Abstract
Individuals with fragile X mental retardation 1 (FMR1) premutation (55 to 200 CGG repeats) are typically unaffected by fragile X syndrome. However, a subgroup of older males with the premutation have developed a neurological syndrome, which usually begins between 50 and 70 years and is associated with a progressive intention tremor and/or ataxia manifested by balance problems, frequent falling, and Parkinsonian symptoms, such as masked facies, intermittent resting tremor, and mild rigidity. This finding has been termed the fragile X-associated tremor/ataxia syndrome (FXTAS) and has brought focus to the aging process in individuals with the FMR1 mutation. The premutation is associated with elevated messenger RNA levels leading to the formation of intranuclear inclusions in neurons and astrocytes associated with FXTAS. This review is a summary of our experience with FXTAS in male carriers of the premutation.
Within the past 3 years, there has been a significant change in understanding the effects of aging in those who carry the Fragile X Mental Retardation 1 (FMR1) premutation (55 to 200 CGG repeats). Individuals with the premutation are usually unaffected intellectually; however, the recent discovery of the fragile X-associated tremor/ataxia syndrome (FXTAS) in a subgroup of older male carriers with the premutation has drawn attention to the process of aging in all individuals with expanded FMR1 alleles. In this paper we summarize the findings of FXTAS at our centers, with a focus on treatment and genetic counseling.
Aging Among Carriers of the FMR1 Premutation
We recently reported a distinctive form of clinical involvement in some older male carriers of the fragile X premutation (Berry-Kravis et al., 2003; Brunberg et al., 2002; R. Hagerman et al., 2001; Jacquemont, Hagerman, Leehey, Grigsby et al., 2003; Leehey et al., 2003a). These carriers, in their 50s and older, develop progressive intention tremor (tremor that occurs with voluntary movement) and ataxia (balance and coordination difficulties). These movement disorders are often accompanied by progressive cognitive and behavioral difficulties, including memory loss, anxiety, executive function deficits leading to dementia, and reclusive or irritable behavior (Jacquemont, Hagerman, Leehey, Grigsby et al., 2003). In addition, patients may have features of Parkinsonism (including masked facies, rigidity in movement, and tremor that occurs at rest), peripheral neuropathy (decreased sensation in the lower extremities to touch and vibration), lower limb proximal muscle weakness, and autonomic dysfunction (urinary/bowel incontinence and impotence). This disorder has been designated FXTAS (Jacquemont, Hagerman, Leehey, Grigsby et al., 2003).
We have also observed a striking neuropathological finding present in all eight brains examined to date from patients who died with FXTAS (Greco et al., 2002; P. Hagerman et al., 2003; Kenneson et al., 2001; Mankodi et al., 2002; Tassone et al., 2001). Intranuclear inclusions in both neurons and astrocytes are present throughout the cortex and brainstem, with the greatest densities of inclusions located in the hippocampus and frontal cortical regions. No inclusions have been detected in the Purkinje cells of the cerebellum, although there is evident Purkinje cell dropout and cerebellar axonal degeneration. The inclusions are polyglutamine-negative, distinguishing FXTAS from the CAG repeat ataxias. Moreover, no tau or α-synuclein proteins have been found within the inclusions (P. Hagerman et al., 2003a).
Recently, a transgenic mouse model with expanded CGG repeats in the premutation range (~100 CGG repeats in the Fmr1 transgene) has been created, and all of the mice develop intra-nuclear inclusions that are morphologically similar to those observed in the FXTAS cases (Willemsen et al., 2003). This confirms that the intranuclear inclusions are directly related to the CGG premutation expansion. Previous work has demonstrated that increased levels of FMR1 messenger RNA (mRNA) (2- to 10-fold elevation) are present in all premutation carriers, despite lowered levels of FMR1 protein in this range (Kenneson et al., 2001; Primerano et al., 2002; Tassone, Hagerman, Taylor, Gane et al., 2000; Tassone et al., 2001). We suspect that the elevated message may be causally related to both FXTAS and to inclusion formation, possibly via a direct, toxic ‘‘gain-of-function’’ effect of the RNA species itself, as has been proposed for myotonic dystrophy (Fininster 2002; Mankodi & Thornton, 2002).
Ascertainment and Inclusion Criteria
This review is a summary of seven articles published on FXTAS (Berry-Kravis et al., 2003; Brunberg et al., 2002; Greco et al., 2002; R. Hagerman et al., 2001; Jacquemont et al., 2002; Jacquemont, Hagerman, Leehey, Grigsby et al., 2003; Jacquemont, Hagerman, Leehey, Hall et al., 2003; Jacquemont, Hagerman, Leehey, Hall, Levine, Brunberg, Zhang, Jardini, Gane, Harris, Herman et al., 2003; Leehey et al., 2003a). The carriers described in this review were recruited almost exclusively through families with known members affected with fragile X. Such carriers were identified either because of symptoms of tremor and/or ataxia or as known carriers who participated in our California family-based penetrance study (Jacquemont, Hagerman, Leehey, Hall et al., 2003). That is, the proportion of affected and unaffected pre-mutation carriers does not reflect the actual penetrance of FXTAS among premutation carriers. The majority of male premutation carriers in this review had been examined by the authors in California (n = 35) or by collaborating neurologists in Denver (n = 7) and Chicago (n = 15) and has been previously reported (as cited above). Thirty additional cases were interviewed and medical records reviewed, but they were not examined directly by the authors. The group of patients described here represents our complete experience with all male carriers of 50 years and older. This is the largest and most comprehensive summary of FXTAS patients reported to date. We have restricted this review of FXTAS to premutation males because FXTAS occurs infrequently in female carriers, limiting our experience to only a few cases (Tassone et al., 2002; R. Hagerman, Tassone et al., 2003).
Clinical Findings
Patient demographics are summarized in Table 1, and the clinical findings for the 87 male carriers are summarized in Table 2. The male carriers were divided into two diagnostic groups, those with FXTAS (n = 64) and those without FXTAS (n = 23). The carrier group without FXTAS had a lower mean age (66 years, standard deviation [SD] = 7.2, range = 51 to 80) than the mean age for the carrier group with FXTAS (M = 71 years, SD = 7.7, range = 51 to 89). Although this difference in mean age is not statistically significant, the trend is likely a reflection of the age-dependent penetrance of FXTAS (Jacquemont Hagerman, Leehey, Grigsby et al., 2003). In this review, FXTAS encompasses patients with pre-mutation alleles who meet the criteria previously reported by Jacquemont, Hagerman, Leehey, Grigsby et al. (2003) in all three categories of involvement: definite, probable, and possible FXTAS as outlined in Table 3. In those with FXTAS, the most frequent and prominent complaints are directly related to the principal diagnostic criteria: gait ataxia (91%) and/or intention tremor (88%). Patients also reported other, more variable symptoms, such as: Parkinsonism (resting tremor in 42%), numbness and/or pain in the lower extremities (28%), and autonomic dysfunction (impotence, 39%; urinary and/or bowel incontinence, 34%).
Table 1.
Patient Demographics (N = 87)
| Demographic | % |
|---|---|
| Ethnicity | |
| White | 78 |
| Hispanic | 7 |
| African American | 2 |
| Native American | 3 |
| Pacific Islander | 1 |
| Examined | 66 |
| Interviewed only | 34 |
Table 2.
Clinical Presentation (N = 87) (in %)
| Presentation | FXTASa (n = 64) | No FXTAS (n = 23) |
|---|---|---|
| Neurological | ||
| Tremor, intention | 88 | 9 |
| Tremor, resting | 42 | 0 |
| Gait ataxia | 91 | 0 |
| Falling | 48 | 9 |
| Walking aid | 52 | 0 |
| Sensorimotor | ||
| Loss of sensation in feet | 28 | 22 |
| Lower limb weakness | 34 | 9 |
| Behavior/emotional | ||
| Anxiety | 33 | 39 |
| Depression | 36 | 9 |
| Other medical problem | ||
| Incontinence | 34 | 0 |
| Impotence | 39 | 9 |
| Heart disease | 34 | 9 |
| Stroke | 8 | 9 |
| Diabetes | 14 | 9 |
| Hearing loss | 25 | 22 |
| Hypertension | 34 | 13 |
| CHFb | 8 | 4 |
| Family history | ||
| Neurological disorder | 20 | 35 |
| Parkinson’s disease | 14 | 9 |
| Dementia | 8 | 4 |
Note. Inclusion of patients from the following publications: Hagerman et al., 2001; Brunberg et al., 2002; Greco et al., 2002; Leehey et al., 2002; Berry-Kravis et al., 2003; Jacquemont, Hagerman, Leehey, Hall et al., 2003; Leehey et al., 2003b.
Fragile X-associated tremor/ataxia syndrome.
Congestive heart failure.
Table 3.
FXTAS Diagnostic Criteria
| Definite | Probable | Possible |
|---|---|---|
| 1 radiology majora + | 2 clinical major or 1 | 1 radiology minor + 1 |
| 1 clinical majorb | radiology major + 1 clinical minor | clinical major |
Note. FXTAS = fragile X-associated tremor/ataxia syndrome. Mandatory criterion CGG repeat >55 and <200.
Radiology major = MRI white matter lesions: middle cerebellar peduncles. Radiology minor = MRI white matter lesions: cerebral white matter; moderate to severe generalized atrophy.
Clinical major = intentional tremor, gait ataxia. Clinical minor = Parkinsonism.
Tremor
Tremor usually involved both upper extremities and was progressively disabling, frequently resulting in moderate to severe disability. Although present primarily with action, or intention and posture, a resting tremor of the same cadence was common. When tremor type was systemically dissected on a formal rating scale, it was clear that rest, postural, and kinetic tremor often co-existed in FXTAS, attesting to a multidimensional tremor phenotype that produces functional impairment in motor tasks and daily activities (Berry-Kravis et al., 2003). Intention tremor usually started in the dominant hand and progressed to the contralateral hand in the following years. The intention tremor was frequently activated by certain postures or writing and has been described in some cases as dystonic.
Gait ataxia
Disturbances of gait were related to several clinical features, including a dominant cerebellar component, Parkinsonism, lower limb muscle weakness, and distal neuropathy. Gait ataxia usually manifested initially as a wide-based gait and difficulty with tandem walking. As the ataxia progressed, the patient developed poor balance, with frequent falls, requiring walking aids or the use of a wheelchair. Lower limb distal neuropathy was also present in 48% of cases, generally comprising abolished ankle reflexes, impairment of vibration sense and pinprick discrimination, decreased pain sensation, and decreased pro-prioceptive (toe position sense) response. Although muscle weakness was a common complaint, it was clinically confirmed in the lower limb proximal muscle groups in only a few cases.
Parkinsonism
Parkinsonism manifests as bradykinesia (slow movements), increased tone, postural instability, and resting tremor, with tremor and rigidity being the most prominent features. In FXTAS, hypomimea (reduction in facial expression) and decreased arm swing when ambulating were generally seen early in the illness. Usually, the Parkinsonism in FXTAS was mild, although symptoms were moderate in some cases. Forty-two percent of the patients had a symmetric resting tremor, which typically began later than the intention tremor.
Cognition
Data on cognitive decline in FXTAS is still limited, but certain patterns of impairment seem to emerge from neuropsychological assessments performed on 29 individuals (Brunberg et al., 2002; R. Hagerman et al., 2001; Jacquemont, Hagerman, Leehey, Grigsby et al., 2003). Deficits in executive cognitive functioning were especially noteworthy because they were consistent with a behavioral pattern that includes disinhibition, distractibility, and inappropriate jocularity. Formal assessment has demonstrated deficits in behavioral self-regulation, control of attention, the capacity for self-monitoring, and verbal fluency (Grigsby, Hill et al., 2002; Grigsby, Leehey et al., 2002). Apart from impaired executive functioning, the most commonly observed cognitive deficits were impairment of working memory and the speed and capacity of information-processing. Difficulty in nonverbal tasks reflects both the effects of the movement disorder (because many of the tests involved manipulation of the materials and timing of performance) and impairment of working memory. Language was relatively spared, and verbal IQ scores were less affected in patients who had otherwise lost complete autonomy due to dementia. The IQs for 29 patients show that 21% of males with FXTAS had Full Scale IQs under 85, which is reflective of significant IQ decline or dementia. Ten percent had verbal IQs < 85, and 28% had performance IQs < 85. The average Full Scale IQ was 102, the average Verbal IQ was 106, and the average Performance IQ was 98.
Neuroimaging
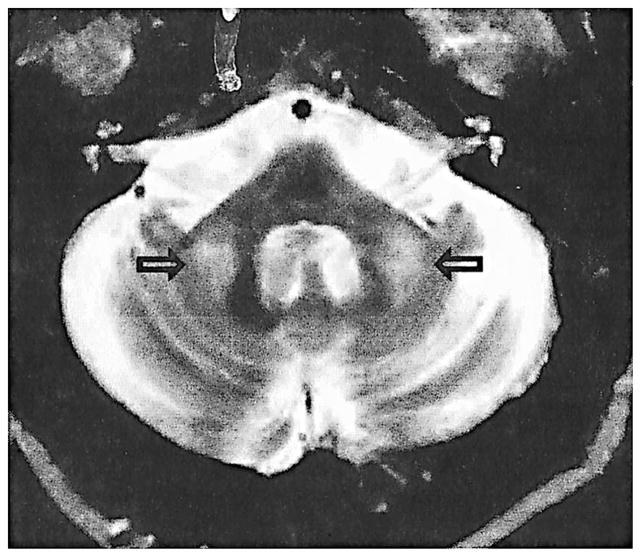
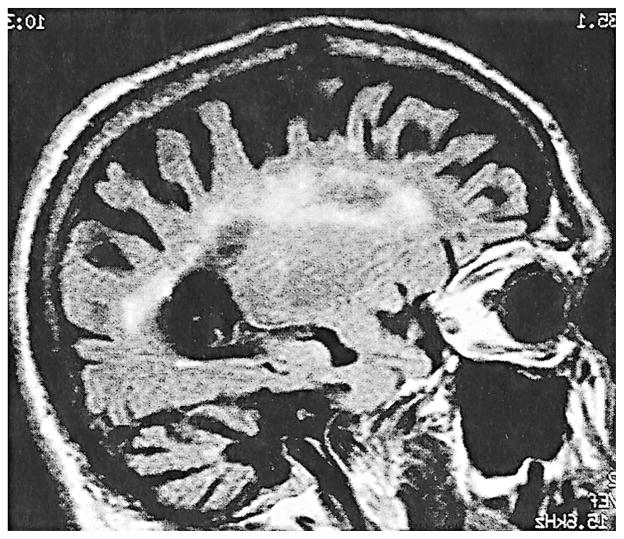
Magnetic Resonance Imaging (MRI) is an important tool for the diagnosis of FXTAS. Patient MRIs typically displayed symmetric increases in T2-weighted signal intensity in the middle cerebellar peduncles (MCP) and adjacent cerebellar white matter (Brunberg et al., 2002) (Figure 1). This unusual radiological finding is visible in 27 out of 46 premutation carriers with FXTAS (59%) who have had brain MRIs and is not seen in carriers without clinical features of FXTAS. This MCP finding, thus, serves as a major diagnostic feature of FXTAS (Jacquemont, Hagerman, Leehey, Grigsby, 2003). In addition, we observed nonspecific areas of increased T2 signal intensity in the subependymal and deep white matter of the frontal and parietal lobes (Figure 2). Some patients demonstrated more diffuse white matter T2 hyperintensities extending from the periventricular areas to the entire adjacent white matter. In addition to the white matter changes, mild to moderate cerebellar and cerebral cortical volume loss was seen in almost all cases. The volume loss can be severe in a limited number of individuals (Figure 2).
Figure 1.
T2 axial cut of the cerebellum and pons showing hyperintensity in the middle cerebellar peduncle; found in 59% of the MRIs (27 out of 46).
Figure 2.
Inversed recovery sagittal image showing severe atrophy and white matter lesion throughout the cerebral white matter.
There is significant variability in the severity and progression of FXTAS. Many individuals may remain relatively stable for years or even decades whereas others have a rapid downhill course (within 5 to 6 years), which also includes dementia. Out of the 64 male carriers with FXTAS, mean age of onset for gait instability in 46 of the affected individuals was 62 years. Of the 25 patients who used a walking aid, use of a cane and wheelchair occurred, on average, 5 and 8 years, respectively, after the onset of gait instability. Out of the 87 carriers, mean age of onset for 50 who experienced tremor was 63 years. Eleven patients had a more severe form, with completely impaired writing skills (Table 2). Overall, the life expectancy of patients affected with FXTAS appears to be shortened. We have gathered medical information on 13 deceased patients. The mean age at death was 73 years as opposed to 83 years for an American Caucasian male who has previously reached the age of 60 years. Dysphagia and aspiration problems are common as individuals become bedridden. Our data concerning mortality are preliminary, however, and further studies are required.
Molecular Data
Among 64 carriers with FXTAS for whom molecular studies were performed, CGG repeats ranged from 55 to 160 (M = 83, SD = 15.8). Only one patient had fewer than 65 repeats (55 repeats), and this individual presented with isolated resting tremor and normal MRI findings; thus, he only met criteria for possible FXTAS. This is the only patient without a family history of fragile X syndrome. There is no correlation between the CGG repeat size and the age of onset or severity of FXTAS. Using the technique described by Willemsen and Oostra (2000), we found that in 30 patients studied, the FMRP levels were normal or mildly deficient (M = 85, SD = 11.7, range = 61% to 100%). In addition, all 30 patients studied had elevated FMR1 levels (M = 3.5, SD = 1.4, range = 2 to 7 times normal) using methodology described by Tassone, Hagerman, Taylor, and Gane et al. (2000).
We have hypothesized (Greco et al., 2002; R. Hagerman et al., 2001; Jacquemont, Hagerman, Leehey, Grigsby et al., 2003) that the molecular mechanism leading to FXTAS may be related to a toxic effect of the elevated FMR1 mRNA levels found in carriers of premutation alleles (Kenneson et al., 2001; Tassone, Hagerman, Taylor, Gane et al., 2000a; Tassone, Hagerman, Taylor, Hagerman, 2000). because FXTAS has not been observed in older adults with fragile X syndrome, who do not express the FMR1 protein (FMRP). This RNA gain-of-function is analogous to the mechanism proposed for myotonic dystrophy, which is based on the demonstration of a myotonia phenotype in transgenic mice expressing an expanded (untranslated) CUG repeat element in a reporter gene (Mankodi et al., 2000; Wasielewski et al., 1998). However, the degree of involvement from FXTAS does not appear to correlate with the degree of FMR1 mRNA elevation, although this analysis is based on only 30 patients. Other factors, genetic and/or environmental, may be necessary in addition to the toxic effects of mRNA to trigger the development of FXTAS symptomatology.
Treatment Approaches for FXTAS
A variety of approaches exist for treatment of the symptoms of FXTAS; however, all of the approaches are based on studies of other neurological conditions with similar symptoms, and their efficacies vary widely. Here, we review some of the treatments used in patients with FXTAS to target intention tremor, ataxia, Parkinsonism, cognitive changes, and emotional symptoms. Table 4 summarizes the medical interventions found to be helpful (typically by history) in a group of 64 patients with FXTAS.
Table 4.
Medical Information (N = 87)
| Symptom/Treatment | n patients |
|---|---|
| Tremor (n = 15) | |
| Beta-blocker (propanolol, atenolol) | 14 |
| Primidone | 4 |
| Phenytoin | 3 |
| Trihexyphenidyl | 2 |
| Parkinsonism (n = 22) | |
| Amantadine | 4 |
| Levodopa | 12 |
| Pramipexole | 3 |
| Neuropathy | |
| Gabapentin (n = ??) | 2 |
| Emotional/cognitive (n = 25) | |
| SSRIs (paroxetine, fluoxetine, sertraline, citalopram) | 19 |
| Clonazepam | 1 |
| Benzodiazepines (alprazolam, amytriptyline) | 3 |
| Tricyclic antidepressants | 4 |
| Buproprion | 2 |
| Donepezil hydrochloride | 4 |
| Venlafaxine | 3 |
Tremor
Therapeutic strategy for action tremor involves trials with medications that are known to be efficacious for cerebellar or essential tremor. Although the medical treatment of cerebellar tremor is generally unrewarding, amantadine, clonazepam, carbamazepine, and cholinergic agents, such as physotigmine, have been shown to have limited favorable results in open studies or case reports (Wasielewski et al., 1998). Essential tremor may be effectively treated with primidone and beta-adrenergic blockers, especially propranolol. Primidone reduces arm tremor by more than 60% in many patients and has fewer long-term side effects than do beta blockers. The major adverse effect is sedation. Beta-adrenergic blocking agents reduce tremor in about half of patients with essential tremor. Adverse effects include bradycardia, bronchospasm, fatigue, depression, and gastrointestinal disturbances. Beta blockers are contraindicated in certain cardiovascular conditions (Jankovic et al., 2002; Wasielewski et al., 1998). One individual with FXTAS was found to show improvement in dystonic tremor symptoms after scopalamine infusion and then continued to show substantial benefits when treated with trihexyl-phenidyl (Artane) over a 7-month period. The main side effect of this treatment was mild sedation.
Botulinum toxin-A (Botox) intramuscular injections have been shown to reduce hand tremor (Jankovic et al., 1991) by blocking the release of acetylcholine at peripheral nerve endings. Although effectiveness is limited by the relatively common occurrence of excessive weakness, this treatment may benefit patients resistant to standard medical therapy.
One FXTAS patient underwent bilateral Vim thalamic deep brain stimulation implantation (Leehey et al., 2003a). Tremor was reduced by an associated microthalamotomy effect, but gait ataxia worsened significantly. Surgical treatment of tremor in FXTAS should be approached cautiously because these patients are prone to gait ataxia and cognitive impairment.
Cerebellar ataxia
Cerebellar symptoms in general are difficult to treat (Perlman, 2000). Amantadine was studied in a double-blind trial of patients with Friedreich’s ataxia and olivopontocerebellar atrophy (Botez et al., 1996). Although treatment did not result in functional improvement, patients had improvement in movement, speed, and reaction time. In our experience, some patients with FXTAS respond to amantadine at standard doses. A randomized double-blind study showed that buspirone, which acts on serotonin 1A receptors, resulted in mild improvement of ataxia, but this has not been studied in patients with FXTAS.
Parkinsonism
Therapies for Parkinsonism involve multiple strategies, from enhancement of dopaminergic transmission and influencing non-dopaminergic neurotransmitters to surgical approaches. Side-effect profiles, and the symptoms seen in a particular patient, can change therapeutic decisions dramatically. Parkinsonism in FXTAS is best treated with levodopa. Patients with FXTAS may have cognitive impairment or autonomic dysfunction, which can be exacerbated by dopamine agonists or anticholinergic medications. It is possible that selegiline, an MAO-B inhibitor, used for motor fluctuations in Parkinson’s disease, could be helpful in FXTAS and might also slow disease progression based on neuroprotective effects observed in experimental models of neural toxicity. The benefit of Parkinsonian agents is reflective of the neuropathology described in the substantia nigra, including the high frequency of inclusions and atrophy (Greco et al., 2002).
Recently, researchers have reported that a reduced level of coenzyme Q10 (CoQ10) is associated with neuronal degeneration in patients with Parkinson’s. Further, treatment with CoQ10 through oral consumption was well-tolerated and reduced the rate of functional decline in patients with the disease as compared to placebo (Shults et al., 2002). Studies are required to determine any potential therapeutic effect it may have for FXTAS patients.
Emotional, cognitive, and pain symptoms
In our experience, anxiety and depression can be treated effectively with selective serotonin reuptake inhibitors (SSRIs), such as sertraline, fluoxetine, or citalopram. Venlafaxine blocks serotonin and nor-epinephrine reuptake, so it can be helpful for anxiety, attention deficit, and possibly executive function deficits. We have found a low dose (37.5 mg) of the long-acting form (Effexor XR) to be helpful in 3 patients (Table 4). It is interesting that the hippocampus also has a high frequency of intranuclear inclusions in neurons and astrocytes in FXTAS (Greco et al., 2002). The involvement of the hippocampus may be related to the problems of anxiety and depression that are common in FXTAS. Volumetric MRI studies demonstrate that the size of the hippocampus in individuals with FXTAS correlates inversely with the severity of mood symptoms and with level of FMR1 mRNA levels (Hessl et al., 2003)
We have treated 4 patients who had documented dementia, and the overall preliminary responses are favorable. Two patients, who were somnolent during the day and did not recognize family members, experienced remarkable improvement on donepezil (Aricept). They became more alert, with improved memory function that included recognition of family members. Donepezil is well-tolerated at a single, daily dose of 5 to 10 mg. Further studies are warranted, and earlier intervention with donepezil should be considered. Finally, 2 patients have had significant improvement of extremity pain with gabapentin.
Future studies should be focused on molecular interventions that may block the process of apoptosis that may lead to brain atrophy. An interesting candidate for future studies is minocycline, a second-generation tetracycline antibiotic and antiinflammatory agent that has been shown to delay apoptosis in mouse models of amyotrophic lateral sclerosis (ALS) (Kriz et al., 2002). It has also had therapeutic effects in Huntington’s disease, Parkinson’s disease, and multiple sclerosis (Chen et al., 2000). By directly inhibiting the release of cytochrome c, minocycline prevents downstream processes related to cell death, resulting in a delayed onset of motor neuron degeneration and muscle strength decline (Friedlander, 2003).
Genetic Counseling
The existence of FXTAS should be addressed in genetic counseling. It is important that families be aware that FXTAS may occur in approximately 30% of male premutation carriers (Jacquemont, Hagerman, Leehey, Hall et al., 2003). Further research is required in order to identify those factors (genetic and/or environmental) that may influence penetrance and modify the severity of the symptoms and the progression of FXTAS. Although progressive, FXTAS differs from many other neurodegenerative diseases in that there is significant variability in its course, penetrance is incomplete, and onset of symptoms occurs later in life. Due to these distinguishing features, as well as the family testing protocol for fragile X syndrome, the guidelines for presymptomatic testing in neurodegenerative diseases (e.g., as applied to Huntington’s disease) may not be appropriate for male FMR1 premutation carriers.
Identification of families with fragile X syndrome usually occurs via a presenting case that is a male with mental retardation. Once the proband is identified, FMR1 gene testing is indicated for other family members (McIntosh et al., 2000). It is well-documented that FMR1 testing in child siblings and other young relatives of the proband is appropriate (McIntosh et al., 2000). Such testing will identify others who are at risk of carrying either premutation or full mutation alleles. Young premutation carriers commonly experience problems with anxiety, attention deficit hyperactivity disorder (ADHD), learning disabilities (LD), and (less commonly) mental retardation (MR) and/or autism (Aziz et al., 2003; Goodlin-Jones et al., 2002; R. Hagerman et al., 1996). Because these forms of clinical involvement can benefit from early treatment, it is important to identify those individuals who harbor premutation alleles. Moreover, it is important to test the grandparent(s) in order to determine the familial inheritance pattern and, therefore, other members of the extended family who are at risk of carrying expanded FMR1 alleles (McIntosh et al., 2000). Such testing will identify premutation carriers who are, therefore, at risk of developing FXTAS but can also receive treatment if needed for learning and/or emotional problems. It is the role of the genetic counselor to address these issues and to make the families aware of the risk–benefit ratio associated with FMR1 gene testing.
Conclusions
The study of aging in individuals with the FMR1 mutation has revealed a new syndrome, FXTAS, which occurs in approximately 30% of older male carriers of the premutation and in occasional female carriers. Because the premutation is relatively common in the general population, approximately 1 in 800 males and 1 in 250 females (Dombrowski et al., 2002; Rousseau et al., 1996), FXTAS may be one of the most common causes of tremor and/or ataxia in older adults. Further research is needed regarding aging in those with fragile X syndrome to determine whether similar neurological problems ever appear among individuals with full mutation alleles. Once the pathophysiology of this neurodegenerative process in aging is understood, new treatments that target key processes leading to FXTAS can be developed.
Contributor Information
S. Jacquemont, M.I.N.D. Institute, University of California, Davis, Medical Center
F. Farzin, M.I.N.D. Institute, University of California, Davis, Medical Center
D. Hall, University of Colorado Health Sciences Center
M. Leehey, University of Colorado Health Sciences Center
F. Tassone, University of California, Davis
L. Gane, M.I.N.D. Institute, University of California, Davis, Medical Center
L. Zhang, University of California, Davis
J. Grigsby, University of Colorado, Health Sciences Center
T. Jardini, M.I.N.D. Institute, University of California, Davis, Medical Center
F. Lewin, RUSH-Presbyterian-St. Luke’s Medical Center (Chicago)
E. Berry-Kravis, RUSH-Presbyterian-St. Luke’s Medical Center (Chicago)
P. J. Hagerman, University of California, Davis
R. J. Hagerman, M.I.N.D. Institute, University of California, Davis, Medical Center
References
- Aziz ME, Stathopulu M, Callias C, Taylor J, Turk B, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations & intermediate alleles. American Journal of Medical Genetics. 2003;121B(1):119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Lewin F, Wuu J, Leehey M, Hagerman R, Hagerman P, Goetz CG. Tremor and ataxia in fragile X pre-mutation carriers: Blinded videotape study. Annals of Neurology. 2003;53(5):616–623. doi: 10.1002/ana.10522. [DOI] [PubMed] [Google Scholar]
- Botez MI, Botez-Marquard T, Elie R, Pedraza OL, Goyette K, Lalonde R. Amantadine hydrochloride treatment in her-edodegenerative ataxias: A double blind study. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;61(3):259–264. doi: 10.1136/jnnp.61.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis E, Grigsby J, Leehey M, Tassone F, Brown WT, Greco C, Hagerman PJ. Fragile X premutation carriers: Characteristic MR imaging findings in adult males with progressive cerebellar and cognitive dysfunction. American Journal of Neuroradiology. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- Chen M, Ova VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Medicine. 2000;6(7):797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Dombrowski C, Levesque ML, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10,572 males from the general population: Loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Human Molecular Genetics. 2002;11(4):371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- Fininster J. Myotonic dystrophy type 2. European Journal of Neurology. 2002;9:441–447. doi: 10.1046/j.1468-1331.2002.00453.x. [DOI] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. New England Journal of Medicine. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones BL, Spence S, Albrect L, Bacalman S, Tassone F, Gane L, Harris SW, Hagerman PJ, Hagerman R. The fragile X premutation and autism. Presented at the 8th International Fragile X Conference; Chicago. 2002. Jul 17–21, [Google Scholar]
- Greco C, Hagerman RJ, Tassone F, Chudley A, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Hills J, Wilson R, Leehey M, Hagerman RJ, Tassone F, Hagerman PJ. Dysexecutive syndrome in older men with action tremor and the fragile X premutation. Journal of the International Neuropsychological Society. 2002;8(2):282. [Google Scholar]
- Grigsby J, Leehey MA, Hagerman RJ, Epstein JH, Wilson R, Jacquemont S, Greco CM, Brunberg JA, Tassone F, Hagerman PJ. Dementia and a tremor-ataxia disorder among older male carriers of the fragile X premutation. Journal of the American Geriatrics Society. 2002;50(Suppl):S48. [Google Scholar]
- Hagerman P, Iwahashi C, Babineau B, Yasui D, Greco C, Duncan N, Graw S, Kim F, Hagerman R. Fragile X–associated tremor/ataxia syndrome (FXTAS): A common heritable neuronal inclusion disorder. Paper presented at the 55th annual meeting of the American Academy of Neurology. 2003;60(Suppl 1):A469. [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, Parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Staley LW, O’Connor R, Lugenbeel K, Nelson D, McLean SD, Taylor A. Learning-disabled males with a fragile X CGG expansion in the upper premutation size range. Pediatrics. 1996;97(1):122–126. [PubMed] [Google Scholar]
- Hagerman RJ, Zhang L, Brunberg J, Hessl D, Jacquemont S, Tassone F, Harris S, Jardini T, Hagerman PJ. Two female cases of the fragile X premutation tremor/ataxia syndrome: Cognitive, molecular, and radiological studies. Paper presented at the 55th annual meeting of the American Academy of Neurology.2003. [Google Scholar]
- Hessl D, Cohen S, DeCarli C, Tong-Turn-beaugh R, Jacquemont S, Gane L, Jardini T, Wegelin J, Tassone F, Hagerman PJ. Structural neuroimagine and molecular correlates of psychopathology in adult males with the FMR1 premutation. American Journal of Human Genetics, 53rd Annual Meeting; Los Angeles, CA. 2003. p. A562.p. 266. [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Greco C, Brunberg J, Tassone F, Gane LW, Jardini T, Harris SW, Zhang L, Grigsby J, Des Portes V, Berry-Kravis E, Brown WT, Hagerman PJ. Characterization of a progressive neurological condition in older adult male carriers of the fragile X premutation. American Journal of Human Genetics (Suppl 71, A) 2002;107(4):185. [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. American Journal of Human Genetics. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco C, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X–associated tremor/ataxia syndrome (FXTAS) in a pre-mutation carrier population: Initial results from a California family-based study. American Journal of Human Genetics, 53rd annual meeting; Los Angeles. 2003. p. A10.p. 163. [Google Scholar]
- Jankovic J, Schwartz K. Botulinum toxin treatment of tremors. Neurology. 1991;41:1185–1188. doi: 10.1212/wnl.41.8.1185. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Tolosa E. Parkinson’s disease and movement disorders. Philadelphia: Lippincott, Williams, & Wilkins; 2002. [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CJ, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Human Molecular Genetics. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Kriz J, Nguyn MD, Julien J. Minocycline slows disease progression in a mouse model of amyotropic lateral sclerosis. Neurobiology of Disease. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- Leehey M, Hagerman RJ, Landau WM, Grigsby J, Tassone F, Hagerman PJ. Tremor/ataxia syndrome in fragile X carrier males. Movement Disorders. 2002;17(4):744–745. [Google Scholar]
- Leehey M, Munhoz R, Lang AE, Brunberg J, Grigsby J, Greco C, Jacquemont S, Tassone F, Lozano A, Hagerman P, Hagerman R. The fragile X premutation presenting as essential tremor. Archives of Neurology. 2003a;60(1):117–121. doi: 10.1001/archneur.60.1.117. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Munhoz RP, Lang AE, Brunberg JA, Grigsby J, Greco C, Jacquemont S, Tassone F, Lozano AM, Hagerman PJ, Hagerman RJ. The fragile x premutation presenting as essential tremor. Archives of Neurology. 2003b;60:117–121. doi: 10.1001/archneur.60.1.117. [DOI] [PubMed] [Google Scholar]
- Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, Krym M, Thornton C. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289(5485):1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- Mankodi A, Thornton CA. Myotonic syndromes. Current Opinion in Neurology. 2002;15:545–552. doi: 10.1097/00019052-200210000-00005. [DOI] [PubMed] [Google Scholar]
- McIntosh N, Gane LW, McConkie-Rosell A, Bennett RL. Genetic counseling for fragile x syndrome: Recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling. 2000;9:303–325. doi: 10.1023/A:1009454112907. [DOI] [PubMed] [Google Scholar]
- Perlman S. Cerebellar ataxia. Current Treatment Options in Neurology. 2000;2(3):215–224. doi: 10.1007/s11940-000-0004-3. [DOI] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile x patients with premutations. RNA. 2002;8:1482–1488. [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Morel M-L, Rouillard P, Khandjian EW, Morgan K. Surprisingly low prevalence of FMR1 premutation among males from the general population. American Journal of Human Genetics. 1996;59(Suppl):A188, 1069. [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal F, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M a. t. P. S. Group. Effects of co-enzyme Q10 in early Parkinson disease. Archives of Neurology. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in fragile X syndrome. American Journal of Human Genetics. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Hagerman PJ. Transcriptional activity in fragile X males with full mutation alleles. Paper presented at the 7th International Fragile X Conference; Los Angeles. 2000. Jul 19–23, [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Hagerman PJ. Fragile X males with methylated, full mutation alleles have significant levels of FMR1 messenger RNA. Journal of Medical Genetics. 2001;38:0–3. doi: 10.1136/jmg.38.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Orrico A, Galli L, Jacquemont S, Hagerman R, Hagerman P. Spastic parapresis and ataxia in two sisters with the fragile x premutation. National Fragile X Foundation’s 8th International Fragile X Conference; Chicago. 2002. [Google Scholar]
- Wasielewski PG, Burns JM, Koller WC. Pharmacologic treatment of tremor. Movement Disorders. 1998;13(3):90–100. doi: 10.1002/mds.870131316. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, Van Unen L, Tassone F, Hoogeveen AT, Hagerman PJ, Mientjes EJ, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions: Implications for the cerebellar tremor/ataxia syndrome. Human Molecular Genetics. 2003;12(9):949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Mohkamsing S, de Vries B, Devys D, van den Ouweland A, Mandel JL, Galjaard H, Oostra B. Rapid antibody test for fragile X syndrome. Lancet. 1995;345(8958):1147–1148. doi: 10.1016/s0140-6736(95)90979-6. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Oostra BA. FMRP detection assay for the diagnosis of the fragile X syndrome. American Journal of Medical Genetics. 2000;97(3):183–188. doi: 10.1002/1096-8628(200023)97:3<183::AID-AJMG1035>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]