Abstract
Adolescents are more likely to experiment with and become addicted to drugs of abuse. A number of studies indicate that the developmental forebrain may be responsible for making adolescents vulnerable to the addictive properties of such drugs. The aim of this study was to first compare behavioral responses to novelty and cocaine between juvenile and adult rats and then compare levels of the immediate-early gene zif268 activation in several forebrain areas via in situ hybridization. We found that juveniles demonstrated higher locomotion scores and required a higher dose of cocaine than adults in order to establish a conditioned place preference. Additionally, at this higher dose, juvenile rats exhibited higher levels of zif268 mRNA in the prefrontal cortex compared to adults. A developmental effect for increased zif268 mRNA was also observed in the striatum and nucleus accumbens, but there was no interaction with the cocaine dose. These findings hold interesting implications for the study of the molecular mechanisms underlying juvenile drug addiction.
Keywords: adolescent, conditioned place preference, addiction, zif268, prefrontal cortex
1. Adolescence is an ontogenetic phase during which neuronal and hormonal systems undergo maturational arrangements. Human adolescents as well as their counterparts in other species exhibit certain characteristic behaviors that may help them survive the transition between childhood and adulthood (Oppenheim 1981). Relative to individuals at other ages, human adolescents as a group exhibit an increased amount of peer-directed social interaction, risk-taking behaviors, and sensation and novelty-seeking (Adriani and Laviola 1998). These behavioral modifications and vulnerability are consistent with the need of the adolescent to explore novel and often risky areas in order to establish new social relationships and achieve independence. These behaviors, however, have the potential to become pathological. One such example is drug addiction, a chronic disorder most often initiated during adolescence (Spear 2000). As most experimentation with illicit drugs occurs during adolescence (SAMHSA, 1999; Beck et al., 2007;) and a disproportionate number of experimenters within this age-group become addicted (Grant and Dawson, 1998; O’Brien and Anthony, 2005), it is becoming increasingly clear that adolescence represents a critically vulnerable period for drug addiction in humans.
Animal research supports the notion that adolescence is a period of altered sensitivity to environmental stimuli, including drugs of abuse. Indeed, juvenile rats show increased novelty-seeking behavior (Bronstein, 1972), increased reward to natural stimuli (Vaidya et al 2004), and enhanced vulnerability to the addictive properties of drugs of abuse (Balda et al., 2006). In both humans and in animal models, these behaviors are due to a combination of biological factors including maturational changes occurring in the adolescent brain (Spear 2000; Chambers et al., 2003). It may be the case that such transient neuronal features may predispose adolescents to commence the use and alter the rewarding properties of drugs, leading to the onset of substance abuse. Unfortunately, the mechanisms behind this vulnerability remain unclear.
In animal studies, conditioned place preference (CPP) is used as a rodent model of drug reward-like behaviors in which a drug (such as cocaine) is paired with a distinct context (Carlezon 2003). Previous works investigating the effects of cocaine on juvenile CPP have found mixed results. Several studies found that juveniles formed a conditioned place preference at a lower dose than adults, suggesting a heightened sensitivity to the conditioning properties of cocaine in juveniles (Badanich, Adler, & Kirstein, 2006; reviewed in Doremus-Fitzwater et al., 2009). Others found no difference in CPP between adults and juveniles (Campbell et al., 2000; Schramm-Sapyta et al., 2004), while a recent study found that a 10 mg/kg dose of cocaine induced CPP in adult rats but not juveniles (Aberg et al., 2007). With no discernable behavioral explanation for why juveniles are vulnerable to drugs of abuse, these findings point to possible neural adaptations from adolescence to adulthood that facilitate susceptibility to drugs of abuse. As the induction of immediate early genes (IEG) has been consistently associated with neuronal activation and adaptation (e.g. Brandon and Steiner, 2003; Shram et al., 2007), they present a logical target for comparison between juveniles and adults. One such IEG, zif268 (also known as egr-1, krox24, NGFI-A, and zenk) is a constitutively expressed transcription factor whose expression levels correlate with neuronal activation (Mutschler et al., 2000). Examining changes in Zif268 is useful for investigating neuronal effects following cocaine exposure because Zif268 mRNA levels can be rapidly altered by cocaine or cocaine-associated cues and have been linked to synaptic plasticity (Covington et al., 2005; Thomas et al., 2003; Mutschler et al., 2000). While there have been several studies that investigated the effects of cocaine on zif268 protein levels, these experiments were carried out in adult rodents (Valjent et al., 2006; Thomas et al., 2003). Recently, there have been studies which examined the relative expression of zif268 levels between adults and juveniles immediately following an acute administration of cocaine (Caster and Kuhn, 2009). The purpose of our study was to use zif268 to discern anatomical disparities in neuronal activation that may underlie differences to the rewarding effects of cocaine. To this end, we compared behavioral responses to novelty and to the rewarding properties of cocaine between juvenile and adult male Sprague-Dawley rats using locomotion chambers and the conditioned place preference test (respectively). We then quantified Zif268 mRNA in the prefrontal cortex, dorsal striatum, nucleus accumbens, and hippocampus (PFC, dStr, NAc, and Hpc respectively) in both groups to obtain a measure of neuronal activation following exposure to cocaine-induced place preference.
2. Experimental Procedures
In all experiments, the rats were housed 2 per cage in clear Plexiglas cages (48 × 27 × 20 cm.) All animals had access to food and water ad libitum and were kept in a 12 h light cycle (lights on at 7am). All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Florida State University. Thirty-two male Sprague-Dawley rats ordered from Harlan (Indianapolis, IN, USA) were used in this experiment. Sixteen rats were adults, weighing between 250–275g and sixteen were 21-day old juveniles. An additional twelve rats (six adults and six 21-day old juveniles) were used as age-matched controls and sacrificed in basal conditions for the evaluation of developmental differences in Zif268 mRNA levels. All animals were allowed to habituate to the vivarium for four days at which point they were handled and weighed.
2.1 Response to novelty
Subsequent to habituation, animals’ locomotor response to novelty was tested in circular activity chambers (Med Associates Inc., St. Albans, Vermont) for one hour during the first four hours of the light cycle. Four photo-beam sensors at equal distances recorded each rats’ crossings between adjacent quadrants. Photo-beam breaks were recorded and a locomotor score was assigned to each rat as previously described (Dietz et al., 2005).
2.2 Conditioned Place Preference
After locomotion, animals were exposed to the conditioned place preference apparatus as previously described (Dietz et al., 2007). Briefly, the Conditioned Place Preference (CPP) boxes are composed of three chambers: a black compartment, a white compartment, and a small, neutral gray area between the two. The apparati were connected to a computer with MedPC software (Med Associates). When the adolescent rats reached postnatal day 28, a 20 minute pre-test was performed in which animals freely explored the chambers and the time spent in the black and white compartments was recorded as well as the locomotion activity of each animal in each chamber. All rats-both juvenile and adults, received drug injections in the least-preferred chamber. Each animal received two injections per day for four consecutive days, consisting of either a 5 or 10 mg/kg injection of cocaine and a saline (1ml/kg) injection (n=8 for each dose and age). Both injections were followed by a 30-minute session in either the drug-paired chamber (when given cocaine) or the saline-paired compartment and the animal’s locomotion activity was recorded. Groups were counterbalanced for time of day in regards to pairing and order (drug/saline). On day 5, rats did not receive an injection and were allowed to freely explore the drug-paired and saline-paired compartments for 20 minutes. The time spent in each and their activity was recorded. Conditioned place preference was established if the rats spent more time in the drug-paired compartment after the conditioning than during the pre-test. After this test, animals were promptly decapitated and their brains removed and snap frozen in 2-methylbutane (Fisher Scientific, Fairlawn, NJ). All brains were then stored at −80°C until further processing.
2.3 In situ hybridization
Five brains per experimental group were used. Each brain was sectioned on a cryostat at 14 µm, and a series of sections were mounted on poly-L-lysine-coated slides. Sections were taken at 100-µm intervals. The sections were fixed in 4% paraformaldehyde for 1 h, followed by three washes in 2 × saline sodium citrate (SSC). The sections were then placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1M, pH 8) for 10 min at room temperature, rinsed in distilled water and dehydrated through graded alcohols (50, 75, 85, 95 and 100%). After air-drying, the sections were hybridized with a 35S-labeled cRNA probe. The rat zif268 cDNA cloned in our lab yielded a 382-nt cRNA probe (Stack et al., 2010). The probe was labeled in a reaction mixture consisting of 1µg of linearized plasmid, 5 × transcription buffer (Epicenter Technologies, Madison, WI, USA), 125µCi [35S]UTP, 125µCi [35S]CTP, 150µM each of ATP, and GTP, 12.5mM dithiothreitol, 20U RNase inhibitor, and 6U polymerase. The reactions were incubated for 90min at 37°C. The probe was then separated from unincorporated nucleotides over Bio-Rad Micro Bio Spin Chromatography Columns (Bio-Rad Hercules, CA, USA). The probe was diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 20 × SSC, 50 mM sodium phosphate buffer, pH 7.4, 50 × Denhardt's solution, 0.1mg/ml yeast tRNA and 10mM dithiothreitol) to yield 106d.p.m./70µl. The sections were coverslipped and placed inside a humidified box overnight at 55°C. Following hybridization, the coverslips were removed and the sections rinsed and washed twice in 2 × SSC for 5min each, then incubated for 1h in RNase (200µg/ml in Tris buffer containing 0.5M NaCl, pH 8) at 37°C. The sections were washed in increasingly stringent solutions of SSC, 2×, 1× and 0.5×, for 5 min each, followed by incubation for 1h in 0.1 × SSC at 65°C. After rinsing in distilled water, the sections were dehydrated through graded alcohols, air-dried and exposed to a Kodak XAR film (Eastman Kodak, Rochester, NY, USA) for 4–7 days.
2.4 Quantification of the Radioactive Signal
As a way to standardize optical density measurements, an outline was developed for each brain region based on the shape and size of the region. Using those outlines, optical density measurements were taken for each brain region from the left and right sides of the brain from rostral/caudal sections. According to the Paxinos and Watson Atlas, the mPFC was sampled from Bregma 3.7mm to Bregma 2.2mm, the striatum was sampled from Bregma 1.7mm to Bregma −0.4mm and dorsal hippocampus from Bregma −2.12mm to Bregma −4.52mm. Accordingly, eight sections per brain region per rat were used. Optical density values were corrected for background, multiplied by the area sampled to produce an integrated density measurement, and then averaged to produce one data point for each brain region for each animal. These data points were averaged per group and compared statistically. Optical density measurements were quantified from X-ray film using Automated Imaging Software (AIS; Imaging Research, St Catherine's, ON, Canada).
2.5 Real-time polymerase chain reaction (RT-PCR)
Zif268 mRNA levels were examined by qRT-PCR in the striatum, nucleus accumbens, and hippocampus of control animals. Six brains per group were used. Brains were sectioned on a freezing cryostat at 200µm and then tissue-punched at 1.0mm for those areas according to the aforementioned Brain Atlas coordinates. 1 µg of total RNA extracted from each brain area of each rat was processed for cDNA synthesis using random hexamers with Invitrogen First-Strand cDNA synthesis kit. cDNA was then used in Bio-Rad real-time PCR reactions in triplicate using iQ SYBR GREEN supermix (Bio-Rad Laboratories). Reactions were monitored using the BioRad iCycler machine, with SyberGreen incorporation as a tracking dye. Amplification specificity was verified with melting curve analysis and all quantification data were processed with an internal standard curve and then normalized to the housekeeping gene NADH and further analyzed via one-way ANOVA. For the striatum and accumbens, one sample in the juvenile group was excluded as an outlier for the analysis. The primer sequences were designed as follows: For Zif268 5’-TGCACCCACCTTTCCTACTC-3’ (Fwd) and 5’-AGGTCTCCCTGTTGTTGTGG-3’ (Rev). For NADH 5’-CTATTAATCCCCGCCTGACC-3’ (Fwd) 5’-GGAGCTCGATTTGTTTCTGC-3’ (Rev).
2.6 Statistical Analyses
Locomotor activity in response to novelty was analyzed using one-way analysis of variance (ANOVA). Conditioned place preference was first analyzed using two-way repeated measures ANOVAs. The within-subjects factor was the test day. The between-subjects factors were age (adult or juvenile) and cocaine dose (5 mg/kg or 10mg/kg). Within each age group, we then employed one-way repeated measures ANOVAs filtered by significant terms to further investigate differences in CPP for each dose. Locomotor sensitization was analyzed using two-way repeated measures ANOVAs with days as the within-subjects factor and age and cocaine dose as the between-subjects factors. In situ Zif268 mRNA levels were analyzed by two-way ANOVAs (age × cocaine dose) for each brain region. Bonferroni post-hoc tests were performed where appropriate.
3. Results
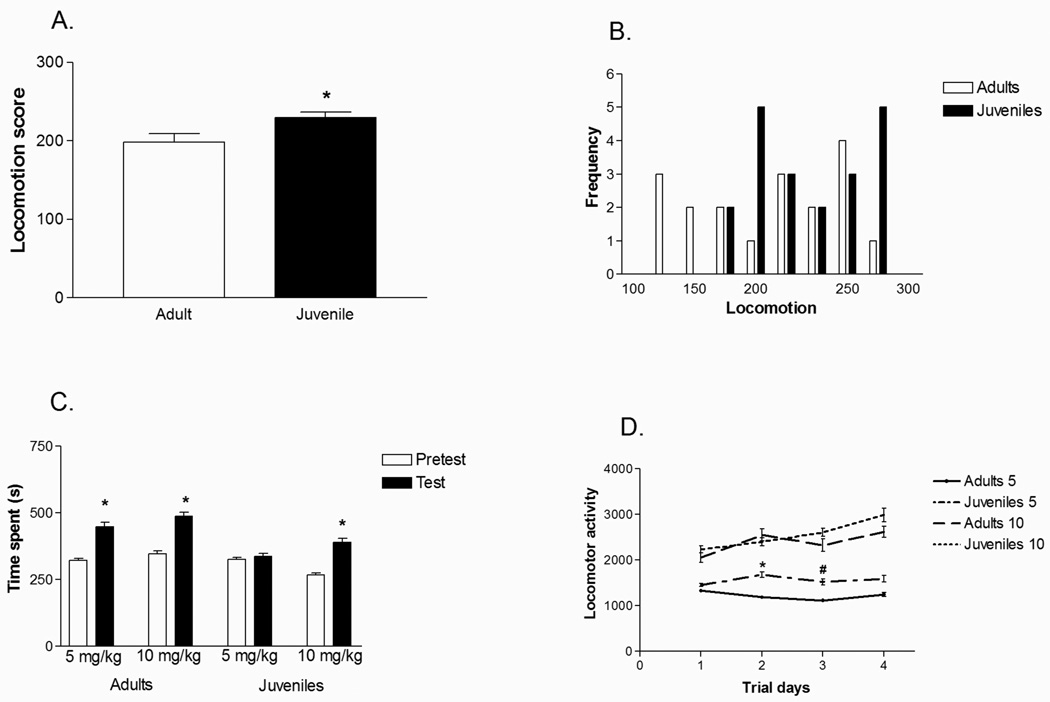
During the screening for locomotor activity, juvenile rats had significantly higher locomotor scores than their adult counterparts [F(1, 34) = 5.76; p < 0.05] (Figure 1A). Adults had a wider distribution of locomotor activity, whereas juvenile rats’ scores clustered towards the upper half of the adult distribution (Figure 1B), demonstrating that juveniles had a uniformly higher response to novelty than adults.
Figure 1.
Behavior in response to novelty and cocaine administration between juveniles and adults. (A) Juveniles have significantly higher locomotor activity than adults in a novel environment. (B) Adults have a wider distribution of locomotor scores, representing a mix of high and low locomotor activity. Juveniles have a more narrow distribution, clustering towards the higher values. (C) Only adults establish a place preference at the 5 mg/kg dose of cocaine, while both juveniles and adults establish a preference at the higher 10 mg/kg dose. (D) Juveniles have significantly higher locomotion during the place preference test in response to 5mg/kg cocaine than adults, while both adults and juveniles have similar locomotor activity at the 10 mg/kg dose. *p <0.05; #p = 0.06; Error bars represent SEM.
A conditioned place preference is said to be established when subjects spend significantly more time in the drug-paired compartment during the test when compared to the pre-test. Analysis by two-way, repeated measures ANOVA revealed a significant difference between our age groups [F(1,28) = 5.49; p<0.05]. Further analysis found that adult rats established a cocaine conditioned place preference at doses of 5 mg/kg [F(1,7) = 6.37; p<0.05] and 10 mg/kg [F(1,7) = 13.92; p<0.01] (Figure 1C). Juveniles, however, only established a preference for cocaine at the higher 10 mg/kg dose [5 mg/kg dose: F(1,7) = 0.085; p=0.78; 10 mg/kg dose: F(1,7) = 9.71; p<0.05] (Figure 1C).
There was a significant effect of cocaine dose for locomotion sensitization [F(1,28) = 20.93; p<0.0001], with further analysis indicating that juveniles exhibited higher locomotion than adults at the 5 mg/kg cocaine dose F(1, 14) = 4.56 ; p<0.05 ] (Figure 1D), but the same level of locomotion at the 10 mg/kg dose (Figure 1D). There was no difference in locomotion during the saline conditioning (data not shown).
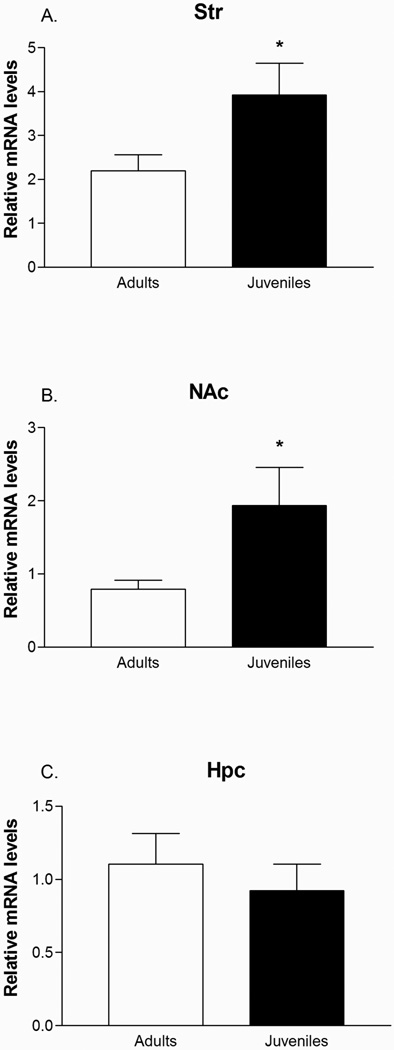
Following the twenty minute CPP test, animals were sacrificed and Zif268 mRNA was quantified in several brain regions via in situ hybridization (Figure 2A). In the dorsal PFC, we found a significant age by dose interaction [F(1,16) = 4.59; p<0.05] and a significant age effect in the ventral PFC [F(1,16) = 5.29; p<0.05]. In both the dorsal and ventral PFC, we found significantly higher Zif268 mRNA in juveniles compared to adults at the 10 mg/kg dose of cocaine [dorsal: F(1, 8) = 7.12 ; p < 0.05; ventral: F(1,8) = 6.985; p<0.05](Figure 2B). There was no difference in Zif268 mRNA between adults and juveniles in the 5 mg/kg dose groups. Within juveniles, there was a trend towards significantly increased Zif268 in the 10 mg/kg dose compared to the 5mg/kg group, whereas within adults there was a trend towards significantly increased Zif268 levels in the 5 mg/kg group [F(1,16) = 3.543; p = 0.076 and F(1,16) = 3.39; p = 0.08, respectively]. In the dorsal striatum, we found an age effect for increased Zif268 mRNA in juveniles versus adults [F(1, 16) = 19.23 ; p < 0.001], but no dose effects within either adult or juvenile group (Figure 2C). As our initial analysis of nucleus accumbens core and shell found no significant differences in Zif268 levels between the two regions, we combined these data for analysis. In doing so, we found an age effect for increased Zif268 in juveniles in the nucleus accumbens [F(1, 16) = 22.268 ; p < 0.0001], but again no dose effect within either adult or juvenile group (Figure 2D). Finally, in the hippocampus, we found no difference in Zif268 levels between adults and juveniles at either cocaine dose or within groups (Figures 2E–G). The age effect in the accumbens and striatum was unexpected and we were interested to know if this was a consequence of development or some aspect of our CPP test. We therefore examined Zif268 levels in the striatum, accumbens, and hippocampus of adult and juvenile rats that had not been exposed to CPP or cocaine and had been sacrificed in basal conditions. We performed quantitative real-time pcr on these areas and found that in basal conditions, juvenile rats did indeed have significantly higher levels of Zif268 mRNA in the striatum [F(1,9) = 5.175; p < 0.05] and nucleus accumbens [F(1,9) = 6.86; p<0.05], but not in the hippocampus [F(1,10) = 0.438; p = 0.52] (Figures 3A–C).
Figure 2.
A: Representative in situ hybridization images of quantified brain areas. Zif268 levels in adult medial prefrontal cortex, striatum, nucleus accumbens, and hippocampus (Top) were compared with juveniles (bottom). Juveniles that formed a place preference at the 10 mg/kg dose had significantly higher Zif268 levels in the dorsal and ventral prefrontal cortex than adults (B). Additionally, there was an age effect where juveniles had significantly higher Zif268 mRNA levels in the striatum (C) and nucleus accumbens (D). There were no significant differences in the CA1 (E), CA3 (F), or DG (G). *p < 0.05
Figure 3.
qRT-PCR found developmental differences between juveniles and adults in levels of zif268 mRNA in the striatum (A), and nucleus accumbens (B), but not in the hippocampus (C). *p<0.05.
4. Discussion
Our results hold several implications for further understanding the effects of cocaine on the juvenile brain.
First, we showed that juveniles have a higher basal response to novel stimuli than adults, as evidenced by a uniform clustering to the right in the juvenile groups towards the upper tail of locomotor scores for the adults. This finding is widely supported in the literature (reviewed in Spear 2000; Doremus-Fitzwater et al., 2009) where juvenile rats and mice showed greater hyperactivity and exploration in novel environments, as well as increased peer social interaction and consummatory behaviors (reviewed in Spear 2000). As motivation to seek out new experiences has been linked the propensity to use drugs of abuse (Kelly 2006), the increased novelty-seeking behavior observed in juveniles may play a major role in facilitating juvenile addiction.
We examined locomotor activity during both cocaine and saline administration over the course of four days. We found that juveniles and adults displayed similar activity levels during saline administration and in response to 10 mg/kg cocaine, but we found significantly higher locomotor activity in juveniles in response to 5 mg/kg cocaine, indicating a heightened sensitivity in juveniles to the activating effects of cocaine. Our data demonstrate that while adults establish a clear place preference for both low (5 mg/kg) and higher (10 mg/kg) doses of cocaine, juveniles only exhibit a preference for the higher dose, suggesting that the locomotor-activating effects of at low doses of cocaine are independent of the rewarding effects in juvenile rats. Such dissociation between the locomotor effects and reward has been previously demonstrated in other works (reviewed in Di Chiara, 2002). Our finding also supports previous studies (Campbell et al., 2000; Schramm-Sapyta et al., 2004) that indicate that juveniles may have an increased threshold for the rewarding effects of cocaine compared to adults. Such an increased threshold may require juveniles to take a drug more frequently or at higher doses to experience its rewarding effects, thus increasing their potential to become addicted.
Conditioned place preference provides a measure of the rewarding properties of drugs by assessing the animal’s ability to associate drug-induced effects with environmental cues, thus providing important models of neuroadaptations that accompany the addiction process. Regulation of gene expression is widely implicated in the mechanism by which drugs of abuse produce long-lasting changes in the brain (Kelley, 2004; Nestler, 2004). As such, it has been proposed that unique molecular responses to cocaine, and other drugs of abuse, could underlie juvenile vulnerability to addiction. The induction of the immediate-early gene, Zif268, is acknowledged as playing a role in the neuroadaptations required for the formation of a conditioned place preference (Valjent et al., 2006) and could therefore provide some insight into differences in cocaine sensitivity during adolescence. Surprisingly, only a few studies have compared adult and juvenile regional induction of zif268 (Caster and Kuhn, 2009) or other IEGs (Cao et al., 2007; Kosofsky et al., 1995) and these focused on the immediate effects of acute cocaine exposure. The present study is unique in that we explored zif268 induction in animals in a drug-free state during the test portion of the CPP procedure, thereby implicating potential sites of activation and potential neuroplastic changes in association with the perceived rewarding effects of cocaine in juveniles versus adults, rather than the actual drug itself. Our findings point to the prefrontal cortex as one such site, where juveniles that had established a place preference for the 10 mg/kg dose exhibited significantly higher levels of zif268 in the PFC than both adults at the same dose and juveniles at the lower dose that had not formed a place preference. This suggests a link between zif268 activation in the PFC and the formation of a rewarding drug-stimulus association in juveniles. Previous work has delineated a role for the PFC in controlling drug-paired associations via influence on the nucleus accumbens in adults (Ventura et al., 2007). Additionally, zif268 in the PFC has previously been implicated in the formation of drug-associated memories in adult animals receiving cocaine noncontingently (Thomas et al., 2003). As the PFC undergoes extensive development during maturation, and is primed to respond to strong stimuli (Brenhouse et al., 2010), it is conceivable that induction of zif268 in this region during adolescence may represent a molecular vulnerability to the rewarding effects of cocaine.
We also compared zif268 mRNA levels in the dorsal striatum and nucleus accumbens core and shell of adult and juvenile animals. We found that juveniles exhibited higher levels of zif268 in these regions, regardless of place preference formation or cocaine dose, and thus hypothesized that this difference could be an artifact of development. We therefore examined zif268 mRNA in these regions in animals under basal conditions and replicated our previous findings, confirming that there does indeed appear to be a developmental increase in zif268 in these regions during adolescence that is unrelated to cocaine exposure. These results are consistent with those of Caster and Kuhn (2009), who also found that adolescents had higher basal levels of zif268 in the striatum and cortex compared to adults (Caster and Kuhn, 2009). Whether this increase also plays a role in the vulnerability to drug addiction during adolescence is a matter for further investigation.
Finally, we found no differences in hippocampal zif268 at either dose or age following both CPP or under basal conditions. This is consistent with previous studies that found that the processing of discrete conditioned cues, such as might be present in a place preference experiment, did not involve hippocampal activation (Burns et al., 1993; LeDoux, 2000; Hall et al., 2001; Thomas et al., 2003).
Adolescence appears to be a critical window for initiating and acquiring an addiction to drugs of abuse. The manner in which this window is opened, via either heightened or reduced juvenile sensitivity to the rewarding effects of drugs, has been contested in the literature. Some studies indicate that juveniles are more sensitive to the place conditioning effects of cocaine, establishing a preference with lower doses than adults (Badanich et al., 2006; Brenhouse and Andersen, 2008; Brenhouse et al., 2008; reviewed in Schramm-Sapyta et al., 2009). The dosage used in these studies and others that claimed to find no difference in sensitivity (Schramm-Sapyta et al., 2004) may play a role in the interpretation of the results, as the low dose used was the same (10 mg/kg) or higher (20 mg/kg) than that used in our study (but see Zakharova et al., 2009). By including a lower dose, our study was able to detect differences in preference. Other discrepancies in the literature may be the result of different training and housing procedures. For example, Campbell and colleagues presented data that indicate no difference in preference for similar low and high doses of cocaine between adolescents and adults, but only gave two training sessions prior to testing instead of four (Campbell et al., 2000). Other groups found that adults established a place preference for lower doses of cocaine than juveniles and exhibited a marked increase in hyperactivity that was not seen in juveniles (Balda et al., 2006; Laviola et al., 1995; reviewed in Schramm-Sapyta et al., 2009). Additionally, the mechanisms behind such vulnerability to drug abuse remain unclear. Our study addresses both aspects of this issue by combining behavior and activation of gene expression following a high and low dose of cocaine between juveniles and adults. With this approach, we found a strong correlation between zif268 expression and juvenile response to the rewarding effects of cocaine that indicate juveniles require a higher dose of cocaine to form a conditioned place preference, but also exhibit higher activation of zif268 in the PFC. This correlation suggests a role for zif268 activation in the PFC that may result in a heightened molecular vulnerability to the rewarding effects of cocaine.
Highlights.
We examine juvenile susceptibility to cocaine addiction.
We found juveniles are more exploratory than adults.
Juveniles require higher doses of cocaine to form a preference.
This preference is correlated with zif268 levels in the prefrontal cortex.
Acknowledgments
This work was funded by two National Institute of Mental Health (NIMH) grants (R21 MH081046-0182 and R01 MH087583-01A1). F. Hollis was supported by the Florida State University Department of Biomedical Sciences Graduate program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badanich KA, Adler KJ, et al. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, et al. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51(2):341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Beck F, Godeau E, et al. [Drug consumptions by the young adolescents: 1. Epidemiological data] Med Sci (Paris) 2007;23(12):1162–1168. doi: 10.1051/medsci/200723121162. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, et al. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111(1):25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bozon B, Davis S, et al. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40(4):695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J Neurosci. 2003;18(6):1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122(2):460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Dumais K, et al. Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;169(2):628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein PM, Spear NE. Acquisition of a spatial discrimination by rats as a function of age. J Comp Physiol Psychol. 1972;78:208–212. doi: 10.1037/h0032188. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, et al. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68(4):487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, et al. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32(11):2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Caster JM, Kuhn CM. Maturation of coordinated immediate early gene expression by cocaine during adolescence. Neuroscience. 2009;160(1):13–31. doi: 10.1016/j.neuroscience.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara Di. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(1–2):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dietz D, Wang H, et al. Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res. 2007;181(2):287–291. doi: 10.1016/j.bbr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Tapocik J, et al. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86(3):347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, et al. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2009;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 2006;189(1):17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosofsky BE, Genova LM, et al. Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain. J Comp Neurol. 1995;351(1):27–40. doi: 10.1002/cne.903510104. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, et al. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275(1):345–357. [PubMed] [Google Scholar]
- Laviola G, Adriani W. Evaluation of unconditioned novelty-seeking and d amphetamine-conditioned motivation in mice. Pharmacol Biochem Behav. 1998;59:1011–1020. doi: 10.1016/s0091-3057(97)00531-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 Suppl 1:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30(5):1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, et al. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 2004;173(1–2):41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, et al. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206(1):1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, et al. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418(3):286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex Differences in Social Interaction in Rats: Role of the Immediate-Early Gene zif268. Neuropsychopharmacology. 2010;35(2):570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, et al. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17(9):1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann N Y Acad Sci. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, et al. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26(18):4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Morrone C, et al. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104(12):5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, et al. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198(1):45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziółkowska B, Kiełbiński M, et al. Regulation of the immediate-early genes arc and zif268 in a mouse operant model of cocaine seeking reinstatement. Journal of Neural Transmission. 2011;118(6):877–887. doi: 10.1007/s00702-011-0583-z. [DOI] [PubMed] [Google Scholar]