Abstract
The present fMRI study examined cortical activity to repeated vibrotactile sequences in 11 early blind and 11 sighted participants. All participants performed with >90% accuracy and showed practice induced improvement with faster reaction times in identifying matched and unmatched vibrotactile sequences. In blind only, occipital/temporal and parietal/somatosensory cortices showed practice induced reductions in positive BOLD amplitudes that possibly reflected repetition induced learning effects. The significant findings in occipital cortex of blind indicated that perceptual processing of tactile inputs in visually deprived cortex is dynamic as response amplitudes changed with practice. Thus, stimulus processing became more efficient. It was hypothesized that the changes in occipital cortex of blind reflected life-long skill in processing somatosensory inputs. Both groups showed activity reductions with practice in mid/posterior ventrolateral prefrontal cortex. These activity reductions suggested common stimulus-response learning associations for vibrotactile sequences in mid/posterior ventrolateral prefrontal cortex.
Keywords: human occipital cortex, magnetic resonance imaging, touch, blindness
1. INTRODUCTION
The primary study objective was to determine whether neural activation patterns in different cortical regions of blind and sighted participants changed with repeated trials that required distinguishing paired vibrotactile temporal sequences. We hypothesized that both groups would show reductions in response amplitudes after repeated practice trials. In occipital cortex, blind would probably show effects throughout occipital cortex based on prior reports of activation with tactile stimulation in early blind (Amedi et al., 2010; Burton et al., 2004; Burton et al., 2006; Gizewski et al., 2003; Kujala et al., 1995a; Merabet et al., 2004; Pascual-Leone and Hamilton, 2001; Ptito et al., 2005; Sadato et al., 1996; Sinclair et al., 2011; Uhl et al., 1991). In sighted, we expected effects to be limited to parts of occipital cortex activated by tactile stimulation: primary visual (Burton et al., 2004; Burton et al., 2006; Burton et al., 2010; Sinclair et al., 2011), lateral occipital (Amedi et al., 2002; Amedi et al., 2010; James et al., 2002; Pietrini et al., 2004), and middle temporal (Hagen et al., 2002). Finding the probable implicit memory effects of practice only in primary visual cortex (V1) of blind participants would bolster the idea of a role for V1 in memory processes (Amedi et al., 2003; Azulay et al., 2009; Raz et al., 2005). However, finding practice effects throughout occipital cortex of early blind would suggest a more general and dynamic involvement in somatosensory processing. The absence of comparable changes in the identified visual areas responsive to tactile inputs in sighted would bolster the idea of a special occipital cortex role for somatosensory processing in blind that possibly involved implicit learning. In somatosensory cortex, we expected blind and sighted to show similar effects of repetition practice.
In the present study sighted and blind learned vibrotactile sequences through repeated discrimination trials of paired matching or non-matching sequences that consisted of short bursts of vibration and gaps of varying durations. Participants had to remember the vibrotactile sequences across multiple trials that spanned several minutes. One possible outcome was no change in response amplitudes across the trials. Cortical areas with comparable response amplitudes per trial might indicate processing that echoed vibration attributes for vibrotactile sequence features (Wheeler et al., 2000). Another outcome might be decreased response amplitudes because resource allocations diminished with practice. Areas showing significant reductions in response amplitudes across trials possibly suggest learning (Leon-Carrion et al., 2010; Saggar et al., 2010; Salimpoor et al., 2010).
Repetition priming tasks also cause declines in response amplitudes. Correlated with response suppressions are better performance accuracy and reaction times with repetition of stimulus-response or stimulus-decision associations (Buckner and Koutstaal, 1998; Dobbins et al., 2004; Horner and Henson, 2008; Race et al., 2009; Race et al., 2010). In the present study, participants learned to associate one of two button push responses with matched or unmatched vibrotactile sequences. A secondary task prompted by instruction involved deep encoding of sequences for later recognition (Sinclair et al., 2011). However, the overt pair-comparison task involved implicit learning of sequences. Consequently, learning these stimulus-response associations might reduce activation in cognitive systems in ventrolateral prefrontal and middle temporal cortex of both groups as previously reported with repetition priming effects on learned stimulus-response associations (e.g., conceptual priming) (Boettiger and D'Esposito, 2005; Buckner et al., 1998; Buckner and Koutstaal, 1998; Cole et al., 2010; Dobbins et al., 2004; Henson, 2003; Henson and Rugg, 2003; Horner and Henson, 2008; Maccotta and Buckner, 2004; Race et al., 2009; Race et al., 2010; Raichle et al., 1994; Salimpoor et al., 2010).
The results showed that practice led to reduced response amplitudes. In occipital cortex, these effects were widespread and only occurred in blind. Somatosensory cortex also showed response reductions in the blind. Ventrolateral prefrontal cortex showed reduced responses with practice in both groups.
2. RESULTS
2.1. Task Performance
Repetition during scanning led to significantly faster reaction times (RT) in distinguishing between matched and un-matched vibrotactile sequences across the trials [Kruskal-Wallis one-way ANOVA, p <.003 in each group). RT was significantly faster by the 7th compared to the 1st trial (Post hoc paired Dunn’s Test, p <.01). However, RT declined less on subsequent trials with no significant difference between the RTs of the 7th compared to 20th trial. In early blind, RT on the 7th trial declined to a mean of 949 ms, ±117 SEM compared to a 1st trial RT average of 1529 ms, ±131 SEM. In sighted, RT declined to 903 ms, ±107 compared to 1275 ms, ±116. A two-way ANOVA revealed significantly faster RTs in sighted compared to early blind (F(1, 1161) = 16.92, p <.0001), especially during the last quartile of trials (F(19,1161) = 2.95, p <.0001).
Both groups had similar hit and false positive rates for identified matching vibrotactile sequence pairs (early blind mean hit rate = 88%, SEM = .01; mean false positive rate = 10.7%, SEM = .005; sighted mean hit rate = 89%, SEM = .06, mean false positive rate = 13.3%) resulting in similar signal detection d’ values (early blind mean d’ = 2.47 and sighted mean d’ = 2.4) that did not significantly differ (two-tailed unpaired t = 1.06, df=76, p = .29).
In the analyses that follow, statistical assessments of variations within a session considered trial data grouped into quartiles.
2.2 ROIs in Occipital Cortex
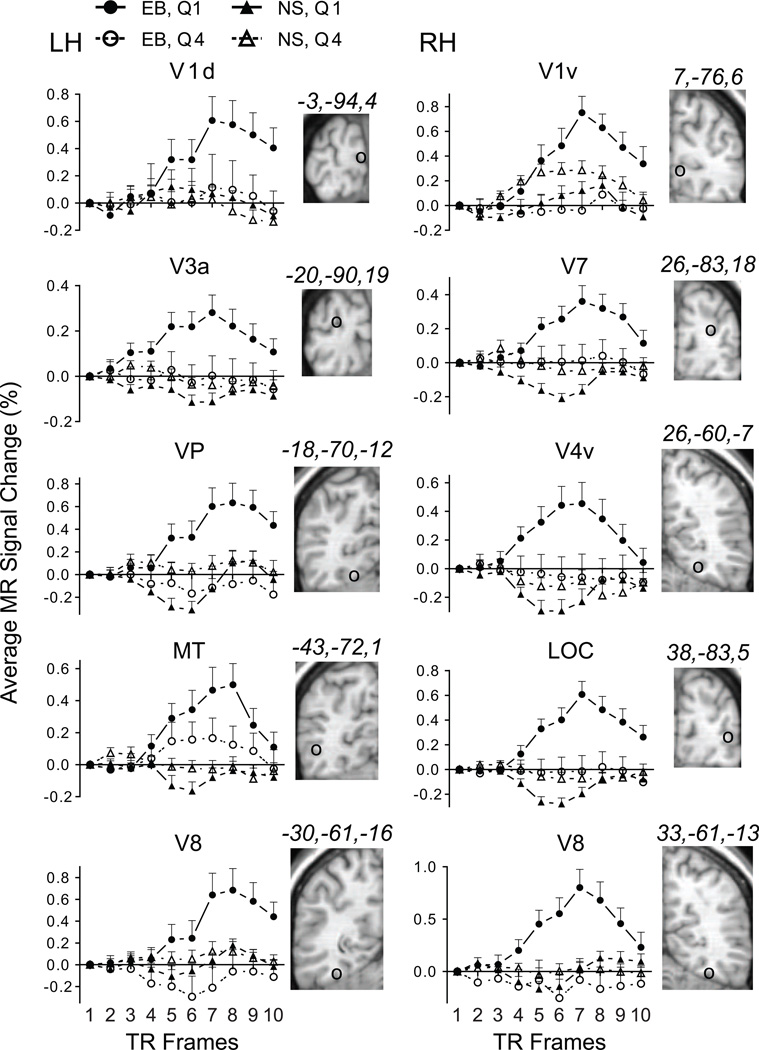
In early blind, occipital ROI showed positive BOLD responses during Quartile 1 (trials 1–5); and amplitudes were often indistinguishable from baseline activity during Quartile 4 (trials 16–20). Positive BOLD amplitudes initially rose at TR 4, reached a peak by TR 7 or 8, and declined towards baseline thereafter during Quartile 1 (Figure 2). Not shown were similar BOLD time courses with reduced amplitudes for Quartile 2 (trials 6–10) and Quartile 3 (trials 11–15). Activation occurred bilaterally in occipital cortex. Figure 2 illustrates example responses from calcarine sulcal (row 1), superior occipital (row 2), lingual (row 3), lateral occipital/posterior inferior temporal (row 4), and fusiform gyral cortex (row 5). In reference to visual areas in sighted (Grill-Spector and Malach, 2004), Figure 2 illustrates the location of affected occipital ROI in lower tier V1 (row 1), dorsal and ventral stream areas (rows 2 and 3), object and motion detection areas (row 4), and higher level visual association areas (row 5). In 11 of 15 occipital ROI, the F-ratio probabilities passed the Bonferroni correction threshold (p=.0023) for significantly greater BOLD amplitudes during Quartile 1 compared to Quartile 4 (Table 1). In the 4 ROI where differences between Quartile 1 and Quartile 4 responses did not meet the correction threshold, amplitudes were still larger during Quartile 1. These ROI generally had modest BOLD responses during Quartile 4 (e.g., MT in Figure 2), which reduced the differences between the quartiles.
Figure 2.
BOLD response time course plots in early blind and sighted for selected ROI in occipital cortex. Brain slices to the right of each plot show the ROI location marked by a circle (○) and corresponding atlas coordinates. Each time point shows the group average and SEM for quartiles 1 and 4 (separately for 11 early blind and 11 sighted participants). See also Table 1. Abb: dorsal primary visual area, V1d; ventral primary visual area, V1v; visual area 3a, V3a; visual area 7, V7; visual posterior area, VP; visual area 4 ventral, V4v; middle temporal area, MT; lateral occipital cortex, LOC, visual area 8, V8; early blind, EB; sighted, NS; Quartile 1, Q1; Quartile 4, Q4; left hemisphere, LH; right hemisphere, RH.
Table 1.
Quartile contrasts of BOLD response amplitudes per ROI in early blind and sighted.
| EB | NS | |||||
|---|---|---|---|---|---|---|
| Q1 vs. Q4 |
df 1/107 | Q1 vs.Q4 |
df 1/77 | |||
| Cortex | ROI | X,Y,Z | F value | Pr > F | F value | Pr > F |
| Occipital | V1d | −3,−93,4 | 11.4 | .001 | 2.2 | NS |
| V1v | 7,−76,6 | 32.9 | <.0001 | .7 | NS | |
| V2d | −5,−94,13 | 17.9 | <.0001 | .9 | NS | |
| V2d/V3 | 12,−90,17 | 8.3 | .005 | .6 | NS | |
| V3a | −20,−88,19 | 15.8 | .0001 | 2.5 | NS | |
| V3a | 21,−90,20 | 8 | .006 | .1 | NS | |
| V7 | 26,−83,18 | 18.6 | <.0001 | 8.3 | .005 | |
| VP | −18,−70,−12 | 23.5 | <.0001 | 22.8 | <.0001 | |
| V4v | 26,−60,−7 | 18.8 | <.0001 | 2.5 | NS | |
| V8 | −30,−61,−16 | 23.5 | <.0001 | 4.3 | NS | |
| V8 | 33,−61,−13 | 36.6 | <.0001 | .8 | NS | |
| LOC | −40,−82,−6 | 7.8 | .006 | 3.9 | NS | |
| LOC | 38,−83,5 | 32 | <.0001 | 15.9 | .0002 | |
| MT | −43,−72,1 | 6 | .01 | .7 | NS | |
| MT | 43,−69,−1 | 17.2 | <.0001 | 0 | NS | |
| Parietal | PCG, BA3 | −43,−29,50 | 3.8 | NS | .7 | NS |
| PCG, BA3 | 51,−18,44 | 8.5 | .004 | 2.2 | NS | |
| IPS, BA7 | 31,−45,53 | 7.8 | .006 | .6 | NS | |
| Frontal | midVLPFC | −49,−31,15 | 1.8 | NS | 12.8 | .0006 |
| postVLPFC | −44,11,25 | 36.3 | <.0001 | 22.8 | <.0001 | |
| Temporal | mid temporal | −52,−43,2 | 26.3 | <.0001 | .82 | NS |
| FG, BA37 | −42,−62,−25 | 24.6 | <.0001 | 0.4 | NS | |
In sighted, positive BOLD amplitudes were absent from all occipital ROI except during Quartile 1 for the right V1v (Figure 2). The Quartile 1 vs. Quartile 4 contrast, however, was not significant (Table 1). Several higher tier visual areas showed greater negative amplitude BOLD responses during Quartile 1. The time course of these negative responses involved an initial decline at TR 4, a nadir at TR 5 or 6, and a recovery towards baseline thereafter (Figure 2). Most responses during Quartile 4 in sighted were at or near baseline levels (Figure 2). In 2 occipital ROI, BOLD responses during Quartile 1 were significantly more negative compared to Quartile 4 (Table 1, left VP and right LOC).
All occipital ROI had significantly larger positive BOLD amplitudes during Quartile 1 in early blind compared to sighted (Table 2).
Table 2.
Group contrast per ROI for Quartile 1 (ANOVA results)
| Q1 | ||||||
|---|---|---|---|---|---|---|
| EB vs. NS |
df 1/92 | |||||
| Cortex | ROI | X,Y,Z | F value | Pr > F | ||
| Occipital | V1d | −3,−93,4 | 13.4 | .0004 | ||
| V1v | 7,−76,6 | 13 | .0005 | |||
| V2d | −5,−94,13 | 9.4 | .002 | |||
| V2d/V3 | 12,−90,17 | 63.6 | <.0001 | |||
| V3a | −20,−88,19 | 26.2 | <.0001 | |||
| V3a | 21,−90,20 | 29.2 | <.0001 | |||
| V7 | 26,−83,18 | 40 | <.0001 | |||
| VP | −18,−70,−12 | 33.1 | <.0001 | |||
| V4v | 26,−60,−7 | 26.1 | <.0001 | |||
| V8 | −30,−61,−16 | 11.8 | .0009 | |||
| V8 | 33,−61,−13 | 25.2 | <.0001 | |||
| LOC | −40,−82,−6 | 16.8 | <.0001 | |||
| LOC | 38,−83,5 | 60.6 | <.0001 | |||
| MT | −43,−72,1 | 16.2 | .0001 | |||
| MT | 43,−69,−1 | 31.3 | <.0001 | |||
| Parietal | PCG, BA3 | −43,−29,50 | 0 | NS | ||
| PCG, BA3 | 51,−18,44 | 5.2 | NS | |||
| IPS, BA7 | 31,−45,53 | 6.7 | NS | |||
| Frontal | midVLPFC | −49,−31,15 | .9 | NS | ||
| postVLPFC | −44,11,25 | 0 | NS | |||
| Temporal | mid temporal | −52,−43,2 | 20.7 | <.0001 | ||
| FG, BA37 | −42,−62,−25 | 4.2 | NS | |||
2.3 ROIs in Parietal Cortex
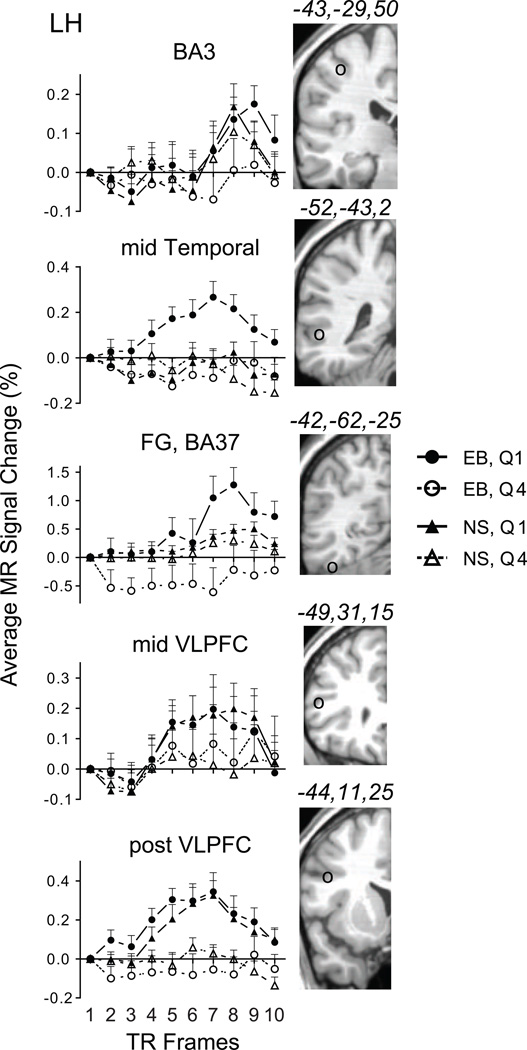
The BOLD response in primary somatosensory cortex (BA3) contralateral to the stimulated hand showed a rise in amplitude delayed to TR6 in early blind (Figure 3). The Quartile 1 response in early blind was larger compared to Quartile 4 (Figure 3), but without a significant difference across TR5 to TR9 (Table 1). The time course for BOLD responses from the right hemisphere BA3 and intraparietal sulcal BA7 ROIs resembled activation in occipital cortex in showing an initial increase in BOLD amplitude at TR4 and a peak at TR7 during Quartile 1. Note that button presses were with the left hand. BOLD amplitudes were near baseline during Quartile 4 (data not shown). The Quartile 1 vs. Quartile 4 response difference was significantly different only with a more lenient correction threshold (Table 1).
Figure 3.
BOLD response time course plots in early blind and sighted for selected ROI in parietal, temporal, and prefrontal cortex. Brain slices to the right of each plot show the ROI location marked by a circle (○) and corresponding atlas coordinates. Each time point shows the group average and SEM for quartiles 1 and 4 (separately for 11 early blind and 11 sighted participants). See also Table 1. Abb: Brodmann area 3 of primary somatosensory area, BA3; middle temporal cortex, mid Temporal; fusiform gyrus, Brodmann area 37, FG, BA37; middle ventrolateral prefrontal cortex, mid VLPFC; posterior ventrolateral prefrontal cortex, post VLPFC; early blind, EB; sighted, NS; Quartile 1, Q1; Quartile 4, Q4; left hemisphere, LH.
In sighted, the left BA3 ROI showed BOLD time courses similar to those during Quartile 1 in early blind. However, the activity during Quartile 4 completely overlapped the Quartile 1 BOLD time course (Figure 3). Bold responses in the right BA3 and BA7 ROI (not shown) resembled activation in occipital cortex of blind (Figure 2) in showing an initial increase in BOLD amplitude at TR4 and a peak at TR7. The BOLD responses during Quartile 1 and Quartile 4 in sighted for all parietal ROI overlapped resulting in no significant differences (Table 1).
No parietal ROI had different BOLD amplitudes during Quartile 1 in early blind compared to sighted (Table 2).
2.4 ROIs in Frontal and Temporal cortex
Several ROI defined from prior findings based on repetition priming tasks showed significant effects from repeated practice trials. There were no responses activated in the anterior ventrolateral prefrontal cortex (VLPFC) ROI in early blind or sighted. In early blind, ROIs with larger amplitude responses during Quartile 1 compared to Quartile 4 were within the superior temporal sulcus in the mid temporal cortex, fusiform gyrus, mid VLPFC, and posterior VLPFC (Figure 3). The positive BOLD amplitudes during Quartile 1 in all but the fusiform gyrus arose at TR4 and had peaks at TR7. The FG ROI showed a BOLD response with a delayed peak. This time course resembled the pattern found in left BA3, contralateral to the stimulated hand. Except for the BOLD responses from the mid VLPFC ROI, the Quartile 1 vs. Quartile 4 amplitude differences in these ROI were significantly different in early blind (Table 1). Greater response variance in the mid VLPFC ROI accounted for lack of significance (Figure 3).
In sighted, significantly different BOLD amplitude for Quartile 1 vs. Quartile 4 occurred in mid and post VLPFC, but not mid temporal or fusiform gyrus ROI (Figure 3, Table 1).
Only the mid temporal ROI had significantly larger BOLD amplitudes during Quartile 1 in early blind compared to sighted (Table 2). The remaining frontal and temporal ROI showed no BOLD amplitude differences between early blind and sighted (Table 2).
3. DISCUSSION
3.1 Performance changes with practice
Accuracies in identifying matched and unmatched vibrotactile sequences in participants from both groups exceeded 90%. Often early blind and sighted participants correctly detected the tactile vibration features that distinguished between vibrotactile sequence pairs during the first practice trials. Despite nearly ceiling performance throughout the practice trials, participants showed evidence of learning with faster reaction times for successive trials. Reaction times changed significantly more during the first quartile of trials and more gradually thereafter. Speeded reaction times suggested more efficient feature monitoring possibly from improved perceptual processing through practice. Monitoring efficiency might have risen because memory for the vibrotactile sequences formed with repetition trials. Consequently, resource allocation to perceptual processing possibly decreased during subsequent trials when assessing detected features against stored information.
The cortical regions where responses changed with practice repetition trials differed between the groups. Prominent were significant reductions in positive BOLD response amplitudes to repeated vibrotactile sequence trials in occipital cortex of early blind compared to sighted participants. In contrast, both groups had relatively similar responses in somatosensory cortex that normally is modality selective for tactile inputs. Cortical regions previously defined using repetition priming tasks in sighted showed more varied response reduction effects in the two groups. The following considers the repetition reduction effects in different cortical regions.
3.2 Response Reduction in Occipital Cortex
In early blind, the cortical regions with repetition practice related response reductions included bilateral lower and higher tier visual areas. The widespread distribution of these effects suggested perceptual processing of vibrotactile sequences throughout occipital cortex, supporting prior findings that occipital cortex plays a role in perceiving tactile inputs in early blind (Amedi et al., 2010; Cohen et al., 1997; Cohen et al., 1999; Hamilton et al., 2000; Kupers et al., 2006; Kupers et al., 2007; Maeda and Yasuda, 2003; Ptito et al., 2008). The current findings especially indicated that perceptual processing of tactile inputs was dynamic as response amplitudes changed with implicit learning through practice. These data also bolstered the idea that activity in occipital cortex directly reflected learning the vibrotactile sequences and contributed to improve perceptual processing of tactile stimulation features. In early blind, implicit learning effects in occipital cortex might indicate reorganization to process somatosensory inputs due to developmentally early visual deafferentation. However, similar effects of practice might occur with auditory stimuli given extensive evidence that auditory stimulation is similarly effective in activating occipital cortex of blind (Arno et al., 2001; Gougoux et al., 2004; Gougoux et al., 2005; Kujala et al., 1995b; Leclerc et al., 2000; Röder et al., 1996; Röder et al., 2001; Weeks et al., 2000).
An implication from the practice induced response reductions in occipital cortex of early blind participants is that this change arose from sharpened neural representations and fewer neural resources when processing learned vibrotactile sequences. This effect in early blind compared to sighted participants possibly arose because the blind have different learning strategies that involve greater utilization of sensory information (Hötting and Röder, 2004; Pring, 1988; Röder and Rösler, 2004).
In the present study, identification of a single attribute possibly sufficed in distinguishing paired vibrotactile sequences (e.g., increasing duration stimulation intervals in one vibrotactile sequence). Thus, an implicit memory for a vibration attribute as opposed to the specific stimulus properties might extensively activate occipital cortex in the early blind. A future experiment in the blind that engaged tactile properties such as the orientation or contour of different size objects may reveal perceptual priming effects only in higher tier visual areas as previously observed with viewed objects in sighted (Buckner et al., 1998; Buckner and Koutstaal, 1998; Henson and Rugg, 2003; Horner and Henson, 2008; Maccotta and Buckner, 2004; Saggar et al., 2010; Salimpoor et al., 2010).
Practice repetition effects in occipital cortex did not significantly correlate with faster reaction times. However, the matching/non-matching task may have been insufficiently challenging because performance accuracy was near ceiling even during the first quartile of trials. Thus, we did not observe functional/behavioral correlations previously reported with repetition priming tasks (Buckner et al., 2000). The current negative findings were not unique. A prior study in sighted using visual stimuli also noted the absence of a correlation between behavioral reaction time improvements and the neural effects of stimulus repetition (Race et al., 2009). Race and colleagues suggested such negative findings might reflect the multi-process nature of repetition effects, which make it difficult to observe “one-to-one mappings” between neural effects and behavioral changes.
Sighted showed no significant response reduction to repeated vibrotactile sequences in occipital cortex despite some evidence of positive BOLD responses in calcarine sulcal cortex. However, negative BOLD responses during Quartile 1 in the ROI located in right LOC and left VP showed significant reductions in negative amplitudes. Prior studies also reported negative BOLD responses to tactile stimulation in higher tier visual areas of sighted (Burton et al., 2004; Burton et al., 2006; Burton et al., 2010). As in these studies, negative BOLD responses potentially indicated processing a modality (e.g., tactile) normally not selectively engaged within a target cortex (e.g., visual) (Gusnard and Raichle, 2001; Haxby et al., 1994).
3.3 Response Reduction in Somatosensory Cortex
Neither group showed significant response reduction to repeated vibrotactile sequence trials in primary somatosensory cortex (S1) contralateral to the stimulated finger. However, response amplitudes during the first quartile were larger in early blind and overlapped for Quartiles 1 and 4 in all somatosensory regions of sighted. Thus, sighted especially, showed no practice effects in somatosensory cortex.
Response time courses, contralateral to the stimulated finger in left S1 in both groups, were delayed with an initial amplitude increase at >12sec compared to ~8sec in nearly all other ROI. Long delays were not previously observed with single presentations of a vibrotactile stimulus (Burton et al., 2004; Burton et al., 2008b; Burton et al., 2010; Sinclair et al., 2011). The current study required assessment of two vibrotactile sequences in a trial. Because S1 closely recapitulates the input parameters of 50 Hz vibrations (Mountcastle et al., 1969; Mountcastle et al., 1990a; Mountcastle et al., 1990b) used in creating the vibrotactile sequences, faithful processing of both vibrotactile sequences within S1 might have affected response timing.
3.4 Response Reduction in Pre-defined Repetition Priming Regions
In the present study, participants learned specified key-pad finger presses to respective matched or unmatched vibrotactile sequence pairs. Thus, a stimulus-response association involved learning paired vibrotactile sequences and the required behavioral responses for matched and unmatched pairs. In sighted, perceptual priming tasks evoked repetition suppression for stimulus-response associations in mid temporal and fusiform gyral cortex (Horner and Henson, 2008; Race et al., 2009; Schacter et al., 2007). These occipital/temporal cortex regions normally process abstract, size-invariant features of visual objects in sighted (Henson and Rugg, 2003). Similar response reductions probably did not occur in these regions in sighted because detected vibrotactile sequence features were relatively simple sensory inputs. ROI in these same cortices showed significant response reduction effects only in early blind. These results were like that generally in occipital cortex possibly because these ROI were in multimodal cortices. The fusiform gyral ROI, for example, was hypothetically part of a ventral visual pathway in blind (Büchel et al., 1998) that responded to tactile stimulation. In sighted, Beauchamp and colleagues reported that visual, tactile and auditory stimuli evoked activity in mid temporal cortex within the superior temporal sulcus (Beauchamp et al., 2007; Beauchamp et al., 2008). In blind, we previously reported that this multi-modal cortex responded when embossed tactile surfaces moved across a fingertip (Burton et al., 2006). Consequently, this mid temporal ROI potentially also showed practice repetition effects comparable to the multi-modal responsive occipital cortex in early blind. Hence, in early blind, repetition practice effects for learned vibrotactile sequences were similar in several multi-modal cortices: occipital, posterior fusiform gyral, and mid temporal.
Both groups showed significant response reductions from Quartile 1 to 4 in posterior ventrolateral prefrontal and sighted showed significant effects in middle ventrolateral prefrontal cortex. Prior repetition priming studies reported response suppression in these same regions for stimulus-response association and stimulus-decision learning (Horner and Henson, 2008; Race et al., 2009; Schacter et al., 2007). Thus, the observed repetition practice effects in mid/ posterior ventrolateral prefrontal ROI occurred in the same locations where conceptual priming tasks with visual stimulation provoked repetition suppression in sighted. A probable commonality was that the current practice task and prior findings with repetition priming involved learning repeated stimulus-response associations. In the current study, the response changes in these regions possibly reflected practice effects in making stimulus-response associations with whether the vibrotactile sequence was matched or unmatched. However, there was no need to decide on a different stimulus response association to the same matched or unmatched stimulus features. Consequently, neither group showed activation to repeated vibrotactile sequence trials in anterior ventrolateral prefrontal cortex where the effects of stimulus-decision associations predominate (Race et al., 2009; Race et al., 2010).
3.5 Summary
Early blind showed response reduction in occipital/temporal cortex normally activated by visual stimuli in sighted with repeated practice in identifying vibrotactile sequences. Trial repetition effects on reducing activation occurred in all normally visuotopic areas and regions in the middle temporal and fusiform gyrus that process features of visual objects in sighted. These cortical regions showed no response reduction effects in sighted. The larger amplitude Quartile 1 responses might reflect processing “new” vibrotactile patterns because these sequences do not pop out like words or pictures. This supports the notion that occipital areas in blind process tactile inputs, perhaps aiding memory consolidation, retrieval, or discrimination of these vibotactile temporal patterns.
Reduced responses with repeated practice trials in early blind that occurred in the mid/posterior ventrolateral prefrontal regions possibly reflected a common repetition effect based on stimulus-response associations in these regions. The similarity of current results in these regions with those based on repetition priming of stimulus-decision learning in sighted (Dobbins et al., 2004; Horner and Henson, 2008; Race et al., 2009; Schacter et al., 2007) possibly reflected a common repetition effect based on stimulus-response associations.
4. METHODS
4.1. Participants
Eleven early blind (6 female; mean age = 38.6. SE 4.4 years, min 22, max 59) and 11 sighted participants (6 female; mean age = 34.2, SE 3.5 years, min 22, max 57) provided informed consent in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and guidelines approved by the Human Studies Committee of Washington University. In early blind, blindness resulted from binocular peripheral pathologies at birth that included retinopathy of prematurity (5), retrolental fibroplasia (3), Leber’s congenital amaurosis (2), and retinoblastoma (1). Four early blind retained negligible light sensitivity but had no pattern perception.
4.2. Vibrotactile Sequences
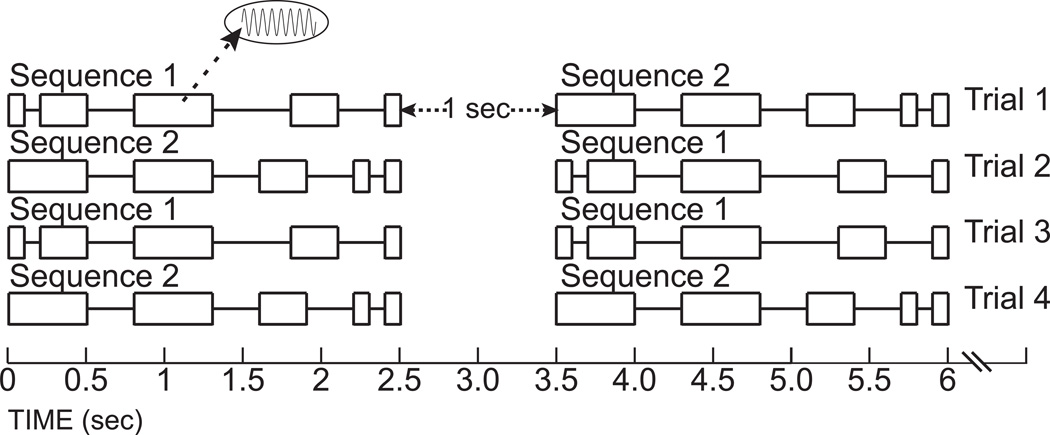
We applied vibrotactile stimulation to the right index finger tip using a previously described MR compatible vibrator (Burton et al., 2004). Each vibrotactile temporal sequence consisted of five intervals of sinusoidal 50Hz vibrations at 40µm peak-to-trough amplitude that were interspersed by four intervals with no stimulation (gaps). Each stimulation-gap sequence was unique by combining discreet durations of stimulation and intervening gaps (100, 300, or 500 ms). For example, the vibrotactile temporal sequence 1 in Figure 1 had intervals of increasing then decreasing stimulation and gaps (stimulation from 100 to 500 and then a decrease to 100ms; gaps were from 100 to 500 and then back to 300ms). Sequence 2 in Figure 1 had successively decreased stimulation and gap durations from 500 to 100ms. Each sequence was 2500ms duration. Synchronization of sequence presentation was to the beginning of a 2000ms imaging frame (see below).
Figure 1.
Vibrotactile sequences relative to imaging frames. Boxed areas show intervals of 40µm 50Hz vibrotactile stimulation. Gaps between boxes indicate intervals without stimulation. Two different sequences provided two unmatched (Trials 1 and 2) and two matched (Trials 3 and 4) sequence pairs.
During each imaging run, participants learned to identify two different sequences by performing a same or different discrimination during each of twenty trials. Each sequence followed itself (same) on one-fourth of trials. Both sequences were presented in the remaining 10 trials (different) with half starting with each of the two sequences (Figure 1). Thus, each combinatorial sequence pair repeated five times in a run. One second separated the presentation of the two sequences within a trial for a total 6 seconds per trial. Participants pressed one of two optical response keys with left hand fingers to indicate discrimination choices. In addition to the same-different task, participants had to memorize the vibrotactile temporal sequences for identification in an immediately following recognition test, the results of which appear elsewhere (Sinclair et al., 2011). Participants received task training and practice trials prior to entering the scanner and again while in the scanner.
4.2. Image acquisition and processing
We acquired whole brain images with a Siemens 3 Tesla TRIO scanner (Erlangen, Germany) and a twelve-element RF head matrix coil. MRI headphones and ear plugs dampened scanner noise and a vacuum cushion stabilized the head. All participants had their eyes covered by a blindfold.
A gradient recalled echo-planar sequence (EPI: repetition time [TR]=2000ms, echo time [TE]=27ms, flip angle=90°) imaged blood oxygenation level-dependent (BOLD) contrast responses across 33 contiguous odd-even interleaved 4 mm axial slices. In-plane resolution was 4×4 mm. All slices aligned to the anterior-posterior commissural plane. Preprocessing of the EPI images from each participant compensated for head movements and corrected slice-dependent time shifts. Signal intensity differences that arose from interleaved slice acquisition were normalized to a global mean mode of 1000 across EPI runs (Burton et al., 2010).
Structural images included a T1-weighted structural magnetization prepared rapid gradient echo (MP-RAGE) sequence acquired in each participant across 176 sagittal slices (TR=2100ms; TE=3.93ms; flip angle=7°; inversion time [TI]=1000ms; 1 × 1 × 1.25 mm voxels). An additional T2-weighted structural image across 33 axial slices (TR=8430ms, TE=98ms, 1.33 × 1.33 × 3 mm voxels) aided registration of the EPI to the sagittal MP-RAGE images after computing 12 parameter affine transforms between an average from the first frames of each EPI run (Ojemann et al., 1997).
Prior to any statistical analysis, we realigned EPI slices and resampled them to 2mm cubic voxels. Slice registration was to a spatially normalized (Lancaster et al., 1995) Talairach atlas template (Talairach and Tournoux, 1988). The atlas template was an average of MP-RAGEs using structural images obtained from age and gender matched sighted and early blind participants similar to the study sample.
4.3. Statistical analyses
Each run of 156 EPI volume acquisitions contained 146 sequential TRs for 20 trials with event durations of 6 to 11 TRs. The event duration distribution followed a negative exponential with short durations more frequent (e.g., event durations of 6, 7, 8, 9, 10, and 11 TRs, respectively, repeated 8, 5, 3, 2, 1, and 1 times). Different durations were pseudo-randomly spliced with no duration successively repeated >2 times. The overlap of event durations was entered into the design matrix with an assumed average duration of 10 TRs (20s) that spanned overlap of jittered intervals (Miezin et al., 2000; Ollinger et al., 2001). Initial dummy frames built into the EPI sequence software and one excluded initial frame allowed for magnetization equalization. The next four frames and five after the last trial furnished images of baseline activity with no stimulation.
The atlas realigned, 2mm3 volumes were spatially smoothed with a 2-voxel Gaussian kernel (4mm FWHM) prior to using a General Linear Model to estimate MR signal per voxel for each event. The GLM included regressors for time points per quartile partition, baseline activity, linear drift, and a high-pass filter (0.014Hz). Signal magnitude at each time point was an average across 5 trials per quartile (e.g., trials 1–5, 6–10, 11–15, and 16–20, respectively, for Quartiles 1, 2, 3, and 4). Separate GLM analyses evaluated data from each run.
The analysis evaluated time courses of signal magnitudes extracted and averaged across all voxels within 4mm radii spheres centered on coordinates defined in prior studies in blind and sighted participants (Table 1). Extracted signals were percent MR signal change per voxel relative to baseline. The predefined ROI in occipital cortex included areas previously activated in blind participants with a variety of somatosensory tasks (Amedi et al., 2010; Bonino et al., 2008; Burton et al., 2002; Burton et al., 2004; Burton et al., 2006; Burton et al., 2010; Hötting and Röder, 2009; Melzer et al., 2001; Merabet et al., 2007; Sadato et al., 1996; Sadato et al., 1998; Sadato et al., 2004; Sathian, 2005; Sathian and Stilla, 2010; Sinclair et al., 2011). These occipital cortex ROI in sighted were within lower and upper tier retinotopic visual areas (Grill-Spector and Malach, 2004) from the calcarine sulcus to superior occipital dorsally, lateral occipital anteriorly, and fusiform gyrus ventrally. The somatosensory regions also were previously identified using tactile stimulation in blind and sighted (Bodegård et al., 2001; Braun et al., 2000; Burton, 2001; Burton et al., 2004; Burton et al., 2008a; Burton et al., 2008b; Kurth et al., 1998; Kurth et al., 2000; Maldjian et al., 1999a; Maldjian et al., 1999b). The ROI in ventrolateral prefrontal and inferior temporal cortex were sites activated using repetition priming tasks in sighted (Buckner et al., 2000; Dobbins et al., 2004; Race et al., 2009). The a priori defined ROI allowed study of practice effects in areas previously found responsive to somatosensory stimulation in blind or repetition priming effects to visual stimulation in sighted. The between and within group statistical assessments of practice effects in defined ROI occurred in cortical areas not already known to show practice effects with somatosensory stimuli using a whole-brain analysis. Analyzing data extracted from regions based on a whole-brain analysis of significant differences by quartiles between or within group has two drawbacks. It suffers from doing statistics on cortex already shown with these response differences (e.g., statistics upon statistics). In addition, regions of interest might be missed using stringent multiple comparison corrections based on whole brain voxel counts.
A MATLAB (version 7.5) script tabulated time courses per ROI for each participant that consisted of 10 time points (TP) with signal magnitudes at each time point based on averages across 5 trials within a quartile per run; TP1 values were subtracted from each subsequent TP to anchor responses to zero average signal magnitudes. Plots of extracted time courses per ROI (GraphPad Software, San Diego, CA) and participant showed response averages and variance (SEM) per quartile in each group at each time point across runs. Statistical assessment utilized repeated measures ANOVAs (PROC GLM, Statistical Analysis System version 9.1.3, SAS Institute, Carey, NC) per selected ROI. The dependent variable was signal magnitudes from TP 5–9 per participant. The repeats were three BOLD runs. An ANOVA by region for each group assessed the within-group factor of responses during two quartiles (Quartile 1 vs. Quartile 4). Another ANOVA by region examined the between-group factor of responses during Q1. All cited probabilities for F-ratios in the ANOVAs for within group quartile and between group effects were Bonferroni corrected for 22 selected ROI (threshold p=.0023).
Research Highlights.
An fMRI study in early blind and sighted to encoding vibrotactile sequences
Repeated practice trials enhanced learning to distinguish matched and unmatched sequences
Occipital cortex regions showed repetition induced response reductions only in early blind
Somatosensory cortex showed no significant repetition reduction effects in either group
Response suppression occurred in prefrontal cortex affected by stimulus-response associations in repetition priming tasks
ACKNOWLEDGEMENTS
Contract grant sponsor: NIH; Contract grant number: NS37237; Contract grant sponsor:
Mr. S. Dixit assisted with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
REFERENCES
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Azulay H, Malach R, Zohary E. Cortical activity during tactile exploration of objects in blind and sighted humans. Restor Neurol Neuros. 2010;28:143–156. doi: 10.3233/RNN-2010-0503. [DOI] [PubMed] [Google Scholar]
- Arno P, De Volder AG, Vanlierde A, Wanet-Defalque MC, Streel E, Robert A, Sanabria-Bohorquez S, Veraart C. Occipital activation by pattern recognition in the early blind using auditory substitution for vision. Neuroimage. 2001;13:632–645. doi: 10.1006/nimg.2000.0731. [DOI] [PubMed] [Google Scholar]
- Azulay H, Striem E, Amedi A. Negative BOLD in sensory cortices during verbal memory: a component in generating internal representations? Brain Topogr. 2009;21:221–231. doi: 10.1007/s10548-009-0089-2. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Kishan N, Ro T. Human MST but not MT responds to tactile stimulation. J Neurosci. 2007;27:8261–8267. doi: 10.1523/JNEUROSCI.0754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. NeuroImage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegård A, Geyer S, Grefkes C, Zilles K, Roland PE. Hierarchical processing of tactile shape in the human brain. Neuron. 2001;31:317–328. doi: 10.1016/s0896-6273(01)00362-2. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, D'Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino D, Ricciardi E, Sani L, Gentili C, Vanello N, Guazzelli M, Vecchi T, Pietrini P. Tactile spatial working memory activates the dorsal extrastriate cortical pathway in congenitally blind individuals. Arch Ital Biol. 2008;146:133–146. [PubMed] [Google Scholar]
- Braun C, Schweizer R, Elbert T, Birbaumer N, Taub E. Differential activation in somatosensory cortex for different discrimination tasks. J Neurosci. 2000;20:446–450. doi: 10.1523/JNEUROSCI.20-01-00446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci (USA) 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in a modal components of priming. Brain. 2000;123 Pt 3:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Burton H. Brain imaging studies of human somatosensory cortical regions: results from brain imnaging studies in humans. In: Nelson RJ, editor. The Somatosensory System: Deciphering the Brain's Own Body Image. Vol. Boca Raton: CRC Press; 2001. pp. 27–72. [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002;87:589–611. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair R, McLaren D. Cortical activity to vibrotactile stimulation: a fMRI study in blind and sighted individuals. Hum Brain Mapp. 2004;23:210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, McLaren DG, Sinclair RJ. Reading embossed capital letters: a fMRI study in blind and sighted individuals. Hum Brain Mapp. 2006;27:325–339. doi: 10.1002/hbm.20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair R, Wingert J, Dierker D. Multiple parietal operculum subdivisions in humans: Tactile activation maps. Somatosen & Mot Res. 2008a;25:149–162. doi: 10.1080/08990220802249275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical network for vibrotactile attention: A fMRI study. Hum Brain Mapp. 2008b;2:207–221. doi: 10.1002/hbm.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, Dixit S. Working memory for vibrotactile frequencies: comparison of cortical activity in blind and sighted individuals. Hum Brain Mapp. 2010;31:1686–1701. doi: 10.1002/hbm.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Faiz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann. Neurol. 1999;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bagic A, Kass R, Schneider W. Prefrontal dynamics underlying rapid instructed task learning reverse with practice. J. Neurosci. Meth. 2010;30:14245–14254. doi: 10.1523/JNEUROSCI.1662-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Gasser T, de Greiff A, Boehm A, Forsting M. Cross-modal plasticity for sensory and motor activation patterns in blind subjects. Neuroimage. 2003;19:968–975. doi: 10.1016/s1053-8119(03)00114-9. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Lepore F, Lassonde M, Voss P, Zatorre RJ, Belin P. Pitch discrimination in the early blind. Nature. 2004;430:309. doi: 10.1038/430309a. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3:324–333. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Ann. Rev. Neurosci. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, McGlone F, Essick G, Dancer C, Pardo JV. Tactile motion activates the human middle temporal/ V5 (MT/ V5) complex. Eur J Neurosci. 2002;16:957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Hamilton R, Keenan JP, Catala M, Pascual-Leone A. Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuroreport. 2000;11:237–240. doi: 10.1097/00001756-200002070-00003. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hötting K, Röder B. Hearing cheats touch, but less in congenitally blind than in sighted individuals. Psychol Sci. 2004;15:60–64. doi: 10.1111/j.0963-7214.2004.01501010.x. [DOI] [PubMed] [Google Scholar]
- Hötting K, Röder B. Auditory and auditory-tactile processing in congenitally blind humans. Hear Res. 2009;258:165–174. doi: 10.1016/j.heares.2009.07.012. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 2002;40:1706–1714. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Kekoni J, Hamalainen H, Reinikainen K, Salonen O, Standertskjold-Nordenstam CG, Naatanen R. Auditory and somatosensory event-related brain potentials in early blind humans. Exp Brain Res. 1995a;104:519–526. doi: 10.1007/BF00231986. [DOI] [PubMed] [Google Scholar]
- Kujala T, Huotilainen M, Sinkkonen J, Ahonen A, Alho K, Hamalainen M, Ilmoniemi R, Kajola M, Knuutila J, Lavikainen J, Salonen O, Simola J, Standerskjold-Nordenstam C-G, Tiitinen H, Tissari S, Naatanen R. Visual cortex activation in blind humans during sound discrimination. Neurosci Letts. 1995b;183:143–146. doi: 10.1016/0304-3940(94)11135-6. [DOI] [PubMed] [Google Scholar]
- Kupers R, Fumal A, de Noordhout AM, Gjedde A, Schoenen J, Ptito M. Transcranial magnetic stimulation of the visual cortex induces somatotopically organized qualia in blind subjects. Proc Natl Acad Sci (USA) 2006;103:13256–13260. doi: 10.1073/pnas.0602925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Pappens M, de Noordhout AM, Schoenen J, Ptito M, Fumal A. rTMS of the occipital cortex abolishes Braille reading and repetition priming in blind subjects. Neurology. 2007;68:691–693. doi: 10.1212/01.wnl.0000255958.60530.11. [DOI] [PubMed] [Google Scholar]
- Kurth R, Villringer K, Mackert BM, Schwiemann J, Braun J, Curio G, Villringer A, Wolf KJ. fMRI assessment of somatotopy in human Brodmann area 3b by electrical finger stimulation. Neuroreport. 1998;9:207–212. doi: 10.1097/00001756-199801260-00006. [DOI] [PubMed] [Google Scholar]
- Kurth R, Villringer K, Curio G, Wolf KJ, Krause T, Repenthin J, Schwiemann J, Deuchert M, Villringer A. fMRI shows multiple somatotopic digit representations in human primary somatosensory cortex. Neuroreport. 2000;11:1487–1491. [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp. 1995;3:209–223. [Google Scholar]
- Leclerc C, Saint-Amour D, Lavoie ME, Lassonde M, Lepore F. Brain functional reorganization in early blind humans revealed by auditory event - related potentials. Neuroreport. 2000;11:545–550. doi: 10.1097/00001756-200002280-00024. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J, Izzetoglu M, Izzetoglu K, Martin-Rodriguez JF, Damas-Lopez J, Martin JMBy, Dominguez-Morales MR. Efficient learning produces spontaneous neural repetition suppression in prefrontal cortex. Behav Brain Res. 2010;208:502–508. doi: 10.1016/j.bbr.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Maeda K, Yasuda H. Braille alexia during visual hallucination in a blind man with selective calcarine atrophy. Psychiat Clin Neuros. 2003;57:227–229. doi: 10.1046/j.1440-1819.2003.01105.x. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Detre JA, Alsop DC. The sensory somatotopic map of the human hand demonstrated at 4 Tesla. NeuroImage. 1999a;10:55–62. doi: 10.1006/nimg.1999.0448. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Pincus D, Detre JA, Alsop DC. Mapping of secondary somatosensory cortex activation induced by vibrational stimulation: an fMRI study. Brain Res. 1999b;824:291–295. doi: 10.1016/s0006-8993(99)01126-9. [DOI] [PubMed] [Google Scholar]
- Melzer P, Morgan VL, Pickens DR, Price RR, Wall RS, Ebner FF. Cortical activation during Braille reading is influenced by early visual experience in subjects with severe visual disability: A correlational fMRI study. Hum Brain Mapp. 2001;14:186–195. doi: 10.1002/hbm.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet L, Thut G, Murray B, Andrews J, Hsiao S, Pascual-Leone A. Feeling by sight or seeing by touch? Neuron. 2004;42:173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, McMains SA, Halko MA, Amedi A, Pascual-Leone A, Somers DC. Combined activation and deactivation of visual cortex during tactile sensory processing. J Neurophysiol. 2007;97:1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter -vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R. Cortical neuronal periodicities and frequency discrimination in the sense of flutter. Cold Spring Harb Symp Quant Biol. 1990a;55:861–872. doi: 10.1101/sqb.1990.055.01.081. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R. Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci. 1990b;10:3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI I. The method. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Hamilton R. The metamodal organization of the brain. Prog Brain Res. 2001;134:427–445. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Ricciardi E, Gobbini MI, Wu WH, Cohen L, Guazzelli M, Haxby JV. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci (USA) 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring L. The 'reverse-generation' effect: A comparison of memory performance between blind and sighted children. Br J Psychol. 1988;79:387. doi: 10.1111/j.2044-8295.1988.tb02297.x. [DOI] [PubMed] [Google Scholar]
- Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- Ptito M, Fumal A, De Noordhout AM, Schoenen J, Gjedde A, Kupers R. TMS of the occipital cortex induces tactile sensations in the fingers of blind Braille readers. Exp Brain Res. 2008;184:193–200. doi: 10.1007/s00221-007-1091-0. [DOI] [PubMed] [Google Scholar]
- Race EA, Shanker S, Wagner AD. Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. J Cogn Neurosci. 2009;21:1766–1781. doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race EA, Badre D, Wagner AD. Multiple forms of learning yield temporally distinct electrophysiological repetition effects. Cereb Cortex. 2010;20:1726–1738. doi: 10.1093/cercor/bhp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Raz N, Amedi A, Zohary E. V1 Activation in congenitally blind humans is associated with episodic retrieval. Cereb Cortex. 2005;15:1459–1468. doi: 10.1093/cercor/bhi026. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Hennighausen E, Näcker F. Event -related potentials during auditory and somatosensory discrimination in sighted and blind human subjects. Cognit. Brain Res. 1996;4:77–93. [PubMed] [Google Scholar]
- Röder B, Rösler F, Neville HJ. Auditory memory in congenitally blind adults: a behavioral- electrophysiological investigation. Cognit. Brain Res. 2001;11:289–303. doi: 10.1016/s0926-6410(01)00002-7. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F. Compensatory plasticity as a consequence of sensory loss. In: Calbert G, Spence C, Stein BE, editors. Handbook of Multisensory Processing. Vol. Cambridge, MA: The MIT Press; 2004. pp. 719–747. [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Deiber MP, Ibanez V, Hallett M. Neural networks for Braille reading by the blind. Brain. 1998;121:1213–1229. doi: 10.1093/brain/121.7.1213. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Kubota K, Yonekura Y. Tactile discrimination activates the visual cortex of the recently blind naive to Braille: a functional magnetic resonance imaging study in humans. Neurosci Letts. 2004;359:49–52. doi: 10.1016/j.neulet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Saggar M, Miikkulainen R, Schnyer DM. Behavioral, neuroimaging, and computational evidence for perceptual caching in repetition priming. Brain Res. 2010;1315:75–91. doi: 10.1016/j.brainres.2009.11.074. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Chang C, Menon V. Neural basis of repetition priming during mathematical cognition: Repetition suppression or repetition enhancement? J Cogn Neurosci. 2010;22:790–805. doi: 10.1162/jocn.2009.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K. Visual cortical activity during tactile perception in the sighted and the visually deprived. Dev Psychobiol. 2005;46:279–286. doi: 10.1002/dev.20056. [DOI] [PubMed] [Google Scholar]
- Sathian K, Stilla R. Cross-modal plasticity of tactile perception in blindness. Restor Neurol Neuros. 2010;28:271–281. doi: 10.3233/RNN-2010-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr. Opin. Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Dixit S, Burton H. Recognition memory for vibrotactile rhythms: An fMRI study in blind and sighted individuals. Somatosen & Mot Res. 2011 doi: 10.3109/08990220.2011.602765. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. Vol. New York: Thieme Medical; 1988. [Google Scholar]
- Uhl F, Franzen P, Lindinger G, Lang W, Deecke L. On the functionality of the visually deprived occipital cortex in early blind persons. Neurosci Letts. 1991;124:256–259. doi: 10.1016/0304-3940(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci (USA) 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]