Abstract
The O-type forkhead domain transcription factor (FOXO) is involved in many biological processes such as aging, the oxidative stress response, and growth regulation. FOXO activity is tightly controlled within cells. In particular, growth factor signaling pathways and the oxidative stress response can both stimulate nuclear translocation of this transcription factor. Here, we show that tetrahydrocurcumin (THC), a curcumin metabolite, regulates the oxidative stress response and aging via FOXO. In NIH3T3 cells, THC induced nuclear accumulation of FOXO4, a member of the FOXO family of transcription factors, by inhibiting phosphorylation of protein kinase B (PKB)/Akt. In Drosophila melanogaster, THC attenuated the oxidative stress response, an effect that was blocked in a foxo mutant background. THC also extended the life span of Drosophila under normal conditions, and loss of either foxo or Sir2 activity eliminated this effect. Based on these results, THC may regulate the aging process via an evolutionarily conserved signaling pathway that includes both foxo and Sir2.
Keywords: tetrahydrocurcumin, Drosophila, NIH-3T3-FOXO4 cell line, anti-aging, FOXO, resveratrol
INTRODUCTION
The insulin/IGF-1 signaling pathway regulates aging processes in Drosophila melanogaster, Caenorhabditis elegans, and mammals [1, 2]. Phosphoinositide-3-kinase (PI3K) and Akt kinase are critical downstream components of the insulin/IGF-1 pathway, and regulate the transcriptional activity of O-type forkhead domain transcription factors (FOXOs) [1]. There is a large family of mammalian FOXO transcription factors, which includes FOXO1, FOXO3, FOXO4, and FOXO6 [3, 4]. These proteins are involved in many cellular events, such as cell cycle arrest, apoptosis, DNA repair, glucose metabolism, anti-oxidative stress response, and longevity [5-7].
The activity and nuclear translocation of FOXO proteins are tightly regulated by post-translational modifications, including phosphorylation, acetylation, ubiquitination, and protein/protein interactions [4]. Oxidative stress stimulates the nuclear localization of FOXO3a via the sirtuins (SirTs), members of the silent information regulator 2 (Sir2) family of class III histone/protein deacetylases [8, 9]. Given that Sir2 family members regulate aging, the FOXO/SirT regulatory network may be a key factor for understanding the relationship between oxidative stress and life span [9].
Curcumin (C) is a yellow dye found in the crude drug turmeric (Curcumae Rhizoma), which comes from the rhizome of Curcuma longa L. (Zingiberaceae). C exhibits anti-oxidative [10], anti-inflammatory [11], liver-protective [12], anti-spastic [13], anti-tumor [14], and anti-allergic [15] effects. Tetrahydrocurcumin (THC) is an active metabolite of C [10, 11]. Orally ingested C is metabolized into THC by a reductase found in the intestinal epithelium [16, 17]. THC possesses extremely strong anti-oxidant activity compared to other curcuminoids [18, 19]. The anti-oxidant role of THC has been implicated in recovery from renal injury in mice [20] and in anti-inflammatory responses [18]. However, the literature concerning the anti-aging mechanism of THC is limited to a single survival study in mice [21]. In our current study, we found that THC regulated the nuclear localization of FOXO in cultured cells and inhibited phosphorylation of protein kinase B (PKB)/Akt kinase. Furthermore, genetic analyses in Drosophila revealed that foxo and Sir2 mediated the effects of THC on life span and the oxidative stress response. These results suggest that THC regulates the oxidative stress response and aging via an evolutionally conserved signaling pathway.
RESULTS
THC regulates nuclear localization of FOXO4 protein in NIH3T3 cells
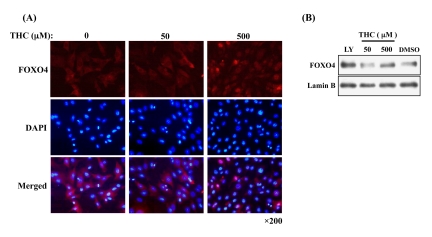
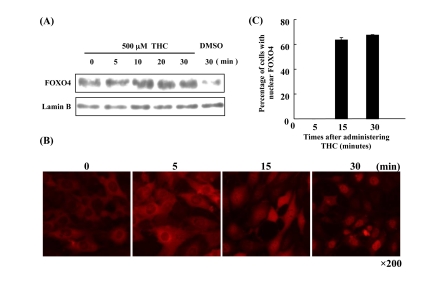
It has been shown that THC regulates the oxidative stress response in cells [22]. Nuclear accumulation of FOXO4 is a molecular marker for activation of the oxidative stress response [23]. We asked, therefore, whether THC affects the nuclear localization of FOXO4 in NIH3T3 cells. Immunocytochemistry revealed that THC treatment increased the nuclear levels of FOXO4 in a dose-dependent manner (Figure 1A). We confirmed this result through cell fractionation experiments. Levels of FOXO4 protein in the nuclear fraction were clearly elevated by THC treatment (Figure 1B). Next, we performed a time course analysis and detected FOXO4 in the nucleus within 30 min of THC treatment (Figure 2). These results indicate that THC regulates the nuclear translocation of FOXO4.
Figure 1. THC-induced nuclear accumulation of FOXO4.
(A) Dose-dependent effects of THC on nuclear accumulation of FOXO4 in NIH-3T3-FOXO4 cells. Cy3 (red) and DAPI (blue) label FOXO4 and nuclei, respectively. (B) FOXO4 levels increase in nuclear fractions from NIH-3T3-FOXO4 cells. Lamin B was used as a nuclear marker and the Akt inhibitor LY294002 (LY) was used as a positive control. Anti-AFX (N-19) primary antibody was used in both analyses.
Figure 2. Time course of FOXO4 localization in response to THC.
(A) FOXO4 protein levels increase in the nuclear fraction of NIH-3T3-FOXO4 cells within minutes of THC treatment. Lamin B was used as a nuclear marker, DMSO as a negative control. (B) Time course of FOXO4 nuclear translocation in NIH-3T3-FOXO4 cells following administration of THC. Red indicates FOXO4 protein. (C) The number of cells with nuclear FOXO was quantified at the indicated times following THC administration.
Effect of THC on signaling pathways that regulate FOXO4 localization in NIH-3T3 cells
To investigate the molecular mechanisms by which THC affects the nuclear localization of FOXO4, we analyzed the mitogen-activated protein kinase (MAPK) signaling pathway and the insulin-Akt signaling pathway. Both have been shown to regulate the nuclear translocation of FOXO4. Activation of ERK1/2 (a member of the MAPK subfamily) has been shown to increase the nuclear accumulation of FOXO4 [24]. We asked, therefore, whether THC affects phospho-ERK1/2 proteins in the NIH-3T3-FOXO4 cell line, and found that THC did not inhibit ERK1/2 phosphorylation (Supplemental Figure 1).
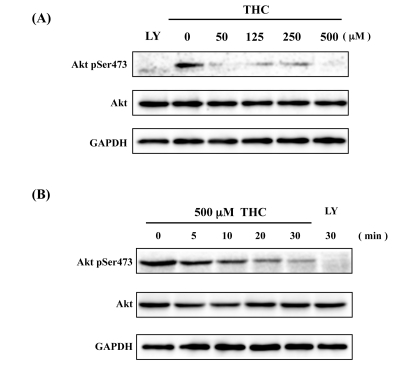
PKB/Akt kinase, acting as a downstream component of the insulin signaling pathway, phosphorylates FOXO4, and inhibits its nuclear translocation. This activity is thought to inhibit aging processes in multi-cellular organisms [25]. We focused, therefore, on the phosphorylation state of Akt using a phospho-specific Akt antibody (AktSer473). We found that THC treatment significantly inhibited phosphorylation of Akt in a dose-dependent manner. The effects of THC on phospho-Akt were similar to those observed with the positive control, LY294002 (Figure 3A). Time-course analysis indicated that THC treatment inhibited Akt phosphorylation within 30 min (Figure 3B). This result was consistent with the time-course analysis of FOXO4 nuclear localization (Figure 2). These analyses suggest that THC promotes translocation of FOXO4 to the nucleus at least in part through inhibition of Akt phosphorylation.
Figure 3. THC inhibits Akt phosphorylation.
Dose-dependence (A) and time-course (B) analyses indicate that THC inhibits phosphorylation of AktSer473. LY294002 (LY) was used as a positive control, Akt and GAPDH antibodies as loading controls.
THC represses the oxidative stress response in Drosophila via foxo
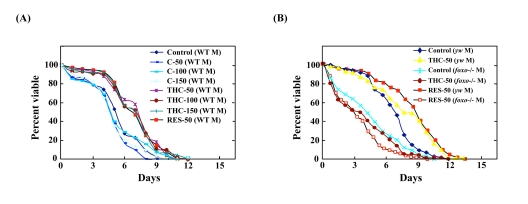
To determine whether FOXO mediates the effect of THC in vivo, we turned to Drosophila as a model organism. It has been shown that Drosophila foxo is involved in the oxidative stress response [26]. We first investigated, therefore, whether THC affects the oxidative stress response in Drosophila. Flies were fed 7.5 mM paraquat, a superoxide-generating agent. Under these conditions, all control flies were dead after 12 d of treatment. THC treatment led to a significant restoration of survival (~28%) (Figure 4A, Table 1). Interestingly, C did not increase tolerance to oxidative stress (Figure 4A, Table 1). Given that both C and THC have anti-oxidant activity, the effect of THC on life span may not be attributable to an effect on scavenging of reactive oxygen species [18, 19].
Figure 4. THC regulates oxidative stress response in Drosophila via foxo activity.
Effects of THC, RES, and C on the life span of wild-type (WT, Oregon-R) (A) and foxo mutant (yw background) (B) flies under oxidative stress conditions. Oxidative stress was induced by adding 7.5 mM paraquat to the food. Life span studies were carried out at 25°C with newly eclosed males (M) in each group (20 per vial; 3-4 vials were counted). Survivors were transferred every 2-3 days to fresh vials containing THC, RES, or C (50-150 μM). The statistical analysis of the data is summarized in Table 1.
Table 1. Results of Drosophila life span experiment.
| Mean lifespan | Log-rank test | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial | Food | Strain | Treatment | N | days | χ2 | P | % change |
| 1 | 7.5 mM (Paraquat) | Oregon-R (Male) | Control | 99 | 7 | |||
| RES-50 | 95 | 8 | 10.47 | 0.0012 | +14.3* | |||
| THC-50 | 97 | 9 | 12.24 | 0.0001 | +28.5* | |||
| THC-100 | 106 | 9 | 9.556 | 0.0020 | +28.5* | |||
| THC-150 | 91 | 8 | 7.448 | 0.0063 | +14.3* | |||
| C-50 | 106 | 6 | 2.728 | 0.0986 | − 14.3ns | |||
| C-100 | 99 | 6 | 1.179 | 0.2775 | − 14.3ns | |||
| C-150 | 103 | 6 | 3.141 | 0.0764 | − 14.3ns | |||
| 2 | 7.5 mM (Paraquat) | yellow white foxo−/− (Male) | Control | 71 | 5 | |||
| RES-50 | 62 | 5 | 0.570 | 0.4499 | 0.0ns | |||
| THC-50 | 57 | 6 | 0.660 | 0.4147 | +20.0ns | |||
| 3 | STD | yellow white (Female) | Control | 79 | 47 | |||
| THC-20 | 80 | 54 | 10.510 | 0.0012 | +14.8* | |||
| THC-50 | 78 | 57 | 11.509 | 0.0034 | +21.2* | |||
| RES-50 | 72 | 49 | 11.95 | 0.0026 | +5.2* | |||
| yellow white (Male) | Control | 75 | 38 | |||||
| THC-50 | 80 | 45 | 11.05 | 0.0009 | +18.4* | |||
| Organ-R (Male) | Control | 70 | 46 | |||||
| C-20 | 73 | 44 | 0.2584 | 0.6114 | − 4.3ns | |||
| C-150 | 74 | 44 | 0.2845 | 0.5938 | − 4.3ns | |||
| 4 | STD | foxo−/− (yw) (Male) | Control | 76 | 33 | |||
| RES-50 | 76 | 25 | 1.559 | 0.2899 | − 24.2ns | |||
| THC-50 | 77 | 30 | 1.123 | 0.2118 | − 9.1ns | |||
| d4EBP−/− (yw) (Male) | Control | 79 | 31 | |||||
| RES-50 | 70 | 28 | 7.466 | 0.0063 | − 9.6* | |||
| THC-50 | 77 | 33 | 0.029 | 0.8136 | +6.5ns | |||
| 5 | STD | Sir2−/df (yw) (Male) | Control | 78 | 32 | |||
| RES-50 | 80 | 27 | 0.2766 | 0.0787 | − 15.6ns | |||
| THC-50 | 78 | 27 | 3.0910 | 0.5990 | − 15.6ns | |||
indicates significant difference from control (P < 0.01)
represented no significant difference (P > 0.05)
STD: Standard, RES: resveratrol, THC: tetrahydrocurcumin, C: curcumin
As shown above, THC regulated FOXO nuclear translocation in mammalian cells. We investigated, therefore, the effects of THC on the oxidative stress response in a foxo-null mutant background. Although THC extended the life span of wild-type Drosophila, it could not extend the life span of foxo-null flies under oxidative stress conditions (Figure 4B, Table 1). These results suggest that THC regulates the oxidative stress response via foxo.
Resveratrol (RES) is a chemical compound that activates SIRT1 deacetylase activity and suppresses the oxidative stress response in mammalian cells [27]. It has been shown that SIRT1 regulates the state of acetylation of FOXO and induces nuclear localization of FOXO [9]. Indeed, we confirmed addition of RES to the culture medium resulted in nuclear accumulation of FOXO4 (Supplemental Figure 2). We also showed that RES treatment prolonged the life span of Drosophila under oxidative stress conditions (Figure 4A; Table 1). RES activity under oxidative stress conditions was inhibited in the foxo mutant background (Figure 4B; Table 1). These results suggest that RES and THC regulate the oxidative stress response in Drosophila via foxo.
THC extends the life span of Drosophila
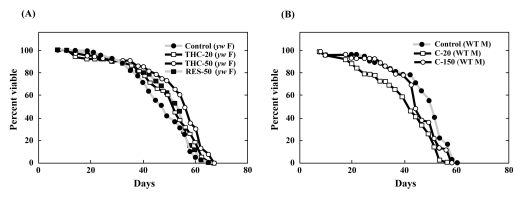
foxo regulates life span through the insulin signaling pathway [1, 28, 29]. We asked, therefore, whether THC also regulates life span under normal conditions. Before performing this analysis, we determined that THC does not affect the growth or eating habits of Drosophila (Supplemental Figures 3, 4). Life-span analysis showed that THC extended the mean but not maximum life span in both female flies (by ~21%) (Figure 5A; Table 1). We also found that RES prolonged the life span of Drosophila (Figure 4A; Table 1), although the effect was smaller than that seen with THC. This result differs from the report [30], that did not detect a significant effect of RES on Drosophila life span.
Figure 5. THC, but not C, extends life span in Drosophila under normal conditions.
The effect of THC and RES (A) was analyzed in a yw background, and C (B) was analyzed in Oregon-R (WT) flies. Life span studies were carried out at 25°C with newly eclosed male (M) or female (F) flies for each longevity experiment (20 per vial; 4 vials were counted). Survivors were transferred to fresh vials containing THC, RES, or C (20—150 μM) every 2—3 days. The statistical analysis of the data is summarized in Table 1.
Foxo and d4E-BP activity is required for life-span extension by THC
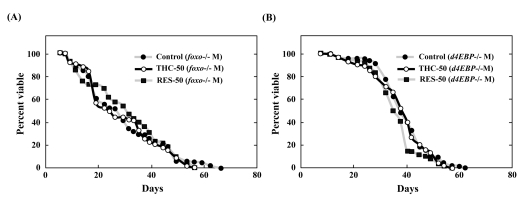
We next tested the ability of THC to extend the life span of foxo-null mutant flies. Compared to wild-type Drosophila, foxo mutants were short-lived. THC exposure, however, did not extend the life span of these foxo mutants (Figure 6A; Table 1). To confirm these results, we analyzed the effect of THC on a null mutant for the gene encoding eukaryotic initiation factor 4E-binding protein (d4E-BP), which acts downstreamof foxo to mediate aging and oxidative stress responses [31-33]. THC did not alter the life span of d4E-BP-null mutant flies (Figure 6B; Table 1). The effect of RES on life span was similarly abrogated in both foxo and d4E-BP mutant backgrounds (Figure 6; Table 1). These data indicate that THC and RES regulate the life span of Drosophila through foxo and d4E-BP activity.
Figure 6. THC and RES do not extend the life span of foxo or d4E-BP mutants.
Effects of THC or RES (50 μM) on the life span of foxo−/− (A) or d4E-BP−/− (B) mutant Drosophila under natural conditions. Life span studies were carried out at 25°C with a total of around 80 newly eclosed male (M) flies (yw background). The statistical analysis of the data is summarized in Table 1.
Sir2 activity is required for life-span extension by THC
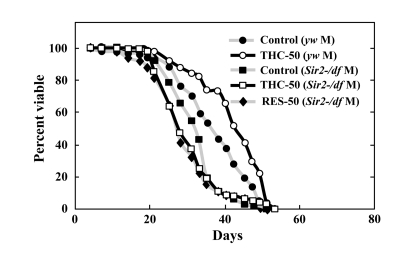
In addition to phosphorylation, acetylation is also known to control the nuclear localization of FOXO [9]. Sirtuin1, an NAD+-dependent deacetylase, plays a particularly important role in regulating the acetylation state of FOXO, and has been shown to affect the nuclear localization FOXO under oxidative stress in mammalian cells [9]. We therefore tested whether Drosophila Sir2 was required for THC to affect life span. We found that Sir2-null mutants were short-lived and that THC did not extend their life span (Figure 7; Table 1). These results suggest that THC extends the Drosophila life span via a mechanism that is dependent on foxo and Sir2. Similarly, RES did not affect the life span of Sir2 mutants, which supports the hypothesis that RES upregulates Sir2 activity (Figure 7; Table 1).
Figure 7. THC and RES do not extend the life span of Sir2 mutants.
Effects of THC or RES (50 μM) on the life span of Sir2-/df mutant Drosophila under natural conditions. Life span studies were carried out at 25°C with a total of around 80 newly eclosed male (M) flies (yw background). The statistical analysis of the data is summarized in Table 1.
DISCUSSION
We present here the first evidence that the small chemical compound THC is associated with the anti-oxidative stress response and extension of life span via the FOXO transcription factor. Using a mammalian cell culture system, we found that THC regulated FOXO4 nuclear translocation (Figure 1). Akt may be involved in this effect, since THC treatment caused Akt dephosphorylation. Phospho-Akt normally prevents the nuclear localization of FOXO (Figure 3). To support this analysis, we found that THC extended the life span of Drosophila under oxidative stress conditions and that this effect was foxo-dependent (Figure 4). Furthermore, THC extended the life span of Drosophila under normal conditions, and this extension required foxo and d4E-BP activity (Figure 6). These findings support the idea that oxidative stress may correlate with life-span extension. Notably, the effect of THC on life span also seemed to depend on Sir2 activity (Figure 7), suggesting that THC regulates aging processes via an evolutionally conserved regulatory network of genes that includes both foxo and Sir2.
Recently, chemical biology approaches have enabled researchers to analyze complicated biological processes such as aging using vast arrays of chemical compounds. As a result, plant-derived phenolic compounds have garnered a great deal of attention, because RES, a phenolic compound found in red wine, has been reported to extend life span in yeast, nematodes, and mice [34-36]. An important component of RES function is its ability to activate the SirTs [37-39]. Although there are indications that RES may not extend life span in Drosophila, our studies support the hypothesis that RES indeed regulates life span through Sir2 activity [30] (Figure 7). We speculate that the difference between these studies may have resulted from experimental variation or the source of THC.
Here, we found that another plant-derived phenolic compound, THC, regulates animal aging and the oxidative stress response via specific biological networks. Both C and THC have previously been reported to display anti-oxidant effects [18, 19]. In our current study, however, only THC affected the oxidative stress response and life span in Drosophila (Figure 5A). These results support another previous study in which C metabolites had more potent biological effects than C itself [40]. C has also been shown to inhibit the histone-modifying enzyme CBP/p300 [41]. This may explain why C actually appeared to have a rather toxic effect in Drosophila (Figure 5B).
THC treatment resulted in the nuclear localization of FOXO4 and dephosphorylation of Akt (Figs. 1, 3). Akt inhibits nuclear localization of FOXO4 and plays a key role in regulating its activity. These results suggest that THC regulates nuclear localization of FOXO protein by affecting either acetylation or ubiquitination. Our genetic analyses using Drosophila suggest that the relationship between THC and FOXO is evolutionarily conserved. Notably, THC activity seemed to depend on d4E-BP, a downstream target of foxo (Figure 6). This result suggests that THC affects the foxo-d4E-BP pathway, consistent with a recent study showing that the foxo-d4E-BP system regulates animal life span by affecting proteostasis [33]. We also observed that THC activity depended on Sir2 (Figure 7). Although we cannot exclude the possibility that the foxo, d4E-BP, and Sir2 mutations cause non-specific cellular toxicity that negatively affects life span (and that THC regulates this effect), our combined results in cultured mammalian cells and Drosophila suggest that THC may be involved in specific events that regulate an organism's life span.
Caloric restriction is known to have an anti-oxidative effect and to extend life [42]. The life-extending effect of caloric restriction is associated with increased level and activity of Drosophila Sir2 histone deacetylase and its mammalian ortholog, SIRT1 [35, 43]. SIRT1 also seems to be involved in the oxidative stress response by regulating the nuclear localization of FOXO [27]. We demonstrated here that both RES and THC depend on foxo and Sir2 to extend the life span of Drosophila. Although we do not know whether THC and RES share a common target in order to regulate longevity, we expect that THC and RES regulate very similar down-stream effectors. THC may regulate FOXO, which might be involved in Sir2-dependent life-span extension.
Finally, it should be noted that THC-treated mice also survive for an extended period of time [21]. Interestingly, it was shown that the average mouse life span, but not the maximum life span, is extended by THC treatment. As THC also increased the average but not maximum life spans of Drosophila (Figure 4), these studies suggest that the effect of THC on longevity may be an evolutionarily conserved process.
MATERIALS AND METHODS
Curcumin, tetrahydrocurcumin, and resveratrol
C and THC were kindly provided by House Foods Industry (Okinawa, Japan). Resveratrol (RES) was purchased from Wako Pure Chemical Industries (Osaka, Japan).
FOXO4 immunostaining in NIH-3T3 cells
NIH-3T3 cells (3 × 104) were seeded in 48-well plates and incubated for 2 days. Cells were incubated 10 min in 500 μl of fresh culture medium and then 2.5 μl of 20 μM insulin growth factor (IGF) was added to each well. Subsequently, 0, 50, 200, or 500 μM resveratrol (RES) or THC was added. The Akt inhibitor LY294002 was used as a positive control (5 μl of 2 mM LY294002 per well). Cells were incubated for 30 min at room temperature and then 250 μl of 3% paraformaldehyde in PBS was added for 15 min at room temperature to attach the cells to the wells. The supernatant was gently removed with a pipette, and 200 μl of 0.5% Triton/CSK-buffer (Cytoskeletal buffer: 20 mM HEPES, 50 mM NaCl, 3 mM MgCl2 and 300 mM Sucrose) was added to each well. Cells were washed with PBS-T (PBS+0.05% Tween 20), then 100 μl of anti-AFX (N-19) antibody (diluted 1:100 in PBS-T) was added to each well, and cells were incubated for 1 h at room temperature. Cells were washed twice in PBS-T, then 100 μl of CY3-labeled secondary antibody (diluted 1:200 in PBS-T) was added to each well, and cells were incubated at room temperature in the dark for 1 h. Cells were washed twice with PBS-T, 200 μl of PBS containing DAPI (Roche Applied Science, USA) was added to each well (1μg/mL), and cells were observed using a fluorescence microscope (Axio version II, Carl Zeiss Inc. Germany).
Western blot analysis
NIH-3T3 cells (1 × 106 cells) were seeded in 90-cm dishes and incubated for 2 d. RES and THC were then added at doses of 0, 50, 200, and 500 μM for the appropriate times. For a positive control, 5 μl of 2 mM LY294002 was added. To prepare total protein samples, cells were harvested in RIPA buffer containing 1% phosphatase and 2% protease inhibitor. After the centrifugation the supernatant was collected and used as the total protein sample. To prepare nuclear and cytoplasmic protein samples, cells were harvested in PBS buffer and washed twice. After the centrifugation the pellet was resuspended in 400 μl of NP-40 buffer (20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% nonidet P-40, 2 mM EDTA). After incubation on ice for 5 min, samples were centrifuged 5 min at 2500 rpm (600× g) at 4°C. The supernatant was removed and used as the cytoplasmic protein sample. Pellets were washed twice with NP-40 buffer and resuspended in 200 μl high-salt buffer at 4°C for 30 min. After centrifugation at 15,000 rpm (20400× g) at 4°C for 5 min, the supernatant was collected and used as the nuclear protein sample. The protein concentration assay was performed using a BAC kit (Thermo Science, Pierce Company, USA). SDS-PAGE was performed using 30 μg protein, and proteins were transferred onto a PVDF membrane. The membrane was incubated with the appropriate primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies, and the antigen was visualized using a chemiluminescent substrate (Amersham, GE Healthcare, Tokyo, Japan). Primary antibodies used for immunoblotting were anti-AFX1 (goat polyclonal; Santa Cruz Biotechnology, California, USA), anti-phospho-FOXO4 (Ser193) (rabbit polyclonal; Santa Cruz Biotechnology, California, USA), anti-Akt (rabbit polyclonal) and anti-phospho-Akt (Ser473) (Cell Signaling Technology, Boston, USA), mouse anti-human lamin B (Oncogene Research Products, CA, USA), rabbit anti-GAPDH (Abcam, Tokyo, Japan), and anti-β-tubulin (Sigma, St. Louis, USA). Secondary antibodies were horseradish peroxidase-conjugated anti-goat IgG, anti-mouse IgG, and anti-rabbit IgG (Amersham, GE Healthcare, Tokyo, Japan).
Drosophila strains, medium, and life span assay
The wild-type D. melanogaster strain was Oregon-R, and yellow white flies (y1,w1118) were used as a second control line. To create foxo-null mutant flies, yw; FRT82B, foxo25/TM6B was crossed with yw; foxo21/TM6B. Both maleand female progeny were analyzed in these experiments. To create Sir2-null mutant flies, yw; Sir217/CyO was crossed with yw; Df(2L)BSC30/CyO. Foxo, Sir2 and d4E-BPnull lines were kindly provided by Drs. Hafen, Helfand and Lasko, respectively.
Drosophila medium consisted of 0.7% agar, 10% glucose, 4.5% corn powder, and 4% dry yeast. C and THC in EtOH were added to melted aliquots of medium at final concentrations of 0, 20, 50, and 150 μM (5% EtOH). EtOH alone was used as a control. Fresh medium was prepared weekly. For survival assays, newly eclosed flies were maintained at 20 flies/vial on standard laboratory food at 25°C. Flies were transferred to fresh vials every 2-3 days and scored for survival. Each experiment was conducted with at least 200 flies of each genotype. All the analyses of life span are summarized in Table 1.
Anti-oxidative stress analysis
To induce the oxidative stress response in Drosophila, adult flies (1 week old) were exposed to 7.5 mM paraquat. Twenty flies were starved in vials containing 2 ml of 1% agar for 6 h before paraquat treatment. Flies were then transferred to vials containing 1% agar with 7.5 mM paraquat (methyl viologen, Sigma, St. Louis, USA) and 5% sucrose. Data are presented as mean ± standard error.
Statistical analysis
Significant differences between groups in all experiments were determined by Kaplan-Meier survival analysis using the biostatistics software GraphPad Prism 5. Curves were compared using the log-rank test. A p value < 0.05 was considered significant.
SUPPLEMENTAL FIGURES
Dose-dependent effects of THC on MAPK phospho-rylation in NIH-3T3-FOXO4 cells. Anti-p44/42 or phospho-p44/42 was used. Anti-GAPDH was used as a loading control. LY: LY294002, an Akt inhibitor.
(A) Dose-dependent effects of RES on FOXO4 nuclear localization. (B) RES increases the level of FOXO4 in the nuclear fraction from NIH-3T3-FOXO4 cells. LY290042 (LY) and DMSO were used as positive and negative controls, respectively. Anti-GAPDH was used as a loading control. (C) Dose-dependent effects of RES on Akt phosphorylation (Ser473) are shown. LY was used as a positive control. Anti-Akt and Anti-GAPDH were used as loading controls.
Compared to 5% EtOH controls, THC treatment (50 μM) did not affect puparium formation in Drosophila (see Supplemental Materials and Methods).
EtOH (5%) or THC (50 μM in 5% EtOH) was mixed with [32P]dCTP and fed to 14-d wild-type (Oregon-R) virgin males or females (20 animals/vial, totally 60 flies). The uptake of [32P]dCTP was measured by scintillation counting for 4 min/sample (see Supplemental Materials and Methods).
Acknowledgments
We gratefully thank Drs. E. Hafen, S.L. Helfand, and P.F. Lasko, as well as the Drosophila Stock Center (Kyoto Institute of Technology, Japan), for gifts of Drosophila strains. We also thank Dr. Yanagino for gifts of reagents for biochemical analyses and for helpful advice. This work was supported in part by grants from the Novartis Foundation (Japan) for the Promotion of Science (A.M.), Grant-in-Aid from the Ministry of Education and Scientific Research for Priority Areas (Y.-M.L. and L.T.), the Ministry of Health, Labor, and Welfare for Comprehensive Research on Aging and Health (W.M.), and Health Sciences of National Institute of Biomedical Innovation (Japan) for the Promotion of Fundamental Studies (W.M.).
Glossary
- THC
tetrahydrocurcumin
- C
curcumin
- ROS
reactive oxygen species
- FOXO
O-type forkhead domain transcription factor
- MnSOD
Mn superoxide dismutase
- RES
resveratrol
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
- CR
caloric restriction
- PKB
protein kinase B)
- IGF
insulin growth factor
Footnotes
The authors of this manuscript have no conflict of interest to declare.
REFERENCES
- Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ziv E, Hu D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Aging Res. Rev. 2011;10:201–204. doi: 10.1016/j.arr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sainz N, Rodriguez A, Catalan V, Becerril S, Ramirez B, Gomez-Ambrosi J, Fruhbeck G. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1a in ob/ob mice. PloS One. 2009;4:e6808. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BC, Denu JM. Acetyl-lysine analog peptides as mechanistic probes of protein deacetylases. J. Biol. Chem. 2007;282:37256–37265. doi: 10.1074/jbc.M707878200. [DOI] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- Sreejayan Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharm. Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Kang HC, Nan JX, Park PH, Kim JY, Lee SH, Woo SW, Zhao YZ, Park EJ, Sohn DH. Curcumin inhibits collagen synthesis and hepatic stellate cell activation in-vivo and in-vitro. J. Pharm. Pharmacol. 2002;54:119–126. doi: 10.1211/0022357021771823. [DOI] [PubMed] [Google Scholar]
- Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol. Pharm. Bull. 2005;28:1438–1443. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- Holder GM, Plummer JL, Ryan AJ. Metabolism and excretion of curcumin1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Kawakishi S, Osawa T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetra-hydrocurcumin. Biochem. Pharmacol. 1996;52:519–525. doi: 10.1016/0006-2952(96)00302-4. [DOI] [PubMed] [Google Scholar]
- Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci. Biotechnol. Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- Majeed M, Badmeav V, Uma S, Rajeuderan JR. Curcuminoids: antioxidant phytonutrients. Nutriscience Publishers; New Jersey: 1995. [Google Scholar]
- Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J. Nutr. 2001;131:2090–2095. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- Atsumi T, Fujisawa S, Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005;11:236–242. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Ichino A, Hayashi T, Yamamoto T, Kikekawa U. Regulation of intracellular localization and transcriptional activity of FOXO4 by protein kinase B through phosphorylation at the motif sites conserved among the FOXO family. J. Biochem. 2005;138:485–491. doi: 10.1093/jb/mvi146. [DOI] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am. J. Physiol. Heart. Circ. Physiol. 2010;298:H1048–H1054. doi: 10.1152/ajpheart.00826.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 2005;16:237–243. [PubMed] [Google Scholar]
- Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J. Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. FOXO factors: a matter of life and death. Future Oncol. 2006;2:83–89. doi: 10.2217/14796694.2.1.83. [DOI] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Patridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Aging Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Daitoku H, Fukamizu A. FOXO transcription factors in the regulatory networks of longevity. J. Biochem. 2007;141:769–774. doi: 10.1093/jb/mvm104. [DOI] [PubMed] [Google Scholar]
- Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19:1840–1843. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Perimmon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:4337–4342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcain FJ, Villalba JM. Sirtuin activators. Expert Opin. Ther. 2009;Patents 19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- Ajmo JM, Liang XM, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastroint. Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosh M, Whitaker R, Kroll A, Hosier S, Chang C, Bauer J, Cooper L, Neretti N, Helfand SL. Comparative transcriptional pathway bioinformatic analysis of dietary restriction, Sir2, p53 and resveratrol life span extension in Drosophila. Cell Cycle. 2011;10:904–911. doi: 10.4161/cc.10.6.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Nishiumi S, Mizushina Y, Fujishima Y, Yamamoto K, Masuda A, Mizuno S, Fujita T, Morita Y, Kutsumi H, Yoshida H, Azuma T, Yoshida M. Monoacetylcurcumin strongly regulates inflammatory responses through inhibition of NF-kappa B activation. Int. J. Mol. Med. 2010;25:761–767. doi: 10.3892/ijmm_00000402. [DOI] [PubMed] [Google Scholar]
- Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Calorie restriction decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose-dependent effects of THC on MAPK phospho-rylation in NIH-3T3-FOXO4 cells. Anti-p44/42 or phospho-p44/42 was used. Anti-GAPDH was used as a loading control. LY: LY294002, an Akt inhibitor.
(A) Dose-dependent effects of RES on FOXO4 nuclear localization. (B) RES increases the level of FOXO4 in the nuclear fraction from NIH-3T3-FOXO4 cells. LY290042 (LY) and DMSO were used as positive and negative controls, respectively. Anti-GAPDH was used as a loading control. (C) Dose-dependent effects of RES on Akt phosphorylation (Ser473) are shown. LY was used as a positive control. Anti-Akt and Anti-GAPDH were used as loading controls.
Compared to 5% EtOH controls, THC treatment (50 μM) did not affect puparium formation in Drosophila (see Supplemental Materials and Methods).
EtOH (5%) or THC (50 μM in 5% EtOH) was mixed with [32P]dCTP and fed to 14-d wild-type (Oregon-R) virgin males or females (20 animals/vial, totally 60 flies). The uptake of [32P]dCTP was measured by scintillation counting for 4 min/sample (see Supplemental Materials and Methods).