Abstract
Background
Respiratory infections are a leading cause of death in Africa, especially among HIV-infected patients. Data on the etiology of fatal respiratory diseases are largely based on autopsy studies. We evaluated causes of pneumonia associated with early mortality among hospitalized HIV-infected patients in Kampala, Uganda.
Methods
Prospective cohort study of HIV-infected patients admitted to Mulago Hospital, Kampala, with at least 2 weeks of cough. Consecutively enrolled patients with negative Ziehl Neelsen sputum smears for acid-fast bacilli underwent bronchoscopy with bronchoalveolar lavage and examination for mycobacteria (smear, solid culture), Pneumocystis jirovecii (Giemsa stain), and fungi (KOH mount, India ink stain, Sabouraud culture). Early mortality was defined as death before the 2-month follow-up visit.
Results
Follow-up data were available for 353 (87%) of 407 patients enrolled. Of participants with follow-up data, 112 (32%) died within 2 months. Among patients with early mortality, a diagnosis was confirmed in 74 (66%), including tuberculosis (TB) (56%), cryptococcal pneumonia (1%), Pneumocystis pneumonia (3%), pulmonary Kaposi sarcoma (4%), and pneumonia caused by 2 or more disease processes (3%).
Conclusions
Mortality in HIV-infected TB suspects is high, with TB associated with the largest proportion of deaths. A significant proportion of patients die without a confirmed diagnosis.
Keywords: HIV, tuberculosis, mortality, hospital admission, Africa
INTRODUCTION
Historically, opportunistic pneumonias have been major causes of morbidity and mortality among HIV-infected persons. In the United States and Europe, the range of opportunistic infections and their contribution to morbidity and mortality in HIV-infected persons are well described.1,2 In contrast, few studies have been conducted in Africa, and the causes of death in HIV-infected individuals remain unclear in many cases.
In sub-Saharan Africa, data on causes of death from respiratory infections come largely from several autopsy series.3-6 These studies show that tuberculosis (TB) is a frequent cause of death, whereas Pneumocystis pneumonia (PCP) is relatively uncommon.3 Although informative, autopsy studies have several limitations: deaths are not consecutively sampled, and data revealing which diagnoses were known antemortem are not always provided. Comparatively, cross-sectional studies of consecutively enrolled living patients with respiratory infection are an improvement but also have limitations. They underestimate overall mortality because many patients may experience poor outcomes in the weeks and months after presentation. In addition, they are unable to identify causes of death in about 16%–33% of patients.7-9
To address these issues, we performed a prospective cohort study of consecutively enrolled HIV-infected patients with unexplained cough admitted to a large referral hospital in Uganda. These patients underwent standardized evaluation including bronchoscopy with bronchoalveolar lavage (BAL) to identify the etiology of their respiratory complaints and were followed for 2 months. Our goals were to determine the mortality rate, to describe risk factors for mortality, and to identify diseases associated with early mortality during and after hospitalization.
METHODS
Study Population
We performed a prospective cohort study of HIV-infected adults admitted to Mulago Hospital between September 2007 and July 2008 with cough for at least 2 weeks but less than six months. Patients were excluded from the study if they were receiving anti-TB treatment or had evidence of heart failure.
Patient Evaluation
Clinical and demographic information were collected using a standardized questionnaire. CD4+ T-lymphocyte counts were measured in all patients. Standardized evaluation for pneumonia included chest radiography and 2 sputum specimens (1 spot and 1 early morning) for acid-fast bacilli (AFB) smear and mycobacterial culture (Lowenstein-Jensen media) as previously described.10 If both sputum examinations were negative for AFB on Ziehl Neelsen smear, patients were referred for bronchoscopy with BAL. Bronchoscopic inspection was performed to assess for tracheobronchial Kaposi sarcoma (KS) lesions, and BAL fluid was examined for mycobacteria (AFB smear and culture), Pneumocystis jirovecii (modified Giemsa stain), and other fungi (potassium hydroxide smear, India ink stain, and culture on Sabouraud agar).
Vital status was assessed in all patients either by telephone or in-person 2 months after enrollment. Patients who returned in-person answered a clinical questionnaire and underwent physical examination. In addition, another chest radiograph and an additional sputum test (smear and culture) were performed if patients had no clinical improvement.
We assigned the following confirmed diagnoses: (1) Culture-positive TB—positive sputum or BAL fluid mycobacterial culture results; (2) Smear-positive TB—positive sputum Ziehl Neelsen AFB smear but negative mycobacterial cultures; (3) Fungal pneumonia—positive BAL fluid smear (KOH or India ink stain), or positive fungal culture; (4) Pulmonary KS—presence of typical lesions in the tracheobronchial tree during airway examination; and (5) PCP—positive BAL fluid Giemsa stain. Patients were categorized as having an unknown diagnosis if there was no confirmed etiology of their symptoms, or if the diagnostic evaluation was incomplete. Patients in the unknown category included those who improved on either empiric anti-TB therapy (presumed TB), or empiric antibiotic therapy (presumed bacterial pneumonia), and those who did not receive specific treatment for any respiratory illness.
Statistical Analysis
The primary outcome was 2-month mortality. We compared categorical variables using the χ2 or Fisher exact test and non-normally distributed continuous variables using the Mann–Whitney rank sum test. We defined significance in reference to the probability of a 2-tailed, type I error (P value) less than 0.05. Although the sample size arose from convenience, we determined the precision of all associations using 95% confidence intervals in lieu of specific power calculations. We carried out sensitivity analyses to determine the distribution of, and mortality related to, the various final diagnoses; first assuming all patients who were lost to follow-up were alive at the 2-month follow-up visit, and then assuming all were dead. We used STATA 10 (College Station, TX) for all analyses.
Ethics Approval
The Institutional Review Board of Mulago Hospital, the Makerere University Faculty of Medicine Research Ethics Committee, the Uganda National Council for Science and Technology, and the Committee on Human Research at the University of California, San Francisco, all approved the study protocol.
RESULTS
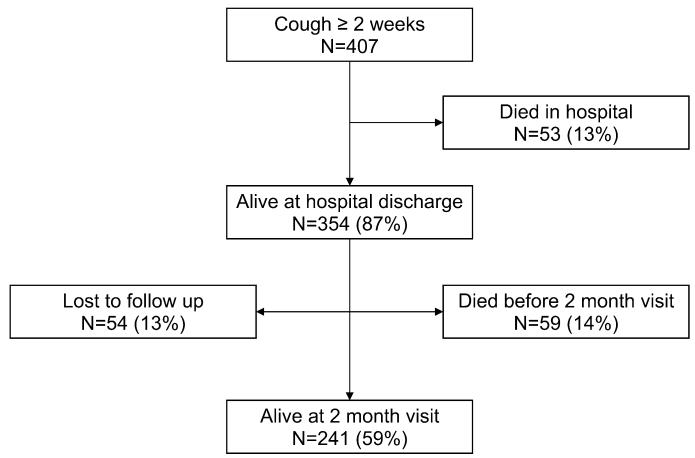
Four hundred seven patients met eligibility criteria and were enrolled in the study (Fig. 1). Of these, 54 (13%) patients were lost to follow-up at 2 months and were excluded from the analysis. Of the 407 patients, 354 (87%) patients survived hospitalization, 53 (13%) patients died in the hospital, and 59 (14%) patients died within 2 months after hospitalization. There were no significant differences in baseline demographic characteristics between patients included and those excluded from the analysis (data not shown).
FIGURE 1.
Study population. All percentages presented as a proportion of the total number of patients enrolled. Percentages may not sum to 100% because of rounding.
Mortality
Of 353 patients included in the analysis, 112 (32%) died within 2 months of follow-up. Among those who died, the median survival time was 12 days [interquartile range (IQR): 3.5–36.5 days] (Fig. 2). Fifty-three patients (47%) died during hospital admission, and their median survival time was 3 days (IQR: 2–7 days). The median duration of hospitalization for all patients was 5 days (IQR: 3–8 days).
FIGURE 2.
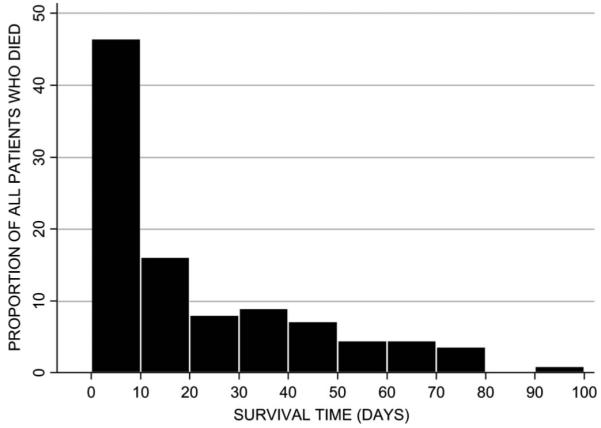
Survival time in days in patients who died.
Risk Factors for Mortality
Compared with patients alive at 2 months, those who died had lower median CD4+ T-lymphocyte counts (24 vs. 58 cells/uL, P < 0.001), but were more likely to have known that they were HIV infected before admission (79% vs. 68%, P = 0.027) (Table 1). Mortality in patients whose CD4 counts were less than 50 cells per microliter was more than 3 times greater than in those whose CD4 count was greater than 200 cells per microliter, and this difference was statistically significant (38% vs. 11%; P < 0.001). In addition, patients who died were less likely to have had chest pain (54% vs. 65%, P = 0.038), but had higher median respiratory rates (28 vs. 25 breaths per minute, P = 0.001), and lower median baseline oxygen saturations (94.5% vs. 97%, P < 0.001) (Table 1).
TABLE 1.
Baseline Demographic and Clinical Characteristics, Stratified by Mortality at 2 Months
| Characteristic, n (%)* | Total, n = 353 | Alive, n = 241 | Dead, n = 112 | P |
|---|---|---|---|---|
| Demographics | ||||
| Female | 197 | 140 (58) | 57 (51) | 0.21 |
| Age (years), median (IQR) | 33 (28–40) | 33 (27–39) | 33 (28–40) | 0.86 |
| HIV history | ||||
| CD4-count†, median (IQR) | 45.5 (12–149) | 58 (17–184) | 24 (7–67) | <0.001 |
| Previous positive HIV test | 253 | 164 (68) | 89 (79) | 0.027 |
| ART use | 56 | 34 (14) | 22 (20) | 0.19 |
| PCP prophylaxis | 200 | 131 (54) | 69 (62) | 0.59 |
| History of present illness | ||||
| Fever | 335 | 227 (94) | 108 (96) | 0.37 |
| Weight loss >5 kg | 254 | 166 (69) | 88 (79) | 0.059 |
| Sputum production | 302 | 207 (86) | 95 (85) | 0.79 |
| Chest pain | 217 | 157 (65) | 60 (54) | 0.038 |
| Dyspnea | 192 | 132 (55) | 60 (54) | 0.83 |
| Hemoptysis | 89 | 59 (24) | 30 (27) | 0.64 |
| Antibiotic use for present illness‡ | 238 | 170 (71) | 68 (61) | 0.090 |
| Clinical findings | ||||
| Temperature, median (IQR)§ | 36.7 (35.6–37.8) | 36.7 (35.7–37.9) | 36.7 (35.5–37.7) | 0.99 |
| Heart rate, median (IQR) | 104 (89–120) | 104 (89–120) | 105 (92–122) | 0.77 |
| Respiratory rate, median (IQR) | 26 (20–32) | 25 (20–30) | 28 (24–38) | 0.001 |
| Oxygen saturation, median (IQR) | 96 (92–98) | 97 (93–98) | 94.5 (86–98) | <0.001 |
Unless otherwise stated, all characteristics given as n (%).
Nine observations missing.
Twelve observations missing.
§Two observations missing.
Final Diagnoses
Overall, a confirmed diagnosis was obtained in 226 (64%) patients (Table 2). Among these 226 patients, 190 (54%) had a positive culture for Mycobacterium tuberculosis; 136 of the 190 culture-positive TB patients were smear positive on sputum (n = 131) or BAL examination (n = 5). Eleven (3%) had smear-positive culture-negative TB, 4 (1%) had PCP, 10 (3%) had pulmonary cryptococcosis, and 5 (1%) had typical KS lesions in the airways. Multiple microbiologic diagnoses were obtained in 6 (2%) patients. One hundred twenty-seven of 353 (36%) patients were classified as having an unknown final diagnosis as follows: 30 (8%) responded to empiric anti-TB treatment; 48 (14%) improved on empiric antibiotic treatment; and 49 (14%) received no specific therapy.
TABLE 2.
Final Diagnoses of All Patients and Among Those Who Died Within 2 Months of Admission
| Diagnosis | Total, n (%) | Died, n (%) |
|---|---|---|
| Confirmed diagnosis | 226 (64) | 74 (66) |
| Culture-positive TB | 190 (54) | 59 (53) |
| Smear-positive culture-negative TB | 11 (3) | 4 (4) |
| PCP | 4 (1) | 3 (3) |
| Cryptococcal pneumonia | 10 (3) | 1 (1) |
| Pulmonary KS | 5 (1) | 4 (4) |
| Multiple diagnoses* | 6 (2) | 3 (3) |
| Unknown | 127 (36) | — |
| Presumed TB | 30 (8) | NA |
| Presumed bacterial pneumonia | 48 (14) | NA |
| Received no specific treatment | 49 (14) | 38 (34) |
| Total | 353 (100) | 112 (100) |
There were 6 patients with more than 1 diagnosis: 4 with culture-positive TB and either PCP (1), cryptococcal pneumonia (2), or pulmonary KS (1); 1 with PCP and cryptococcal pneumonia; and 1 with cryptococcal pneumonia and pulmonary KS.
Causes of Death
Pulmonary TB (smear positive or culture positive) was the leading cause of mortality, accounting for 63 (57%) deaths (Table 2). A total of 52 patients, including 24 who died in hospital, were not diagnosed with TB until after hospital discharge. Mortality associated with PCP, fungal pneumonia, and pulmonary KS was also high, but these diseases were uncommon and accounted for only 8% of all deaths. For 38 (34%) of the patients who died, a confirmed diagnosis could not be identified. In sensitivity analyses, we found no difference in the distribution of diagnoses or causes of mortality when all patients lost to follow-up were assumed to have died or when they were all assumed to be alive.
Reasons for Unknown Antemortem Diagnoses
Of the 38 (34%) patients who died with an unknown diagnosis, 28 did not complete the diagnostic evaluation (26 did not undergo bronchoscopy and 2 did not produce sputum) (Table 3). The remaining 10 patients did complete the diagnostic evaluation and were started on broad-spectrum antibiotics, but all 10 died before a response-to-treatment could be measured.
TABLE 3.
Reasons for Missing Diagnosis of 42 Patients Who Died Without a Confirmed Diagnosis
| Incomplete evaluation: 28 patients* |
| 2 died before specimens were collected |
| 26 had no bronchoscopy done |
| 16 died before procedure |
| 5 were considered too ill |
| 2 patients refused |
| 3 left hospital before procedure |
| Inconclusive evaluation:10 patients† |
| 2 died in hospital before discharge |
| 8 received broad-spectrum antibiotics and died before treatment response could be measured |
In an incomplete evaluation, not all specimens were collected.
In an inconclusive evaluation, all tests were performed but were nondiagnostic.
Treatment Initiation and Mortality
Twenty-four pulmonary TB patients died in hospital, and for these patients, information on TB treatment initiation was not available. Of the 177 total TB patients who survived hospitalization, mortality at the 2-month follow-up visit was higher among the 35 patients who did not initiate anti-TB treatment (n = 11, 31%) than among the 142 who did initiate anti-TB treatment (n = 28, 20%), although this difference was not statistically significant (P = 0.13). Of 75 patients who started ART pre-enrollment (n = 56) or at hospital discharge (n = 19), 29 (39%) died within 2 months, which was not significantly higher than the 83 of 278 (30%) patients who died within 2 months while not on ART (P = 0.15).
DISCUSSION
This study found that in a large referral hospital in sub-Saharan Africa, 32% of HIV-positive patients with respiratory complaints and a known outcome status died within 2 months of admission. A large proportion of patients who died did not have a microbiologically or bronchoscopically confirmed diagnosis before death, most often because they died before diagnostic specimens could be collected. An equally important finding was that half of the patients who died within 2 months of admission died after hospital discharge. An additional 13% were lost to follow-up, and it is possible that a proportion of these patients died as well.
These results suggest that faster and more accurate diagnostic tests are urgently needed to allow early identification of the underlying cause of respiratory illness, specifically TB, and initiation of specific treatment. Previously, we reported that bronchoscopy increases the sensitivity of diagnostic testing for TB and that BAL AFB smears provide a faster diagnosis of TB than sputum AFB cultures.11 In addition, the high proportion of confirmed and presumed TB in this cohort suggests that empiric anti-TB therapy in TB prevalent settings such as this should be considered.12
Several factors may have contributed to the high mortality in this group of patients. Twenty-eight percent did not know their HIV status, and for those who did know, 78% were not receiving ART and 21% were not taking cotrimoxazole prophylaxis. Earlier initiation of ART is associated with decreased mortality,13 and the use of cotrimoxazole prophylaxis has been shown to reduce mortality, delay the first AIDS-defining illness by 6–12 months and reduce the risk for bacterial pneumonia.14-16 Another factor contributing to high mortality is that most patients also presented with advanced HIV/AIDS and a CD4+ T-lymphocyte count below 50 cells per microliter. Although there was a greater than 3-fold increase in mortality in patients with CD4 counts less than 50 cells per microliter compared with those with CD4 counts greater than 200 cells per microliter, implying that severe immunosuppression was a risk factor for mortality, mortality in patients with CD4 counts greater than 200 cells per microliter was still greater than 10%. Thus, early diagnosis, access to treatment for HIV infection and the underlying cause of respiratory illness, and active screening and preventive treatment for TB are essential for all patients, regardless of CD4 count, to prevent the advanced stage of disease and mortality that we found in this group.
This study confirms the findings of previous studies, including autopsy studies done in sub-Saharan Africa, which showed that TB is the leading cause of death in HIV-infected patients with respiratory complaints, though it is likely to be under reported.3,4,7,13,17 Previously, we found that clinical and radiographic findings were not able to diagnose smear-negative TB in our cohort.18 We found that 2% of patients had more than 1 diagnosis, which is slightly lower than the proportions of 3%–13% that have been described in other studies.1,8,9,19 This is an important finding because infections can mimic each other clinically and radiologically and be potentially fatal if not recognized promptly.
Thirty-six percent of the study population (127 of 353) had an unconfirmed diagnosis, which is similar to proportions of 16%–33% that are described in other studies from sub-Saharan Africa.7,8 The mortality among patients without a diagnosis was extremely high. This demonstrates the need for new, rapid, highly sensitive, and specific point-of-care diagnostic tests, especially in HIV-seropositive patients. Such tests are likely to decrease mortality, especially in this population with advanced HIV and AIDS.
Study Limitations
A limitation of our study was that TB drug susceptibility testing was not performed. Although HIV-infected patients with multidrug-resistant TB are twice as likely to die as those with drug-sensitive TB,20 the prevalence of drug resistance among new TB cases in Uganda is only 0.5%,21 and therefore drug resistance is unlikely to have contributed significantly to the high mortality we observed.
CONCLUSIONS
Early mortality was high in this HIV-seropositive population hospitalized with unexplained cough. As a result of our prospective cohort study design, we were able to demonstrate that more than half of all deaths occurred after discharge, a finding which suggests that there may be opportunities to intervene earlier to reduce mortality. Newer and more rapid diagnostic tests are needed to facilitate diagnosis and treatment of respiratory disease, but empiric treatment of smear-negative TB suspects with anti-TB drugs should also be considered in settings with high TB prevalence. Deaths from pulmonary infections, including TB, will continue in such settings until a combination of effective preventive measures for HIV and TB and improved diagnosis and early treatment of TB and other common life-threatening opportunistic infections are implemented.
Acknowledgments
The authors A.C., (K23 HL094141) J.L.D., (K23 AI080147) and L.H. (K24 HL087713 and R01 HL090335) have received support from the National Institutes of Health for this research.
REFERENCES
- 1.Afessa B, Green W, Chiao J, et al. Pulmonary complications of HIV infection: autopsy findings. Chest. 1998;113:1225–1229. doi: 10.1378/chest.113.5.1225. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 4.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Martinson NA, Karstaedt A, Venter WD, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 6.Abouya YL, Beaumel A, Lucas S, et al. Pneumocystis carinii pneumonia. An uncommon cause of death in African patients with acquired immunodeficiency syndrome. Am Rev Respir Dis. 1992;145:617–620. doi: 10.1164/ajrccm/145.3.617. [DOI] [PubMed] [Google Scholar]
- 7.Batungwanayo J, Taelman H, Lucas S, et al. Pulmonary disease associated with the human immunodeficiency virus in Kigali, Rwanda. A fiberoptic bronchoscopic study of 111 cases of undetermined etiology. Am J Respir Crit Care Med. 1994;149:1591–1596. doi: 10.1164/ajrccm.149.6.8004318. [DOI] [PubMed] [Google Scholar]
- 8.Malin AS, Gwanzura LK, Klein S, et al. Pneumocystis carinii pneumonia in Zimbabwe. Lancet. 1995;346:1258–1261. doi: 10.1016/s0140-6736(95)91862-0. [DOI] [PubMed] [Google Scholar]
- 9.Aderaye G, Bruchfeld J, Aseffa G, et al. Pneumocystis jiroveci pneumonia and other pulmonary infections in TB smear-negative HIV-positive patients with atypical chest X-ray in Ethiopia. Scand J Infect Dis. 2007;39:1045–1053. doi: 10.1080/00365540701474508. [DOI] [PubMed] [Google Scholar]
- 10.Cattamanchi A, Davis JL, Worodria W, et al. Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2009;13:1130–1136. [PMC free article] [PubMed] [Google Scholar]
- 11.Worodria W, Davis L, Cattamanchi A, et al. Bronchoscopy is useful for diagnosing smear negative tuberculosis in HIV-infected patients. Eur Res J. 2010 doi: 10.1183/09031936.00010210. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Improving the Diagnosis and Treatment of Smear Negative Pulmonary and Extrapulmonary Tuberculosis Among Adults and Adolescents: Recommendations for HIV-Prevalent and Resource-Constrained Settings. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 13.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings—the case of Cote d’Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 15.Hoover DR, Saah AJ, Bacellar H, et al. Multicenter AIDS Cohort Study Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. N Engl J Med. 1993;329:1922–1926. doi: 10.1056/NEJM199312233292604. [DOI] [PubMed] [Google Scholar]
- 16.Hirschtick RE, Glassroth J, Jordan MC, et al. Pulmonary Complications of HIV Infection Study Group Bacterial pneumonia in persons infected with the human immunodeficiency virus. N Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 17.MacPherson P, Moshabela M, Martinson N, et al. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103:588–593. doi: 10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Davis JL, Worodria W, Kisembo H, et al. Clinical and radiological factors do not predict smear negative tuberculosis in HIV-infected adults. PLoS ONE. 2010;5(3) doi: 10.1371/journal.pone.0009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro JG, Manzi G, Espinoza L, et al. Concurrent PCP and TB pneumonia in HIV infected patients. Scand J Infect Dis. 2007;39:1054–1058. doi: 10.1080/00365540701472056. [DOI] [PubMed] [Google Scholar]
- 20.Manosuthi W, Chottanapand S, Thongyen S, et al. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Global Tuberculosis Control: Epidemiology, Strategy, Financing. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]