with MLA or lipid A (100 ng/ml) for 5 hours. Results are the average of triplicate wells ± SEM from four independent experiments (A and E) or are representative of four (B and F) or five (C and D) independent experiments. n.s., not significant; *P < 0.05; **P < 0.01.
Upon analyzing the relative contributions of MyD88 and TRIF to the increased abundance of Il1b mRNA in lipid A–treated DCs, we discovered that expression of Il1b to its fullest extent (that is, similar to that in wild-type DCs) required both of the adaptor proteins (Fig. 2E). Although Il1b induction was thought to be strictly MyD88-dependent, this was based on a study performed before the full extent of the complexity of the priming, assembly, and activation of inflammasomes was understood, and before new and more sensitive techniques for the detection of transcripts were developed (36). In the absence of Z-YVAD-FMK, MyD88 substantially contributed to the production of pro–IL-1β, with TRIF being less critical (Fig. 2F), which may reflect, in part, the requirement for MyD88 in IL-1R1 signaling that could potentiate the production of pro–IL-1β.
TLR4-dependent secretion of IL-1β in the absence of ATP depends on MyD88 and TRIF
Previous studies have reported the weak production of IL-1β by MLA (5, 6). We confirmed this finding in myeloid DCs in experiments in which we used synthetic MLA to stimulate the cells in the absence of ATP, which showed weak production of IL-1β at all doses relative to the extent of induction by lipid A (Fig. 3A). When we assessed the relative dependencies of IL-1β production on MyD88 and TRIF, we found that the amounts of secreted IL-1β and cell-associated, mature IL-1β were reduced in the absence of either signaling adaptor compared to those in wild-type cells (Fig. 3, B to E). In addition, MLA remained less potent than lipid A at inducing IL-1β production in either TRIFlps2 or MyD88−/− DCs. We were unable to directly detect caspase-1 maturation in cells treated with TLR4 agonist alone; however, the caspase inhibitor experiments described earlier showed that the 17-kD product corresponding to mature IL-1β was dependent on caspase activity. These data show that the low amounts of mature IL-1β produced in response to TLR4 activation alone, in the absence of exogenous ATP, were dependent on both TRIF and MyD88 signaling. Moreover, the different extents of secretion of mature IL-1β induced by MLA and lipid A did not appear to result from differences in the abundances of Asc or procaspase-1, because both proteins were constitutively present and similarly abundant (Fig. 3D), which has been previously reported (13).
Fig. 3.
Generation of mature IL-1β by TLR4 agonists alone is codependent on MyD88 and TRIF. (A to C) ELISA analysis of IL-1β in culture supernatants from 2 × 105 WT, TRIFlps2, or MyD88−/− myeloid DCs activated for 6 hours with MLA or lipid A (100 ng/ml). (D and E) Western blotting analysis and quantification of the amounts of pro–IL-1β or Asc and procaspase-1 present in 2 × 106 WT, TRIFlps2, or MyD88−/− myeloid DCs activated for 5 hours with MLA or lipid A (100 ng/ml). Results are the average of triplicate wells ± SEM from three independent experiments (A to C) or are representative of four (D, top, and E) or three (D, middle and bottom) independent experiments. n.s., not significant; *P < 0.05.
MyD88 is critically required for ATP-dependent IL-1β production
Although we found that the production of mature IL-1β in response to TLR4 stimulation, in the absence of ATP, depended on both MyD88 and TRIF, we speculated that ATP could compensate for activities induced downstream of either adaptor. We tested this by stimulating wild type, TRIFlps2, or MyD88−/− cells with TLR4 agonists and then providing ATP to stimulate the assembly and activation of the inflammasome. We found that lipid A–primed TRIFlps2 DCs showed only a slight decrease in IL-1β production relative to that of wild-type cells in the presence of ATP, whereas MyD88 appeared critical for ATP-induced IL-1β production, which correlated with the abundance of released active caspase-1 (Fig. 4, A to C). MLA-primed DCs still produced substantially less IL-1β than did DCs primed with lipid A. It was previously reported that ATP induces the secretion of both pro–IL-1β and mature IL-1β (37). To determine the relative contributions of these forms of IL-1β to the total amounts that we detected by ELISA (Fig. 4A), we analyzed the culture supernatants of MLA- or lipid A–primed, ATP-treated DCs by Western blotting (Fig. 4D). Although ATP markedly increased the amount of total IL-1β secreted by MLA-primed DCs, relative to that of cells that were not treated with ATP (Fig. 4A), we found that ~99% of the IL-1β released by ATP was unprocessed pro–IL-1β (Fig. 4E). This confirmed the inability of MLA to activate caspase-1 even in the presence of ATP (5). Collectively, these data show a stringent requirement for MyD88, and to a much lesser degree TRIF, in ATP-triggered production of IL-1β by cells primed by lipid A through TLR4. Additionally, MLA-primed DCs were unable to secrete the mature form of IL-1β even when given a strong secondary stimulus to activate the inflammasome.
Fig. 4.
MyD88 is required for ATP-stimulated IL-1β production. (A to C) ELISA analysis of the amount of IL-1β in culture supernatants from 2 × 105 WT, TRIFlps2, and MyD88−/− myeloid DCs activated for 6 hours with MLA or lipid A (100 ng/ml) and then treated for 20 min with ATP (5 mM). (D) Western blotting analysis of IL-1β and p10 caspase-1 performed with equal volumes of supernatants from the samples (A to C) that were treated with agonist (100 ng/ml). (E) Densitometric analysis of the ratios of the amounts of pro–IL-1β to mature IL-1β from (D) resulted in calculation of the amounts of pro–IL-1β and mature IL-1β as percentages of total IL-1β. Results are the average of triplicate wells ± SEM from three independent experiments (A to C), are representative of three independent experiments (D), or are the average of three independent experiments ± SEM (E). n.s., not significant; *P < 0.05.
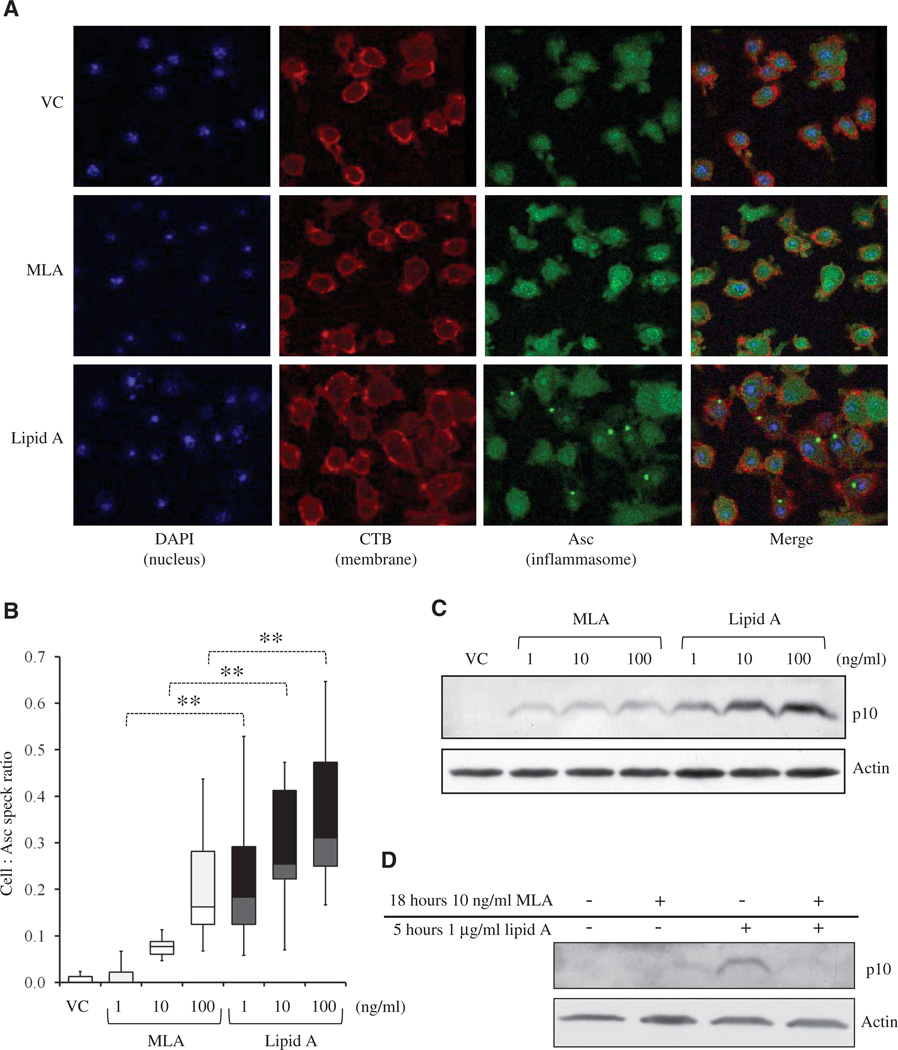
MLA-primed myeloid DCs display reduced Asc speck formation and caspase-1 activation
The almost complete failure of MLA to support IL-1β maturation in the presence of ATP suggested that the formation of higher-order inflammasome structures might be impaired. Such structures are commonly detected by imaging a constitutively expressed inflammasome component, Asc, after exogenous ATP is provided to cells primed with TLR4 agonists. Asc visualization under these conditions shows diffuse and then highly focused aggregation into Asc “specks,” which reflect the assembly and activation of the inflammasome induced by ATP in primed cells (12). To test for the competence of MLA-primed cells to support the formation of these structures, we treated MLA- or lipid A–primed DCs briefly with ATP before visualizing Asc specks by confocal microscopy. Lipid A–primed DCs displayed significantly more Asc specks than did cells primed with MLA (Fig. 5A, P < 0.01), with MLA-primed DCs forming fewer Asc specks at all of the tested doses (Fig. 5B). The potency shift revealed by the dose-response experiment suggested that the potency of MLA was ~1% of that of lipid A. Further, the formation of Asc specks correlated with the amount of the p10 subunit of mature caspase-1 that was generated, with MLA-primed DCs generating markedly less p10 than those primed with lipid A, upon addition of ATP (Fig. 5C). These data show that the activation of TLR4 by MLA resulted in impaired inflammasome assembly and caspase-1 activation even in the presence of an exogenous secondary stimulus for inflammasome activation and even with some priming at the level of pro–IL-1β.
Fig. 5.
MLA-primed myeloid DCs have fewer Asc specks and less active caspase-1 than those of lipid A–primed myeloid DCs. (A and B) WT myeloid DCs (5 × 104) were activated for 5 hours with MLA and lipid A and then treated for 5 min with ATP (5 mM). (A) Representative images from confocal microscopic analysis showing nuclei (stained with DAPI, blue), plasma membrane (CTB, red), and inflammasomes (Asc, green). Images are representative of at least two independent experiments. (B) Box-and-whisker plots of enumerated cells and Asc specks of images from at least two independent experiments containing ~550 cells per treatment. (C) Western blotting analysis of the p10 subunit of caspase-1 from 1 × 106 WT myeloid DCs activated for 5 hours with MLA or lipid A. Data are representative of three independent experiments. (D) Western blotting analysis of the p10 subunit of caspase-1 from 1 × 106 WT myeloid DCs pretreated with MLA (10 ng/ml) or VC for 18 hours before activation for 5 hours with lipid A (1 µg/ml) followed by a 5-min treatment with ATP (5 mM). Image is representative of two independent experiments. **P < 0.01.
Pretreatment with a low concentration of MLA prevents lipid A–induced inflammasome activation in the presence of ATP
Because MLA has protective effects against IL-1β–mediated pathology, such as in ischemic reperfusion injury and endotoxic shock (21–26), we wanted to see whether stimulating myeloid DCs with low concentrations of MLA could inhibit subsequent lipid A–induced inflammasome activation in the presence of ATP. Indeed, whereas treatment of myeloid DCs with lipid A for 5 hours after overnight stimulation with vehicle resulted in the robust activation of casapse-1, pretreatment of cells with a low concentration of MLA did not. Pretreatment with MLA effectively inhibited the activation of the ATP-induced inflammasome by lipid A (Fig. 5D), indicating that MLA may be potentially used for protection from IL-1β–mediated pathology.
NLRP3 induction is weak in MLA-treated cells
Because MLA-primed DCs failed to form Asc specks and activate caspase-1 upon addition of ATP, we considered whether other components of the inflammasome might be involved in the MLA-associated deficiency in IL-1β production. Production of NLRP3 protein during priming is required for IL-1β production and is critical for the activation of caspase-1 because NLRP3 acts as a scaffold for formation of the higher-order structures that are represented by Asc specks (8, 38). We measured the abundance of NLRP3 mRNA in response to MLA or lipid A and found that MLA was an inefficient inducer of this critical inflammasome component (Fig. 6A). Western blotting analysis of the production of NLRP3 protein provided consistent results (Fig. 6, B and C). Therefore, MLA-primed DCs may have failed to form Asc specks and activate caspase-1, even in the presence of ATP, because inflammasome priming at the level of de novo NLRP3 synthesis was defective.
Fig. 6.
Induction of NLRP3 mRNA and protein in WT, TRIFlps2, and MyD88−/− myeloid DCs activated with MLA or lipid A. (A) Real-time RT-PCR analysis of the abundance of NLRP3 mRNA from 1 × 106 WT myeloid DCs activated with MLA or lipid A (100 ng/ml). (B and C) Western blotting analysis and quantification of NLRP3 from 2 × 106 WT myeloid DCs activated for 5 hours with MLA or lipid A. (D) Real-time RT-PCR analysis of the abundance of NLRP3 mRNA from 1 × 106 WT, TRIFlps2, or MyD88−/− myeloid DCs activated for 1 hour with MLA or lipid A (100 ng/ml). (E) Western blotting analysis of NLRP3 from 2 × 106 WT, TRIFlps2, or MyD88−/− myeloid DCs activated for 5 hours with MLA or lipid A (100 ng/ml). (F) Western blotting analysis of NLRP3 and pro–IL-1β from 2 × 106 WT myeloid DCs activated for 5 hours with various doses of poly(I:C) or with lipid A (100 ng/ml). Data are the averages of triplicate wells ± SEM from four independent experiments (A and D) or are representative of four (B, C, and E) or two (F) independent experiments. n.s., not significant; *P < 0.05; ***P < 0.001.
MyD88 is required for NLRP3 induction during lipid A priming
We next tested the possibility that the distinct TLR4-dependent signaling stimulated by MLA was the cause of its weak ability to induce the expression of NLRP3. Comparison of the contributions made by TRIF and MyD88 to the increased abundance of NLRP3 mRNA revealed that MyD88, but not TRIF, was required for the full induction of NLRP3 expression (Fig. 6D). Analysis of the generation of NLRP3 protein showed the same MyD88 dependence, with no detectable increases in the amount of NLRP3 protein in lipid A–primed MyD88−/− DCs relative to that of vehicle-treated control cells, and full induction in TRIFlps2 DCs (Fig. 6E). The discovery that the increased synthesis of NLRP3 protein in response to TLR4 signaling depends on MyD88 provides a mechanistic basis to understand why MLA fails to support IL-1β maturation.
Stimulation of TLR3-TRIF signaling by poly(I:C) does not induce expression of NLRP3
The TLR3 agonist polyinosine-polycytosine [poly(I:C)] fails to induce IL-1β production unless it is delivered to the cytosol of previously primed cells by transfection (39). Because this is suggestive of a lack of NLRP3 induction by the TLR3-TRIF signaling pathway, we decided to test directly the extent to which poly(I:C) primes inflammasome activity at the level of production of NLRP3 protein. We stimulated myeloid DCs with poly(I:C) from two separate sources and found that although poly(I:C) was a potent inducer of pro–IL-1β, NLRP3 was not produced. The ability of poly(I:C) to induce the production of pro–IL-1β, but not NLRP3, supports our previous findings that, whereas TRIF can contribute to the priming of the inflammasome at the level of production of pro–IL-1β, MyD88 is strictly required for the induction of NLRP3.
DISCUSSION
There is a need for safe vaccine adjuvants that stimulate the immune system without inducing inflammatory side effects. We previously characterized MLA, which is used clinically in vaccine preparations, as a TRIF-biased agonist of TLR4 (6, 7). Some studies have led to the conclusion that the failure of MLA to induce the production of IL-1β is responsible for the decreased toxicity of MLA compared to that of its parent molecule lipid A (5). Here, we showed that altered TLR4 signaling by MLA was upstream of IL-1β and was in fact responsible for failure to produce IL-1β. Activation of TLR4 by MLA resulted in efficient priming at the level of pro–IL-1β, but weak induction of NLRP3, which required intact MyD88 signaling.
The only other adjuvant approved for use in humans is alum, which is a potent activator of the NLRP3 inflammasome and induces both T helper 1 (TH1)– and TH2-type antibody responses (40–42); however, whether NLRP3 activation and IL-1β production are required to initiate protective immune responses is disputed (43). In fact, the induction by alum of specific TH1-type immune responses, which are most effective at protecting against intracellular viral and bacterial infections, is independent of NLRP3 and IL-1β (40–42). If IL-1β production is not critical for adjuvant-established protective immunity, then MLA, which fails to support production of this inflammatory cytokine, is preferable to alum or an admixture of both. Collateral activation of the inflammasome during vaccination would merely increase the likelihood of pathogenic side effects. Additional evidence for the dispensability of IL-1β in generating protective immunity shows that NLRP3 activation and IL-1β production in antigen-presenting cells are actively suppressed by effector and memory T cells during recall responses (44).
In addition to reconciling the causality of IL-1β loss with TRIF-biased signaling, we have clarified the contributions of TRIF and MyD88 to inflammasome priming and activation downstream of TLR4 signaling alone, or in the presence of exogenous ATP. In the absence of an exogenous stimulus that activates the inflammasome, MyD88 signaling induced an increase in the amounts of pro–IL-1β and NLRP3, whereas TRIF signaling promoted some increase in the amount of pro–IL-1β production and the maturation of small amounts of IL-1β through an as yet undefined mechanism. Although the contribution of TRIF to inflammasome activation was demonstrated in mice deficient in autophagic factors (45), more studies are required to fully define its role in TLR4-dependent IL-1β production. Our elucidation of the TRIF and MyD88 signaling pathways responsible for priming and activating the NLRP3 inflammasome provides a knowledge base for the development of signaling therapies for IL-1β–mediated pathologies—a current aim of many investigators and clinicians (10, 15, 46–50). Additionally, the small amounts of IL-1β produced in response to TLR4 stimulation alone may be clinically relevant to inflammatory conditions that are mediated by endogenous activators of TLR4 (14–20).
Even in the presence of exogenous ATP, MLA-primed cells were unable to accomplish inflammasome assembly, caspase-1 activation, or production of mature IL-1β because of a critical deficiency at the level of NLRP3. Additionally, pretreatment with MLA inhibited lipid A–induced inflammasome activation at the level of production of p10 caspase-1 in the presence of ATP. This is intriguing considering that MLA is used to prevent ischemic reperfusion injury (21–26), whose pathology is mediated in part by IL-1β (17). The effectiveness of MLA at inducing a tolerogenic state in which cells are unresponsive to further stimulation through TLR4 has been demonstrated in humans and several animal models (51–54). Tolerance of this kind is also achievable with endotoxin (LPS or lipid A); however, these TLR4 agonists induce a toxic inflammatory response before the acquisition of tolerance (55, 56). Clinically, intravenous administration of MLA is well tolerated (53), and it is an attractive alternative to endotoxin for initiating tolerance, because the initial induction of proinflammatory cytokines is greatly decreased. Preconditioning with MLA seems to result in a type of “inflammasome tolerance,” which could be critically valuable for conditions in which IL-1β–mediated pathology occurs after reperfusion of ischemic tissue, such as in organ transplantation, myocardial infarct, and stroke, and the subsequent release of inflammasome-activating ATP from stressed or damaged cells.
Here, we have presented the first thorough study of the signaling events involved in TLR4-dependent priming of the NLRP3 inflammasome for IL-1β production and described a possible mechanism for the protective effects of MLA that are observed in therapeutic settings, such as in the prevention of ischemic reperfusion injury. Further studies will be required to reveal the extent of such tolerance induced by MLA and whether TRIF-biased TLR4 signaling can be used effectively as a preconditioning regimen for IL-1β–mediated pathology.
MATERIALS AND METHODS
Mice and cell culture
C57BL/6, TRIFlps2, and IL-1RItmlRoml (referred to as IL-1RI−/−) mice were purchased from Jackson Laboratories. MyD88−/− mice were a gift from S. Akira (through R. Kedl, 3M Corporation). All mice were kept in a specific pathogen-free animal facility at the University of Louisville under the supervision of its Institutional Animal Care and Use Committee. Myeloid DCs were derived as previously described (7). Femurs and tibiae of mice were flushed of bone marrow plugs with a 22½-gauge needle and sterile Hanks’ balanced salt solution (HBSS). After centrifugation, bone marrow cells were resuspended at 2 × 106 cells/ml in complete R10F medium [RPMI 1640 medium (Gibco) supplemented with 1 mM sodium pyruvate, penicillin (50 U/ml), streptomycin (50 µg/ml), 2 mM l-glutamine, recombinant murine granulocyte-macrophage colony-stimulating factor (GMCSF; 5 ng/ml, R&D Systems), 50 µM 2-mercaptoethanol, and 10% heat-inactivated fetal bovine serum (Valley Biomedical)]. Cells were cultured in 100-mm bacteriological petri dishes and given fresh media on days 3, 6, and 8, with the culture lasting 10 days. Nonadherent cells were harvested and washed in R10F, resuspended, and analyzed by flow cytometry for cell surface markers. This culture system yields a myeloid DC phenotype that is >97% positive for CD11b (and >83% double-positive for CD11b and CD11c) and <5% positive for CD4, CD8, CD19, B220, and GR1. R10F medium supplemented with only l-glutamine was used in experiments. DCs were rested at 37°C and 5% CO2 in a humidified incubator for 2 hours before activation.
Reagents
Synthetic lipid A (Peptides International) and synthetic MLA (Invivogen) derived from the Escherichia coli structures of LPS were reconstituted in dimethyl sulfoxide (DMSO), aliquoted, and stored at −80°C. Reported mass-to-charge ratios for the lipid A structures were confirmed by mass spectrometry. Paired lots of lipid A and MLA were used for experiments, and each aliquot went through only one freeze-thaw cycle. Poly(I:C) from Sigma and Invivogen was reconstituted in ribonuclease-free water (Qiagen). ATP (Roche) and Z-YVAD-FMK (R&D Systems) were used at concentrations of 5 mM and 50 µM, respectively.
Real-time reverse transcription–polymerase chain reaction assays
RNA isolated from whole-cell lysates with the Qiagen RNeasy Mini Kits was reverse-transcribed into complementary DNA (cDNA) with Quanta qScript cDNA Synthesis Kits according to the manufacturer’s protocol. cDNA was used with 0.5 µMtarget (Cox2, Ifit2, Asc, Il1b) or control (Actin) QuantiTect Primer Assays from Qiagen. Primers for NLRP3 (forward, 5′-ATGGTATGCCAGGACAG-3′; reverse, 5′-ATGCTCCTTGACCAGTTGGA-3′) were synthesized by Sigma. Quantitative real-time polymerase chain reaction (PCR) was performed on a CFX96 Real-Time System C1000 Thermal Cycler (Bio-Rad) with Power SYBR Green Master Mix (Applied Biosystems). Relative mRNA abundance was calculated by the comparative cycle method (ΔΔCt).
Western blotting analysis
Cells were washed with ice-cold HBSS containing 50 µM NaF and lysed with radioimmunoprecipitation buffer [50 mM tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS] supplemented with Complete Mini Protease Inhibitor Cocktail Tablets (Roche). Equal protein amounts as determined by bicinchoninic acid kits (Pierce Biotechnology) from clarified lysates were separated on 8 or 12% polyacrylamide gels under reducing conditions. Resolved proteins were transferred to nitrocellulose membranes (GE Healthcare) that were then blocked in 5% nonfat dry milk or bovine serum albumin (BSA). Membranes were incubated overnight at 4°C with primary antibody and then incubated for 1 hour with horseradish peroxidase (HRP)–conjugated secondary antibody. ECL (Amersham) was used for band visualization by film or a FujiFilm LAS-4000(mini) Luminescent Image Analyzer. Densitometric analysis of bands was performed with Quantity One 4.6.6 software (Bio-Rad). Antibodies used in Western blotting analysis included polyclonal rabbit antibody against IL-1β (Abcam), monoclonal antibody against NLRP3 (Cryo-2, Adipogen), rabbit antibody against caspase-1 p10 (M-20, Santa Cruz Biotechnology), rabbit antibody against Asc (N-15-R, Santa Cruz Biotechnology), rabbit antibody against caspase-1/ICE (Upstate Cell Signaling Solutions), and antibody against β-actin (I-19, Santa Cruz Biotechnology). These antibodies were detected with HRP-conjugated secondary antibodies against rabbit, mouse, and goat antibodies, which were obtained from Jackson ImmunoResearch.
Cytokine detection
Culture supernatants were analyzed with OptEIA mouse IL-6 ELISA sets (BD Biosciences) or mouse IL-1β Ready-Set-Go! ELISA sets (eBioscience) according to the manufacturers’ protocols. Optical density (OD) values were obtained with a Molecular Devices Emax Precision Microplate Reader and SoftMax Pro.
Immunofluorescence confocal microscopy
For each treatment, myeloid DCs were activated in chamber slides (Nalge Nunc International) for 5 hours with TLR4 agonists and then treated with 5 mM ATP for 5 min. Chamber slides were placed on ice and incubated with Alexa Fluor 594–conjugated cholera toxin subunit B (CTB, Invitrogen) for an additional 5 min before they were fixed and permeabilized with a 1:1 solution of methanol/acetone. Nonspecific binding sites were blocked with Image-iT FX Signal Enhancer (Invitrogen) for 1 hour before overnight incubation at 4°C with polyclonal antibody against Asc (N-15-R). Slides were then incubated with Alexa Fluor 488–conjugated goat antibody against rabbit immunoglobulin G (IgG; Invitrogen) for 1 hour before being prepared with VectaShield Hard-Mount Medium and 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Images were captured by a Leica TCS SP5 II confocal microscope with LAS AF 2.2.0 software. Cells and Asc specks were enumerated with ImageJ 1.43 analysis software.
Statistics and sample normalization
Each independent experiment used separate myeloid DC cultures derived from individual mice, and each graph is representative of at least three independent experiments. The differences between groups in time-course experiments were statistically analyzed with balanced one-way analysis of variance (ANOVA). Individual data points were analyzed by paired twosample for means t test with a hypothesized mean difference of zero and a confidence level of 0.05. A P value of <0.05 was considered statistically significant. Data points in ELISA and real-time reverse transcription–PCR (RT-PCR) assays are the average of triplicate wells ± SEM. All data from real-time RT-PCR assays were normalized to the abundance of actin mRNA, and cells treated with vehicle alone were used as reference points for the calculation of fold increase.
Acknowledgments
We thank C. Casella, P. Chilton, and M. Martin for their expert advice; D. Hadel for animal husbandry and genotyping; R. Higashi and B. Bogdonov for mass spectrometry analysis of lipid A structures; and E. Yolcu, C. Daep, and E. Roy for confocal microscopy assistance. Funding: This study was funded by the American Heart Association (predoctoral grant to C.A.E.), the Crohn’s and Colitis Foundation of America (Career Development Award to L.F.), and the NIH (R01 AI071047 to T.C.M. and R01 1AR052756/AI063331 to G.N.).
Footnotes
Author contributions: C.A.E. performed all experimentation and wrote the preliminary manuscript draft. C.A.E., L.F., G.N., and T.C.M. contributed to experimental design and interpretation and manuscript revision.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Taking toll: Lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10:S32–S37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 3.Ribi E. Beneficial modification of the endotoxin molecule. J. Biol. Response Mod. 1984;3:1–9. [PubMed] [Google Scholar]
- 4.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 5.Okemoto K, Kawasaki K, Hanada K, Miura M, Nishijima M. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1β or activation of caspase-1. J. Immunol. 2006;176:1203–1208. doi: 10.4049/jimmunol.176.2.1203. [DOI] [PubMed] [Google Scholar]
- 6.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 7.Cekic C, Casella CR, Eaves CA, Matsuzawa A, Ichijo H, Mitchell TC. Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J. Biol. Chem. 2009;284:31982–31991. doi: 10.1074/jbc.M109.046383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchi L, Eigenbrod T, Núñez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing caspase recruitment domain. J. Immunol. 2009;182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJ-F, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin T-T, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA, Tocci MJ. A novel heterodimeric cysteine protease required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 14.Davis BK, Ting JP. NLRP3 has a sweet tooth. Nat. Immunol. 2010;11:105–106. doi: 10.1038/ni0210-105. [DOI] [PubMed] [Google Scholar]
- 15.Mitroulis I, Skendros P, Ritis K. Targeting IL-1β in disease; the expanding role of NLRP3 inflammasome. Eur. J. Intern. Med. 2010;21:157–163. doi: 10.1016/j.ejim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Toledo-Pereyra LH. Interleukin 1 and tumor necrosis factor production as the initial stimulants of liver ischemia and reperfusion injury. J. Surg. Res. 1994;57:253–258. doi: 10.1006/jsre.1994.1140. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, Li B, Wang N, Xie Y, Wang L, Yuan Q, Zhang F, Qin J, Peng Z, Ning W, Wang L, Hu G, Li J, Tao L. Fluorofenidone protects mice from lethal endotoxemia through the inhibition of TNF-α and IL-1β release. Int. Immunopharmacol. 2010;10:580–583. doi: 10.1016/j.intimp.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Torres R, Macdonald L, Croll SD, Reinhardt J, Dore A, Stevens S, Hylton DM, Rudge JS, Liu-Bryan R, Terkeltaub RA, Yancopoulos GD, Murphy AJ. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann. Rheum. Dis. 2009;68:1602–1608. doi: 10.1136/ard.2009.109355. [DOI] [PubMed] [Google Scholar]
- 19.Voronov E, Dayan M, Zinger H, Gayvoronsky L, Lin JP, Iwakura Y, Apte RN, Mozes E. IL-1β-deficient mice are resistant to induction of experimental SLE. Eur. Cytokine Netw. 2006;17:109–116. [PubMed] [Google Scholar]
- 20.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado C, Stadelmann WK, Ramirez S, Quan EE, Barker JH. Preconditioning of latissimus dorsi muscle flaps with monophosphoryl lipid A. Plast. Reconstr. Surg. 2003;111:267–274. doi: 10.1097/01.PRS.0000033066.12439.C0. [DOI] [PubMed] [Google Scholar]
- 22.Sharony R, Frolkis I, Froylich D, Wildhirt SM, Shapira I, Reichart B, Nesher N, Uretzky G. Pharmacological preconditioning with monophosphoryl lipid A improves post ischemic diastolic function and modifies TNF-α synthesis. Eur. J. Cardiothorac. Surg. 2005;27:501–507. doi: 10.1016/j.ejcts.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Xiuhua L, Xudong W, Yue H, Chaoshu T, Jingyi S. Signaling pathway of cardioprotection induced by monophosphoryl lipid A in rabbit myocardium. Pathophysiology. 2002;8:193–196. doi: 10.1016/s0928-4680(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita N, Hoshida S, Otsu K, Taniguchi N, Kuzuya T, Hori M. Monophosphoryl lipid A provides biphasic cardioprotection against ischaemia-reperfusion injury in rat hearts. Br. J. Pharmacol. 1999;128:412–418. doi: 10.1038/sj.bjp.0702809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida T, Engelman RM, Engelman DT, Rousou JA, Maulik N, Sato M, Elliott GT, Das DK. Preconditioning of swine heart with monophosphoryl lipid A improves myocardial preservation. Ann. Thorac. Surg. 2000;70:895–900. doi: 10.1016/s0003-4975(00)01508-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhu HQ, Jiang JL, Lu R, Zhang XH, Deng HW, Li YJ. The protective effects of monophosphoryl lipid A on the ischemic myocardium and endothelium in rats. Cardiovasc. Drugs Ther. 2003;17:311–318. doi: 10.1023/a:1027335321530. [DOI] [PubMed] [Google Scholar]
- 27.Shikama Y, Kuroishi T, Nagai Y, Iwakura Y, Shimauchi H, Takada H, Sugawara S, Endo Y. Muramyldipeptide augments the actions of lipopolysaccharide in mice by stimulating macrophages to produce pro-IL-1β and by down-regulation of the suppressor of cytokine signaling 1 (SOCS1) Innate Immun. 2011;17:3–15. doi: 10.1177/1753425909347508. [DOI] [PubMed] [Google Scholar]
- 28.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2–NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferwerda G, Kramer M, de Jong D, Piccini A, Joosten LA, Devesaginer I, Girardin SE, Adema GJ, van der Meer JW, Kullberg BJ, Rubartelli A, Netea MG. Engagement of NOD2 has a dual effect on proIL-1β mRNA transcription and secretion of bioactive IL-1β. Eur. J. Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 30.Vacheron F, Guenounou M, Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect. Immun. 1983;42:1049–1054. doi: 10.1128/iai.42.3.1049-1054.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, Yamamoto M, Akira S, Nakanishi K, Noda T, Taniguchi S. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–1067. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 32.Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-κB activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 2009;183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Planillo R, Franchi L, Miller LS, Núñez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Nunez G, Suzuki T. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-κB signaling. J. Immunol. 2010;184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 35.Taxman DJ, Zhang J, Champagne C, Bergstralh DT, Iocca HA, Lich JD, Ting JP. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. J. Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 37.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 38.Huang MT, Taxman DJ, Holley-Guthrie EA, Moore CB, Willingham SB, Madden V, Parsons RK, Featherstone GL, Arnold RR, O’Connor BP, Ting JP. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J. Immunol. 2009;182:2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 39.Rajan JV, Warren SE, Miao EA, Aderem A. Activation of the NLRP3 inflammasome by intracellular poly I:C. FEBS Lett. 2010;584:4627–4632. doi: 10.1016/j.febslet.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 42.Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur. J. Immunol. 2010;40:638–642. doi: 10.1002/eji.200940039. [DOI] [PubMed] [Google Scholar]
- 44.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 45.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1α production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 46.Naik E, Dixit VM. Modulation of inflammasome activity for the treatment of autoinflammatory disorders. J. Clin. Immunol. 2010;30:485–490. doi: 10.1007/s10875-010-9383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook GP, Savic S, Wittmann M, McDermott MF. The NLRP3 inflammasome, a target for therapy in diverse disease states. Eur. J. Immunol. 2010;40:631–634. doi: 10.1002/eji.200940162. [DOI] [PubMed] [Google Scholar]
- 48.Wanderer AA. Rationale for IL-1β targeted therapy for ischemia-reperfusion induced pulmonary and other complications in sickle cell disease. J. Pediatr. Hematol. Oncol. 2009;31:537–538. doi: 10.1097/MPH.0b013e3181acd89d. [DOI] [PubMed] [Google Scholar]
- 49.Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, Wu R, Mellis S, Radin A. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: Results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann. Rheum. Dis. 2009;68:1613–1617. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinarello CA. Interleukin-1β and the autoinflammatory diseases. N. Engl. J. Med. 2009;360:2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- 51.Wy CA, Goto M, Young RI, Myers TF, Muraskas J. Prophylactic treatment of endotoxic shock with monophosphoryl lipid A in newborn rats. Biol. Neonate. 2000;77:191–195. doi: 10.1159/000014215. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton-Davies C, Webb AR. Monophosphoryl lipid A and endotoxin tolerance. Crit. Care Med. 1995;23:1789–1790. doi: 10.1097/00003246-199510000-00032. [DOI] [PubMed] [Google Scholar]
- 53.Astiz ME, Rackow EC, Still JG, Howell ST, Cato A, Von Eschen KB, Ulrich JT, Rudbach JA, McMahon G, Vargas R, Stern W. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: A prospective, double-blind, randomized, controlled trial. Crit. Care Med. 1995;23:9–17. doi: 10.1097/00003246-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Gustafson GL, Rhodes MJ, Hegel T. Monophosphoryl lipid A as a prophylactic for sepsis and septic shock. Prog. Clin. Biol. Res. 1995;392:567–579. [PubMed] [Google Scholar]
- 55.Lüderitz O, Staub AM, Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol. Rev. 1966;30:192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]