Abstract
Drug selection is widely used in transgene studies of microbial pathogens, mammalian cell and plant cell lines. Drug selection of transgenic schistosomes would be desirable to provide a means to enrich for populations of transgenic worms. We adapted murine leukemia retrovirus (MLV) vectors - widely used in human gene therapy research - to transduce schistosomes, leading to integration of transgenes into the genome of the blood fluke. A dose-response kill curve and lethal G418 (geneticin) concentrations were established: 125 to 1,000 μg/ml G418 were progressively more toxic for schistosomules of Schistosoma mansoni with toxicity increasing with antibiotic concentration and with duration of exposure. By day 6 of exposure to ≥500 μg/ml, significantly fewer worms survived compared with non-exposed controls and by day 8, significantly fewer worms survived than controls at ≥250 μg/ml G418. When schistosomules were transduced with MLV encoding the neomycin resistance (neoR) transgene and cultured in media containing G418, the neoR transgene rescued transgenic schistosomules from the antibiotic; by day 4 in 1,000 μg/ml and by day 8 in 500 μg/ml G418, significantly more transgenic worms survived the toxic effects of the antibiotic. More copies of neoR were detected per nanogram of genomic DNA from populations of transgenic schistosomes cultured in G418 than from transgenic schistosomes cultured without G418. This trend was G418 dose-dependent, demonstrating enrichment of transgenic worms from among the schistosomules exposed to virions. Furthermore, higher expression of neoR was detected in transgenic schistosomes cultured in the presence of G418 than in transgenic worms cultured without antibiotic. The availability of antibiotic selection can be expected to enhance progress with functional genomics research on the helminth parasites responsible for major neglected tropical diseases.
Keywords: Schistosome, Transgene, Antibiotic selection, Neomycin, G418, Geneticin, Retrovirus, neoR
1. Introduction
Schistosomiasis is considered the most important of the helminth diseases in terms of morbidity and mortality with more than 200 million infected people and a further 800 million at risk. Treatment and control of this neglected tropical disease relies on a single drug, praziquantel. New interventions including vaccines, drugs and diagnostics are needed as a global health priority (Hotez et al., 2008; Brindley et al., 2009). Genome sequences for Schistosoma japonicum and Schistosoma mansoni were reported recently; landmark events that ushered in the post-genomic era for schistosomiasis (Berriman et al., 2009; Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium, 2009). Analysis of target genes to underpin new interventions for schistosomiasis would be aided by functional genomics to validate the essentiality of gene functions to be targeted with novel drugs or vaccines. For Caenorhabditis elegans, methods for genetic manipulation are well advanced. In noteworthy recent progress on the technical toolkit for C. elegans, drug selection with antibiotics, neomycin and puromycin has been demonstrated utilizing resistance genes perpetuated in the transgenic worms as extrachromosomal arrays (Giordano-Santini et al., 2010; Semple et al., 2010). A molecular toolkit would also enhance our capacity to perform genetic manipulations of schistosomes and other parasitic helminths and recently there has been progress in this endeavor (Grevelding, 2006; Castelletto et al., 2009; Suttiprapa et al., 2011a). For example, murine leukemia retrovirus (MLV) vectors - widely used in human gene therapy research – have been adapted to transduce schistosomes, leading to chromosomal integration of reporter transgenes and transgene expression (Kines et al., 2008). As noted in regard to advances with C. elegans, genetic selection systems using antibiotics in combination with antibiotic resistance genes are a mainstay of molecular genetics research (Chamberlin, 2010). These flexible tools allow stringent, conditional selection of experimentally manipulated individuals. Such a system might also facilitate rapid progress with functional genomics of schistosomes.
Here we investigated drug selection of transgenic schistosomes in order to provide a means to enrich for populations of transgenic worms in virion-exposed parasites. We determined that schistosomes were sensitive in culture to the aminoglycoside antibiotic geneticin (= G418), at doses similar to those reported for other eukaryotes including mammalian cell lines and free living nematodes (Tavoloni, 1997; Yallop and Svendsen, 2001; Giordano-Santini et al., 2010). Schistosomes that were transduced by retrovirus encoding neoR, the gene encoding resistance to neomycin, were rescued in culture from G418 toxicity, and the retroviral transgene copy number was enriched in comparison to transgenic schistosomes cultured in the absence of the antibiotic.
2. Materials and methods
2.1. Schistosomes
Biomphalaria glabrata snails infected with S. mansoni were supplied by Dr. Fred Lewis, Biomedical Research Institute, Rockville, MD, USA. Schistosomules were obtained from cercariae released from the snails. Cercariae were concentrated by centrifugation (980 g/15 min) and washed in DMEM supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, 500 ng/ml amphotericin B and 10 mM HEPES (wash medium). Cercarial tails were removed by 20 passes through a 22 G emulsifying needle, after which schistosomule bodies were separated from tails by Percoll gradient centrifugation (Lazdins et al., 1982). Schistosomula were cultured at 37°C under 5% CO2 in Basch’s medium (Mann et al., 2010).
2.2. Establishing a G418/geneticin dose response curve for schistosomules
Schistosomules were transferred to 8 μm mesh polyethylene terephthalate insert membranes and holders (BD Biosciences, USA) inserted into 24-well tissue culture plates. Schistosomules, 500–1,000 per well, were cultured in 125, 250, 500 or 1,000 μg/ml G418 (Geneticin, Invitrogen, USA) or without G418 in Basch’s medium for 10 days. Media were changed every second day. In some cases, the detergent Triton X-100 was included at 0.1% or 0.5% (Semple et al., 2010).
2.3. Assessment of viability of schistosomules
Viability of schistosomules was monitored and scored visually. To accomplish this, four or more micrographs of the schistosomules were taken on days 2, 4, 6, 8 and 10 of culture of each treatment condition using a Zeiss Axio Observer A.1 inverted microscope fitted with a 10 X magnification objective lens and a digital camera (AxioCam ICc3, Zeiss, Germany). Manipulation of digital images was undertaken with the AxioVision release 4.6.3 software (Zeiss). Each of the micrographs for each G418 concentration recorded non-overlapping regions of the culture plates.
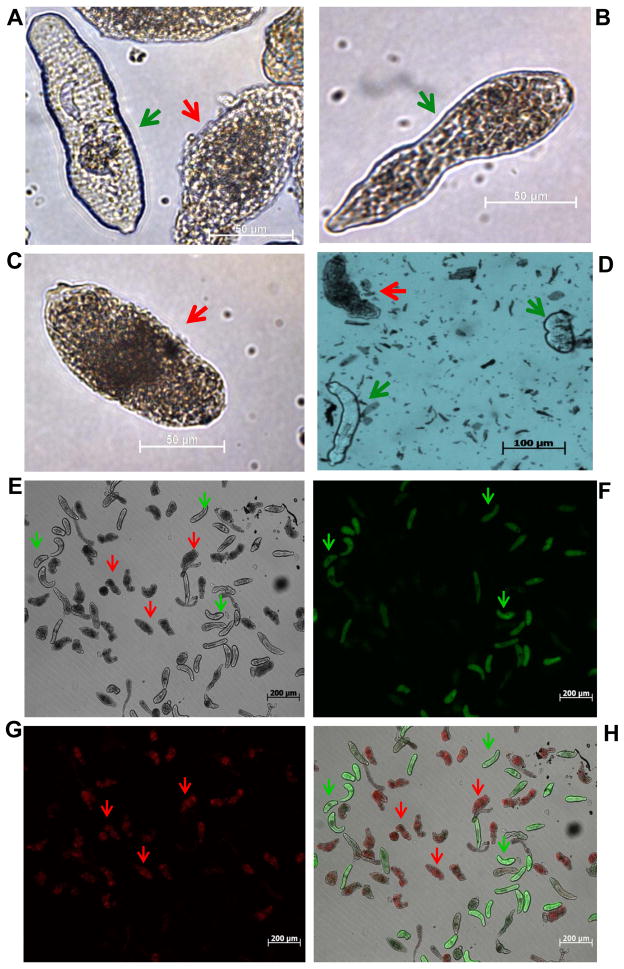
To score the effect of G418 on cultured schistosomes, 150 to 200 schistosomules for each treatment and time-point group were scored using non-overlapping micrographs. Schistosomules were scored as either live or dead, based on appearance (Clegg and Smithers, 1972; Cottrell et al., 1989). Fig. 1 presents representative images of live and dead schistosomules. Live worms were defined as light or lighter in color, usually with obvious internal organs including the nascent gut and frequently with an elongate, vermiform appearance (Fig. 1A, B). By contrast, dead schistosomules were defined as dark colored worms (brown or black), with a granular appearance and often with a more compact, rounded appearance rather than elongate (Fig. 1C) (Cottrell et al., 1989). In some of experiments with G418, aliquots of schistosomules were removed from the culture and incubated in the vital dye Trypan blue (Invitrogen) at 0.1% in PBS for 10 min at 37°C, 5% CO2, after which they were scored as dead if stained blue or live if not stained and/or stained weakly and still moving (Fig. 1C and Supplementary Fig. S1) (Cottrell et al., 1989; Gold, 1997). In a similar fashion, in some experiments aliquots of schistosomules were removed from the culture and incubated in the fluorophores fluorescein diacetate (FDA), which stains live schistosomules, and propidium iodide (PI), which stains dead schistosomules, using methods reported by Peak et al. (2010). Assessing viability by (i) visual monitoring of standard cultures, (ii) Trypan blue, or (iii) differential staining by FDA/PI of aliquots of cultured schistosomules, all gave similar results (Fig. 1, Supplementary Fig. S1). Consequently, we employed visual inspection since this procedure allowed maintenance in culture of the entire populations, which could be monitored repeatedly by capturing several micrographs (10X objective) of non-overlapping fields every 2 days.
Fig. 1.
Evaluation of vitality of schistosomules of Schistosoma mansoni cultured in vitro and/or in the presence of the aminoglycoside antibiotic G418 (= geneticin). (A–D) Schistosomules cultured in the presence of G418. Representative micrographs of live (A, green arrow; B) and dead (A, red arrow; C) schistosomules. (D) Images of representative Trypan blue stained schistosomules. (E–H) Representative schistosomules in culture for 8 days and co-stained with propidium iodide (PI, 2.0 μg/ml) and fluorescein diacetate (FDA, 0.5 μg/ml). Epi-fluorescence and bright field microscopy was used to monitor uptake of fluorophores and examine schistosomule morphology (E, bright field; F, live schistosomules detected using a 494 nm filter; G, dead schistosomula detected with a 536 nm filter; H, merge of images in E–G). Red arrows indicate representative dead schistosomules and green arrows indicate representative live schistosomules. Scale bars, 50 μm (A–C), 100 μm (D), 200 μm (E–H).
2.4. Transduction of schistosomules with pseudotyped virions
Vesicular stomatitis virus glycoprotein (VSVG)-pseudotyped murine leukemia virus (MLV) virions were produced in GP2-293 cells transfected with retroviral constructs, pLNHX_SmAct-Luc or pLNHXΔD70, both of which carry the neoR antibiotic resistance gene (Kines et al., 2008; Suttiprapa et al., 2011b). Viral titers were determined using two complementary approaches, a functional (biological) and second a quantitative real-time PCR (qRT-PCR; Retro-X™ qRT-PCR Titration Kit, Clontech, USA) (Mann et al., 2011; Rinaldi et al., 2011). Two days after transformation from cercariae, schistosomules were transduced with virions. Briefly, ~10,000 schistosomules were exposed to virions in 1 ml of medium, 8 μg/ml of polybrene (Sigma-Aldrich); two virion titers were employed, 8 × 105 colony forming units (cfu) (~108 virions estimated by qPCR and 2.4 x106 cfu (~109 virions) (Mann et al., 2011). Schistosomules exposed to polybrene but without virions were included as controls. Schistosomes were incubated with the virions for 18 h at 37°C, 5% CO2, washed, divided into groups and transferred into media containing G418 at 0, 125, 250, 500 or 1,000 μg/ml. Media including G418 were replaced every second day for 10 days. Micrographs of the schistosomes were collected on days 1, 4, 6, 8 and 10. In addition, aliquots of schistosomules were removed at days 6 and 10 and stored as wet pellets at −80°C for analysis of transgene copy number and expression.
2.5. Estimation of the transgene copy number
Genomic DNAs (gDNAs) were extracted from virion transduced and control schistosomules and the concentration determined by spectrophotometer. qPCRs were performed using TaqMan probes and primers specific for neomycin phosphotransferase II (neoR) (forward primer, 5′-GGA GAG GCT ATT CGG CTA TGA C-3′; reverse primer, 5′-CGG ACA GGT CGG TCT TGA C-3′; probe, 5′-/56-FAM/CTG CTC TGA TGC CGC CGT GTT CCG/3IABIk_FQ/-3′). Reactions were performed using 200 ng of template gDNA in 20 μl of Perfecta qPCR FastMix, UNG (Quanta Bioscience, USA) and a primer-probe set. qPCRs were performed in triplicate, with a denaturation step at 95°C of 3 min followed by 40 cycles of 30 s at 95°C and 30 s at 55°C, in a thermal cycler (iCycler, Bio-Rad, USA) fitted with a real time detector (iQ5, Bio-Rad). Absolute quantification was undertaken using a standard curve with serial dilutions of plasmid pLNHX (Kines et al., 2010), from 9.9 × 105 copies to 9.9 × 109 copies. The copy number of diluted plasmid was established through the relationship between the molecular mass of pLNHX and the Avogadro constant. Absolute copy numbers of the neoR transgene per ng of schistosome gDNA were determined by interpolation of the sample PCR signals from a standard curve (see Ginzinger, 2002). Results were plotted as the absolute neoR copy number per ng of gDNA (Kines et al., 2010).
2.6. Analysis of transgene expression
Total RNA was extracted from virion-transduced and control schistosomules using the RNAqueous-4PCR kit (Ambion). Residual DNA was removed with DNase (TurboDNase, Ambion). cDNAs were synthesized from 60 ng of total RNA using the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). neoR gene expression was investigated by endpoint reverse transcription-PCR and by qRT-PCR. End-point reverse transcription-PCR was performed using neoR gene-specific primers (F: 5′-TGT GCT CGA CGT TGT CAC TGA A-3′; R:5′-ATG AAT CCA GAA AAG CGG CCA-3′); expected amplicons size, 383 bp. Expression of the S. mansoni actin gene (GenBank Accession Number U19945) was used as an internal control using the primers, F: 5′-CAG TGT TCC CTT CCA TCG TT-3′; R: 5′-GGA CAG GGT GTT CTT CTG GA-3′, expected amplicons size, 224 bp. PCR conditions included an initial denaturation at 94°C for 30 s followed by 35 cycles of 60 s at 94°C, 60 s at 52°C, 90 s at 72°C and a final extension at 72°C for 10 min. Amplification products were separated by electrophoresis through 1% agarose, stained with ethidium bromide, visualized under UV illumination and digital images captured (Gene-Doc, Bio-Rad). qRT-PCR was performed using a TaqMan probe and primers specific for neoR (above) and for S. mansoni glyceraldehyde 3-phosphate dehydrogenase (SmGAPDH) (GenBank Accession Number M92359) as follows: forward primer: 5′-TGT GAA AGA GAT CCA GCA AAC -3′; reverse primer: 5′-GAT ATT ACC TGA GCT TTA TCA ATG G -3′; probe: 5′-/56-FAM/AAG ACT CCA GTA GAC TCA ACG ACA T/3IABIk_FQ/-3′. qPCRs were performed in triplicate using 96-well plates (Bio-Rad), with denaturation at 95°C for 3 min followed by 40 cycles of 30 s at 95°C and 30 s at 55°C. The relative quantification assay 2−ΔΔCt method was employed (Livak and Schmittgen, 2001), with results plotted as normalized fold expression of neoR gene relative to the reference gene SmGAPDH, considering 1 = neo relative expression level measured in control schistosomules not exposed to virions (calibrator sample).
2.7. Statistical analysis
Bars on curves represent 1 S.D. of the mean. Levels of statistical significance among treatments were determined using ANOVA and Student’s t-test; P-values of ≤0.05 were considered to be significant.
3. Results
3.1. Schistosomules are sensitive to geneticin (G418)
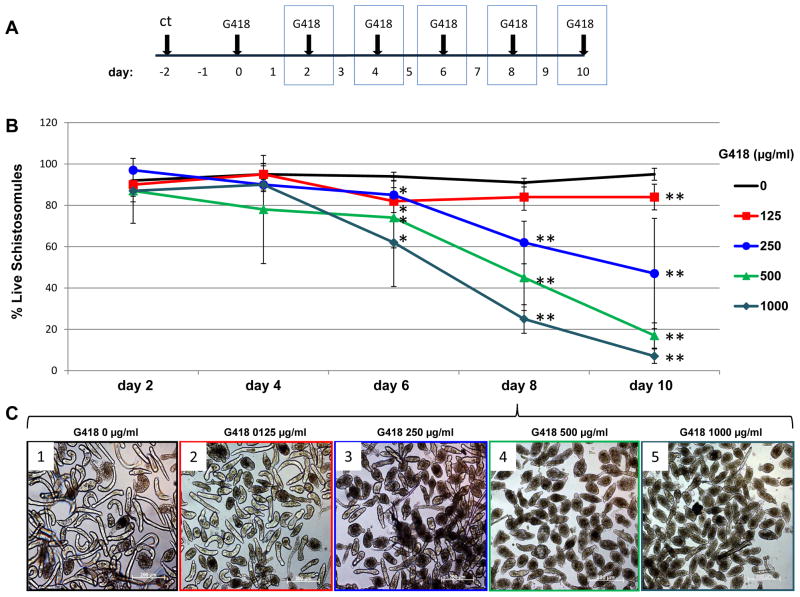
To establish the sensitivity of S. mansoni to geneticin (G418), an antibiotic that inhibits protein synthesis (Colbere-Garapin et al., 1981), schistosomules were cultured in media with or without G418 at 125, 250, 500 and 1,000 μg/ml (experimental design, Fig. 2A). Micrographs of cultures were recorded every second day from day 2 to 10 from which viability of the schistosomules was determined by scoring the appearance of the worms and percentages of live schistosomules were plotted over time. At day 2, no differences were evident among the groups. Significant differences were apparent by day 6 and onwards among schistosomules in 500 μg/ml, e.g., 75% and 45% of live parasites by days 6 and 8, respectively; 1,000 μg/ml, 63 % and 25% of live parasites by days 6 and 8, respectively, and control schistosomules cultured without G418. By days 8 and 10, all of the G418 treated groups of parasites showed significant mortality compared with the controls (Fig. 2B). Even at lower antibiotic concentrations assayed, e.g. 250 μg/ml G418, significantly fewer schistosomules survived at day 8 (61% live) and day 10 (46% live) compared with the control schistosomules cultured without G418 (>90% live). Fig. 2C and Supplementary Fig. S2 present representative images of schistosomules cultured in the absence or presence G418 at several drug concentrations. In addition to Fig. 2, replicate dose response assays were carried out; similar findings were seen with each of the three replicates (Supplementary Fig. S2 and not shown).
Fig. 2.
Dose response curve for schistosomules of Schistosoma mansoni to the aminoglycoside antibiotic, G418/geneticin. (A) Schematic showing the experimental design. Micrographs were captured on days highlighted with the blue boxes. ct: cercarial transformation to produce schistosomules. (B) Survival curve showing percentage of live schistosomules plotted against time in culture. Concentrations of G418 are indicated. Bars on curves represent 1 S.D. of the mean. ANOVA and Student’s t-test were performed among groups cultured in the presence of the indicated concentration of G418 and controls without the antibiotic. * P ≤ 0.05, ** P ≤ 0.01. (C). Representative images of schistosomules cultured in the presence of G418 for 8 days; 1: 0 μg/ml, 2: 125 μg/ml, 3: 250 μg/ml, 4: 500 μg/ml, 5: 1000 μg/ml G410. Scale bars, 200 μm.
Since detergents appear to facilitate access of the antibiotic puromycin in C. elegans, markedly improving the selection of drug resistance (Semple et al., 2010), we tested inclusion of Triton X-100 in the cultures. Schistosomules died soon (less than 1 day) after transfer to medium containing 0.1% or 0.5% Triton X-100, with or without G418 (Supplementary Fig. S2F).
3.2. Transgenic schistosomules rescued by neoR transgene from G418 antibiotic
Given that G418/geneticin was lethal for cultured schistosomules under the conditions tested here, we investigated whether VSVG-MLV transduced schistosomules might be rescued (selected for) in the presence of G418/geneticin since neomycin phosphotransferase II (encoded by neoR) endows resistance to G418 as well as to neomycin and other aminoglycosides (Smith and Baker, 2002; Vakulenko and Mobashery, 2003; Padilla and Burgos, 2010). Schistosomules were transduced with virions generated from plasmid pLNHXΔD70 (Suttiprapa et al., 2011b). Control groups of schistosomes not exposed to virions were also included in the analysis.
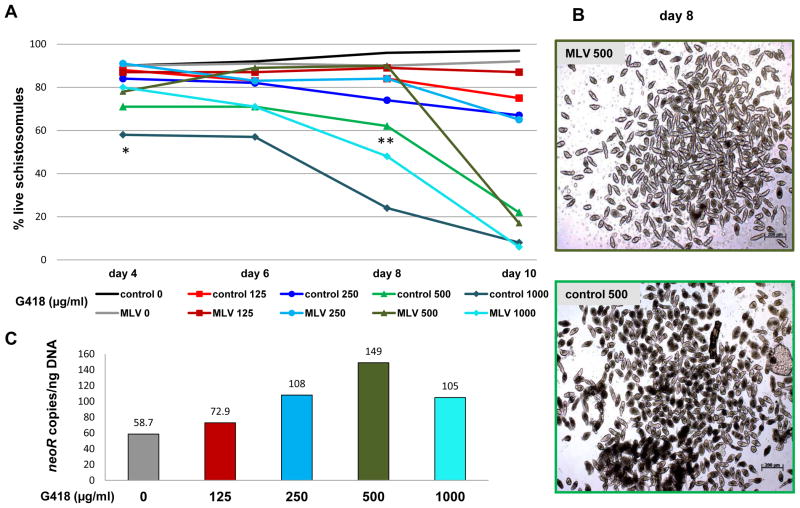
Percentages of live schistosomules within each treatment group were plotted against time in culture in G418-containing media (Fig. 3A): from day 4 or 6 onwards, differences in survival were evident among all groups (F = 38.1, F crit = 2.8, P ≤ 0.05 within each time point). Additionally, at each time point and G418 concentration, more MLV transduced parasites survived than did the cognate group (control) worms not transduced with virions. For example, by day 4 in 1,000 μg/ml G418, 80% of the VSVG-MLV transduced parasites were alive compared with 58% of the control parasites; P ≤ 0.05. By day 8 in 500 μg/ml, 90% of the MLV transduced parasites survived whereas only 62% of the control schistosomules survived (P ≤ 0.01) (Fig. 3B). The experiment was repeated three times with similar outcomes (not shown).
Fig. 3.
Survival of vesicular stomatitis virus glycoprotein (VSVG) pseudotyped murine leukemia virus (MLV) transduced schistosomules in the presence of the aminoglycoside antibiotic G418/geneticin. (A) Percentage of live, VSVG MLV-transduced schistosomules and control non-virion exposed schistosomules cultured for 10 days in increasing concentrations of G418. Concentrations of G418 are indicated with discrete colors. ANOVA and Student’s t-test were performed among retrovirus transduced and control schistosomules cultured in the indicated concentration of G418. * P ≤ 0.05, ** P ≤ 0.01; control, control schistosomules; MLV, VSVG-MLV transduced schistosomules. (B) representative images of MLV transduced schistosomules (MLV 500) and control schistosomules (control 500) cultured for 8 days in the presence of 500 μg/ml G418. Scale bar, 200 μm. (C) neomycin resistance gene (neoR) copy number per ng of genomic DNA from VSVG-MLV transduced schistosomules cultured for 6 days in increasing concentrations of G418.
3.3. Antibiotic selection enriches populations of transgenic schistosomules
Given that VSVG-MLV transduced schistosomules were rescued in the presence of G418, we investigated whether this rescue was reflected by enrichment of transgenic schistosomes in populations cultured in the antibiotic (Semple et al., 2010). To investigate this, we analyzed the transgene copy numbers in virion-transduced schistosomules cultured in increasing concentrations of G418. gDNAs were extracted from worms cultured in G418 for 6 and 10 days (from the experiment in Fig. 3A). neoR transgene copy numbers per ng of gDNA estimated by qPCR from these schistosomes are presented in Fig. 3C. By day 6, there was an increased number of neoR transgenes in chromosomes of the parasites and the copy number increased directly in relation to increasing concentration of G418. Thus, a neoR copy number of 59 copies/ng of gDNA was detected in gDNA from MLV transduced schistosomules cultured without G418, whereas at concentrations of 125, 250, 500 and 1,000 μg/ml G418, copy numbers for neoR of 73, 108, 149 and 105, respectively, were detected. A similar trend where copy number increased as G418 concentration increased was also evident in schistosomules cultured for 10 days (not shown).
3.4. Transgene expression in virion-transduced parasites reflects enrichment at the RNA level
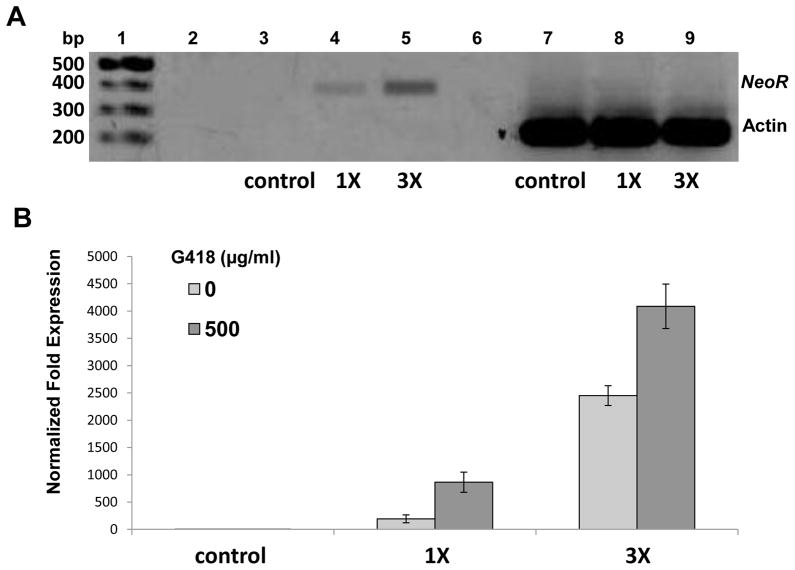
End-point reverse transcription-PCR and qRT-PCR were used to investigate transcription activity of the neoR gene in the VSVG-MLV transduced schistosomules. Parasites not transduced with MLV virions (control), and schistosomules transduced with two increasing concentrations of virions (8 × 105 cfu [=1X] and 2.4 x106 cfu [=3X] were cultured in 500 μg/ml of G418 for 10 days. Analysis by ethidium bromide-stained gels of the end-point PCR products revealed a signal at 383 bp in RNA from schistosomules transduced with the MLV virions, and indeed the signal strength increased in relation to the viral titer employed to transduce the schistosomules: worms transduced with the 3X titer showed much stronger neoR transcript signals than schistosomules transduced with 1X titer virions (Fig. 4A). Signals at 224 bp representing the control target actin transcript were present in each of the three groups, indicating the integrity of RNA preparations (Fig. 4A). No bands were detected in control groups where RNA was used as a template, indicating the absence of contaminating gDNAs in templates (not shown).
Fig. 4.
Expression of neomycin resistance transgene (neoR) in retrovirus virion transduced schistosomules. (A) End-point, reverse transcription-PCR showing reporter gene expression from schistosomules not transduced with virions (control), or 1X and 3X concentrations of vesicular stomatitis virus glycoprotein pseudotyped murine leukemia virus (VSVG-MLV) virions (8 × 105 colony forming units (cfu) [=1X] and 2.4 x106 cfu [=3X]). Molecular size standards are shown at the left, while amplicons of the expected sizes for neoR and the reference gene actin were apparent. (B) neoR expression relative to the endogenous Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase (Sm GAPDH) observed in indicated group of VSVG-MLV transduced parasites. Bars, ± 1 S.D.
Expression of neoR relative to the endogenous reference gene SmGAPDH from VSVG-MLV transduced schistosomules was investigated by qRT-PCR. In a similar fashion to the outcome of the end-point PCR, reverse transcription-PCR analysis detected higher levels of neoR transcripts in RNA from schistosomules transduced with higher compared with lower titers of virions (Fig. 4B). In addition, neoR expression was elevated in schistosomules cultured in G418 compared with the virion-transduced worms cultured without G418, a trend seen with both high and low titers of virions (Fig. 3B, dark blue versus light blue bars). This finding may reflect, at the RNA level, the enrichment of neoR transgenes and selection of transgenic schistosomes by the antibiotic. Indeed, in this experiment significantly more virion treated than non-virion schistosomes survived at day 10 (not shown).
4. Discussion
The availability of drug selection of transgenic schistosomes would be welcomed because it would provide a means to enrich for the presence of transgenic worms in transgene-exposed populations of parasites. Given that the retroviral transgene constructs employed here carried the neoR gene, we investigated the sensitivity of schistosomes to geneticin (G418), resistance to which is encoded by the neoR gene. G418 was toxic for schistosomules at each concentration tested from 125 – 1,000 μg/ml, a toxicity profile comparable to C. elegans and several other free-living nematodes and mammalian cells (Tavoloni, 1997; Yallop and Svendsen, 2001; Giordano-Santini et al., 2010). G418 is derived from Micromonospora rhodorangea; it is an aminoglycoside antibiotic similar to neomycin and kanamycin, all of which are widely used for selection of resistant cells. In addition to the bactericidal effect, the aminoglycosides affect mitochondrial ribosomes of mammalian cells, blocking polypeptide synthesis leading to protein misreading (Smith and Baker, 2002; Padilla and Burgos, 2010).
Whereas other methods have been reported for determination of drug sensitivity of cultured schistosomes, including differential uptake of fluorophores (Peak et al., 2010), movement based (Smout et al., 2010) and calorimetry based (Manneck et al., 2011) assays, direct observation and counting of up to 200 schistosomules per treatment allowed us to establish toxicity of G418 and rescue of transgenic worms. This method allows the observer to appreciate the detail and anatomy of the schistosomules. This is tractable when the numbers to be analyzed are not overwhelming, when very high throughput might not be needed and when the system has to be validated. Given that this appears to be the first report of analysis of sensitivity of schistosomes to G418, direct observation was a judicious approach. (We observed little or no differences in outcomes when scoring schistosomules as dead or live by direct observation of unstained cultures, staining with Trypan blue (Supplementary Fig. S1) and/or differential staining with PI and FDA (Fig. 1 and not shown.))
We tested whether transgene neoR could rescue schistosomules cultured in G418. There was a clear trend with each concentration of G418 examined; the retroviral transgene rescued the virion exposed worms from the antibiotic. For example, at day 4 in 1,000 μg/ml of G418, 80% of the MLV transduced parasites survived compared with 58% of the controls (P ≤ 0.05); and at day 8 in 500 μg/ml of G418, 90% of the retrovirus-transduced parasites remained alive compared with 62% of the control parasites (62%) (P ≤ 0.01). Transgene copy number and transgene expression may reflect an enrichment effect at DNA and RNA levels, respectively. In addition to the selection of antibiotic-resistant schistosomes following retroviral transduction, we examined gDNA from the worms exposed to G418 for 6 and 10 days. Increasingly higher copy numbers of transgenes were present at each drug concentration from 125 to 1,000 at both 6 and 10 days. The findings confirmed the enrichment of the neoR copy number – increasing density of the transgene was detected per ng of gDNA as the concentration of G418 increased. Enrichment of the neoR copy number is clearly anticipated because non-transgenic flukes will be progressively eliminated from the cultured population. In addition, transcription of neoR increased in virion-exposed schistosomules cultured on G418 compared with virion-exposed schistosomules cultured without the antibiotic. This likely reveals enrichment of transgenic schistosomules within the population of transduced parasites subjected to G418 pressure (Yu et al., 1996). It likely reflects enrichment at the DNA level, not a conditional response to the antibiotic (Yallop and Svendsen, 2001).
The levels of enrichment achieved with the neomycin resistance gene cassette in the static (i.e. non-replicating) populations of schistosomules were statistically significant, but they were modest (e.g. by day 8 in 500 μg/ml, 90% of the MLV transduced worms survived whereas only 62% of the control schistosomules survived) compared with the robust levels of enrichment reported with the puromycin selectable marker in replicating cultures of C. elegans (Semple et al., 2010). Given that those populations of transgenic C. elegans were established from eggs transfected with an antibiotic resistance gene, all or most of the cells of the transgenic C. elegans population would exhibit the drug resistance phenotype. By contrast, in the non-replicating cultures of schistosomules described here, individual schistosomules would likely exhibit differential numbers of transgenic cells and indeed each schistosomule would be a mosaic of transgenic and non-transgenic tissues. Accordingly, whereas the results are not directly comparable given that schistosomules unlike C. elegans do not self-replicate in culture, differences in the two models likely explain the robust enrichment of drug-resistant worms in C. elegans. Selective efficiency of the present system might be enhanced, however, by modification to the expression cassette, including incorporation of stronger promoters and/or chromosomal insulators of the transgene (Suttiprapa et al., 2011b). Furthermore, other aminoglycosides (e.g., kanamycin, gentamicin or tobramycin) may be more efficient antibiotics for selection of transgenic schistosomules.
Inclusion in culture media of 0.1% Triton X-100 strikingly improved the selection of drug-resistant C. elegans; the detergent facilitated access of the puromycin through the nematode cuticle (Semple et al., 2010). However, this detergent-based advance with C. elegans remained unavailable for schistosomes since Triton X-100 rapidly killed schistosomules. Given that the tegument of the schistosomes includes a double membrane covering a syncytial, metabolically active cytoplasm, and is of crucial importance for modulation of the host response and parasite survival (Gobert et al., 2003; Skelly and Wilson, 2006; Van Hellemond et al., 2006), it may not be surprising that exposure of schistosomules even to dilute concentrations of detergent rapidly killed the parasites.
Schistosomules rather than other stages were examined in this investigation of antibiotic resistance because this stage can be cultured in vitro for several weeks and in addition can be scored for viability using direct observation and/or differential staining with vital dyes. However, given that schistosome developmental stages are differentially sensitive to other drugs including praziquantel (Sabah et al., 1986), analysis of sensitivity of the adult and egg stages to G418 should be instructive. Indeed if eggs of S. mansoni were sensitive to aminoglycosides, experimental approaches involving transduction of eggs by MLV (Kines et al., 2010) in tandem with antibiotic selection of the maturing, transgenic eggs may enable the isolation and establishment of lines of transgenic schistosomes (Mann et al., 2011).
Antibiotic-resistance genes are commonly used as markers to monitor the introduction of exogenous genetic material into cells. Although widely used for genetic manipulation of cultured eukaryotic cells (Yallop and Svendsen, 2001), yeast and bacteria (Agaphonov et al., 2010), antibiotic selection systems have not yet been used for helminth parasites and were only recently reported with C. elegans and related free living nematodes (see Giordano-Santini and Dupuy, 2011). With C. elegans, most genetic markers for transgenesis are based on readily scored visual phenotypes. In general such markers fail to provide a selective advantage, making the enrichment and maintenance of transgenic populations time-consuming. For antibiotic selection in C. elegans, Semple et al. (2010) predicted that, as with single-celled microbes, transgene endowed puromycin resistance will have wide-ranging potential for screening applications. Selection is also possible with resistance markers for other antibiotics (Semple et al., 2010). As such, drug selection should be broadly applicable and constructive to the community of platyhelminth researchers.
Supplementary Material
Trypan blue staining of schistosomules. Representative micrographs of Trypan blue stained (A, B) or non-stained schistosomules (C, D) cultured for 10 days without the aminoglycoside antibiotic G418 (A, C) or in 500 μg/ml G418 (B, D). Green arrows indicate live and red arrows indicate dead schistosomules. Scale bars, 200 μm.
G418 dose response curve for schistosomules. (A – E) Representative micrographs of schistosomules cultured in the presence of the aminoglycoside antibiotic G418 (various concentrations) but without Triton X-100. (F) Images of schistosomules cultured in 0.5% Triton X-100 without G418. Scale bars, 200 μm.
Acknowledgments
We thank Dr. Victoria H. Mann for critical review of the manuscript and the anonymous reviewers for the insightful recommendations to improve the report. Schistosome-infected snails were supplied by Dr. Fred A. Lewis, Biomedical Research Institute, Rockville, MD, USA under National Institutes of Health (NIH), National Institute of Allergy and Infectious Disease (NIAID) contract HHSN272201000005I. These studies were supported by NIH-NIAID award R01AI072773 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH).
Footnotes
Note: Supplementary files associated with this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agaphonov M, Romanova N, Choi ES, Ter-Avanesyan M. A novel kanamycin/G418 resistance marker for direct selection of transformants in Escherichia coli and different yeast species. Yeast. 2010;27:189–195. doi: 10.1002/yea.1741. [DOI] [PubMed] [Google Scholar]
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley PJ, Mitreva M, Ghedin E, Lustigman S. Helminth genomics: The implications for human health. PLoS Negl Trop Dis. 2009;3:e538. doi: 10.1371/journal.pntd.0000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelletto ML, Massey HC, Jr, Lok JB. Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathog. 2009;5:e1000370. doi: 10.1371/journal.ppat.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin HM. C. elegans select. Nat Methods. 2010;7:693–695. doi: 10.1038/nmeth0910-693. [DOI] [PubMed] [Google Scholar]
- Clegg JA, Smithers SR. The effects of immune rhesus monkey serum on schistosomula of Schistosoma mansoni during cultivation in vitro. Int J Parasitol. 1972;2:79–98. doi: 10.1016/0020-7519(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F, Horodniceanu F, Kourilsky P, Garapin AC. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981;150:1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cottrell B, Pye C, Butterworth A. Cytotoxic effects in vitro of human monocytes and macrophages on schistosomula of Schistosoma mansoni. Parasite Immunol. 1989;11:91–104. doi: 10.1111/j.1365-3024.1989.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- Giordano-Santini R, Dupuy D. Selectable genetic markers for nematode transgenesis. Cell Mol Life Sci. 2011;68:1917–1927. doi: 10.1007/s00018-011-0670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano-Santini R, Milstein S, Svrzikapa N, Tu D, Johnsen R, Baillie D, Vidal M, Dupuy D. An antibiotic selection marker for nematode transgenesis. Nat Methods. 2010;7:721–723. doi: 10.1038/nmeth.1494. [DOI] [PubMed] [Google Scholar]
- Gobert GN, Stenzel DJ, McManus DP, Jones MK. The ultrastructural architecture of the adult Schistosoma japonicum tegument. Int J Parasitol. 2003;33:1561–1575. doi: 10.1016/s0020-7519(03)00255-8. [DOI] [PubMed] [Google Scholar]
- Gold D. Assessment of the viability of Schistosoma mansoni schistosomula by comparative uptake of various vital dyes. Parasitol Res. 1997;83:163–169. doi: 10.1007/s004360050227. [DOI] [PubMed] [Google Scholar]
- Grevelding CG. Transgenic flatworms. In: Maule AG, Marks NJ, editors. Parasitic Flatworms. Molecular Biology, Biochemisrty, Immunolgy and Physiology. CABI; Wallingford, UK: 2006. pp. 149–173. [Google Scholar]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines KJ, Morales ME, Mann VH, Gobert GN, Brindley PJ. Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus. Faseb J. 2008;22:2936–2948. doi: 10.1096/fj.08-108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines KJ, Rinaldi G, Okatcha TI, Morales ME, Mann VH, Tort JF, Brindley PJ. Electroporation facilitates introduction of reporter transgenes and virions into schistosome eggs. PLoS Negl Trop Dis. 2010;4:e593. doi: 10.1371/journal.pntd.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins JK, Stein MJ, David JR, Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982;53:39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mann VH, Morales ME, Rinaldi G, Brindley PJ. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology. 2010;137:451–462. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VH, Suttiprapa S, Rinaldi G, Brindley PJ. Establishing transgenic schistosomes. PLoS Negl Trop Dis. 2011;5:e1230. doi: 10.1371/journal.pntd.0001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneck T, Braissant O, Haggenmuller Y, Keiser J. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J Clin Microbiol. 2011;49:1217–1225. doi: 10.1128/JCM.02382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla IM, Burgos L. Aminoglycoside antibiotics: structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep. 2010;29:1203–1213. doi: 10.1007/s00299-010-0900-2. [DOI] [PubMed] [Google Scholar]
- Peak E, Chalmers IW, Hoffmann KF. Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability. PLoS Negl Trop Dis. 2010;4:e759. doi: 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi G, Suttiprapa S, Brindley PJ. Quantitative retrotransposon anchored PCR confirms transduction efficiency of transgenes in adult Schistosoma mansoni. Mol Biochem Parasitol. 2011;177:70–76. doi: 10.1016/j.molbiopara.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JI, Garcia-Verdugo R, Lehner B. Rapid selection of transgenic C. elegans using antibiotic resistance. Nat Methods. 2010;7:725–727. doi: 10.1038/nmeth.1495. [DOI] [PubMed] [Google Scholar]
- Skelly PJ, Wilson RA. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- Smith CA, Baker EN. Aminoglycoside antibiotic resistance by enzymatic deactivation. Curr Drug Targets Infect Disord. 2002;2:143–160. doi: 10.2174/1568005023342533. [DOI] [PubMed] [Google Scholar]
- Smout MJ, Kotze AC, McCarthy JS, Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiprapa S, Rinaldi G, Brindley PJ. Genetic manipulation of schistosomes - progress with integration competent vectors. Parasitology. 2011a doi: 10.1017/S003118201100134X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiprapa S, Rinaldi G, Brindley PJ. Prototypic chromatin insulator cHS4 protects retroviral transgene from silencing in Schistosoma mansoni. Transgenic Research. 2011b doi: 10.1007/s11248-011-9556-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoloni N. A simple procedure to determine the biological titer of recombinant retroviral vectors. Gene Ther. 1997;4:150–155. doi: 10.1038/sj.gt.3300370. [DOI] [PubMed] [Google Scholar]
- Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hellemond JJ, Retra K, Brouwers JF, van Balkom BW, Yazdanbakhsh M, Shoemaker CB, Tielens AG. Functions of the tegument of schistosomes: clues from the proteome and lipidome. Int J Parasitol. 2006;36:691–699. doi: 10.1016/j.ijpara.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Yallop CA, Svendsen I. The effects of G418 on the growth and metabolism of recombinant mammalian cell lines. Cytotechnology. 2001;35:101–114. doi: 10.1023/A:1017550902771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DC, Wang AL, Wang CC. Stable coexpression of a drug-resistance gene and a heterologous gene in an ancient parasitic protozoan Giardia lamblia. Mol Biochem Parasitol. 1996;83:81–91. doi: 10.1016/s0166-6851(96)02752-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trypan blue staining of schistosomules. Representative micrographs of Trypan blue stained (A, B) or non-stained schistosomules (C, D) cultured for 10 days without the aminoglycoside antibiotic G418 (A, C) or in 500 μg/ml G418 (B, D). Green arrows indicate live and red arrows indicate dead schistosomules. Scale bars, 200 μm.
G418 dose response curve for schistosomules. (A – E) Representative micrographs of schistosomules cultured in the presence of the aminoglycoside antibiotic G418 (various concentrations) but without Triton X-100. (F) Images of schistosomules cultured in 0.5% Triton X-100 without G418. Scale bars, 200 μm.